Abstract
Background
Widespread implementation of ABO incompatible (ABOi) living donor kidney transplantation (LDKT) has been proposed as a means to partially ameliorate the national shortage of deceased donor kidneys. The acceptance of this practice has been encouraged by reports from experienced centers demonstrating acute rejection (AR) rates similar to those obtained with ABO compatible (ABOc) LDKT. AR rate and graft survival following ABOi LDKT on a national level have yet to be fully determined.
Study Design
Adult (>18years) LDKT recipients from 2000 to 2015 reported to the Scientific Registry of Transplant Recipients were studied. AR rates in the first post-transplant year (modified Poisson regression) and graft survival (Cox proportional hazards) were assessed by ABO compatibility status (ABOi: 930; ABOc: 89,713).
Results
Patients undergoing ABOi LDKT were found to have AR rate of 19.4% compared to 10.5% for ABOc recipients (p<0.0001). After adjusting for recipient and donor related risk factors, patients undergoing ABOi LDKT were found to have a 1.76-fold greater risk for AR within 1-year of transplant compared to ABOc LDKT recipients (adjusted Relative Risk (aRR): 1.76; 95%CI 1.54-2.01). Moreover, there was a 2.34-fold greater risk of death-censored graft loss at 1-year post-transplant among ABOi vs. ABOc LDKT recipients (aHR: 2.34; 95% CI 1.85-2.96).
Conclusions
Based on these findings, the low rates of AR and excellent short-term graft survival presented in single center series may not be sustainable on a national level. These findings highlight the potential utility for identification of centers of excellence and regionalization of ABOi LDKT.
Keywords: ABO incompatible, Kidney transplantation, Acute rejection, Graft survival, Patient survival
Introduction
Despite efforts aimed to increase the availability of kidney transplantation, organ scarcity remains a prevalent issue in the United States (US), with nearly 90,000 patients on the kidney transplant waiting list (1). Of the 17,000 kidney transplants performed annually in the US, only one-third are from living donors(1). Blood group incompatibility (ABOi) represents a major barrier to living kidney donor transplantation (LDKT) as more than one-third of persons who come forward to donate are found to be ABOi with their intended recipients(2). Advances in technology and development of desensitization protocols now allow for transplantation across this barrier in select cases, and as such, ABOi LDKT has been proposed as a solution to address the national organ scarcity through increasing access to LDKT.
After bleak preliminary results for ABOi LDKT were published(3), concerns for hyper-acute rejection initially stifled further pursuits of incompatible transplantation. Then, during the 1980s, reports of successful ABOi LDKT in Japan (4-6) sparked a renewed interest in the US. Several single-center center studies in the US have since demonstrated equivalent outcomes for patients undergoing ABOi LDKT compared to ABO compatible (ABOc) LDKT, with similar graft-survival rates (7-9). Advances in immunosuppression regimens, with more efficacious agents, and desensitization protocols, including plasmapheresis (PP) and intravenous immunoglobulin (IVIg) without the need for splenectomy, also led to further propagation of kidney transplantation across the ABO barrier(10, 11). Perhaps as a result of single centers' promising experiences, the utilization of ABOi LDKT in US has become more widespread over the past few decades, with 120 centers performing at least one ABOi LDKT as of 2010 (12).
In their retrospective analysis from 1995-2010, Montgomery et. al. found higher rates of graft loss for ABOi recipients in the first two weeks post-transplant, but found no significant long term differences in patient or allograft survival, endorsing the utility of ABOi LDKT in the absence of an available compatible donor(12). However, the national experience after broader implementation of ABOi LDKT has not been widely described, specifically with regard to acute rejection (AR) rates. In order to address this issue, we sought to evaluate the landscape of ABOi LDKT in the US from 2000-2015 using data obtained from the Scientific Registry of Transplant Recipients (SRTR). The goals of the study were to assess national outcome trends following ABOi LDKT, including risk for AR, graft loss, and mortality. We hypothesized that AR rates following ABOi LDKT on a national level may be higher than had previously been reported in single-center studies, and that these higher AR rates would translate to worse graft and patient survival for ABOi LDKT recipients compared to their ABOc counterparts.
Methods
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This study received approval from the University of Alabama at Birmingham Institutional Review Board.
Study Design
A retrospective cohort study was carried out among all adult (≥18years) LDKT recipients between January 1, 2000 and December 31, 2015. Transplant recipients were classified according to recipient-donor ABO mismatch, i.e., either compatible (ABOc) or incompatible (ABOi). Because donor blood type A2 kidneys have reduced antigen expression, and thus have outcomes equivalent with ABOc, we classified all transplants of these donor kidneys as ABOc(9, 13). The primary outcome was AR within 1-year of transplant. Secondary outcomes included 1-and 3-year patient mortality, all cause graft failure, and death censored graft failure.
AR was determined by review of follow-up records through the first year post-transplant. Recipients were classified as having had AR if any episode of AR was reported, both treated and untreated with anti-rejection agents, or AR was listed as the reason for graft failure, or AR had been confirmed by biopsy. Other variables of interest included donor age (≤ 50 years or > 50 years), recipient age (≤ 50 years or > 50 years), sex, race (White, African American, Asian, Other/Unknown), pre-transplant maximum panel reactive antibody (PRA) > 80%, number of human leukocyte antigen (HLA) mismatches ≥ 3, and history of previous kidney transplant.
Statistical Analysis
Acute Rejection
Chi-square tests were used to test for significant differences for variables of interest between ABOi and ABOc groups. A modified Poisson regression was used to estimate crude and adjusted risk ratios (aRR) and corresponding 95% confidence intervals (CI) were calculated. Multivariate models included donor age, and recipient age, race, PRA >80%, HLA ≥ 3 mismatches, and previous kidney transplant. All models included Transplant Center ID to adjust for potential confounding at the center-level. Potential interactions were examined using cross-product terms of ABOi status and covariates within adjusted models, and assessing the Wald statistic for statistical significance (p<0.05).
Secondary Outcomes
Outcomes were censored for administrative end of study (December 31, 2016). Patient death was defined as the time from transplantation to death. Death indicators were supplemented by linkage to the Social Security Death Master File; death and graft loss were supplemented by linkage to data from the Centers for Medicare and Medicaid Services. All-cause graft failure was defined as the time from transplantation to the earliest of the following: death, graft loss, or return to dialysis. Death-censored graft failure was defined as the time from transplantation to graft loss and/or return to dialysis, censored for death.
Recipient characteristics included age at transplant, race, diabetes present at transplant, maximum PRA, HLA mismatch, and dialysis years. Other covariates included donor age, transplant center, and transplant year. For all covariates other than ABOi status, the proportional hazards assumption was assessed using time dependent variables, and if not met, extended Cox models were used with the addition of time dependent variables. Multiplicative interactions with ABOi status and other covariates were assessed using cross-product terms for secondary outcomes in Cox proportional hazard models.
Results
There were 930 ABOi and 89,713 ABOc recipients identified. The incidence of ABOi LDKT increased steadily in the early 2000s, peaked around 2010, and most recently has plateaued. As of 2015, ABOi LDKT accounted for 1.3% of all LDKT performed in the US (Figure 1).
Figure 1.
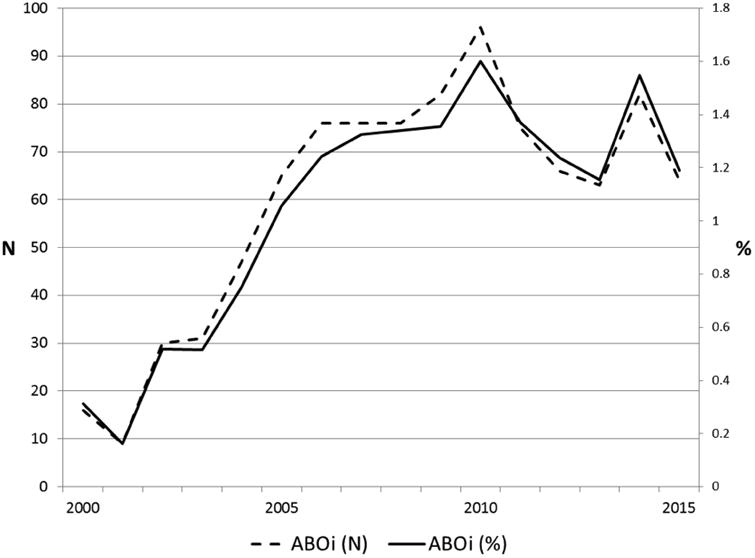
ABO-Incompatible live donor kidney transplantation, 2000 to 2015.
ABOi recipients were more likely to be older than age 50 years at the time of transplantation (30.2% vs. 22.9%, p <0.0001) and were also more likely to have received kidneys from donors greater than 50 years (48.5% vs. 43.9%, p=0.01). There were no differences in gender between the two groups. A larger proportion of ABOi LDKT recipients were African American (17.1% vs. 14.2, p=0.02), and were more commonly blood type O (68.5% vs. 44.4%, p<0.0001). More ABOi recipients had maximum PRA >80% (11.3% vs. 4.2, p<0.0001), but there was no difference in HLA mismatch. However, more ABOi recipients had history of prior LDKT (17.6% vs 10.5%, p < 0 .0001) (Table 1).
Table 1. Demographic and Clinical Characteristics of Adult Live Donor ABO-Incompatible and ABO-Compatible Recipients, 2000 to 2015.
| Characteristic | ABOi (n=930) | ABOc (n=89,713) | p Value |
|---|---|---|---|
| Donor age > 50 y | 281 (30.2) | 20,573 (22.9) | <0.0001 |
| Recipient age > 50 y | 449 (48.3) | 39,398 (43.9) | 0.0076 |
| Male recipient | 559 (60.1) | 54,355 (60.6) | 0.7656 |
| Recipient race | 0.0219 | ||
| White | 713 (76.7) | 72,030 (80.3) | |
| Black | 159 (17.1) | 12,700 (14.2) | |
| Asian | 48 (5.2) | 3,771 (4.2) | |
| Other/unknown | 10 (1.1) | 1,212 (1.4) | |
| Recipient ABO | <0.0001 | ||
| A | 129 (13.9) | 33,376 (37.1) | |
| A1 | 7 (0.8) | 1,031 (1.2) | |
| A1B | 0 (-) | 86 (0.1) | |
| A2 | 0 (-) | 199 (0.2) | |
| A2B | 0 (-) | 16 (0.0) | |
| AB | 0 (-) | 3,405 (3.8) | |
| B | 157 (16.9) | 11,762 (13.1) | |
| O | 637 (68.5) | 39,838 (44.4) | |
| Maximum PRA > 80% | 105 (11.3) | 3,719 (4.2) | <0.0001 |
| HLA mismatches ≥ 3 | 659 (71.3) | 61,824 (69.5) | 0.2372 |
| Previous kidney transplant | 164 (17.6) | 9,541 (10.6) | <0.0001 |
| Acute rejection w/in 1-year | 180 (19.4) | 9,383 (10.5) | <0.0001 |
ABOc, ABO-compatible; ABOi, ABO-incompatible; HLA, human leukocyte antigen; PRA, panel reactive antibody
The AR rate was 19.4% in the first year following LDKT for ABOi recipients, compared to 10.5% for ABOc. Compared to ABOc LDKT recipients, ABOi LDKT recipients had a 1.76-fold increased risk for AR within the first year of transplantation (aRR: 1.76; 95%CI 1.54-2.01). Other significant factors that were associated with increased AR risk were high PRA (aRR: 1.51; 95%CI 1.40-1.64) and HLA mismatch (aRR: 1.60; 95% CI 1.53-1.68). Donor age was not independently predictive of AR, but recipient age greater than 50 was found to be protective, with a decreased risk of AR at one year (aRR: 0.71; 95% CI 0.68-0.74) (Table 2). For acute rejection, there was no significant interaction on a multiplicative scale between recipient age, ABOi and AR risk (p=0.14).
Table 2. Crude and Adjusted Relative Risks and 95% Confidence Intervals for the Association Between Acute Rejection Within 1-Year of Transplantation and ABOi and Select Risk Factors, 2000 to 2015.
| Risk factor | Relative risk (95% CI) | Adjusted relative risk (95% CI)1 |
|---|---|---|
| ABOi | 1.85 (1.62, 2.12) | 1.76 (1.54, 2.01) |
| Donor age > 50 y | 1.15 (1.10, 1.20) | 1.23 (1.18, 1.29) |
| Recipient age > 50 y | 0.74 (0.71, 0.77) | 0.71 (0.68, 0.74) |
| Recipient race | ||
| White | Ref | Ref |
| Black | 1.16 (1.10, 1.22) | 1.12 (1.06, 1.18) |
| Asian | 0.74 (0.66, 0.83) | 0.73 (0.66, 0.82) |
| Other/unknown | 0.96 (0.81, 1.13) | 0.97 (0.82, 1.15) |
| Maximum PRA > 80% | 1.51 (1.40, 1.63) | 1.51 (1.40, 1.64) |
| HLA mismatches ≥ 3 | 1.58 (1.51, 1.66) | 1.60 (1.53, 1.68) |
| Previous kidney transplant | 1.14 (1.08, 1.21) | 1.02 (0.99, 1.09) |
Adjusted for recipient race and age at transplant, HLA mismatches GT 3, maximum PRA, previous kidney transplant, and donor age.
ABOi, ABO-incompatible; aRR, adjusted relative risk; HLA, human leukocyte antigen; PRA, panel reactive antibody.
Compared to ABOc LDKT recipients, there was a statistically significant increased risk for mortality at 1-year post-transplant among ABOi LDKT recipients (aHR: 1.74; 95%CI 1.15-2.63), which persisted at 3-years post-transplant (aHR: 1.51; 95%CI 1.15-1.99). Similar findings were observed for all-cause graft failure for ABOi LDKT recipients at 1 and 3-years post-transplant (aHR: 2.27; 95%CI 1.76-2.93 and aHR: 1.70; 95%CI 1.41-2.06 respectively). Likewise compared to ABOc LDKT recipients, there was a 2.34-fold increased risk for death-censored graft failure at 1-year post-transplant for ABOi recipients (aHR: 2.34; 95%CI 1.85-2.96), which also persisted 3-years post-transplant (aHR: 1.82; 95% CI 1.45-2.27) (Table 3). At 3-years post-transplant there were significant interactions between recipient age and ABOi, and risks for graft failure (p=0.02) and for death censored graft failure (p=0.01). For example, stratified by recipient age, among age 50 and older at transplant, ABOi recipients had an increased relative risk for 3-year graft failure (aHR: 2.04; 95 CI 1.57- 2.65) and 3-year death censored graft failure (aHR: 2.81; 95 CI 1.98-3.99); whereas, among less than 50 years of age at transplant, ABOi recipients had similar risks for 3-year graft failure (aHR: 1.09; 95 CI 0.77-1.54) and 3-year death censored graft failure (aHR: 1.05; 95 CI 0.71-1.54).
Table 3. Crude and Adjusted Hazard Ratios and 95% Confidence Intervals for Live Donor ABO-Incompatible Transplantation 2000 to 2015, Patient Survival, All-Cause Graft Failure, and Death Censored Graft Failure.
| Follow-up | Patient survival | All-cause graft failure | Death censored graft-failure | |||
|---|---|---|---|---|---|---|
| Crude HR (95% CI) | aHR (95% CI)* | Crude HR (95% CI) | aHR (95% CI)† | Crude HR (95% CI) | aHR (95% CI)† | |
| 1-y | 1.81 (1.26-2.60) | 1.74 (1.15-2.63)* | 2.23 (1.78-2.79) | 2.27 (1.76-2.93)‡ | 2.42 (1.85-3.17) | 2.34 (1.85-2.96)‖ |
| 3-y | 1.55 (1.21-1.98) | 1.51 (1.15-1.99)† | 1.71 (1.44-2.03) | 1.70 (1.41-2.06)§ | 1.83 (1.47-2.26) | 1.82 (1.45-2.27)‖ |
Adjusted for recipient race and age at transplant, HLA mismatches GT 3, maximum PRA, previous kidney transplant, dialysis years, presence of diabetes at transplant, donor age, transplant center and transplant year.
Adjusted for recipient race and age at transplant, maximum PRA, previous kidney transplant, dialysis years, presence of diabetes at transplant, donor age, transplant center and transplant year.
Adjusted for recipient race, HLA mismatches GT 3, maximum PRA, dialysis years, presence of diabetes at transplant, donor age, transplant center and transplant year;
Adjusted for recipient race, HLA mismatches GT 3, maximum PRA, previous kidney transplant, dialysis years, presence of diabetes at transplant, donor age, transplant center and transplant year;
Adjusted for recipient race, HLA mismatches GT 3, maximum PRA, dialysis years, donor age, transplant center and transplant year.
aHR, adjusted Hazard Ratio; HLA mismatches GT3, human leukocyte antigen greater than 3; PRA, panel reactive antibody.
Discussion
The results from this national study of 90,643 LDKT recipients from 2000 through 2015 demonstrate an increased risk of AR for ABOi recipients in the US, with an observed AR rate nearly double that of the ABOc cohort. Moreover, ABOi LDKT recipients were 76% more likely to develop AR within the first year following transplantation. The risk of AR for ABOi LDKT recipients was found to be greater than the risk posed by either high level of PRA or HLA mismatch. The ABOi recipient cohort did have more elderly recipients and donors and a larger AA population, but even after accounting for these demographic differences, the discrepancy in AR based on ABO compatibility status persisted. Importantly, the observed higher rate of AR after ABOi LDKT translated into worse 1 and 3-year post-transplant patient and graft survival for ABOi LDKT recipients compared to their ABOc counterparts. These overall findings support previous reports of higher risks for graft failure and rejection early after ABOi LDKT and highlight the need for increased surveillance during the critical period following ABOi LDKT(12, 14). Moreover, when exploring the effect of older recipient age on outcomes among the cohort of ABOi LDKT recipients through effect modification analyses, we found statistically significant multiplicative interactions between older age and receipt of an ABOi LDKT for graft loss at 3 years. This suggests that ABOi LDKT recipients over the age of 50 years are at even greater risk for graft loss than their younger counterparts.
While the results of this study demonstrate worse outcomes among ABOi LDKT recipients, without question the ABO barrier continues to contribute to the gap between organ supply and demand, as more than a third of living donors will be blood group incompatible with their intended recipient (2). Therefore, identifying mechanisms for overcoming ABO incompatibility are paramount. Kidney paired donation (KPD) is one such method, and helps facilitate LDKT by taking two incompatible donor-recipient pairs (e.g., D1-R1 and D2-R2) and “swapping” donor kidneys. For example, D1 simply donates a kidney in honor of his/her intended recipient (R1) to another recipient in need (R2), and in return that recipient's living donor (D2) gives a kidney to their original recipient (D1). The end result generates two compatible LDKTs (15). Prior studies have demonstrated the efficacy and utility of KPD programs, and suggest that more than two-thirds of incompatible pairs could find a match within a national KPD program (2, 16, 17).
However, not every patient will find a match via KPD secondary to blood group imbalances (too few blood group O donors), high sensitivity (PRA greater than 95%), or positive cross-matches with potential ABO compatible donors (2); and as such, desensitization and subsequent transplantation across the ABO barrier remains an important option for patients unable to obtain a match in a KPD. Current desensitization regimens consist of removing anti-ABO antibodies, through PP or immunoadsorption, and immunomodulation by IVIg, accompanied by maintenance immunosuppression (16). Although our findings demonstrate worse overall outcomes with ABOi LKDT compared to ABOc LDKT, opting for desensitization and crossing the blood group barrier when attempts to find a compatible LDKT via KPD have proven unsuccessful may be associated with a survival benefit over waiting on dialysis for a compatible deceased donor kidney to become available (18-20).
In contrast to our study findings, Montgomery et. al. demonstrated no difference in graft survival 1-year post-transplant among ABOi compared to ABOc LDKT recipients. It is important to note that Montgomery and colleagues classified transplants from A2 donors into non-A recipients as ABOi, while in our study these pairs were treated as ABOc (12). Currently, there is greater consensus that A2 donors are functionally similar to blood type O donors, and as such, non-A recipients of kidney from A2 donors typically do not require desensitization prior to transplant. In fact, the new kidney allocation system for deceased donor transplantation allows A2 donor kidneys to be routinely offered to non-A (13). It is likely that the divergent methods of classifying A2 donors could have contributed to the difference in graft survival observed in our study compared to Montgomery et. al. Furthermore, the discrepancies in outcomes for ABOi LDKT recipients on a national level observed in our study compared to single center series further emphasize the importance of potentially identifying centers of excellence for ABOi LDKT and warrant consideration for regionalization of ABOi LDKT.
Although the large sample size from the SRTR database allows for the assessment of national trends in ABOi LDKT, there are limitations due to the retrospective analyses of a secondary data source, with unknown factors that could potentially confound our findings. Additionally, certain granular information is not attainable from the data. Specifically, desensitization methods cannot be assessed using SRTR data, which may explain the discrepancies observed between our national study and single center reports of outcomes after ABOi LDKT. In fact, in a 2010 national survey of transplant programs, only 50% of centers in which ABOi KT were performed utilized desensitization for all ABOi KT, while up to 80% reported desensitization for at least one blood type (21). Such variability in desensitization methods on a national level could account for some of the increased levels of AR observed in our study. Finally, we could not account for differences in degree of ABO incompatibility, measured by isoagglutinin titer levels pre-transplant, as that information was not available from the OPTN database.
Conclusions
In summary, there are currently disparities in outcomes for ABOi compared with ABOc LDKT recipients in the US. Based on these findings, it appears that the low rates of AR and excellent short-term graft and patient survival presented in single center series may not be sustainable on a national level. Consequently, at this time, broad implementation without standardization of ABOi LDKT protocols should be approached with caution. Furthermore, the results of this study also highlight the potential utility for identification of centers of excellence and regionalization of ABOi LDKT, in addition to supporting the value of KPD in this patient population.
Acknowledgments
This project was supported by the National Institutes of Health (NIH)- National Research Service Award, through Grant Award Number T32 DK007545 (PI Mustian, mentored), and the National Institute of Diabetes and Digestive and Kidney Diseases – R01 DK113980 (PI Locke) and K23 DK103918 (PI Locke, mentored). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This work was also supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Disclosures outside the scope of this work: Dr Locke is paid by Sanofi for lectures and developing educational presentations.
Support: This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): T32 DK007545 (PI Mustian, mentored), R01 DK113980 (PI Locke), and K23 DK103918 (PI Locke, mentored), and in part by Health Resources and Services Administration (HRSA) contract 234-2005-37011C.
Disclaimer: The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, NIDDK, HRSA or the US Government.
Abbreviations
- ABOc
ABO compatible
- ABOi
ABO incompatible
- AR
Acute Rejection
- aRR
Adjusted Risk Ratio
- aHR
Adjusted Hazard Ratio
- HLA
Human Leukocyte Antigen
- IVIg
Intravenous Immunoglobulin
- LDKT
Living Donor Kidney Transplantation
- OPTN
Organ Procurement and Transplantation Network
- PRA
Panel Reactive Antibody
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 129th Annual Meeting, Hot Springs, VA, December 2017.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69:A4. doi: 10.1053/j.ajkd.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Segev DL, Gentry SE, Warren DS, et al. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293:1883–1890. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 3.Hume DM, Merrill JP, Miller BF, Thorn GW. Experiences with renal homotransplantation in the human: report of nine cases. J Clin Investigation. 1955;34:327–382. doi: 10.1172/JCI103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama T, Konno O, Kihara Y, et al. Clinical outcomes and results of pathological findings of 1-year protocol biopsy in recipients of ABO-incompatible living donor kidney transplantants. Transplantation Proc. 2016;48:831–835. doi: 10.1016/j.transproceed.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Saito K, Takahara S, et al. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4:1089–1096. doi: 10.1111/j.1600-6143.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 6.Okumi M, Toki D, Nozaki T, et al. ABO-incompatible living kidney transplants: evolution of outcomes and immunosuppressive management. Am J Transplant. 2016;16:886–896. doi: 10.1111/ajt.13502. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery RA, Locke JE, King KE, et al. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246–1255. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 8.Stegall MD, Dean PG, Gloor JM. ABO-incompatible kidney transplantation. Transplantation. 2004;78:635–640. doi: 10.1097/01.tp.0000136263.46262.0d. [DOI] [PubMed] [Google Scholar]
- 9.Gloor JM, Lager DJ, Moore SB, et al. ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation. 2003;75:971–977. doi: 10.1097/01.TP.0000058226.39732.32. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RA, Locke JE. ABO-incompatible transplantation: less may be more. Transplantation. 2007;84:S8–9. doi: 10.1097/01.tp.0000296032.12974.bb. [DOI] [PubMed] [Google Scholar]
- 11.Segev DL, Simpkins CE, Warren DS, et al. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant. 2005;5:2570–2575. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery JR, Berger JC, Warren DS, et al. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603–609. doi: 10.1097/TP.0b013e318245b2af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes RC, Feurer ID, Shaffer D. A2 incompatible kidney transplantation does not adversely affect graft or patient survival. Clin Transplant. 2016;30:589–597. doi: 10.1111/ctr.12724. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Saito K. ABO-incompatible kidney transplantation. Transplantation Rev (Orlando, Fla) 2013;27:1–8. doi: 10.1016/j.trre.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Ross LF, Rubin DT, Siegler M, et al. Ethics of a paired-kidney-exchange program. N Engl J Med. 1997;336:1752–1755. doi: 10.1056/NEJM199706123362412. [DOI] [PubMed] [Google Scholar]
- 16.Pham TA, Lee JI, Melcher ML. Kidney paired exchange and desensitization: Strategies to transplant the difficult to match kidney patients with living donors. Transplantation Rev (Orlando, Fla) 2017;31:29–34. doi: 10.1016/j.trre.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: integrating paired donation into desensitization protocols. Am J Transplant. 2010;10:449–457. doi: 10.1111/j.1600-6143.2009.03001.x. [DOI] [PubMed] [Google Scholar]
- 18.Lonze BE, Bae S, Kraus ES, et al. Outcomes and risk stratification for late antibody-mediated rejection in recipients of ABO-incompatible kidney transplants: a retrospective study. Transplant Int. 2017 doi: 10.1111/tri.12969. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 20.Kim YC, Yu MY, Lee JP, et al. The effect of desensitization therapy in kidney transplantation. Clin Experimental Nephrol. 2017 doi: 10.1007/s10157-017-1424-7. [DOI] [PubMed] [Google Scholar]
- 21.Garonzik Wang JM, Montgomery RA, Kucirka LM, et al. Incompatible live-donor kidney transplantation in the United States: results of a national survey. Clin J Am Soc Nephrol. 2011;6:2041–2046. doi: 10.2215/CJN.02940311. [DOI] [PMC free article] [PubMed] [Google Scholar]


