Abstract
The current study examined between- and within-subject variability in pain-related symptoms as predictors of pain and fatigue, and identified patient subgroups based upon symptom variability characteristics. Two hundred and fifty-six fibromyalgia (FM) patients completed daily diaries up to a period of 154 days and reported on symptoms of pain intensity, pain unpleasantness, fatigue, anxiety, and depressed mood. Measures of health status, quality of life, and somatic symptoms were obtained at baseline, and hierarchical linear modeling and cluster analyses were employed. Significant intra- and inter-individual variability in daily FM symptoms was observed. Higher levels of pain were associated with greater fluctuations in pain unpleasantness, fatigue, and depressed mood. Similar effects were observed for fatigue and individual variability in anxiety also emerged as a robust predictor. Three FM subgroups were revealed: low variability in symptoms (Cluster 1), high symptom variability (Cluster 2), and a mixed variability group characterized by low fluctuation in pain unpleasantness; moderate pain, fatigue, and depressed mood variability; and high anxiety variability (Cluster 3). Cluster 3 exhibited lower social functioning and higher levels of pain, compared to Cluster 1. These findings support the dynamic nature of FM pain and suggest the presence of FM subgroups based upon variation in mood and pain symptomatology.
Keywords: fibromyalgia, variability, pain, fatigue, mood, anxiety, depression
Introduction
Chronic pain does not follow a static course, but rather is associated with fluctuations in symptoms that can vary within and across days.1, 3, 21, 23, 30 This variable nature of pain can make treatment more challenging and reduce one’s ability to capitalize on adaptive coping resources. Although variation in symptoms is a normal part of chronic pain, most studies have relied on patient recall or self-report of pain at a single time point which may not fully capture the dynamics of the pain experience. Thus, momentary-based assessments examining pain symptoms may be more sensitive to the changes in pain that naturally occur over the course of time.9, 12, 20
Previous studies have identified a number of factors associated with greater variability in pain including depression,35, 48 poor health,48 lower quality of life,39 and higher daily pain intensity.48 While negative pain-related factors (e.g., poor sleep quality, negative affect) may give rise to daily changes in pain, it is also plausible that these effects are bidirectional. Indeed, the stress associated with disease unpredictability may have an adverse impact on psychological and physical functioning and ultimately heighten levels of pain and concomitant symptoms. Supporting this, symptomatic variability in multiple sclerosis has been identified as a contributor to greater levels of depression and fatigue,33 while day-to-day changes in rheumatoid arthritis symptoms are known to predict self-reported interpersonal functioning.7 These effects also extend to adaptive functioning, as Zautra and colleagues found that within-person increases in positive affect contributed to lower daily fatigue in fibromyalgia (FM).49 While these findings highlight the association between symptom variability and health outcomes, there has been limited investigation on the influence of these fluctuations. This represents an important area of inquiry as distinguishing the clinical course of pain and disease-associated symptoms may be a step toward optimizing pain management.
Equally underexplored is whether phenotypic patterns of variability exist across pain symptomatology. To our knowledge, only one study has addressed this2 by characterizing two groups of osteoarthritis patients as high versus low pain variability (measured over a period of 30 days). Although individuals in the high pain variability group exhibited greater levels of pain intensity and employed more emotion-focused rather than problem-focused coping strategies, the opposite was true for the low pain variability group. These findings suggest that clinical symptoms may differ across characteristics of variability, and would align with other studies reflecting distinct subgroups based upon pain symptom presentation.8, 13, 24, 25 However, it is important to note that the authors used a median split to identify their subgroups rather than relying on empirical-driven methods (e.g., cluster analysis) to capture group patterns of variability.
Using a longitudinal, daily diary approach, the objective of the current study was to assess day-to-day variability in pain-related symptoms (i.e., pain intensity, pain unpleasantness, fatigue, depressed mood, anxiety). FM was selected as the target population as it represents a condition often affected by significant variation in pain and fatigue, as well as changes in behavioral and affective symptoms that coincide with symptom fluctuations.26, 49 In particular, we were interested in whether individuals with greater intra-individual fluctuation in symptoms (i.e., pain and unpleasantness, fatigue, mood), and more frequent symptoms on average, reported higher levels of pain and fatigue. We hypothesized that FM patients with greater daily symptom variability would exhibit higher levels of pain and fatigue. A secondary aim of this research was to examine cluster profiles across variability characteristics and identify associations with psychosocial factors.
Methods
Participants and Procedures
A total of 256 FM participants were recruited from the community through flyers, FM support groups, and the outpatient clinics of the University of Florida. All subjects attended a laboratory session where a thorough evaluation of eligibility was conducted. Participants who fulfilled the 1990 American College of Rheumatology (ACR) Criteria47 for FM (confirmed by a rheumatologist [RS]) were asked to complete a battery of psychosocial questionnaires. Subjects were excluded if they had any other significant medical illness besides FM. Chronic painless conditions like controlled hypertension and thyroid conditions were allowed. To assess the natural variability in pain and mood symptoms, subjects were tapered off all analgesic and psychotropic medications except for low dose anti-depressants (<10 mg/day) and muscle relaxers. Low dose trazodone (<15 mg/day) was allowed as a sleep aid. Procedures were approved by the University of Florida Institutional Review Board and all participants provided written informed consent.
Phone Call-In of Daily Ratings
Participants were asked to complete daily symptom ratings for at least 2 weeks and up to 90 days (or until they experienced a disease flare). A unique identification number was provided as well as verbal and written instructions to call a toll-free automated phone line (VoiceGuide, AU) in the morning and report their overall clinical pain intensity, pain unpleasantness, fatigue, depressed mood, and anxiety once daily. Using a phone pad, an automated voice-response system asked subjects to rate their current symptoms using five separate 0–100 numerical scales (NRS). Interpretation of these scales is as follows: Pain Intensity, 0=no pain, 100=most intense pain imaginable; Pain Unpleasantness, 0=no pain unpleasantness, 100=most intense pain unpleasantness imaginable; Fatigue, 0=no fatigue, 100=most intense fatigue imaginable; Depression (i.e., depressed mood), 0=no depression, 100=most intense depression imaginable; Anxiety, 0=no anxiety, 100=most intense anxiety imaginable. During the baseline assessment, participants were provided specific instructions on the interpretation of symptom variables.
Questionnaires
Short Form (36) Health Survey (SF-36)
The SF-3645 is a 36-item measure examining health status and quality of life across eight scaled scores: 1) Energy/Fatigue (4 items), 2) Physical Functioning (10 items), 3) Bodily Pain (2 items), 4) General Health Perceptions (5 items), 5) Physical Role Limitations (4 items), 6) Emotional Role Functioning (3 items), 7) Social Role Functioning (2 items), and 8) Mental Health (5 items). Only the physical functioning (e.g., “Does your health now limit you in walking more than a mile”), social role functioning (e.g., “To what extent has your physical health or emotional problems interfered with your normal social activities with family, friends, neighbors, or groups”), and physical role limitations (e.g., “Have you cut down the amount of time you spent on work or other activities as a result of your physical health”) scales were used for the current study. All scale items were linearly transformed to a 0 to 100 scale, with 100 indicating the highest level of functioning (higher quality of life). The SF-36 is a commonly used instrument and demonstrates good reliability and validity.5, 22
Pennebaker Inventory of Limbic Languidness (PILL)
The PILL29 is a 54-item inventory that examines the frequency of physical symptoms and sensations (e.g., racing heart, ringing in ears, coughing) associated with the tendency to exhibit hypervigilance to somatic stimuli. Respondents recorded the prevalence of each item on a 1 to 5 scale ranging from “have never or almost never experienced” to “more than once every week.” Higher scores indicate a greater frequency of somatic symptoms. The PILL demonstrates high internal consistency and test-retest reliability.29
Data Analysis
Hierarchical linear modeling (HLM) using full maximum likelihood estimation (MIXED procedure in SPSS 23.0) was conducted to examine inter- and intra-individual variability among daily ratings of pain, pain unpleasantness, fatigue, depressed mood, and anxiety. Subject ID was applied as the grouping variable to define Level 2 units. Given the hierarchical nature of daily diary data (i.e., daily observations nested within each participant), HLM is a suitable analytic method as it does not require observations to be independent, ensures missing cases are not excluded, and can correctly model variability between and within individuals using more complex error structures.11, 16, 18 The heterogeneous compound symmetric error covariance matrix was specified as the final model as it had the best fit according to Akaike Information Criterion (AIC). Participants were included in the analysis if they contributed at minimum seven days of symptom ratings, and were excluded if more than 15 days lapsed between symptom monitoring. Daily diary observations from FM participants were analyzed with up to 154 time-points nested within each subject.
Following the conventions of HLM outlined by Singer and Willett as well as Heck and colleagues,16, 36 a series of steps were conducted for modeling procedures. Initially, unconditional models (i.e., null model) with no predictors were estimated to examine between- and within-subject variation in daily FM symptoms (i.e., pain intensity, pain unpleasantness, fatigue, depressed mood, anxiety). Then, separate unconditional growth models were assessed using time as the Level 1 predictor and FM symptoms entered as the dependent variables to examine baseline levels of change over time existing across each outcome. Next, time-varying predictors were group-mean centered for Level 2 variables (each person’s average score of the variable across time) and person-mean centered for Level 1 variables (subtracting each individual’s average score for a variable from the daily rating) to disaggregate the between- and within-subject effects. Thus, Level 2 predictors represented each person’s mean score on a variable, while Level 1 predictors denoted each individual’s daily deviation from their average score on that variable. Afterwards, separate multilevel models were conducted to examine FM symptoms as mean- and within-level predictors of pain intensity and fatigue. Cross-level interaction terms were added to assess whether the effect of time on each outcome (pain intensity, fatigue) differed by the levels of another predictor (using the centered value). Both unadjusted and adjusted (controlling for the effects of the other predictors) models were analyzed for comparison. In the unadjusted models, mean-level predictors were entered separately, while centered predictors were added individually both as a fixed effect and as a random effect.
Hierarchical cluster analysis employing Ward’s clustering method with squared Euclidean distances as the similarity measures was conducted to identify subgroups of individuals that differed across measures of variability. In this analysis, the within-subject variance (i.e., SD’s of pain intensity, pain unpleasantness, fatigue, depressed mood, anxiety) across time was calculated separately for each participant to capture fluctuations about the mean. Agglomeration coefficients were examined to identify the cluster solution that best represented the data, with the optimal number being chosen based upon the point at which the percentage change was the largest between the clusters.27 To ensure the generalizability of the cluster assignment, a cross-validation was conducted by creating a sub-sample of the dataset through a random splitting method (50% of cases), and comparing the cluster solution (i.e., testing dataset) for consistency with the original sample (no differences were found in the cluster solution across the two samples). Multivariate ANOVA’s were then conducted to examine cluster group differences in mean pain intensity and psychosocial characteristics (SF-36 physical functioning, SF-36 social functioning, SF-36 physical role limitations, PILL), controlling for the effects of sex. To obtain effect size estimates associated with F-tests, partial eta-squared (ηp2) was calculated (small=.01, medium=.06, large=.14). Significance was set at p<.05 for all analyses.
Results
Participant characteristics
Table 1 presents demographic and clinical data for the sample. A total of 323 subjects participated in the study; however, 65 were excluded due to incomplete data (i.e., <7 days of monitoring or >15 lapsed days) and two participants were omitted due to having an extreme number of data points (i.e., 223 days of symptom reporting). Therefore, analyses were based upon the final sample of 256 participants. Subjects were mainly female, white/Caucasian, not married, and unemployed. The average age of the sample was 48.5 years and mean educational attainment was 14.1 years. Mean symptom levels at the baseline visit were as follows: pain intensity (M=57.1, SD=19.9), pain unpleasantness (M=56.2, SD=21.8), fatigue (M=59.9, SD=24.1), depressed mood (M=30.0, SD=25.3), anxiety (M=31.9, SD=26.9), SF-36 physical functioning (M=39.2, SD=23.7), SF-36 social role functioning (M=45.1, SD=25.2), SF-36 physical role limitations (M=14.4, SD=28.9), and the PILL (M=91.6, SD=33.5). Table 2 lists the mean symptom levels throughout the duration of the study. The average number of days for symptom rating was 18.8 (Mode=14.0; Range=7.0 to 154.0 days). Participants completed 4,820 days out of 5,586 possible data points, a completion rate of 84.1%. Descriptive statistics and analysis of variance did not reveal any significant differences in the degree of missingness across demographic/clinical characteristics or cluster group membership.
Table 1.
Participant Demographic and Clinical Characteristics
| Total Sample N=256 |
Cluster 1 N=115 |
Cluster 2 N=48 |
Cluster 3 N=93 |
Group Comparison |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Low Variability Group |
High Variability Group |
Mixed Variability Group |
|||||||
|
| |||||||||
| M or N | SD or % | M or N | SD or % | M or N | SD or % | M or N | SD or % | p-value | |
| Age (in yrs) | 48.5 | 12.8 | 46.6 | 12.7 | 46.8 | 12.6 | 50.2 | 11.5 | .22 |
| Sex | .03 | ||||||||
| Female | 239 | 93.7 | 103 | 89.6 | 48 | 100.0 | 88 | 95.7 | |
| Male | 16 | 6.3 | 12 | 10.4 | 0 | 0.0 | 4 | 4.3 | |
| Race | .98 | ||||||||
| White | 223 | 87.1 | 102 | 89.5 | 40 | 88.9 | 81 | 90.0 | |
| Non-White | 26 | 10.2 | 12 | 10.5 | 5 | 11.1 | 9 | 10.0 | |
| Marital Status | .90 | ||||||||
| Married | 118 | 46.1 | 55 | 49.1 | 21 | 45.7 | 42 | 46.7 | |
| Not Married | 130 | 50.8 | 57 | 50.9 | 25 | 54.3 | 48 | 52.4 | |
| Employment | .35 | ||||||||
| Employed | 107 | 41.8 | 52 | 50.5 | 22 | 56.4 | 33 | 42.9 | |
| Not Employed | 112 | 43.8 | 51 | 49.5 | 17 | 43.6 | 44 | 57.1 | |
| Education (in yrs) | 14.1 | 2.0 | 14.2 | 2.0 | 14.2 | 2.0 | 13.9 | 2.0 | .66 |
| Total Pain Duration (in yrs) | 18.5 | 10.2 | 17.3 | 10.7 | 17.7 | 9.6 | 19.5 | 10.5 | .45 |
Table 2.
Estimates for between and within-subject variance in FM symptoms
| Between-Person | Within-Person | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Estimate | % | SE | Z | Estimate | % | SE | Z | M | SD | |
| Pain Intensity | 361.5 | 66.7 | 33.1 | 10.9 | 179.9 | 33.3 | 3.8 | 47.8 | 54.1 | 23.4 |
| Pain Unpleasantness | 401.9 | 64.6 | 36.8 | 10.9 | 218.7 | 35.4 | 4.6 | 47.8 | 53.6 | 25.1 |
| Fatigue | 441.6 | 65.4 | 40.5 | 10.9 | 233.4 | 34.6 | 4.9 | 47.6 | 54.7 | 25.7 |
| Depressed Mood | 575.2 | 77.5 | 51.9 | 11.1 | 166.9 | 22.5 | 3.5 | 47.7 | 29.3 | 27.3 |
| Anxiety | 575.9 | 75.9 | 52.3 | 11.1 | 183.2 | 24.1 | 4.0 | 45.4 | 29.9 | 27.3 |
Individual differences in between- and within-subject variability
Multilevel null models were estimated to examine variances between and within persons for daily ratings of pain intensity, pain unpleasantness, fatigue, depressed mood, and anxiety (Table 2). Each of the outcome variables showed pronounced variation both between and within individuals; thus, there was adequate variability at each level to conduct a multilevel analysis. While most of the variance in FM symptoms was due to the differences between persons in their average levels (Range 64–77%), a statistically significant amount of variance was also explained by day-to-day variation (Range 23–35%) in scores.
Linear growth in outcomes
Next, baseline multilevel models were expanded by entering time as a predictor of each FM symptom to determine whether there was systematic variation over time in each outcome. As seen in Table 3, linear decreases in pain intensity (β= −.17, p=.005) and fatigue (β= −.17, p=.01) were observed, while the decline in pain unpleasantness approached significance (β= −.10, p=.07). There were no significant changes over time in depressed mood (β= −.07, p=.21) and anxiety (β= −.05, p=.47). Based on covariance parameter estimates, there was still significant variance in intercepts and slopes to be explained across individuals for all FM symptoms (all p’s<.001).
Table 3.
Unconditional multilevel models predicting FM symptoms from time
| Pain | Unpleasantness | Fatigue | Depressed Mood |
Anxiety | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| β | SE | β | SE | β | SE | β | SE | β | SE | |
| Fixed Effects | ||||||||||
| Time | −.17 | .06 | −.10 | .06 | −.17 | .07 | −.07 | .05 | −.05 | .06 |
| Random Effects | ||||||||||
| Residual | 159.97 | 3.50 | 199.58 | 4.37 | 204.91 | 4.47 | 147.40 | 3.19 | 156.16 | 3.58 |
| Intercept Variance | 365.19 | 34.94 | 410.53 | 39.49 | 470.70 | 45.28 | 570.48 | 52.28 | 627.07 | 59.43 |
| Slope Variance | .43 | .09 | .34 | .09 | .54 | .11 | .36 | .07 | .62 | .11 |
Predictors of pain and fatigue variability
Tables 4 and 5 present results for unadjusted models, as well as final multilevel models in which each predictor was entered simultaneously (covarying for the effects of other predictors). When entered separately, all mean-level predictors (i.e., pain unpleasantness, fatigue, depressed mood, anxiety) were associated with daily pain intensity (p’s<.001); however, depressed mood was no longer significant in the full model. At the within-person level, symptom increases (across all predictors) were associated with higher levels of pain intensity, but anxiety was no longer a significant predictor after adjusting for all variables (p=.96). There were significant interactions in the unadjusted analyses, signifying that a 1 SD decrease in within-subject variation in both unpleasantness and depressed mood was associated with a .09% and .15% increase in pain intensity over time, respectively.
Table 4.
Multilevel models predicting daily pain intensity from mean and within-person variation in symptoms
| Unadjusted Analysis | Adjusted Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| β | SE | df | t | p | β | SE | df | t | p | ||
| Fixed Effects | |||||||||||
| Level 2 (Between-Subjects) | |||||||||||
| Mn Pain Unpleasantness | .90 | .02 | 245 | 45.95 | <.001 | .87 | .03 | 255 | 32.71 | <.001 | |
| Mn Fatigue | .60 | .04 | 256 | 14.12 | <.001 | .08 | .03 | 257 | 2.86 | <.01 | |
| Mn Depressed Mood | .38 | .04 | 256 | 8.58 | <.001 | .02 | .03 | 277 | .62 | .54 | |
| Mn Anxiety | .33 | .05 | 256 | 7.34 | <.001 | −.06 | .03 | 278 | −2.15 | .03 | |
| Level 1 (Within-Subjects) | |||||||||||
| Time | −.16 | .06 | 109 | −2.86 | <.01 | −.06 | .02 | 44 | −2.83 | <.01 | |
| C_Pain Unpleasantness | .77 | .01 | 214 | 53.40 | <.001 | .73 | .02 | 236 | 47.69 | <.001 | |
| C_Fatigue | .39 | .02 | 215 | 18.00 | <.001 | .05 | .01 | 201 | 4.06 | <.001 | |
| C_Depressed Mood | .40 | .03 | 151 | 14.39 | <.001 | .05 | .01 | 96 | 4.53 | <.001 | |
| C_Anxiety | .27 | .03 | 159 | 9.89 | <.001 | .00 | .01 | 87 | .05 | .96 | |
| Time × C_Pain Unpleasantness | −.00 | .00 | 3856 | −3.12 | <.01 | −.00 | .00 | 3411 | −1.57 | .12 | |
| Time × C_Fatigue | −.00 | .00 | 3492 | −1.34 | .18 | −.00 | .00 | 2574 | −.72 | .48 | |
| Time × C_Depressed Mood | −.00 | .00 | 2186 | −2.20 | .03 | −.00 | .00 | 156 | −.70 | .49 | |
| Time × C_Anxiety | −.00 | .00 | 1205 | −1.29 | .20 | .00 | .00 | 42 | .98 | .33 | |
| β | SE | Z | p | β | SE | Z | p | ||||
|
|
|
||||||||||
| Random Effects | |||||||||||
| Residual | 37.95 | .95 | 40.06 | <.001 | |||||||
| Intercept Variance | 372.08 | 33.34 | 11.16 | <.001 | |||||||
| Slope Variance | .03 | .01 | 2.31 | .02 | |||||||
| C_Pain Unpleasantness | .03 | .00 | 6.68 | <.001 | .03 | .00 | 6.39 | <.001 | |||
| C_Fatigue | .06 | .01 | 6.44 | <.001 | .02 | .00 | 4.84 | <.001 | |||
| C_Depressed Mood | .09 | .02 | 5.39 | <.001 | .00 | .00 | 1.49 | .14 | |||
| C_Anxiety | .08 | .02 | 5.45 | <.001 | .00 | .00 | .61 | .54 | |||
Note: Mn=Mean; C=Centered.
Table 5.
Multilevel models predicting daily fatigue from mean and within-person variability in symptoms
| Unadjusted Analysis | Adjusted Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| β | SE | df | t | p | β | SE | df | t | p | ||
| Fixed Effects | |||||||||||
| Level 2 (Between-Subjects) | |||||||||||
| Mn Pain Intensity | .73 | .05 | 254 | 14.13 | <.001 | .41 | .14 | 256 | 2.86 | <.01 | |
| Mn Pain Unpleasantness | .70 | .05 | 254 | 14.33 | <.001 | .16 | .14 | 257 | 1.12 | .26 | |
| Mn Depressed Mood | .49 | .05 | 255 | 10.76 | <.001 | .08 | .07 | 264 | 1.26 | .21 | |
| Mn Anxiety | .50 | .05 | 256 | 10.92 | <.001 | .23 | .06 | 263 | 3.62 | <.001 | |
| Level 1 (Within-Subjects) | |||||||||||
| Time | −.16 | .06 | 126 | −2.53 | .01 | −.05 | .04 | 52 | −1.37 | .18 | |
| C_Pain Intensity | .48 | .02 | 198 | 19.12 | <.001 | .13 | .03 | 479 | 4.05 | <.001 | |
| C_Pain Unpleasantness | .47 | .02 | 185 | 19.44 | <.001 | .28 | .03 | 374 | 9.47 | <.001 | |
| C_Depressed Mood | .40 | .03 | 177 | 12.48 | <.001 | .16 | .03 | 197 | 5.20 | <.001 | |
| C_Anxiety | .33 | .03 | 174 | 10.48 | <.001 | .12 | .03 | 176 | 4.85 | <.001 | |
| Time × C_Pain Intensity | .00 | .00 | 2291 | 1.15 | .25 | .00 | .00 | 1212 | .77 | .44 | |
| Time × C_Pain Unpleasantness | .00 | .00 | 2540 | .79 | .43 | .00 | .00 | 1043 | 1.18 | .24 | |
| Time × C_Depressed Mood | −.00 | .00 | 2853 | −1.57 | .12 | −.00 | .00 | 1346 | −1.21 | .23 | |
| Time × C_Anxiety | −.00 | .00 | 1870 | −.44 | .66 | .00 | .00 | 1045 | .19 | .85 | |
| β | SE | Z | p | β | SE | Z | p | ||||
|
|
|
||||||||||
| Random Effects | |||||||||||
| Residual | 132.36 | 3.26 | 40.60 | <.001 | |||||||
| Intercept Variance | 454.82 | 41.65 | 10.92 | <.001 | |||||||
| Slope Variance | .09 | .04 | 2.49 | .01 | |||||||
| C_Pain Intensity | .07 | .01 | 5.33 | <.001 | .03 | .01 | 2.62 | <.01 | |||
| C_Pain Unpleasantness | .07 | .01 | 5.58 | <.001 | .03 | .01 | 2.91 | <.01 | |||
| C_Depressed Mood | .13 | .02 | 5.91 | <.001 | .07 | .02 | 4.84 | <.001 | |||
| C_Anxiety | .12 | .02 | 5.89 | <.001 | .05 | .01 | 4.63 | <.001 | |||
Note: Mn=Mean; C=Centered.
As observed with pain intensity, all mean-level predictors were associated with daily fatigue (all p’s<.001) in the unadjusted analyses, signifying that individuals with higher symptoms, on average, displayed greater levels of fatigue. When covarying the effects of the other variables, mean pain intensity (p<.01) and anxiety (p<.001) were the only significant predictors of fatigue. The level 1 (within-person) results demonstrate significant associations between all predictors with daily fatigue and these effects remained significant in the full model (p’s<.001). The cross-level interactions in both the unadjusted and adjusted analyses were all non-significant for fatigue.
Variability Cluster Analysis
All measures of variability (i.e., SD units of pain intensity, pain unpleasantness, depressed mood, anxiety, and fatigue) were subjected to Cluster Analysis to identify empirically-derived classifications based upon variability profiles (Table 6). Three clusters were revealed and characterized by the following: 1) Cluster 1: Low Variability (N=115; 44.9%): low degree of variability among pain intensity, pain unpleasantness, fatigue, and affect; 2) Cluster 2: High Variability (N=48; 18.8%): high degree of variability among pain intensity, pain unpleasantness, fatigue, and affect; and 3) Cluster 3: Mixed Variability (N=93; 36.3%): low degree of variability in pain unpleasantness; moderate variability in pain intensity, fatigue, and depressed mood; and high variability in anxiety.
Table 6.
Descriptive statistics for variability outcomes across cluster profiles
| Cluster 1 N=115 |
Cluster 2 N=48 |
Cluster 3 N=93 |
Inferential Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| Low Variability Group |
High Variability Group |
Mixed Variability Group |
||||||||
| M | SD | M | SD | M | SD | F | df |
p- value |
ηp2 | |
| Pain Intensity Variability | 10.48a | 4.19 | 19.30b | 3.72 | 11.72c | 3.42 | 93.03 | 2,253 | <.001 | .42 |
| Pain Unpleasantness Variability | 11.68a | 3.80 | 21.05b | 4.38 | 12.16a | 3.59 | 78.29 | 2,253 | <.001 | .38 |
| Fatigue Variability | 11.76a | 5.61 | 20.31b | 3.94 | 13.65c | 5.09 | 47.25 | 2,253 | <.001 | .27 |
| Depressed Mood Variability | 5.12a | 3.44 | 18.26b | 5.02 | 14.45c | 5.58 | 178.56 | 2,253 | <.001 | .59 |
| Anxiety Variability | 6.69a | 5.63 | 15.70b | 6.72 | 16.51b | 6.95 | 72.00 | 2,253 | <.001 | .36 |
Note: Means in the same row that do not share a superscript are significantly different at p < .05.
Psychosocial Profiles across Cluster Assignment
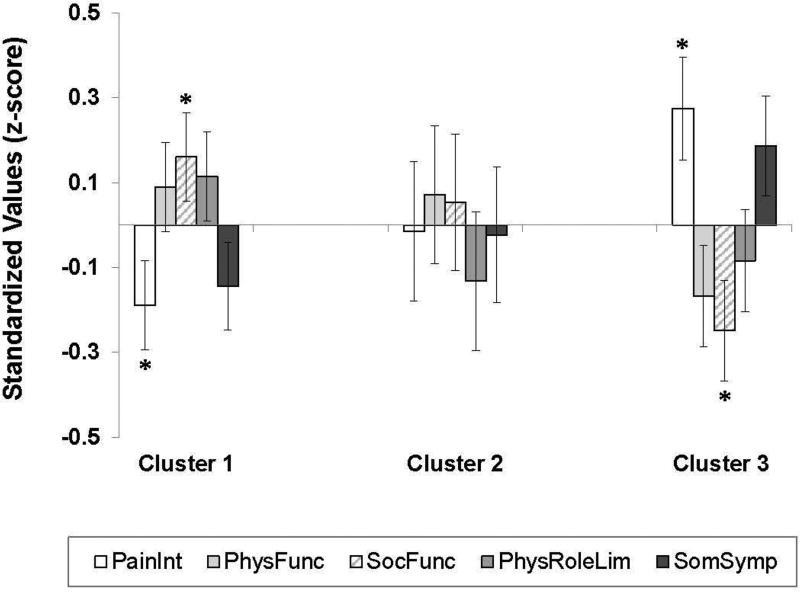
After controlling for sex, significant differences across cluster membership emerged in pain (F [2,195]=4.20, p=.02, ηp2=.04) and social functioning (F [2,195]=3.44, p=.03, ηp2=.03) (Figure 1). Specifically, participants in Cluster 3 (Mixed Variability) reported the highest degree of pain intensity (p<.01) and lower social functioning (p=.01), relative to Cluster 1 (Low Variability). No differences in physical functioning (p=.24), physical role limitations (p=.32), or somatic symptoms (p=.11) were detected across clusters.
Figure 1. Symptom profiles across psychosocial clusters.
Relative to Cluster 1, pain intensity was significantly higher and social functioning was lower in Cluster 3. There were no group differences in physical functioning, physical role limitations, or somatic symptoms. Note: PainInt=Pain Intensity, PhysFunc=Physical Functioning, SocFunc=Social Functioning, PhysRoleLim=Physical Role Limitations, SomSymp=Somatic Symptoms.
Discussion
Traditionally, methods to understand pain and associated symptoms in FM have focused on capturing average or current levels in patients; however, it is known that these experiences are highly dynamic15 and are characterized by frequent highs and lows in symptomatology.9, 20, 41, 44, 49 The purpose of this study was to: 1) identify predictors of pain and fatigue in FM using a longitudinal, ecological momentary approach, and 2) characterize patient subgroups based upon variation in pain and affective symptomatology.
There was pronounced variability in FM symptoms, with up to 77% of the variation in symptomatology occurring across individuals, while a smaller, yet significant portion of the variance was attributed to intra-individual fluctuation in symptoms. We also found that levels of pain intensity, fatigue, and to a lesser extent, pain unpleasantness decreased over time, while depressed mood and anxiety demonstrated greater long-term stability. Such findings provide evidence regarding the temporal trajectory of pain-related symptoms and suggest that factors other than negative emotions may contribute to longitudinal changes in pain and fatigue.
After controlling for other predictors in the model, FM patients with higher mean levels of pain unpleasantness, fatigue, and anxiety reported greater pain intensity. Further, individuals with greater day-to-day variability in fatigue and depressed mood exhibited higher levels of pain. These effects were less robust than those found for pain unpleasantness (as reflected by the beta weights), and ultimately could signify a natural correlation between these two variables as participant’s mean pain unpleasantness and intensity ratings were highly correlated (r=.91). A similar pattern for fatigue was also found, with the exception that patients with greater intra-individual variation in anxiety also reported higher fatigue. This suggests that anxiety may be a stronger predictor of fatigue than pain intensity, and could highlight the role that negative affect has on fatigue as reflected by the size of the effects observed for anxiety and depressed mood. Consistent with these results, Graff and colleagues14 found that psychological distress and lower well-being predicted greater fatigue over a two-year period. Likewise, both fatigue and negative mood (i.e., frustration) predicted within-day increases in one another in a lagged analysis by Hegerty et al.17 In light of the current findings, the inability to flexibly modulate daily mood may be physically taxing and interrupt the ability to self-regulate energy levels. Ultimately, these effects may have a detrimental impact on physical functioning (i.e., fatigue) in FM.
We also found evidence of three distinct subgroups centered on patterns of variability in pain intensity, mood (i.e., anxiety, depressed mood), and fatigue. While our study is distinct in that variability was the primary outcome, these findings are generally consistent with existing research as prior attempts of classification have identified subgroups based upon a number of factors including psychological and physical functioning,8, 10, 13, 24, 25, 31, 34, 43, 46 coping,13, 25, 40, 42 somatic symptomatology,8, 10, 24, 25, 43 medical health comorbidities,10 somatosensory functioning/symptoms,13, 19, 34 biomedical markers,24 and childhood trauma.24 The current findings support the notion that FM subgroups are determined at least, in part, by variability in disease-associated symptoms.
Further investigation revealed that the clusters had distinct characteristics based upon pain intensity and social functioning. For instance, participants with low overall symptom fluctuation (Cluster 1) had lower levels of pain and higher psychosocial functioning. This could signify a form of adaptive functioning with patients having relative stability in their disease symptoms despite current FM diagnosis. Conversely, patients in Cluster 3, characterized by lower variation in pain but high mood variability (namely, anxiety), exhibited poorer psychosocial outcomes. Notably, this group also had the highest degree of pain intensity, and upon additional analysis, also demonstrated higher levels of depressed mood, anxiety, and fatigue. While the specific direction of these effects is unclear, it is tempting to suggest that for this group, the inability to regulate emotions in conjunction with the profile of high symptom scores could add to the disease burden of FM and adversely impact psychological and social functioning.
Symptom variability in chronic pain conditions has long been underappreciated as studies have generally focused on mean values at a single time-point. However, attending to the static features of pain may obscure actual patterns of functioning that exist in the pain experience. Instead, capturing day-to-day dynamic shifts in symptom reporting may promote increased understanding of FM and other pain conditions. While daily diary strategies come with their own limitation of increasing participant burden, such approaches have the added value of highlighting processes that shape the course of chronic pain and provide a rich platform for enhancing clinical care. Indeed, Harris and colleagues15 observed greater responsiveness to analgesics and placebo in individuals characterized by greater pain variation. Thus, identifying variability profiles among patients with ongoing pain may have broad implications for pharmacological intervention and could assist in the identification of treatment responders. Likewise, pinpointing modifiable processes that account for symptom variability and working toward strategies that promote stability in these factors may prove beneficial for reducing disease burden.
Also notable is the demarcation observed for both pain intensity and fatigue suggesting that they are, in fact, distinct entities. While fatigue has been conceptualized as a consequence of pain32 and commonly examined as a secondary outcome, more recent research has revealed that pain and fatigue are independent symptoms6 that uniquely predict pain outcomes.4 As noted in our study, they also may be explainable by discrete predictors given that variability in pain unpleasantness was a more robust predictor of pain intensity, while day-to-day fluctuations in negative affect were more closely associated with fatigue. Gaining a better understanding of the mechanisms that drive the dynamics of daily pain and fatigue may facilitate more directed therapies that target the regulation of FM-related symptoms (e.g., affect). Moreover, given the clear emergence of subgroups based upon temporal patterns of variability, our findings encourage additional examination of day-to-day shifts in FM symptoms. Understanding how fluctuations in symptoms are associated with coping and other psychosocial processes may yield important information that can drive clinical treatments for individuals with chronic pain.
In the context of our findings, a few limitations are worth noting. First, some of the effect sizes for our pain symptoms were relatively modest, and symptoms were measured during the morning which may have limited the ability to observe fluctuations across other time-points. For instance, a number of studies have noted variability in pain symptoms within the day and even on the weekends.1, 3, 17, 37 Second, differences in psychosocial characteristics across our clusters were only compared across two measures (i.e., SF-36 and PILL); therefore, it is possible that other factors not assessed could differ across patterns of variability. Third, a small degree of demographic data was missing. To address this issue, analyses were conducted comparing participants with complete demographic data with individuals who had missing data and results were analogous to those reported. Thus, we have confidence that this did not significantly impact our findings. Fourth, given the variability in symptom reporting, it is possible that participants with greater symptomatology were less compliant with daily reporting, thus creating a potential selection bias. Moreover, we did not collect data on whether patients were involved in ongoing non-pharmacological treatment for their pain, an effect which could have altered pain and mood symptoms. Further research assessing changes in positive affect may also provide some insight into the presentation of pain and fatigue, especially given the adaptive benefits of positive coping.38, 50 Supporting this, Zautra and colleagues49 found that lower levels of fatigue were noted on days with greater positive affect, while daily variations in positive mood were associated with lower fatigue. Additionally, Ong et al28 observed that increases in daily positive emotions predicted decreases in pain catastrophizing, an outcome which was observed in individuals with higher psychological resilience. These limitations notwithstanding, the current study builds upon existing research supporting the fluctuating nature of FM symptoms,26, 49 and highlights the feasibility of ecological momentary assessment to capture daily manifestations of pain symptomatology. Additionally, daily pain and concomitant symptoms were captured in a large FM sample over a prolonged period of time. The findings provide evidence on the heterogeneity of FM, and is the first, to our knowledge, to identify subgroups of patients based upon characteristics of symptom variability.
Taken together, our findings suggest that targeting symptom variability may be an important goal for treatment. For instance, patients in Cluster 3 may benefit from both pharmacological and psychotherapy interventions aimed at reducing overall levels of pain and related symptoms, but also targeting the regulation of mood. For Cluster 1 patients who appear to be relatively well-adjusted despite having symptoms of FM, psychological treatment may be less warranted. Future research may benefit from examining the effectiveness of interventions based upon phenotypic characteristics of symptom variability, and increasing understanding of patient controllability of both pain and mood symptoms.
Highlights.
Fibromyalgia patients experience daily variability in pain, fatigue, and mood.
Differences in pain and social functioning emerged across variability clusters.
Targeting symptom variability may be an important clinical initiative.
Perspective.
Fibromyalgia patients display significant intra- and inter-individual variability in pain, mood, and fatigue. Subgroups in mood and pain-related variability emerged, with phenotypic clusters differing across levels of pain intensity and social functioning. Better understanding of the processes impacting pain variability may facilitate targeted treatments for the control of pain.
Acknowledgments
This work was funded by the National Institute of Nursing Research (R01NR014049) awarded to Dr. Roland Staud. Preparation of the article was supported by the National Institutes of Health/National Institute on Aging (K99AG052642) awarded to Dr. Emily Bartley. The authors would like to thank Marlin Mejia and Taylor Kizer for their valuable contributions to this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no conflicts of interest.
References
- 1.Allen KD, Coffman CJ, Golightly YM, Stechuchak KM, Keefe FJ. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis and Cartilage. 2009;17:1275–1282. doi: 10.1016/j.joca.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Allen KD, Golightly YM, Olsen MK. Pilot study of pain and coping among patients with osteoarthritis: a daily diary analysis. Journal of Clinical Rheumatology. 2006;12:118–123. doi: 10.1097/01.rhu.0000221801.63880.3f. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy N, Sothern RB, Campbell J. Rhythmic variations in pain perception in osteoarthritis of the knee. The Journal of Rheumatology. 1990;17:364–372. [PubMed] [Google Scholar]
- 4.Boggero IA, Rojas-Ramirez MV, Carlson CR. All fatigue is not created equal: the association of fatigue and its subtypes on pain interference in orofacial pain. The Clinical Journal of Pain. 2016;33:231–237. doi: 10.1097/AJP.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brazier JE, Harper R, Jones N, O'cathain A, Thomas K, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie A, Dagfinrud H, Mowinckel P, Hagen KB. Variation in fatigue may be poorly explained by pain: results from a longitudinal, exploratory study. Rheumatology International. 2016;36:279–282. doi: 10.1007/s00296-015-3357-3. [DOI] [PubMed] [Google Scholar]
- 7.Davis MC, Affleck G, Zautra AJ, Tennen H. Daily interpersonal events in pain patients: applying action theory to chronic illness. Journal of Clinical Psychology. 2006;62:1097–1113. doi: 10.1002/jclp.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza JB, Goffaux P, Julien N, Potvin S, Charest J, Marchand S. Fibromyalgia subgroups: profiling distinct subgroups using the Fibromyalgia Impact Questionnaire. A preliminary study. Rheumatology International. 2009;29:509–515. doi: 10.1007/s00296-008-0722-5. [DOI] [PubMed] [Google Scholar]
- 9.de Wit R, van Dam F, Hanneman M, Zandbelt L, van Buuren A, van der Heijden K, Leenhouts G, Loonstra S, Abu-Saad HH. Evaluation of the use of a pain diary in chronic cancer pain patients at home. Pain. 1999;79:89–99. doi: 10.1016/S0304-3959(98)00158-4. [DOI] [PubMed] [Google Scholar]
- 10.Docampo E, Collado A, Escaramis G, Carbonell J, Rivera J, Vidal J, Alegre J, Rabionet R, Estivill X. Cluster analysis of clinical data identifies fibromyalgia subgroups. PloS One. 2013;8:e74873. doi: 10.1371/journal.pone.0074873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enders CK. Applied Missing Data Analysis. Guilford Press; New York, NYY: 2010. [Google Scholar]
- 12.Feldman SI, Downey G, Schaffer-Neitz R. Pain, negative mood, and perceived support in chronic pain patients: a daily diary study of people with reflex sympathetic dystrophy syndrome. Journal of Consulting and Clinical Psychology. 1999;67:776–785. doi: 10.1037//0022-006x.67.5.776. [DOI] [PubMed] [Google Scholar]
- 13.Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, Gracely RH, Clauw DJ. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis and Rheumatism. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 14.Graff LA, Clara I, Walker JR, Lix L, Carr R, Miller N, Rogala L, Bernstein CN. Changes in fatigue over 2 years are associated with activity of inflammatory bowel disease and psychological factors. Clinical Gastroenterology and Hepatology. 2013;11:1140–1146. doi: 10.1016/j.cgh.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis and Rheumatism. 2005;52:3670–3674. doi: 10.1002/art.21407. [DOI] [PubMed] [Google Scholar]
- 16.Heck RH, Thomas SL, Tabata LN. Multilevel and longitudinal modeling with IBM SPSS. Routledge, Taylor & Francis Group; New York: 2014. [Google Scholar]
- 17.Hegarty RS, Treharne GJ, Stebbings S, Conner TS. Fatigue and mood among people with arthritis: carry-over across the day. Health Psychology. 2016;35:492–499. doi: 10.1037/hea0000321. [DOI] [PubMed] [Google Scholar]
- 18.Hox JJ. Multilevel Analysis. Lawrence Erlbaum Associates; Mahwah, NJ: 2002. [Google Scholar]
- 19.Hurtig IM, Raak RI, Kendall SA, Gerdle B, Wahren LK. Quantitative sensory testing in fibromyalgia patients and in healthy subjects: identification of subgroups. The Clinical Journal of Pain. 2001;17:316–322. doi: 10.1097/00002508-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Hutchings A, Calloway M, Choy E, Hooper M, Hunter DJ, Jordan JM, Zhang Y, Baser O, Long S, Palmer L. The Longitudinal Examination of Arthritis Pain (LEAP) study: relationships between weekly fluctuations in patient-rated joint pain and other health outcomes. The Journal of Rheumatology. 2007;34:2291–2300. [PubMed] [Google Scholar]
- 21.Jamison RN, Brown GK. Validation of hourly pain intensity profiles with chronic pain patients. Pain. 1991;45:123–128. doi: 10.1016/0304-3959(91)90176-X. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Quality of Life Research. 1994;3:7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 23.Kratz AL, Murphy SL, Braley TJ. Ecological momentary assessment of pain, fatigue, depressive, and cognitive symptoms reveals significant daily variability in multiple sclerosis. Archives of Physical Medicine and Rehabilitation. 2017 doi: 10.1016/j.apmr.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loevinger BL, Shirtcliff EA, Muller D, Alonso C, Coe CL. Delineating psychological and biomedical profiles in a heterogeneous fibromyalgia population using cluster analysis. Clinical Rheumatology. 2012;31:677–685. doi: 10.1007/s10067-011-1912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukkahatai N, Walitt B, Espina A, Gelio A, Saligan LN. Understanding the association of fatigue with other symptoms of fibromyalgia: development of a cluster model. Arthritis Care & Research. 2016;68:99–107. doi: 10.1002/acr.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez JE, Domingues C, Davolos FJC, Martinez LC, Gozzano JOA. Fibromyalgia patients' quality of life and pain intensity variation. Revista Brasileira de Reumatologia. 2008;48:325–328. [Google Scholar]
- 27.Milligan GW, Cooper MC. An examination of procedures for determining the number of clusters in a data set. Psychometrika. 1985;50:159–179. [Google Scholar]
- 28.Ong AD, Zautra AJ, Reid MC. Psychological resilience predicts decreases in pain catastrophizing through positive emotions. Psychology and Aging. 2010;25:516–523. doi: 10.1037/a0019384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennebaker JW. The Psychology of Physical Symptoms. Springer; New York: 1982. [Google Scholar]
- 30.Peters ML, Sorbi MJ, Kruise DA, Kerssens JJ, Verhaak PF, Bensing JM. Electronic diary assessment of pain, disability and psychological adaptation in patients differing in duration of pain. Pain. 2000;84:181–192. doi: 10.1016/s0304-3959(99)00206-7. [DOI] [PubMed] [Google Scholar]
- 31.Plazier M, Ost J, Stassijns G, De Ridder D, Vanneste S. Pain characteristics in fibromyalgia: understanding the multiple dimensions of pain. Clinical Rheumatology. 2015;34:775–783. doi: 10.1007/s10067-014-2736-6. [DOI] [PubMed] [Google Scholar]
- 32.Pollard L, Choy E, Gonzalez J, Khoshaba B, Scott D. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology. 2006;45:885–889. doi: 10.1093/rheumatology/kel021. [DOI] [PubMed] [Google Scholar]
- 33.Randolph JJ, Arnett PA. Depression and fatigue in relapsing-remitting MS: the role of symptomatic variability. Multiple Sclerosis. 2005;11:186–190. doi: 10.1191/1352458505ms1133oa. [DOI] [PubMed] [Google Scholar]
- 34.Rehm SE, Koroschetz J, Gockel U, Brosz M, Freynhagen R, Tolle TR, Baron R. A cross-sectional survey of 3035 patients with fibromyalgia: subgroups of patients with typical comorbidities and sensory symptom profiles. Rheumatology. 2010;49:1146–1152. doi: 10.1093/rheumatology/keq066. [DOI] [PubMed] [Google Scholar]
- 35.Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE. Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: associations with psychological variables. Pain. 2012;153:813–822. doi: 10.1016/j.pain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford University Press; Oxford; New York: 2003. [Google Scholar]
- 37.Stone AA, Broderick JE, Porter LS, Kaell AT. The experience of rheumatoid arthritis pain and fatigue: examining momentary reports and correlates over one week. Arthritis Care & Research. 1997;10:185–193. doi: 10.1002/art.1790100306. [DOI] [PubMed] [Google Scholar]
- 38.Sturgeon JA, Zautra AJ. Resilience: a new paradigm for adaptation to chronic pain. Current Pain and Headache Reports. 2010;14:105–112. doi: 10.1007/s11916-010-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tupper SM, Rosenberg AM, Pahwa P, Stinson JN. Pain intensity variability and its relationship with quality of life in youths with juvenile idiopathic arthritis. Arthritis Care & Research. 2013;65:563–570. doi: 10.1002/acr.21850. [DOI] [PubMed] [Google Scholar]
- 40.Turk DC, Okifuji A, Sinclair JD, Starz TW. Differential responses by psychosocial subgroups of fibromyalgia syndrome patients to an interdisciplinary treatment. Arthritis Care & Research. 1998;11:397–404. doi: 10.1002/art.1790110511. [DOI] [PubMed] [Google Scholar]
- 41.Vangronsveld KLH, Peters M, Goossens M, Vlaeyen J. The influence of fear of movement and pain catastrophizing on daily pain and disability in individuals with acute whiplash injury: a daily diary study. Pain. 2008;139:449–457. doi: 10.1016/j.pain.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Verra ML, Angst F, Brioschi R, Lehmann S, Keefe FJ, Staal JB, de Bie RA, Aeschlimann A. Does classification of persons with fibromyalgia into Multidimensional Pain Inventory subgroups detect differences in outcome after a standard chronic pain management program? Pain Research & Management. 2009;14:445–453. doi: 10.1155/2009/137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent A, Hoskin TL, Whipple MO, Clauw DJ, Barton DL, Benzo RP, Williams DA. OMERACT-based fibromyalgia symptom subgroups: an exploratory cluster analysis. Arthritis Research & Therapy. 2014;16:463. doi: 10.1186/s13075-014-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walitt B, Fitzcharles MA, Hassett AL, Katz RS, Hauser W, Wolfe F. The longitudinal outcome of fibromyalgia: a study of 1555 patients. The Journal of Rheumatology. 2011;38:2238–2246. doi: 10.3899/jrheum.110026. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 46.Wilson HD, Robinson JP, Turk DC. Toward the identification of symptom patterns in people with fibromyalgia. Arthritis and Rheumatism. 2009;61:527–534. doi: 10.1002/art.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis and Rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 48.Zakoscielna KM, Parmelee PA. Pain variability and its predictors in older adults: depression, cognition, functional status, health, and pain. Journal of Aging and Health. 2013;25:1329–1339. doi: 10.1177/0898264313504457. [DOI] [PubMed] [Google Scholar]
- 49.Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128:128–135. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Zautra AJ, Sturgeon JA. Examining the complexities of affective experience will enhance our understanding of pain and inform new interventions designed to bolster resilience. Pain. 2016;157:1586–1587. doi: 10.1097/j.pain.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]