Abstract
Atrophy of the thymus, the primary site of T lymphocyte generation, is a hallmark of the aging immune system. Age-associated thymic atrophy results in diminished output of new, naïve T cells, with immune sequelae that include diminished responses to novel pathogenic challenge and vaccines, as well as diminished tumor surveillance. Although a variety of stimuli are known to regulate transient thymic atrophy, mechanisms governing progressive age-associated atrophy have been difficult to resolve. This has been due in part to the fact that one of the primary targets of age-associated thymic atrophy is a relatively rare population, thymic stromal cells. This review focuses on changes in thymic stromal cells during aging and on the contributions of periodic, stochastic, and progressive causes of thymic atrophy.
1. Introduction
The thymus is the primary site of generation of new, naïve T lymphocytes, which are generated in the thymus by periodic recruitment of circulating multi-potent thymus seeding progenitors (Bhandoola et al., 2007; Foss et al., 2001; Goldschneider et al., 1986; Luc et al., 2012; Luis et al., 2016). Mutually inductive signaling in the unique stromal microenvironment of the thymus, comprised of a heterogeneous population of thymic epithelial cells (TECs), as well as mesenchymal, neural, vascular, and hematopoietic populations (including dendritic cells, macrophages, and B cells), directs these multi-potent progenitors along a well-characterized program of differentiation, proliferation, and selection to generate a self-tolerant, self-restricted T cell population (Abramson and Anderson, 2017; Klug et al., 1998; Petrie and Zuniga-Pflucker, 2007). However, the thymus begins to atrophy relatively early in life, with concomitant reduction in production of new, naïve T cells (Aspinall et al., 2010; Hale et al., 2006; Hartwig and Steinmann, 1994; Steinmann et al., 1985). This results in shift toward an oligoclonal memory T cell population with diminished T cell receptor diversity (Ernst et al., 1990; Hale et al., 2006; Haynes et al., 2000b; Nikolich-Zugich and Rudd, 2010; Utsuyama et al., 1992). The resulting immunodeficiencies include diminished vaccine responsiveness and tumor surveillance, as well as decreased response to new infections, especially viral infection (including a well characterized loss of immunity against influenza A epitopes) ((Yager et al., 2008), reviewed in (Nikolich-Zugich and Rudd, 2010) (Dicarlo et al., 2009; Montecino-Rodriguez et al., 2013)).
Both the thymic stromal population and the hematopoietic stem cells (HSC) that ultimately give rise to T cells undergo extensive changes with age. Changes in HSCs include altered homing and increased bias toward myeloid, rather than lymphoid development (reviewed in (Chinn et al., 2012; Montecino-Rodriguez et al., 2013; Palmer, 2013)). However, thymic atrophy, which begins at approximately 7 weeks of age in mice (Manley et al., 2011), precedes the initiation of these changes in the hematopoietic population, which begin later in life, at around 7 months of age in mice (Montecino-Rodriguez et al., 2013). A large body of evidence supports the notion that the stromal population in the thymus is a primary target of age-associated thymic dysfunction (recently reviewed in (Lepletier et al., 2015; Masters et al., 2017)). For instance, transplant experiments employing age mismatched donors and hosts support the notion that age-associated defects in thymus size and architecture are primarily a function of the age of the thymic microenvironment rather than the age of the transplanted bone marrow cells (Doria et al., 1997; Mackall et al., 1998; Zhu et al., 2007). Functional assessments of recent thymic emigrants (RTE) in age mismatched bone marrow chimeras showed that the aged thymic microenvironment and lymphoid-intrinsic factors both contribute to age-associated declines in RTE function (Clise-Dwyer et al., 2007), and recent work has shown that transplant of young TECs into aged thymi can increase T cell production (Kim et al., 2015). Consistent with these studies, we, and others (Sutherland et al., 2005), have found that the lymphoid phenotype in the thymus is essentially unchanged at 12 months of age in mice, and that age-associated transcriptional changes predominately occur in stromal, rather than lymphoid populations (Griffith et al., 2012).
2. Histological and transcriptional characteristics of aging thymic stroma
Histological changes in thymus architecture have been observed as early as the first few years of life in humans, including the expansion of perivascular space and adipose tissue relative to functional cortical and medullary tissue (Steinmann et al., 1985), and loss of cortico-medullary organization (Henry and Anderson, 1987). In the mouse, histological changes are obvious by 3 months of age, and also include increases in adipose tissue (Yang et al., 2009), as well as loss of cortico-medullary junction integrity and cortical thinning (Manley et al., 2011), and decreased complexity of medullary islet morphology (Griffith et al., 2012) (and reviewed in (Manley et al., 2011; Montecino-Rodriguez et al., 2013; Taub and Longo, 2005)). In humans, total thymus size is considered to remain relatively constant during aging, though functional lymphopoietic tissue is replaced by perivascular space and adipose tissue over time, whereas in murine studies, declines in total thymus size (weight) are well characterized (Haynes et al., 2000a). Nonetheless, in both mice and humans, as well as many other vertebrate species as discussed below (Kendall, 1981), the volume of lymphopoietic tissue within the thymus and thymic generation of new T cells decline beginning early in life and are progressive during aging.
Transcriptome-based approaches have revealed extensive changes in stromal cells. Murine studies based on microdissected whole thymus tissue showed that the stromal compartment, especially cortical stroma, undergoes broad transcriptional changes within the first year of life, while relatively few changes are apparent in major thymic T cell subsets (Griffith et al., 2012). In these studies, transcriptional changes may reflect altered relative frequencies of stromal subpopulations and/or alterations in gene expression within individual stromal populations. Stromal transcriptional changes include changes in Wnt pathway signaling associated with stem cell expansion and epithelial cell division (Ferrando-Martinez et al., 2015; Kvell et al., 2010; Wei et al., 2015). In particular, studies have found decreased expression of Wnt3a and Wnt4, and increases in Wnt10a and Wnt5b in the aged thymus (Bredenkamp et al., 2014; Griffith et al., 2012; Ki et al., 2014; Kvell et al., 2010; Wei et al., 2015). These results are consistent with known roles for Wnt3a and Wnt4 in cell proliferation (Liu et al., 2010) and Foxn1 expression (Balciunaite et al., 2002; Kvell et al., 2010; Wei et al., 2015), respectively. Increased expression of Wnt 5b is consistent with known roles for Wnt5b in promotion of adipogenesis (van Tienen et al., 2009).
Expression of tissue restricted self-antigens, critical for tolerizing developing T cells (Derbinski et al., 2005; Klein et al., 2014), declines with age in the stromal population as a whole (Griffith et al., 2012), and within the epithelial compartment (Bredenkamp et al., 2014), revealing a potential mechanistic link between aging and age-associated increases in autoimmunity (Muller and Pawelec, 2015). There is also evidence to support the notion that thymus atrophy is associated with declines in negative selection of autoreactive T cells in a mouse model of thymus atrophy induced by Foxn1 deficiency (Coder et al., 2015). In addition, diminished T cell output after thymus atrophy may promote autoimmunity by allowing homeostatic expansion of autoreactive T cells in the periphery (Hakim and Gress, 2007; King et al., 2004).
Studies based on purified stromal populations, including cortical and medullary thymic epithelial cells (cTECs, mTECs), dendritic cells, and fibroblasts, revealed transcriptional changes associated with atrophy as early as 3 months of age in mice (Ki et al., 2014). Among these cell types, most age-associated changes were found in dendritic cells and MHCIIlo mTECs. Within the MHCIIlo mTEC (mTEClo) population, age-associated changes included diminished expression of cell cycle regulators (i.e. Cdc20, Cdc6). In both cTECs and the mTEClo population, significant decreases were found in transcription of targets of the transcription factor E2F3, an important cell cycle regulator. These declines in expression of genes important for proliferation are consistent with evidence that TEC proliferation declines during aging (Gray et al., 2006), and that enforced TEC proliferation increases thymus size in aged mice (Garfin et al., 2013; Robles et al., 1996). In dendritic cells, aging was associated with increased expression of proinflammatory regulators such as Il1a, Il1b, IL6 and Tnf (Ki et al., 2014).
Another critical change that has been identified in the aging thymus is diminished expression of Foxn1 in epithelial cells. Foxn1 is a transcriptional regulator necessary for TEC development and function (Nehls et al., 1994; Vaidya et al., 2016). Expression of Foxn1 diminishes with age in the thymus of mice (Chen et al., 2009; Ortman et al., 2002) and humans (Reis et al., 2015), and overexpression is sufficient to restore not only thymus size (Zook et al., 2011), but also medullary islet complexity and TRA expression, even in 12 month-old mice, highlighting its potential as a therapeutic target for long-term reversal of thymus size and function (Bredenkamp et al., 2014; Gallo et al., 2017; Rode et al., 2015).
3. Mechanisms governing thymic atrophy
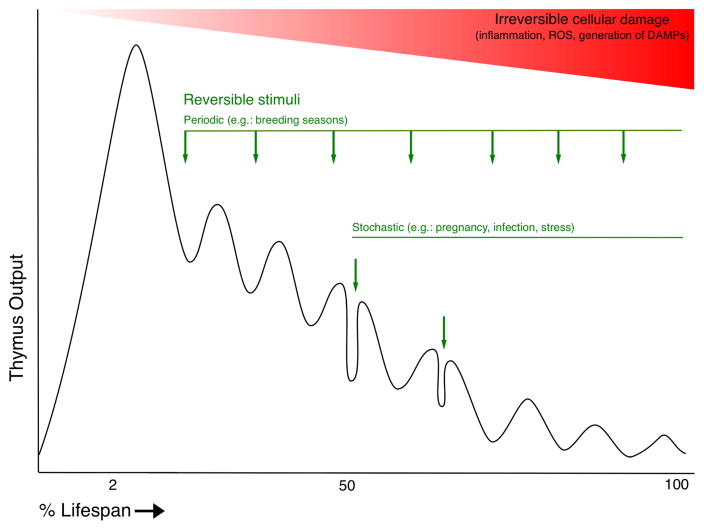
Although broad changes in thymus structure and function during aging have been described, the mechanisms governing age-associated thymic atrophy have been difficult to resolve. This in part due to the diversity of periodic, episodic, and cumulative events that influence thymus size and function over the lifespan (Figure 1). Several key contributors have emerged, as described below.
Figure 1.
Significant reciprocal feedback occurs between the thymus and endocrine system (reviewed in (Montecino-Rodriquez et al., 2005). The thymus has role in regulating reproductive functions (Besedovsky and Sorkin, 1974; Pierpaoli and Sorkin, 1972; Weinstein, 1978), and there is a clear inverse relationship between androgens and thymus size (Olsen and Kovacs, 1996; Olsen et al., 1998). The effects of sex steroids on thymus size are remarkable, including atrophy of the thymus during pregnancy (Tibbetts et al., 1999) and rebound after castration (Heng et al., 2005; Leposavic et al., 1996; Sutherland et al., 2005; Utsuyama and Hirokawa, 1989). However, the transient nature of thymic rebound after castration ((Griffith et al., 2012; Leposavic et al., 1996; Utsuyama and Hirokawa, 1989)), and diminished rebound in older rodents relative to young, support the notion that other age-associated factors are the predominant factors in determining steady state thymus size (Montecino-Rodriquez et al., 2005). This notion is also supported by studies reviewing thymus size in species from diverse vertebrate classes exhibiting seasonal breeding patterns (Osteichthyes, Amphibia, Reptilia, Aves, and Mammalia), indicating that during each breeding season, when sex steroids are present at relatively high levels, the thymus diminishes in size temporarily, regenerating each year, but to a diminished degree, as the organisms age (Kendall, 1981). Growth hormone (GH) and insulin-like growth factor-I (IGF-I) have also been characterized as regulators of age-associated thymic atrophy, as both hormones promote thymus growth (Bar-Dayan and Small, 1994; Kelley et al., 1986; Montecino-Rodriguez et al., 1998), and production of GH declines with age (Lamberts et al., 1997). As with androgens, while the effects of hormone treatment on thymus size are large in relatively young mice, effects in older mice are more limited, suggesting other age-associated factors prevent sustained reversal of thymic atrophy (Montecino-Rodriquez et al., 2005).
The hormonal stimuli discussed above may be periodic, that is, occurring at regular intervals (e.g. breeding season), or sporadic (episodic) (e.g. pregnancy), and while their effects on thymus size can be large in magnitude, they are generally reversible. Sporadic events such as infection-induced inflammation and glucocorticoid release in response to a variety of physiological stressors also negatively affect thymus size (reviewed in (Majumdar and Nandi, 2017; Taub and Longo, 2005)). Recent work has shown that miR-205 expression in thymic epithelial cells (TECs) promotes recovery from episodic stressors such of this type through enhanced expression of Foxn1, as well as critical chemokine transcriptional targets (Hoover et al., 2016).
Over the last several years, immune sensing of metabolic dysregulation has emerged as an important factor in age-associated thymic atrophy, but the mechanisms regulating this association are not yet completely understood. Calorie restriction has been shown to partially mitigate age-associated thymic atrophy (Yang et al., 2009). Conversely, obesity has been associated with decreases in thymus output (Howard et al., 1999). Adipogenesis within the thymus and thymic atrophy are promoted by age-associated decreases in expression of ghrelin, an orexigenic and anti-inflammatory peptide, and ghrelin receptor (growth hormone secretagogue receptor) in thymic stromal cells (Dixit et al., 2007; Youm et al., 2009). Mechanistic links between aging, thymic adiposity, and thymic atrophy have been revealed by recent work showing that lipotoxic “danger-associated molecular patterns” (DAMPs), such as ceramide and free cholesterol, increase during aging (de Mello-Coelho et al., 2017; Youm et al., 2012), and can initiate NLRP3 inflammasome signaling and IL-1β production in thymic myeloid cells (Youm et al., 2012). Il1r expression was found mainly in the TEC compartment, and since IL-1β causes thymic dysfunction (Morrissey et al., 1988a; Morrissey et al., 1988b) these studies suggest that lipotoxic DAMPs inhibit thymus function via IL-1β signaling in TEC (Youm et al., 2012). In agreement with these studies, Ki et al found high levels of Il1r expression in TECs, and also found that the antagonists Il2rn and Il1r2 were preferentially expressed by mTECs (Ki et al., 2014). The authors noted that IL-1 therefore likely has differential effects on the cortex and medulla. Together, these studies support the notion that DAMP-initiated NLRP3 signaling may preferentially trigger IL-1β signaling in the thymic cortex, perhaps contributing to the noted decline in cortical:medullary ratio with age (Brelinska et al., 2008; Griffith et al., 2012; Li et al., 2003; Steinmann et al., 1985; Sutherland et al., 2005). In addition, the prolongevity factor and ketogenic hormone FGF21 has also been shown to decrease thymic adiposity and mitigate thymic atrophy, presumably by diminishing lipotoxic DAMP signals in the thymus during aging (Youm et al., 2016). This is consistent with the observation that mutation of the FGF21 obligate co-receptor, βKlotho, promotes thymic atrophy (Kuro-o et al., 1997).
Thymic stromal cells may be especially sensitive to damage induced by inflammation and DAMPs, such as reactive oxygen species (ROS), for several reasons. First, the early stages of lymphopoiesis that occur in the thymus involve exceptionally high levels of proliferation (Lind et al., 2001; Penit, 1988). While the lymphocytes exit cell cycle, and eventually emigrate from the thymus, stromal cells, especially in the cortex, are constantly juxtaposed to the periodically recruited lymphoid progenitors exhibiting high rates of proliferation (Petrie and Zuniga-Pflucker, 2007), and will be continually exposed to the cell-permeable products thereof, including ROS, such as hydrogen peroxide. We have recently identified a deficiency in the peroxide-quenching enzyme catalase in thymic stromal cells. This deficiency renders thymic stromal cells particularly sensitive to ROS-induced DNA damage, which is found at much higher levels in thymic epithelial cells than in thymic lymphocytes. Accumulated metabolic damage promotes atrophy of the thymus, which can be mitigated by dietary or genetic complementation of antioxidant activity (Griffith et al., 2015). The cellular damage induced by ROS, which may be generated as byproducts of metabolism and/or DAMP-induced (Youm et al., 2012) or dendritic cell-mediated inflammation (Ki et al., 2014), represents an example of a continual, cumulative, and relatively irreversible age-associated change. Because ROS may be generated as a result of inflammation (reviewed in (Laskin et al., 2011)), and may also serve as NLRP3-activating DAMPs (reviewed in (Tschopp and Schroder, 2010)), a feed-forward loop of cellular damage may be established once inflammation and/or ROS emerge.
In addition to the factors discussed above, careful examination of differences in thymic atrophy among 16 recombinant inbred mouse strains have revealed evidence of genetic determinants that may regulate maximal thymus size and pace of atrophy on chromosomes 3 and 9, respectively (Hsu et al., 2003). Recent work has also identified genetic differences in thymic epithelial cell number and lymphocyte:epithelial ratio among CBA/J and PWK/PhJ mouse strains (Nagakubo et al., 2017). These studies highlight the fact that there are genetic factors regulating thymus atrophy during aging that remain to be identified.
4. Conclusions
As illustrated in Figure 1, both transient and cumulative events contribute to declining thymus output during aging. To the best of our knowledge, direct measurements of total lymphocyte cellularity in the human thymus during aging have not been reported. However, careful histological analysis of human thymus during aging (Steinmann et al., 1985) agrees with estimates of thymic output in humans based on T cell receptor excision circle (TREC) analysis (Douek et al., 1998; Jamieson et al., 1999; Thome et al., 2016) and mathematical modeling (Bains et al., 2009), indicating that peak thymus output occurs in the first few years of life, and yet continues to occur through the 9th decade of life (Mitchell et al., 2010). These data informed our estimates corresponding to thymus output in human and experimental models illustrated in Figure 1. Although the thymus retains a remarkable capacity to regenerate after removal of a negative stimulus (Chaudhry et al., 2016; Dudakov et al., 2012; Majumdar and Nandi, 2017; Rode and Boehm, 2012; Sutherland et al., 2005), age progressively limits the size reached at return to steady state. Persistent regeneration of thymus size and function holds great promise for extending the healthspan, but remains a challenge, and approaches aimed at preventing atrophy are relatively unexplored. A more complete understanding of the causes of thymic atrophy will inform studies that aim to reverse or prevent it.
Highlights.
This article reviews the role of thymic stromal cells in age-associated atrophy of the thymus.
Age-associated changes in stromal phenotype, transcriptome, and function are reviewed.
Periodic, stochastic, and progressive causes of atrophy are discussed.
Acknowledgments
We thank Dr. Howard Petrie for helpful discussions. A.V.G. is supported by NIH R01AI121367, and S.C. is supported by NIH R01AI121367-S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson J, Anderson G. Thymic Epithelial Cells. Annual review of immunology. 2017;35:85–118. doi: 10.1146/annurev-immunol-051116-052320. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Pitts D, Lapenna A, Mitchell W. Immunity in the elderly: the role of the thymus. Journal of comparative pathology. 2010;142(Suppl 1):S111–115. doi: 10.1016/j.jcpa.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Bains I, Thiebaut R, Yates AJ, Callard R. Quantifying thymic export: combining models of naive T cell proliferation and TCR excision circle dynamics gives an explicit measure of thymic output. Journal of immunology. 2009;183:4329–4336. doi: 10.4049/jimmunol.0900743. [DOI] [PubMed] [Google Scholar]
- Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Hollander GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nature immunology. 2002;3:1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- Bar-Dayan Y, Small M. Effect of bovine growth hormone administration on the pattern of thymic involution in mice. Thymus. 1994;23:95–101. [PubMed] [Google Scholar]
- Besedovsky HO, Sorkin E. Thymus involvement in female sexual maturation. Nature. 1974;249:356–358. doi: 10.1038/249356a0. [DOI] [PubMed] [Google Scholar]
- Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bredenkamp N, Nowell CS, Blackburn CC. Regeneration of the aged thymus by a single transcription factor. Development. 2014;141:1627–1637. doi: 10.1242/dev.103614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelinska R, Malendowicz LK, Malinska A, Kowalska K. Characteristics of age-related changes in rat thymus: morphometric analysis and epithelial cell network in various thymic compartments. Biogerontology. 2008;9:93–108. doi: 10.1007/s10522-007-9117-3. [DOI] [PubMed] [Google Scholar]
- Chaudhry MS, Velardi E, Dudakov JA, van den Brink MR. Thymus: the next (re)generation. Immunological reviews. 2016;271:56–71. doi: 10.1111/imr.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xiao S, Manley NR. Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood. 2009;113:567–574. doi: 10.1182/blood-2008-05-156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Seminars in immunology. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. Journal of immunology. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- Coder BD, Wang H, Ruan L, Su DM. Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation. Journal of immunology. 2015;194:5825–5837. doi: 10.4049/jimmunol.1500082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello-Coelho V, Cutler RG, Bunbury A, Tammara A, Mattson MP, Taub DD. Age-associated alterations in the levels of cytotoxic lipid molecular species and oxidative stress in the murine thymus are reduced by growth hormone treatment. Mechanisms of ageing and development. 2017;167:46–55. doi: 10.1016/j.mad.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. The Journal of experimental medicine. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicarlo AL, Fuldner R, Kaminski J, Hodes R. Aging in the context of immunological architecture, function and disease outcomes. Trends Immunol. 2009;30:293–294. doi: 10.1016/j.it.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. The Journal of clinical investigation. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria G, Mancini C, Utsuyama M, Frasca D, Hirokawa K. Aging of the recipients but not of the bone marrow donors enhances autoimmunity in syngeneic radiation chimeras. Mechanisms of ageing and development. 1997;95:131–142. doi: 10.1016/s0047-6374(97)01871-x. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MR. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, Hayakawa K, Hardy RR, Weigle WO. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. Journal of immunology. 1990;145:1295–1302. [PubMed] [Google Scholar]
- Ferrando-Martinez S, Ruiz-Mateos E, Dudakov JA, Velardi E, Grillari J, Kreil DP, Munoz-Fernandez MA, van den Brink MR, Leal M. WNT signaling suppression in the senescent human thymus. The journals of gerontology Series A, Biological sciences and medical sciences. 2015;70:273–281. doi: 10.1093/gerona/glu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. The Journal of experimental medicine. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Cirillo E, Giardino G, Pignata C. FOXN1 Deficiency: from the Discovery to Novel Therapeutic Approaches. J Clin Immunol. 2017 doi: 10.1007/s10875-017-0445-z. [DOI] [PubMed] [Google Scholar]
- Garfin PM, Min D, Bryson JL, Serwold T, Edris B, Blackburn CC, Richie ER, Weinberg KI, Manley NR, Sage J, Viatour P. Inactivation of the RB family prevents thymus involution and promotes thymic function by direct control of Foxn1 expression. The Journal of experimental medicine. 2013;210:1087–1097. doi: 10.1084/jem.20121716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I, Komschlies KL, Greiner DL. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. The Journal of experimental medicine. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- Griffith AV, Fallahi M, Venables T, Petrie HT. Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging cell. 2012;11:169–177. doi: 10.1111/j.1474-9726.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith AV, Venables T, Shi J, Farr A, van Remmen H, Szweda L, Fallahi M, Rabinovitch P, Petrie HT. Metabolic Damage and Premature Thymus Aging Caused by Stromal Catalase Deficiency. Cell reports. 2015;12:1071–1079. doi: 10.1016/j.celrep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim FT, Gress RE. Thymic involution: implications for self-tolerance. Methods in molecular biology. 2007;380:377–390. doi: 10.1007/978-1-59745-395-0_24. [DOI] [PubMed] [Google Scholar]
- Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig M, Steinmann G. On a causal mechanism of chronic thymic involution in man. Mechanisms of ageing and development. 1994;75:151–156. doi: 10.1016/0047-6374(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annual review of immunology. 2000a;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunologic research. 2000b;22:253–261. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. Journal of immunology. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- Henry L, Anderson G. Epithelial-cell architecture during involution of the human thymus. J Pathol. 1987;152:149–155. doi: 10.1002/path.1711520303. [DOI] [PubMed] [Google Scholar]
- Hoover AR, Dozmorov I, MacLeod J, Du Q, de la Morena MT, Forbess J, Guleserian K, Cleaver OB, van Oers NS. MicroRNA-205 Maintains T Cell Development following Stress by Regulating Forkhead Box N1 and Selected Chemokines. J Biol Chem. 2016;291:23237–23247. doi: 10.1074/jbc.M116.744508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. The Journal of clinical investigation. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HC, Zhang HG, Li L, Yi N, Yang PA, Wu Q, Zhou J, Sun S, Xu X, Yang X, Lu L, Van Zant G, Williams RW, Allison DB, Mountz JD. Age-related thymic involution in C57BL/6J x DBA/2J recombinant-inbred mice maps to mouse chromosomes 9 and 10. Genes and immunity. 2003;4:402–410. doi: 10.1038/sj.gene.6363982. [DOI] [PubMed] [Google Scholar]
- Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, Marelli D, Koup RA, Zack JA. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, Walker EB. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5663–5667. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall MD. The Thymus gland. Academic Press; London; New York: 1981. p. xii.p. 218. [Google Scholar]
- Ki S, Park D, Selden HJ, Seita J, Chung H, Kim J, Iyer VR, Ehrlich LI. Global transcriptional profiling reveals distinct functions of thymic stromal subsets and age-related changes during thymic involution. Cell reports. 2014;9:402–415. doi: 10.1016/j.celrep.2014.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Miller CM, Shadrach JL, Wagers AJ, Serwold T. Young, proliferative thymic epithelial cells engraft and function in aging thymuses. Journal of immunology. 2015;194:4784–4795. doi: 10.4049/jimmunol.1403158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nature reviews Immunology. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:11822–11827. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kvell K, Varecza Z, Bartis D, Hesse S, Parnell S, Anderson G, Jenkinson EJ, Pongracz JE. Wnt4 and LAP2alpha as pacemakers of thymic epithelial senescence. PLoS One. 2010;5:e10701. doi: 10.1371/journal.pone.0010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- Laskin DL, V, Sunil R, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annual review of pharmacology and toxicology. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepletier A, Chidgey AP, Savino W. Perspectives for Improvement of the Thymic Microenvironment through Manipulation of Thymic Epithelial Cells: A Mini-Review. Gerontology. 2015;61:504–514. doi: 10.1159/000375160. [DOI] [PubMed] [Google Scholar]
- Leposavic G, Karapetrovic B, Obradovic S, Vidiic Dandovic B, Kosec D. Differential effects of gonadectomy on the thymocyte phenotypic profile in male and female rats. Pharmacol Biochem Behav. 1996;54:269–276. doi: 10.1016/0091-3057(95)02165-5. [DOI] [PubMed] [Google Scholar]
- Li L, Hsu HC, Grizzle WE, Stockard CR, Ho KJ, Lott P, Yang PA, Zhang HG, Mountz JD. Cellular mechanism of thymic involution. Scand J Immunol. 2003;57:410–422. doi: 10.1046/j.1365-3083.2003.01206.x. [DOI] [PubMed] [Google Scholar]
- Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. The Journal of experimental medicine. 2001;194:127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Luo M, Xie W, Wells JM, Goodheart MJ, Engelhardt JF. Sox17 modulates Wnt3A/beta-catenin-mediated transcriptional activation of the Lef-1 promoter. American journal of physiology Lung cellular and molecular physiology. 2010;299:L694–710. doi: 10.1152/ajplung.00140.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luc S, Luis TC, Boukarabila H, Macaulay IC, Buza-Vidas N, Bouriez-Jones T, Lutteropp M, Woll PS, Loughran SJ, Mead AJ, Hultquist A, Brown J, Mizukami T, Matsuoka S, Ferry H, Anderson K, Duarte S, Atkinson D, Soneji S, Domanski A, Farley A, Sanjuan-Pla A, Carella C, Patient R, de Bruijn M, Enver T, Nerlov C, Blackburn C, Godin I, Jacobsen SE. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nature immunology. 2012;13:412–419. doi: 10.1038/ni.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis TC, Luc S, Mizukami T, Boukarabila H, Thongjuea S, Woll PS, Azzoni E, Giustacchini A, Lutteropp M, Bouriez-Jones T, Vaidya H, Mead AJ, Atkinson D, Boiers C, Carrelha J, Macaulay IC, Patient R, Geissmann F, Nerlov C, Sandberg R, de Bruijn M, Blackburn CC, Godin I, Jacobsen SEW. Initial seeding of the embryonic thymus by immune-restricted lympho-myeloid progenitors. Nature immunology. 2016;17:1424–1435. doi: 10.1038/ni.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall CL, Punt JA, Morgan P, Farr AG, Gress RE. Thymic function in young/old chimeras: substantial thymic T cell regenerative capacity despite irreversible age-associated thymic involution. European journal of immunology. 1998;28:1886–1893. doi: 10.1002/(SICI)1521-4141(199806)28:06<1886::AID-IMMU1886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Nandi D. Thymic Atrophy: Experimental Studies and Therapeutic Interventions. Scand J Immunol. 2017 doi: 10.1111/sji.12618. [DOI] [PubMed] [Google Scholar]
- Manley NR, Richie ER, Blackburn CC, Condie BG, Sage J. Structure and function of the thymic microenvironment. Frontiers in bioscience. 2011;16:2461–2477. doi: 10.2741/3866. [DOI] [PubMed] [Google Scholar]
- Masters AR, Haynes L, Su DM, Palmer DB. Immune senescence: significance of the stromal microenvironment. Clinical and experimental immunology. 2017;187:6–15. doi: 10.1111/cei.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell WA, Lang PO, Aspinall R. Tracing thymic output in older individuals. Clinical and experimental immunology. 2010;161:497–503. doi: 10.1111/j.1365-2249.2010.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. The Journal of clinical investigation. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Clark R, Dorshkind K. Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology. 1998;139:4120–4126. doi: 10.1210/endo.139.10.6263. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriquez E, Min H, Dorshkind K. Reevaluating current models of thymic involution. Seminars in immunology. 2005;17:356–361. doi: 10.1016/j.smim.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Morrissey P, Charrier K, Bressler L, Alpert A. The influence of IL-1 treatment on the reconstitution of the hemopoietic and immune systems after sublethal radiation. Journal of immunology. 1988a;140:4204–4210. [PubMed] [Google Scholar]
- Morrissey PJ, Charrier K, Alpert A, Bressler L. In vivo administration of IL-1 induces thymic hypoplasia and increased levels of serum corticosterone. Journal of immunology. 1988b;141:1456–1463. [PubMed] [Google Scholar]
- Muller L, Pawelec G. As we age: Does slippage of quality control in the immune system lead to collateral damage? Ageing research reviews. 2015;23:116–123. doi: 10.1016/j.arr.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Nagakubo D, Krauth B, Boehm T. Genetic and non-genetic determinants of thymic epithelial cell number and function. Sci Rep. 2017;7:10314. doi: 10.1038/s41598-017-10746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Current opinion in immunology. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Viselli SM, Fan J, Kovacs WJ. Androgens accelerate thymocyte apoptosis. Endocrinology. 1998;139:748–752. doi: 10.1210/endo.139.2.5729. [DOI] [PubMed] [Google Scholar]
- Ortman CL, Dittmar KA, Witte PL, Le PT. Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. International immunology. 2002;14:813–822. doi: 10.1093/intimm/dxf042. [DOI] [PubMed] [Google Scholar]
- Palmer DB. The effect of age on thymic function. Frontiers in immunology. 2013;4:316. doi: 10.3389/fimmu.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit C. Localization and phenotype of cycling and post-cycling murine thymocytes studied by simultaneous detection of bromodeoxyuridine and surface antigens. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1988;36:473–478. doi: 10.1177/36.5.2895787. [DOI] [PubMed] [Google Scholar]
- Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annual review of immunology. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W, Sorkin E. Alterations of adrenal cortex and thyroid in mice with congenital absence of the thymus. Nat New Biol. 1972;238:282–285. doi: 10.1038/newbio238282a0. [DOI] [PubMed] [Google Scholar]
- Reis MD, Csomos K, Dias LP, Prodan Z, Szerafin T, Savino W, Takacs L. Decline of FOXN1 gene expression in human thymus correlates with age: possible epigenetic regulation. Immun Ageing. 2015;12:18. doi: 10.1186/s12979-015-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles AI, Larcher F, Whalin RB, Murillas R, Richie E, Gimenez-Conti IB, Jorcano JL, Conti CJ. Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7634–7638. doi: 10.1073/pnas.93.15.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode I, Boehm T. Regenerative capacity of adult cortical thymic epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3463–3468. doi: 10.1073/pnas.1118823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode I, V, Martins C, Kublbeck G, Maltry N, Tessmer C, Rodewald HR. Foxn1 Protein Expression in the Developing, Aging, and Regenerating Thymus. Journal of immunology. 2015;195:5678–5687. doi: 10.4049/jimmunol.1502010. [DOI] [PubMed] [Google Scholar]
- Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. Journal of immunology. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunological reviews. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Thome JJ, Grinshpun B, Kumar BV, Kubota M, Ohmura Y, Lerner H, Sempowski GD, Shen Y, Farber DL. Longterm maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts TA, DeMayo F, Rich S, Conneely OM, O’Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12021–12026. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature reviews Immunology. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K. Hypertrophy of the thymus and restoration of immune functions in mice and rats by gonadectomy. Mechanisms of ageing and development. 1989;47:175–185. doi: 10.1016/0047-6374(89)90030-4. [DOI] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, Hashimoto W, Sato K. Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mechanisms of ageing and development. 1992;63:57–68. doi: 10.1016/0047-6374(92)90016-7. [DOI] [PubMed] [Google Scholar]
- Vaidya HJ, Briones Leon A, Blackburn CC. FOXN1 in thymus organogenesis and development. European journal of immunology. 2016;46:1826–1837. doi: 10.1002/eji.201545814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tienen FH, Laeremans H, van der Kallen CJ, Smeets HJ. Wnt5b stimulates adipogenesis by activating PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling pathway together with Wnt5a. Biochemical and biophysical research communications. 2009;387:207–211. doi: 10.1016/j.bbrc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Wei T, Zhang N, Guo Z, Chi F, Song Y, Zhu X. Wnt4 signaling is associated with the decrease of proliferation and increase of apoptosis during age-related thymic involution. Mol Med Rep. 2015;12:7568–7576. doi: 10.3892/mmr.2015.4343. [DOI] [PubMed] [Google Scholar]
- Weinstein Y. Impairment of the hypothalamo-pituitary-ovarian axis of the athymic “nude” mouse. Mechanisms of ageing and development. 1978;8:63–68. doi: 10.1016/0047-6374(78)90007-6. [DOI] [PubMed] [Google Scholar]
- Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. The Journal of experimental medicine. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. Journal of immunology. 2009;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Horvath TL, Mangelsdorf DJ, Kliewer SA, Dixit VD. Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:1026–1031. doi: 10.1073/pnas.1514511113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, Owen JS, Thomas MJ, Francis J, Parks JS, Dixit VD. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell reports. 2012;1:56–68. doi: 10.1016/j.celrep.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Yang H, Sun Y, Smith RG, Manley NR, Vandanmagsar B, Dixit VD. Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J Biol Chem. 2009;284:7068–7077. doi: 10.1074/jbc.M808302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su DM. Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging cell. 2007;6:663–672. doi: 10.1111/j.1474-9726.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- Zook EC, Krishack PA, Zhang S, Zeleznik-Le NJ, Firulli AB, Witte PL, Le PT. Overexpression of Foxn1 attenuates age-associated thymic involution and prevents the expansion of peripheral CD4 memory T cells. Blood. 2011;118:5723–5731. doi: 10.1182/blood-2011-03-342097. [DOI] [PMC free article] [PubMed] [Google Scholar]