Abstract
Adenosine-to-inosine (A-to-I) RNA editing is the most common type of post-transcriptional nucleotide modification in humans, which is catalyzed in ADAR enzymes. Recent genomic studies have revealed thousands of altered RNA editing events in various cancer tissues, leading to diverse functional consequences. A critical role of individual A-to-I RNA editing events in cancer has been reported. Here, we review the current state of our knowledge on key A-to-I RNA editing events in coding and non-coding regions for their roles in cancer development and discuss their potential clinical utility. A better understanding of A-to-I RNA editing and its oncogenic mechanisms may facilitate the development of novel cancer therapeutic strategies.
Keywords: adenosine deaminases acting on RNA (ADARs), cancer driver, miRNA regulation, prognostic markers, predictive markers, therapeutic targets, therapeutics
Introduction
RNA editing is a widespread post-transcriptional mechanism that modifies specific RNA nucleotides without altering its template genomic DNA. The most prevailing RNA editing type in humans is adenosine to inosine (A to I) in which I is recognized as G by the translational machinery. The A-to-I conversion is catalyzed by adenosine deaminases that act on RNA (ADARs). The ADAR enzymes, ADAR1, ADAR2 and ADAR3, are highly conserved across metazoans [1]. ADAR1 and ADAR2 have some distinct but overlapping target specificities [2], and their enzymatic activities have been comprehensively reviewed [3–5]. In contrast, ADAR3 has no known A-to-I RNA editing activity in most tissues, but may play a role in the regulation of specific substrates of RNA editing in mammalian brains [6]. For example, ADAR3 can compete with ADAR2 to inhibit RNA editing by directly binding to GRIA2 pre-mRNA in glioblastoma [7]. The mechanisms for controlling A-to-I editing at specific sites remain largely unknown, although ADAR accessibility to target substrate RNAs and protein–protein interactions that directly change ADAR enzymatic activity have been proposed as major factors [8]. With advances in sequencing technology, recent bioinformatic analyses have revealed a large number of RNA editing sites in the human transcriptome [9,10]. So far, several million high-confidence A-to-I RNA editing sites have been annotated in humans, most of which occur in primate-specific Alu sequences with a low editing level (<1%)[10,11].
Recent studies, especially those conducted through The Cancer Genome Atlas (TCGA) project, have characterized the transcriptomes of various cancer types in a systematic way. Using these large-scale RNA-seq data, we and other groups have characterized the RNA-editing genomic landscape [12–14]. These studies reveal many altered A-to-I RNA editing events in tumor samples relative to matched normal tissues. For most tumor tissues surveyed (e.g., head and neck cancer, breast cancer, and thyroid cancer), the A-to-I RNA editing activities are elevated, which seems to be largely due to the overexpression of ADAR1 in tumors [12,13]. The vast majority of A-to-I editing in cancer takes place in 3′UTRs, introns and intergenic regions, but the absolute number of editing events in coding regions is also significant. Depending on their genomic locations, these RNA editing events could generate diverse functional consequences, including (i) introducing amino acid changes; (ii) affecting miRNA biosynthesis and target recognition; (iii) modifying splicing patterns; (iv) modulating gene regulation; and (v) affecting the lncRNA functions (Figure 1). This review focuses on the current knowledge about how individual A-to-I RNA editing events contribute to cancer development.
Figure 1.
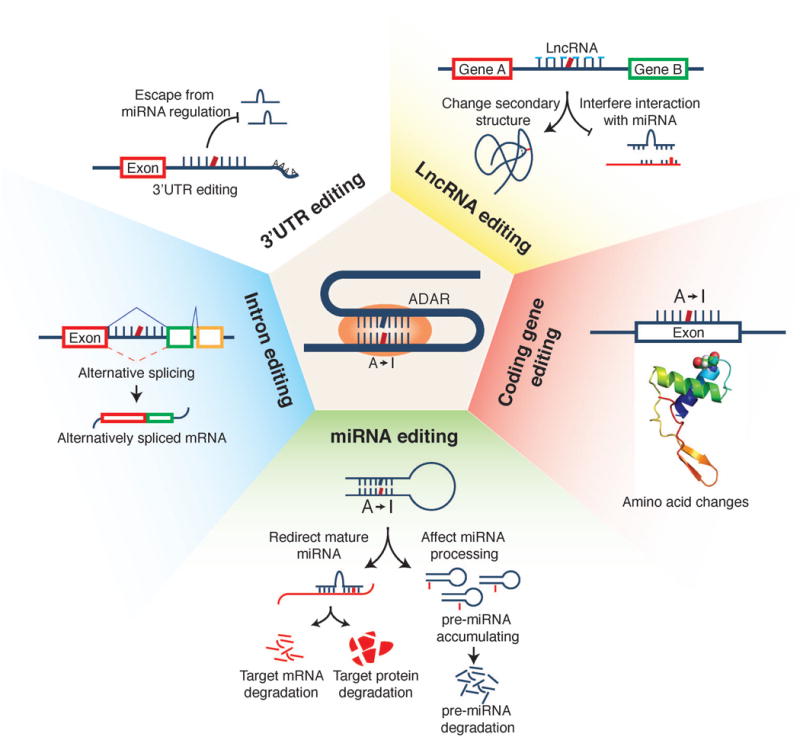
Schematic representation of functional consequences of A-to-I RNA editing.
Missense A-to-I RNA editing in cancer
For A-to-I RNA editing to occur in coding regions, premature mRNA first fold back to form an imperfect dsRNA structure, and then ADAR enzymes act on the editing site complementary sequence to introduce I (decoded as G), thereby causing amino acid substitutions in the protein [1]. Bioinformatic analyses on large-scale cancer mRNA-seq data have identified a considerable number of missense A-to-I editing events, many of which intriguingly show clinically relevant patterns such as differential editing activity among different tumor stages or tumor subtypes and correlations with patient survival times [12,13]. Some RNA editing sites are catalyzed by ADAR1 or ADAR2 separately, whereas others are edited by both enzymes [15].
Several A-to-I RNA editing events have been reported to make a positive contribution to cancer development and progression. One of the best characterized examples is the editing of AZIN1 in liver cancer. Editing of AZIN1 by ADAR1 converts a serine to glycine at residue 367, and the edited protein has a stronger affinity for antizyme and induces cytoplasmic-to-nuclear translocation of AZIN1, leading to more aggressive tumor behaviors [16]. Editing of RHOQ has been suggested to promote the potential of tumor invasion in colorectal cancer and to cause rearrangement of the actin cytoskeleton [17]. Another exciting example is the editing in SLC22A3 catalyzed by ADAR2, which results in the substitution of asparagine 72 to aspartate. This RNA editing is increased in the tumor tissues of familial esophageal cancer and significantly correlates with lymph node metastasis. Functionally, it causes a reduced expression of SLC22A3, a metastasis suppressor in this disease; and deregulation of SLC22A3 facilitates cell invasion and filopodia formation by reducing its direct association with α-actinin-4 (ACTN4)[18]. Moreover, we recently show that another two RNA editing events (GRIA2_R764G and COG3_I635V) can significantly increase cell viability in multiple cell lines but further efforts are required to elucidate their detailed oncogenic mechanisms [12]. In contrast, A-to-I RNA editing can also make a negative contribution via suppression of tumorigenesis and metastasis. ADAR2-mediated RNA editing in CDC14B modulates the Skp2/p21/p27 cell cycle pathway and inhibits the growth of glioblastoma [19]. In gastric cancer, the ADAR2-regulated recoding RNA editing at PODXL codon H241R confers a loss-of-function phenotype that neutralizes the tumorigenic ability of the unedited PODXL [20]. In breast cancer, the edited GABRA3 protein inhibits cell motility, invasion and metastasis by reduction of Akt activation. In addition, intracellular transport of the edited GABRA3 protein is modified, thereby disrupting its localization on cell membranes [21]. In esophageal cancer, ADAR2-mediated editing in IGFBP7 stabilizes the protein by changing the protease recognition site of matriptase, thereby inducing apoptosis [22]. Taken together, these examples indicate that like somatic mutations, missense RNA editing events may play critical roles in cancer development, and their functional effects often depend on tumor contexts.
Effects of A-to-I RNA editing on miRNA functions
MiRNAs are small non-coding RNAs that act as posttranscriptional repressors of gene expression [23]. The stem-loop secondary structures adopted by primary transcripts of miRNA genes (pri-miRNAs) and miRNA precursors (pre-miRNAs) enable the interactions between the A-to-I editing machinery and the miRNA biogenesis pathway [24]. ADARs can suppress miRNA maturation at different processing stages by editing-dependent and editing-independent mechanisms [25].
A-to-I editing of pri-mir-142 at +4 and +5 positions by ADAR1 and ADAR2 inhibits its cleavage by Drosha-GDCR8 and, consequently decreases the expression levels of mature miR-142-5p and miR-142-3p [24]. ADAR1-mediated editing of pri-mir-151 at −1 and +3 positions results in complete blockage of its cleavage by Dicer and accumulation of edited pre-mir-151, such that mir-151-3p expression is inhibited [26]. Editing of pre-let-7g at +4 position by ADAR2 partially prevents Dicer cleavage [27]. Editing of pri-mir-33, pri-mir-133a2 and pri-mir-379 in brain tissue has also been reported to inhibit their cleavage by Drosha [27]. In human melanocytes, editing of pri-mir-455 at the +2 and +17 positions by ADAR1 suppresses the Drosha cleavage step [28]. ADAR2 can edit pre-mir-222/221 and pre-mir-21 and decrease the expression of the corresponding mature miRNAs, which affects glioblastoma cell proliferation and migration [29]. ADAR1 editase activity impairs the miRNA biogenesis of the let-7 family and enhances progenitor self-renewal capacity, resulting in malignant reprogramming of progenitors [30].
If A-to-I RNA editing takes place within the mature miRNA, especially at the seed region of positions 2–8, a specific nucleotide change introduced can largely alter the base-pairing properties of the miRNA, thereby affecting the recognition of its target genes. Intriguingly, several such RNA editing events appear to be critical in cancer. The reduced editing at miR-376a promotes the migration and invasion of glioma cells, and the edited miRNA loses its ability to inhibit the original target RAP2A and acquires a new target AMFR [31]. In melanoma, wild-type miR-455-5p promotes melanoma metastasis through inhibition of a tumor suppressor gene CPEB1, while ADAR1-mediated RNA editing reverses these effects [28]. More recently, we performed a systematic analysis to characterize A-to-I RNA editing events in 20 cancer types using TCGA miRNA-sequencing data. Although numerous RNA editing events were identified, highly recurrent RNA editing sites with a significant editing level (i.e., >5%) in mature miRNAs are relatively limited. We identified and validated 15 such miRNA RNA editing hotspots [32]. Furthermore, in contrast to the behavior of the wild-type miR-200b, the edited miR-200b can promote cell migration and invasion through its impaired ability to inhibit ZEB1/ZEB2 and acquired concomitant ability to repress the new target LIFR, a known metastasis suppressor [32]. These results indicate that dysregulation of RNA editing on key miRNA regulators could make a notable contribution to the malignant phenotype.
A-to-I RNA editing in other non-coding regions
Although the impacts of RNA editing events in the coding regions and mature miRNAs are straightforward, the biological consequences of RNA editing events in other noncoding regions such as 3′UTRs, introns and lncRNAs remain largely unexplored. Because 3′UTRs usually contain key regulatory elements that affect mRNA stability, localization and translatability, A-to-I editing in these elements can modulate the regulation of mRNA expression [33], and several examples have been reported. ADAR1 up-regulates the expression of DHFR in breast cancer by editing the miR-25-3p and miR-125a-3p binding sites in the 3′-UTR of DHFR, which could enhance cellular proliferation and resistance to methotrexate [34]. Increased RNA editing in miRNA target sties in the 3′UTR region of MDM2 may increase its mRNA levels by abolishing microRNA-mediated repression in multiple cancer types [35]. A-to-I RNA editing in introns has been reported to affect gene splicing patterns, and thus dysregulation of RNA editing may affect the splicing isoforms expressed in the tumor context [36]. As for lncRNAs, the potential roles of RNA editing include affecting the secondary structures of lncRNAs and the interactions between miRNA and lncRNAs. A bioinformatic database for exploring this aspect of RNA editing has been developed [37].
Clinical utility of A-to-I RNA editing
Given its diverse functions in cancer, A-to-I RNA editing may represent an exciting theme to provide some utility for cancer treatment (Figure 2). First, several studies have shown a considerable number of RNA editing sites for which the editing level is correlated with patient survival times, suggesting their utility as prognostic markers [12,13,16,20,32]. Other than individual RNA editing sites, Paz-Yaacov et al. developed an Alu editing index (AEI), which represents the weighted average editing level across all expressed Alu sequences, and showed that the increased index is associated with worse prognosis in multiple cancer types [13]. The key related question is whether RNA editing confers additional power given clinical and other molecular makers already used in clinics. Further efforts are needed to evaluate the prognostic power of individual RNA editing events or their global editing index in independent patient cohorts. Second, several studies provide the evidence that a specific RNA editing event could selectively affect the response of cancer therapies, suggesting utility as predictive markers. For example, RNA recoding editing in COG3 and GRIA2 increases sensitivity to MEK inhibitors [12]. Third, like driver somatic mutations, some RNA editing events appear to play a causal role in tumor progression. For example, both in vitro and in vivo experiments show that the edited AZIN1_S367G protein could confer more aggressive tumor behaviors in liver cancer [16]. Thus, such an edited protein may represent potential therapeutic targets for which specific targeted agents may be developed. Finally, some edited miRNAs appear to play a negative role in tumor progression. For example, edited miR-376a has been shown to inhibit tumor migration and invasion by targeting oncogene AMFR [31]. Since there are more than 30 clinical trials using siRNA/shRNA therapeutics [38], such edited small RNA molecules have the potential to treat tumors.
Figure 2. Potential clinical utility of A-to-I RNA editing in cancer.
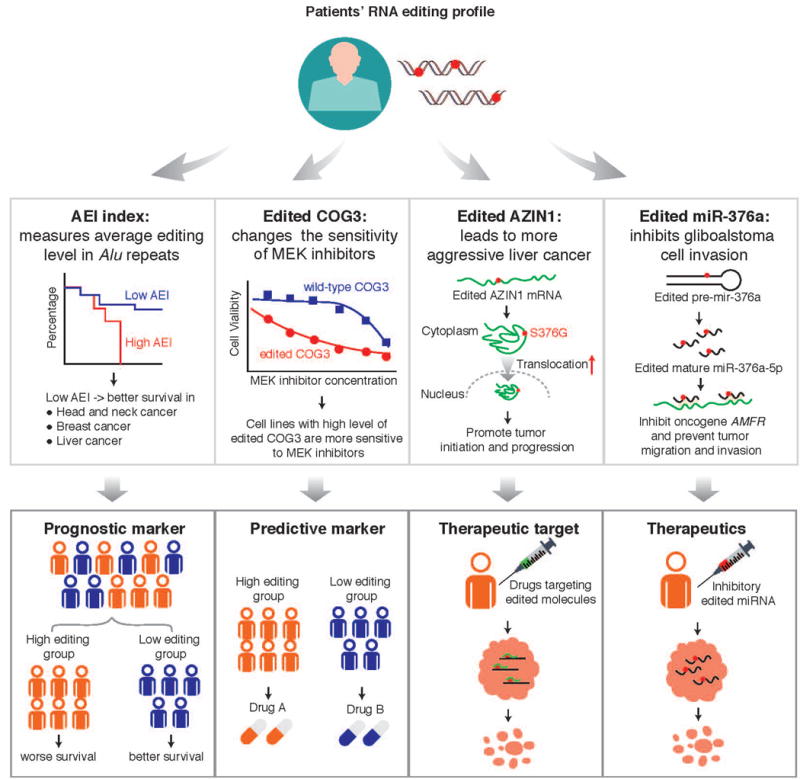
The Alu editing index (AEI) is used to show that the RNA editing profile correlates with patient survival time, suggesting the utility of prognostic markers; COG3 editing is used to show how RNA editing affects drug sensitivity, suggesting the utility of predictive markers; AZIN1 editing is an example of RNA editing driving tumor initiation and growth, thereby representing potential therapeutic targets; and edited miR-376a is used to show that edited miRNA can inhibit tor migration and invasion, thereby representing potential therapeutics.
Concluding remarks
A-to-I RNA editing has emerged as a new player that contributes to cancer development and progression. While A-to-I RNA editing events have been systematically identified in cancer transcriptomes, the RNA editing events that have been functionally demonstrated as relevant to cancer are still limited, yet likely represent only the tip of the iceberg. Given the tremendous complexity of post-transcriptional and translational regulations, it remains unclear to what extent RNA editing events detected by deep sequencing studies can be incorporated into the “functional” version of gene products. Further, since most RNA editing events occur at a low level, functional assessment of their effects in cancer should take their real physiological levels into consideration. Finally, there is a need to elucidate how key RNA editing events interact with other cancer drivers, thereby collectively defining the complexity and therapeutic vulnerability of cancer. A better understanding of “driver-like” A-to-I RNA editing events in cancer and their related oncogenic mechanisms may suggest some unexpected but exciting therapeutic strategies and tools against cancer.
Acknowledgments
This study was supported by the U. S. National Institutes of Health (grant R01CA175486 to H.L.) and Natural Scientific Foundation of China (grant 81572777 to X.X.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
special interest (*) or outstanding interest (**)
- 1.Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem. 2010;79:321–349. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riedmann EM, Schopoff S, Hartner JC, Jantsch MF. Specificity of ADAR-mediated RNA editing in newly identified targets. RNA. 2008;14:1110–1118. doi: 10.1261/rna.923308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishikura K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat Rev Mol Cell Biol. 2016;17:83–96. doi: 10.1038/nrm.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song C, Sakurai M, Shiromoto Y, Nishikura K. Functions of the RNA Editing Enzyme ADAR1 and Their Relevance to Human Diseases. Genes (Basel) 2016;7 doi: 10.3390/genes7120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas JM, Beal PA. How do ADARs bind RNA? New protein-RNA structures illuminate substrate recognition by the RNA editing ADARs. Bioessays. 2017;39 doi: 10.1002/bies.201600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA. 2000;6:755–767. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oakes E, Anderson A, Cohen-Gadol A, Hundley HA. Adenosine Deaminase That Acts on RNA 3 (ADAR3) Binding to Glutamate Receptor Subunit B Pre-mRNA Inhibits RNA Editing in Glioblastoma. J Biol Chem. 2017;292:4326–4335. doi: 10.1074/jbc.M117.779868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deffit SN, Hundley HA. To edit or not to edit: regulation of ADAR editing specificity and efficiency. Wiley Interdiscip Rev RNA. 2016;7:113–127. doi: 10.1002/wrna.1319. [DOI] [PubMed] [Google Scholar]
- 9.Bazak L, Haviv A, Barak M, Jacob-Hirsch J, Deng P, Zhang R, Isaacs FJ, Rechavi G, Li JB, Eisenberg E, et al. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Res. 2014;24:365–376. doi: 10.1101/gr.164749.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramaswami G, Li JB. RADAR: a rigorously annotated database of A-to-I RNA editing. Nucleic Acids Res. 2014;42:D109–113. doi: 10.1093/nar/gkt996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picardi E, D’Erchia AM, Lo Giudice C, Pesole G. REDIportal: a comprehensive database of A-to-I RNA editing events in humans. Nucleic Acids Res. 2017;45:D750–D757. doi: 10.1093/nar/gkw767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Han L, Diao L, Yu S, Xu X, Li J, Zhang R, Yang Y, Werner HM, Eterovic AK, Yuan Y, et al. The Genomic Landscape and Clinical Relevance of A-to-I RNA Editing in Human Cancers. Cancer Cell. 2015;28:515–528. doi: 10.1016/j.ccell.2015.08.013. This study characterizes the genome-wide pattern of A-to-I RNA editing in 17 cancer types, and provides the first evidence that RNA editing could affect drug sensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Paz-Yaacov N, Bazak L, Buchumenski I, Porath HT, Danan-Gotthold M, Knisbacher BA, Eisenberg E, Levanon EY. Elevated RNA Editing Activity Is a Major Contributor to Transcriptomic Diversity in Tumors. Cell Rep. 2015;13:267–276. doi: 10.1016/j.celrep.2015.08.080. This study provides a pan-cancer analysis of A-to-I RNA editing global patterns, and introduces a global editing index for patient survival prediction. [DOI] [PubMed] [Google Scholar]
- 14.Fumagalli D, Gacquer D, Rothe F, Lefort A, Libert F, Brown D, Kheddoumi N, Shlien A, Konopka T, Salgado R, et al. Principles Governing A-to-I RNA Editing in the Breast Cancer Transcriptome. Cell Rep. 2015;13:277–289. doi: 10.1016/j.celrep.2015.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan TH, Lin CH, Qi L, Fei J, Li Y, Yong KJ, Liu M, Song Y, Chow RK, Ng VH, et al. A disrupted RNA editing balance mediated by ADARs (Adenosine DeAminases that act on RNA) in human hepatocellular carcinoma. Gut. 2014;63:832–843. doi: 10.1136/gutjnl-2012-304037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Li Y, Lin CH, Chan TH, Chow RK, Song Y, Liu M, Yuan YF, Fu L, Kong KL, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat Med. 2013;19:209–216. doi: 10.1038/nm.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SW, Kim HP, Shin JY, Jeong EG, Lee WC, Kim KY, Park SY, Lee DW, Won JK, Jeong SY, et al. RNA editing in RHOQ promotes invasion potential in colorectal cancer. J Exp Med. 2014;211:613–621. doi: 10.1084/jem.20132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Fu L, Qin YR, Ming XY, Zuo XB, Diao YW, Zhang LY, Ai J, Liu BL, Huang TX, Cao TT, et al. RNA editing of SLC22A3 drives early tumor invasion and metastasis in familial esophageal cancer. Proc Natl Acad Sci U S A. 2017;114:E4631–E4640. doi: 10.1073/pnas.1703178114. This study describes an intriguing example of A-to-I RNA editing of SLC22A3 that contributes to the early development and progression of familial esophageal cancer in high-risk individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galeano F, Rossetti C, Tomaselli S, Cifaldi L, Lezzerini M, Pezzullo M, Boldrini R, Massimi L, Di Rocco CM, Locatelli F, et al. ADAR2-editing activity inhibits glioblastoma growth through the modulation of the CDC14B/Skp2/p21/p27 axis. Oncogene. 2013;32:998–1009. doi: 10.1038/onc.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan TH, Qamra A, Tan KT, Guo J, Yang H, Qi L, Lin JS, Ng VH, Song Y, Hong H, et al. ADAR-Mediated RNA Editing Predicts Progression and Prognosis of Gastric Cancer. Gastroenterology. 2016;151:637–650 e610. doi: 10.1053/j.gastro.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Gumireddy K, Li A, Kossenkov AV, Sakurai M, Yan J, Li Y, Xu H, Wang J, Zhang PJ, Zhang L, et al. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat Commun. 2016;7:10715. doi: 10.1038/ncomms10715. This study reports A-to-I RNA-edited form of Gabra3 only in non-invasive breast cancers and show that edited Gabra3 suppresses breast cancer cell invasion and metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YB, Liao XY, Zhang JB, Wang F, Qin HD, Zhang L, Shugart YY, Zeng YX, Jia WH. ADAR2 functions as a tumor suppressor via editing IGFBP7 in esophageal squamous cell carcinoma. Int J Oncol. 2017;50:622–630. doi: 10.3892/ijo.2016.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Chendrimada TP, Wang Q, Higuchi M, Seeburg PH, Shiekhattar R, Nishikura K. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat Struct Mol Biol. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negi V, Paul D, Das S, Bajpai P, Singh S, Mukhopadhyay A, Agrawal A, Ghosh B. Altered expression and editing of miRNA-100 regulates iTreg differentiation. Nucleic Acids Res. 2015;43:8057–8065. doi: 10.1093/nar/gkv752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawahara Y, Zinshteyn B, Chendrimada TP, Shiekhattar R, Nishikura K. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO Rep. 2007;8:763–769. doi: 10.1038/sj.embor.7401011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawahara Y, Megraw M, Kreider E, Iizasa H, Valente L, Hatzigeorgiou AG, Nishikura K. Frequency and fate of microRNA editing in human brain. Nucleic Acids Res. 2008;36:5270–5280. doi: 10.1093/nar/gkn479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Shoshan E, Mobley AK, Braeuer RR, Kamiya T, Huang L, Vasquez ME, Salameh A, Lee HJ, Kim SJ, Ivan C, et al. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis. Nat Cell Biol. 2015;17:311–321. doi: 10.1038/ncb3110. This study finds that wild-type miR-455 enhances melanoma growth and metastasis through the tumour suppressor gene CPEB1, whereas the edited form inhibits these effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomaselli S, Galeano F, Alon S, Raho S, Galardi S, Polito VA, Presutti C, Vincenti S, Eisenberg E, Locatelli F, et al. Modulation of microRNA editing, expression and processing by ADAR2 deaminase in glioblastoma. Genome Biol. 2015;16:5. doi: 10.1186/s13059-014-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zipeto MA, Court AC, Sadarangani A, Delos Santos NP, Balaian L, Chun HJ, Pineda G, Morris SR, Mason CN, Geron I, et al. ADAR1 Activation Drives Leukemia Stem Cell Self-Renewal by Impairing Let-7 Biogenesis. Cell Stem Cell. 2016;19:177–191. doi: 10.1016/j.stem.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choudhury Y, Tay FC, Lam DH, Sandanaraj E, Tang C, Ang BT, Wang S. Attenuated adenosine-to-inosine editing of microRNA-376a* promotes invasiveness of glioblastoma cells. J Clin Invest. 2012;122:4059–4076. doi: 10.1172/JCI62925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Wang Y, Xu X, Yu S, Jeong KJ, Zhou Z, Han L, Tsang YH, Li J, Chen H, Mangala LS, et al. Systematic characterization of A-to-I RNA editing hotspots in microRNAs across human cancers. Genome Res. 2017;27:1112–1125. doi: 10.1101/gr.219741.116. This study systematically identifies the A-to-I RNA editing hotspots in miRNAs and show that edited miR-200b switches its role from suppressing to activating tumor invasion by targeting a new target LIFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang H, Landweber LF. Hypothesis: RNA editing of microRNA target sites in humans? RNA. 2007;13:463–467. doi: 10.1261/rna.296407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Nakano M, Fukami T, Gotoh S, Nakajima M. A-to-I RNA Editing Up-regulates Human Dihydrofolate Reductase in Breast Cancer. J Biol Chem. 2017;292:4873–4884. doi: 10.1074/jbc.M117.775684. This study shows that ADAR1 increases the expression of DHFR by editing the miR-25-3p and miR-125a-3p binding sites in the 3′-UTR of DHFR, enhancing cellular proliferation and resistance to methotrexate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Yang CS, Varelas X, Monti S. Altered RNA editing in 3′ UTR perturbs microRNA-mediated regulation of oncogenes and tumor-suppressors. Sci Rep. 2016;6:23226. doi: 10.1038/srep23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flomen R, Knight J, Sham P, Kerwin R, Makoff A. Evidence that RNA editing modulates splice site selection in the 5-HT2C receptor gene. Nucleic Acids Res. 2004;32:2113–2122. doi: 10.1093/nar/gkh536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong J, Liu C, Liu W, Xiang Y, Diao L, Guo AY, Han L. LNCediting: a database for functional effects of RNA editing in lncRNAs. Nucleic Acids Res. 2017;45:D79–D84. doi: 10.1093/nar/gkw835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burnett JC, Rossi JJ, Tiemann K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J. 2011;6:1130–1146. doi: 10.1002/biot.201100054. [DOI] [PMC free article] [PubMed] [Google Scholar]


