Abstract
Chronic immune activation is a harbinger of AIDS-associated non-Hodgkin lymphoma (AIDS-NHL), yet the underlying basis is unclear. Microbial translocation, the passage of microbial components from the gastrointestinal tract into the systemic circulation, is a source of systemic immune activation in HIV infection and may be an important contributor to the chronic B cell activation and subsequent AIDS-NHL development. We measured biomarkers of microbial translocation including bacterial receptors/antibodies, intestinal barrier proteins, and macrophage activation-associated cytokines/chemokines, in serum from 200 HIV-infected men from the Multicenter AIDS Cohort Study prior to their AIDS-NHL diagnosis (mean=3.9 years; SD=1.6 years) and 200 controls. Controls were HIV-infected men who did not develop AIDS-NHL, individually matched to cases on CD4+ T cell count, prior antiretroviral drug use, and recruitment year into the cohort. Biomarkers of bacterial translocation and intestinal permeability were significantly increased prior to AIDS-NHL. Lipopolysaccharide-binding protein (LPB), fatty acid binding protein 2 (FABP2) and soluble CD14 had 1.6-, 2.9-, and 3.7-fold increases in risk for each unit increase on the natural log scale, respectively. Haptoglobin had a 2.1-fold increase and endotoxin-core antibody a 2.0-fold decrease risk for AIDS-NHL (4th versus 1st quartile). Biomarkers of macrophage activation were significantly increased prior to AIDS-NHL: B-cell activation factor (BAFF), IL18, monocyote chemoatractant protein-1 (MCP1), Tumor Necrosis factor-α (TNFα), and CCL17 had 2.2-, 2.0-, 1.6-, 2.8-, and 1.7-fold increases in risk for each unit increase on the natural log scale, respectively. These data provide evidence for microbial translocation as a cause of the systemic immune activation in chronic HIV infection preceding AIDS-NHL development.
Keywords: microbial translocation, and macrophage activation, AIDS-NHL, HIV
Introduction
Non-Hodgkin lymphoma (NHL) is an important cause of morbidity and mortality in people with HIV infection.[1–3] While the incidence of AIDS-associated NHL (AIDS-NHL) has decreased with combination antiretroviral therapy (cART), it still results in 23–30% of AIDS-related deaths in countries with readily accessible cART.[4–7] There are several AIDS-NHL subtypes; all are of B cell origin, yet subtypes are thought to differ in their pathogenesis.[8–10] Primary central nervous system lymphomas (PCNSLs) are less common, but more aggressive, than Burkkit’s lymphoma (BL), diffuse large B cell lymphoma (DLBCL), or other systemic tumors.[11] Two major mechanisms are believed to contribute to the genesis of AIDS-NHL: i) the loss of immunoregulatory control of Epstein Barr virus (EBV) infected B cells, resulting from impaired T cell function; and ii) chronic B cell activation, which leads to DNA modifying events (oncogene mutations and translocations) that contribute to lymphomagenesis.[12–14]
Epidemiologic evidence suggests a role for B cell stimulatory cytokines and molecules in the development of AIDS-NHL. Elevated levels of circulating cytokines (IL6, IL10, CXCL13, Tumor Necrosis factor-α [TNFα], Interferon-γ inducible protein [IP-10]/CXCL10), soluble receptors (sCD23, sCD27, sCD30), and markers of inflammation and immune activation (neopterin, κ and λ immunoglobulin free light chains) have been shown to precede the development of AIDS-NHL by up to 7 years.[11, 15] Examining potential sources of chronic B cell activation in the setting of HIV infection may provide insights into the etiology of AIDS-NHL. Microbial components (e.g., lipopolysaccharide [LPS]) passing across the intestinal epithelial barrier into peripheral circulation (termed microbial translocation) has been shown to contribute to systemic immune activation in chronic HIV infection, and may be a contributor to the chronic B cell activation that predisposes to AIDS-NHL.[16, 17] Microbial translocation and the consequential systemic immune activation is influenced by the loss of integrity and structural damage to the gastrointestinal barrier in chronic HIV infection, which can be measured by circulating barrier proteins including fatty acid binding protein (FABP) 2 and haptoglobin.[18][17] The presence of LPS in the bloodstream leads to consumption of antibodies against LPS (anti-endotoxin-core protein IgM, otherwise known as EndoCAb), increased production of LPS binding protein (LBP) and receptor (sCD14), and increase in activation of macrophages and the secretion of nerous cytokines and chemokines.
In this study, we sought to determine the association between these biomarkers of microbial translocation, intestinal integrity, and macrophage activation and subsequent AIDS-NHL development, to support our hypothesis that microbial translocation contributes to chronic B cell activation and AIDS-NHL risk. We also examined several B cell activation markers to confirm prior reports of significant elevations preceeding AIDS-NHL diagnosis.
Methods
Study population
This is a nested case-control study utilizing serum specimens from participants in the Multicenter AIDS Cohort Study (MACS), a prospective cohort study of the natural and treated history of HIV/AIDS in men who report sex with men. [19, 20] The participants were recruited from four U.S. metropolitan areas (Baltimore/Washington, Chicago, Los Angeles, and Pittsburgh), and study visits have been held at 6-month intervals beginning in 1983 (www.statepi.jhsph.edu/macs/macs.html). A total of 7,350 men have been enrolled in the MACS.
Participants were selected for study on the basis of pathologically confirmed AIDS-NHL after enrollment into the MACS and archival serum specimens available prior to cancer diagnosis (n=200). For each case, one control was selected randomly from all possible HIV-infected MACS participants who had not developed AIDS-NHL as of November 2014, matched on: (i) CD4+ T cell counts (± 200/ul); (ii) prior antiretroviral drug use (ever versus never); and (iii) recruitment year into the cohort (1984–85, 1987–91, or 2001+). Additionally, cases treated with a potent combination of antiretroviral drugs were matched to controls on time since first therapy, and cases who became HIV-infected after recruitment into the cohort were matched to controls by their seroconversion date.
Biomarker determination
Serum levels of haptoglobin and anti endotoxin-core protein IgM were determined by ELISA according to the manufacturer’s directions (R&D Systems and Hycult Biotech, respectively). Serum levels of all other biomarkers were determined using the Luminex platform with custom-made panels produced by R&D systems. Briefly, Luminex microparticles pre-coated with analyte-specific antibodies were incubated with diluted serum samples, followed by a biotin–antibody and by a streptavidin–phycoerythin conjugate. The fluorescence intensity of each analyte’s microparticles was quantified using a Bioplex 200 (Luminex) System Analyzer (Bio-Rad), and the data analyzed using BioPlex Manager (v 4.1.1) software. The lower limit of detection (LLD) for each biomarker was set either as the lowest value that the BioPlex Manager software could calculate using the standard curve, or as the lowest value of the standard curve, whichever was smaller. For quality control, case and control samples were equally distributed across reaction plates, and replicates were included across the reaction plates to calculate coefficients of variation (CVs). All labotaroty personnel were blinded to the case-control status of samples. Additional assay details including LLD, dilution factors, detectable fractions, and CVs, are presented in Supplemental Table 1.
Statistical analysis
We analyzed our biomarkers as natural log-transformed continuous variables. Additionally, in exploratory analyses, we examined biomarker categories by quartiles according to the distribution among the controls. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) representing the risk of AIDS-NHL associated with one log-unit change in biomarker levels or risk of AIDS-NHL associated with each of the 2nd, 3rd, and 4th quartiles compared to the 1st quartile, using conditional logistic regression. In addition to the matching factors, ORs were adjusted for covariates selected a priori for their association with AIDS-NHL in prior studies including age (continuous), race/ethnicity (categorical), and Hepatatis C virus (HCV) infection status. Each biomarker was tested for association with AIDS-NHL in separate regression models. Additionally, we tested for trends across quartiles using a continuous variable with values representing the medians of each category. We examined patterns of AIDS-NHL risk associated with biomarkers according to subgroups of systemic lymphomas or PCNSL. We also examined patterns of AIDS-NHL risk associated with biomarkers according to the time interval between serum sample collection and AIDS-NHL diagnosis. We classified this lag time into two categories: < 4 years or ≥ 4 years. These categories were selected according to the natural distribution of lag times and wanting to ensure approximately equal number of participants in each category. In addition to these stratified analyses, we also tested for statistical interactions between biomarkers and lagtime using interaction terms in the models. Lastly, we calculated pairwise correlations between all biomarkers using Pearson’s correlation coefficient.
Results
Study population description
Cases and controls were similar in their distributions by recruitment year, CD4+ T cell count, and antiretroviral drug therapy, as expected based on the matched design (Table 1). The majority of controls and cases were non-Hispanic white (80.0% and 81.0% respectively). Cases tended to be older than controls, with 44.5% of cases ≥40 years, compared to 38.0% of controls. Cases were more likely to have acute or chronic HCV infection compared to controls (10.2% versus 6.5%). Both groups had relatively high levels of CD4+ T cells, with 51.5% of controls and 46.5% of cases having >400 CD4+ T cells/mm3, and the majority were antiretroviral drug naïve (94.0% of controls and 94.5% of cases). The mean time from serum date to NHL diagnosis was 3.9 years; standard deviation of 1.6 years and a range of 1 month to 12 years. The majority of cases (69.5%) were systemic lymphomas, and DLBCL was the major subtype (48.2%).
Table 1.
Select characteristics of AIDS-NHL cases and controls
| HIV-infected controls (n = 200) | AIDS-NHL cases (n = 200) | |||
|---|---|---|---|---|
|
|
|
|||
| Count | Percentage | Count | Percentage | |
| Recruitment cohort | ||||
| 1984–1985 | 169 | 84.5% | 169 | 84.5% |
| 1987–1991 | 24 | 12.0% | 24 | 12.0% |
| 2001+ | 7 | 3.5% | 7 | 3.5% |
| Race | ||||
| White, non-Hispanic | 160 | 80.0% | 162 | 81.0% |
| Black, non-Hispanic | 23 | 11.5% | 17 | 8.5% |
| Hispanic | 16 | 8.0% | 21 | 10.5% |
| Asian or Pacific Islander | 1 | 0.5% | 0 | |
| Age* | ||||
| < 30 | 31 | 15.5% | 28 | 14.0% |
| 30 – 39 | 93 | 46.5% | 83 | 41.5% |
| 40 – 49 | 61 | 30.5% | 70 | 35.0% |
| ≥ 50 | 15 | 7.5% | 19 | 9.5% |
| Body mass index (mean ± SD)* | 23.7 ± 2.8 | 23.9 ± 3.1 | ||
| HCV status* | ||||
| Negative | 180 | 90.5% | 171 | 86.8% |
| Acute or chronic infection | 13 | 6.5% | 20 | 10.2% |
| Cleared | 6 | 3.0% | 6 | 3.0% |
| Unknown | 1 | 3 | ||
| CD4+ T-cells/mm3* | ||||
| < 200 | 40 | 20.0% | 44 | 22.0% |
| 200 – 399 | 57 | 28.5% | 63 | 31.5% |
| ≥400 | 103 | 51.5% | 93 | 46.5% |
| Unknown | ||||
| Prior HAART exposure* | ||||
| No | 188 | 94.0% | 189 | 94.5% |
| Yes | 12 | 6.0% | 11 | 5.5% |
| Time from serum date to NHL diagnosis, years (mean ± SD) | N/A | 3.9 ± 1.6 | ||
| NHL site | ||||
| Systemic | 139 | 69.5% | ||
| Central Nervous System | 61 | 30.5% | ||
| NHL subtype (systemic only) | ||||
| Diffuse large B-cell lymphoma | 67 | 48.2% | ||
| Burkitt Lymphoma | 23 | 16.5% | ||
| Lymphoplasmacytic lymphoma | 2 | 1.4% | ||
| Peripheral T-cell lymphoma | 2 | 1.4% | ||
| Primary effusion lymphoma | 1 | 0.7% | ||
| Follicular lymphoma | 1 | 0.7% | ||
| NHL, NOS | 43 | 30.9% | ||
| Tumor EBV status | ||||
| Negative | 28 | 31.8% | ||
| Positive | 60 | 68.2% | ||
| Unknown | 88 | |||
NHL, non-Hodgkin lymphoma; SD, standard deviation; HCV, hepatitis C virus; HAART, highly active antiretroviral therapy; EBV, Epstein-barr virus
The reference date for these variables is the blood collection date which was used for testing serum biomarkers
Microbial translocation biomarkers
Levels of sCD14, FABP2, and LBP were associated with AIDS-NHL risk, with 3.71-, 1.64-, and 2.97-fold increases in risk for each unit increase on the natural log scale, respectively (Table 2, Figure 1). Additionally, participants with marker levels in the highest versus lowest quartile for haptoglobin, and EndoCAb, were at 2.18-fold increased, and 2-fold decreased, risk of AIDS NHL, respectively. For some markers (haptoglobin, FABP2, and LBP), there appeared to be a threshold effect for the highest quartile: an increased AIDS-NHL risk was only observed for participants with marker levels in the highest versus lowest quartiles. In comparison, for sCD14, AIDS-NHL risk was elevated similarly across the 2nd, 3rd, and 4th quartiles. Also, AIDS-NHL risk was reduced similarly across the 2nd, 3rd, and 4th quartiles for EndoCAb.
Table 2.
Association between microbial translocation biomarkers and AIDS-NHL risk overall and stratified by time from blood collection to AIDS-NHL diagnosis
| All AIDS-NHL | < 4 years time lag | ≥4 years time lag | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Controls, N | Cases, N | OR | 95% CI | Controls, N | Cases, N | OR | 95% CI | Controls, N | Cases, N | OR | 95% CI | |
| sCD14 ng/mL | 200 | 200 | 3.71 | 1.25 – 11.00 | 103 | 103 | 14.9 | 2.41 – 91.4 | 97 | 97 | 1.40 | 0.31 – 6.36 |
|
| ||||||||||||
| < 1307 | 50 | 26 | 1 | 30 | 10 | 1 | 20 | 16 | 1 | |||
| 1307–1529 | 50 | 56 | 2.03 | 1.06 – 3.87 | 26 | 25 | 3.51 | 1.23 – 10.0 | 24 | 31 | 1.57 | 0.60 – 4.15 |
| 1529–1738 | 50 | 62 | 2.24 | 1.16 – 4.33 | 25 | 38 | 5.39 | 1.90 – 15.3 | 25 | 24 | 0.89 | 0.32 – 2.51 |
| > 1738 | 50 | 56 | 2.08 | 1.05 – 4.11 | 22 | 30 | 5.51 | 1.75 – 17.3 | 28 | 26 | 1.09 | 0.40 – 3.01 |
|
| ||||||||||||
| EndoCAb MMU/mL | 200 | 200 | 0.75 | 0.51 – 1.08 | 103 | 103 | 0.77 | 0.44 – 1.37 | 97 | 97 | 0.70 | 0.42 – 1.18 |
|
| ||||||||||||
| < 39 | 50 | 76 | 1 | 21 | 36 | 1 | 29 | 40 | 1 | |||
| 39–51 | 50 | 34 | 0.44 | 0.24 – 0.80 | 27 | 15 | 0.28 | 0.11 – 0.72 | 23 | 19 | 0.63 | 0.28 – 1.42 |
| 52–73 | 50 | 49 | 0.55 | 0.31 – 0.10 | 32 | 29 | 0.50 | 0.21 – 1.15 | 18 | 20 | 0.80 | 0.33 – 1.97 |
| > 73 | 50 | 41 | 0.50 | 0.28 – 0.90 | 23 | 23 | 0.54 | 0.22 – 1.32 | 27 | 18 | 0.43 | 0.19 – 0.99 |
|
| ||||||||||||
| FABP2 pg/mL | 200 | 200 | 1.64 | 1.13 – 2.38 | 103 | 103 | 1.64 | 0.98 – 2.77 | 97 | 97 | 1.73 | 0.96 – 3.14 |
|
| ||||||||||||
| < 694 | 50 | 37 | 1 | 27 | 20 | 1 | 23 | 17 | 1 | |||
| 694–1009 | 50 | 49 | 1.87 | 0.94 – 3.71 | 27 | 29 | 2.37 | 0.90 – 6.24 | 23 | 20 | 1.60 | 0.54 – 4.73 |
| 1010–1448 | 50 | 35 | 1.05 | 0.53 – 2.07 | 23 | 13 | 0.99 | 0.36 – 2.67 | 27 | 22 | 1.15 | 0.42 – 3.15 |
| > 1448 | 50 | 79 | 2.77 | 1.42 – 5.39 | 26 | 41 | 2.90 | 1.11 – 7.57 | 24 | 38 | 3.37 | 1.16 – 9.79 |
|
| ||||||||||||
| Haptoglobin μg/mL | 200 | 199 | 1.12 | 0.95 – 1.33 | 103 | 102 | 1.14 | 0.89 – 1.46 | 97 | 97 | 1.12 | 0.87 – 1.42 |
|
| ||||||||||||
| < 462 | 50 | 38 | 1 | 21 | 14 | 1 | 29 | 24 | 1 | |||
| 462–657 | 50 | 33 | 0.85 | 0.43 – 1.70 | 26 | 18 | 0.80 | 0.26 – 2.41 | 24 | 15 | 0.75 | 0.28 – 2.01 |
| 657–932 | 50 | 55 | 1.38 | 0.76 – 2.48 | 24 | 27 | 1.90 | 0.63 – 5.74 | 26 | 28 | 1.17 | 0.56 – 2.43 |
| > 932 | 50 | 73 | 2.18 | 1.15 – 4.12 | 32 | 43 | 3.24 | 1.18 – 8.89 | 18 | 30 | 1.63 | 0.65 – 4.13 |
|
| ||||||||||||
| LBP ng/mL | 200 | 200 | 2.97 | 1.61 – 5.48 | 103 | 103 | 7.07 | 2.44 – 20.5 | 97 | 97 | 1.68 | 0.82 – 3.43 |
|
| ||||||||||||
| < 8930 | 50 | 27 | 1 | 27 | 9 | 1 | 23 | 18 | 1 | |||
| 8931–11169 | 50 | 28 | 1.04 | 0.51 – 2.10 | 22 | 15 | 2.28 | 0.70 – 7.43 | 28 | 13 | 0.51 | 0.19 – 1.35 |
| 11169–13777 | 50 | 50 | 1.72 | 0.88 – 3.34 | 28 | 25 | 2.47 | 0.83 – 7.33 | 22 | 25 | 1.41 | 0.55 – 3.62 |
| > 13777 | 50 | 95 | 3.40 | 1.79 – 6.45 | 26 | 54 | 7.32 | 2.43 – 22.1 | 24 | 41 | 2.04 | 0.84 – 4.94 |
N, number; OR, odds ratio; CI, confidence interval. ORs were adjusted for age at serum sample date (continuous), race/ethnicity (categorical), and HCV infection status at serum sample date.
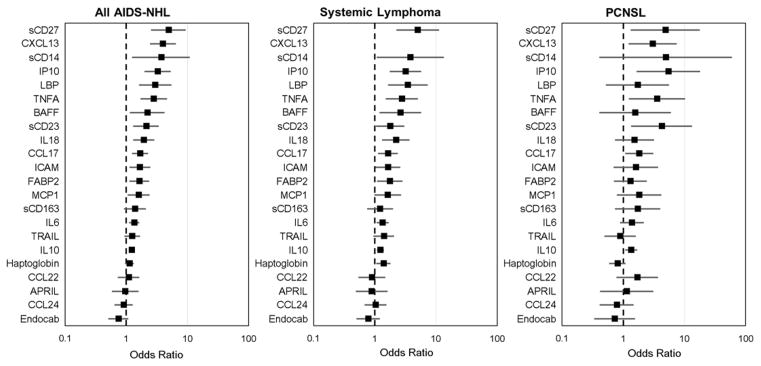
Figure 1.
Overall AIDS-NHL, systemic lymphoma, and PCNSL risk associated with 1-unit increase in biomarkers on the natural log scale (OR=box, 95% CI=bar).
In subgroup analyses by lag time between pre-diagnostic serum collection date and AIDS-NHL diagnosis date, EndoCAb, FABP2, and Haptoglobin demonstrated consistent associations with AIDS-NHL risk across lag times, while other markers were more strongly associated with AIDS-NHL risk when measured close to diagnosis date (< 4 years, sCD14 and LBP, Table 2). There was an association between increased AIDS-NHL risk and higher levels of FABP2, haptoglobin, and LBP, particularly among those men with systemic lymphoma in comparison to those with PCNSL (Figure 1, Supplemental Table 2). Conversely, higher levels of sCD14 were more strongly associated with PCNSL versus systemic lymphoma. EndoCAb appeared to be similarly associated with decreased risk of systemic lymphoma and PCNSL.
Macrophage-associated cytokines and chemokines
BAFF, CCL17, ICAM, IL18, and MCP1 were associated with AIDS-NHL, with 2.21-, 1.68-, 1.66-, 1.93-, and 1.60-fold increases in risk for each unit increase on the natural log scale, respectively (Table 3, Figure 1). When modeled as quartiles, these biomarkers generally displayed dose-response relationships with AIDS-NHL risk (the higher the levels, the higher the risk). For sCD163, participants with levels in the highest versus lowest quartile were at 1.94-fold increased AIDS-NHL risk. CCL17 and MCP1 displayed similar associations with AIDS-NHL risk when measured < 4 years or ≥ 4 years prior to diagnosis date (Table 3). BAFF and IL18, were more strongly associated with AIDS-NHL risk when measured close to diagnosis date (< 4 years), while ICAM and sCD163 displayed stronger associations when measured ≥ 4 years prior to diagnosis. BAFF and IL18 also displayed stronger associations with systemic lymphoma than PCNSL, while CCL17, ICAM, MCP1 (Supplemental Table 2) were similarly associated with systemic and PCNSL risks. TRAIL was unassociated with AIDS-NHL overall, however we observed a statistically significant interaction (p=0.04) between TRAIL and lag time: higher TRAIL levels were associated with AIDS-NHL risk only when measured ≥ 4 years prior to AIDS-NHL diagnosis.
Table 3.
Association between macrophage activation biomarkers and AIDS-NHL risk overall and stratified by time from blood collection to AIDS-NHL diagnosis
| All AIDS-NHL | < 4 years time lag | ≥4 years time lag | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Controls, N | Cases, N | OR | 95% CI | Controls, N | Cases, N | OR | 95% CI | Controls, N | Cases, N | OR | 95% CI | |
| APRIL pg/mL | 200 | 200 | 0.96 | 0.58 – 1.58 | 103 | 103 | 1.15 | 0.56 – 2.34 | 97 | 97 | 0.78 | 0.37 – 1.63 |
|
| ||||||||||||
| < 938 | 50 | 50 | 1 | 29 | 28 | 1 | 21 | 22 | 1 | |||
| 938–1257 | 50 | 54 | 1.12 | 0.64 – 1.97 | 31 | 28 | 0.81 | 0.37 – 1.74 | 19 | 26 | 1.89 | 0.70 – 5.12 |
| 1258–1703 | 50 | 50 | 1.02 | 0.56 – 1.84 | 24 | 26 | 1.54 | 0.66 – 3.58 | 26 | 24 | 0.75 | 0.29 – 1.94 |
| > 1703 | 50 | 46 | 0.91 | 0.48 – 1.72 | 19 | 21 | 1.34 | 0.52 – 3.44 | 31 | 25 | 0.64 | 0.24 – 1.74 |
|
| ||||||||||||
| BAFF pg/mL | 200 | 200 | 2.21 | 1.15 – 4.24 | 103 | 103 | 4.43 | 1.56 – 12.5 | 97 | 97 | 1.23 | 0.46 – 3.30 |
|
| ||||||||||||
| < 1475 | 50 | 26 | 1 | 28 | 14 | 1 | 22 | 12 | 1 | |||
| 1475–1816 | 50 | 38 | 1.33 | 0.69 – 2.58 | 27 | 18 | 1.16 | 0.42 – 3.25 | 23 | 20 | 1.51 | 0.57 – 3.97 |
| 1817–2287 | 50 | 65 | 2.35 | 1.27 – 4.35 | 25 | 29 | 2.52 | 1.00 – 6.34 | 25 | 36 | 2.63 | 1.02 – 6.77 |
| > 2287 | 50 | 71 | 2.88 | 1.48 – 5.60 | 23 | 42 | 4.28 | 1.59 – 11.5 | 27 | 29 | 2.11 | 0.73 – 6.07 |
|
| ||||||||||||
| CCL17 pg/mL | 200 | 200 | 1.68 | 1.25 – 2.26 | 103 | 103 | 1.71 | 1.11 – 2.64 | 97 | 97 | 1.70 | 1.10 – 2.63 |
|
| ||||||||||||
| < 311 | 50 | 32 | 1 | 20 | 18 | 1 | 30 | 14 | 1 | |||
| 311–501 | 50 | 48 | 1.82 | 0.95 – 3.51 | 25 | 20 | 0.85 | 0.33 – 2.22 | 25 | 28 | 3.62 | 1.31 – 10.0 |
| 502–759 | 50 | 38 | 1.26 | 0.66 – 2.38 | 35 | 15 | 0.60 | 0.23 – 1.55 | 15 | 23 | 3.86 | 1.31 – 11.4 |
| > 759 | 50 | 82 | 3.09 | 1.66 – 5.77 | 23 | 50 | 3.60 | 1.33 – 9.70 | 27 | 32 | 3.31 | 1.29 – 8.47 |
|
| ||||||||||||
| CCL22 pg/mL | 200 | 200 | 1.09 | 0.73 – 1.63 | 103 | 103 | 1.31 | 0.74 – 2.32 | 97 | 97 | 0.92 | 0.49 – 1.74 |
|
| ||||||||||||
| < 609 | 50 | 49 | 1 | 24 | 24 | 1 | 26 | 25 | 1 | |||
| 609–784 | 50 | 44 | 0.84 | 0.47 – 1.50 | 24 | 21 | 0.96 | 0.40 – 2.30 | 26 | 23 | 0.68 | 0.29 – 1.57 |
| 785–1031 | 50 | 51 | 1.06 | 0.61 – 1.87 | 31 | 23 | 1.04 | 0.43 – 2.52 | 19 | 28 | 1.18 | 0.54 – 2.59 |
| > 1031 | 50 | 56 | 1.08 | 0.61 – 1.91 | 24 | 35 | 1.87 | 0.77 – 4.54 | 26 | 21 | 0.66 | 0.29 – 1.50 |
|
| ||||||||||||
| CCL24 pg/mL | 200 | 200 | 0.91 | 0.64 – 1.28 | 103 | 103 | 0.93 | 0.56 – 1.56 | 97 | 97 | 0.91 | 0.56 – 1.47 |
|
| ||||||||||||
| < 595 | 50 | 43 | 1 | 27 | 26 | 1 | 23 | 17 | 1 | |||
| 595–1010 | 50 | 74 | 1.99 | 1.11 – 3.59 | 27 | 33 | 1.52 | 0.66 – 3.49 | 23 | 41 | 3.87 | 1.47 – 10.2 |
| 1011–1475 | 50 | 27 | 0.57 | 0.29 – 1.12 | 26 | 16 | 0.58 | 0.22 – 1.56 | 24 | 11 | 0.61 | 0.22 – 1.68 |
| > 1475 | 50 | 56 | 1.50 | 0.80 – 2.81 | 23 | 28 | 1.63 | 0.65 – 4.09 | 27 | 28 | 1.60 | 0.63 – 4.05 |
|
| ||||||||||||
| ICAM ng/mL | 200 | 200 | 1.66 | 1.12 – 2.48 | 103 | 103 | 1.55 | 0.84 – 2.87 | 97 | 97 | 2.10 | 1.07 – 4.12 |
|
| ||||||||||||
| < 148 | 51 | 32 | 1 | 23 | 15 | 1 | 28 | 17 | 1 | |||
| 148–196 | 49 | 32 | 0.90 | 0.43 – 1.88 | 25 | 17 | 0.96 | 0.34 – 2.66 | 24 | 15 | 0.94 | 0.29 – 2.99 |
| 196–289 | 50 | 65 | 1.81 | 0.99 – 3.31 | 29 | 36 | 1.97 | 0.80 – 4.85 | 21 | 29 | 2.54 | 0.98 – 6.60 |
| > 289 | 50 | 71 | 2.00 | 1.09 – 3.67 | 26 | 35 | 1.84 | 0.77 – 4.38 | 24 | 36 | 3.41 | 1.23 – 9.45 |
|
| ||||||||||||
| IL18 pg/mL | 200 | 200 | 1.93 | 1.30 – 2.88 | 103 | 103 | 2.06 | 1.12 – 3.79 | 97 | 97 | 1.66 | 0.94 – 2.93 |
|
| ||||||||||||
| < 978 | 50 | 27 | 1 | 25 | 10 | 1 | 25 | 17 | 1 | |||
| 978–1418 | 50 | 47 | 1.83 | 0.94 – 3.56 | 23 | 24 | 2.84 | 0.85 – 9.42 | 27 | 23 | 1.22 | 0.50 – 2.96 |
| 1419–1987 | 50 | 59 | 2.12 | 1.10 – 4.10 | 33 | 34 | 2.23 | 0.71 – 7.04 | 17 | 25 | 1.86 | 0.73 – 4.77 |
| > 1987 | 50 | 67 | 2.55 | 1.32 – 4.92 | 22 | 35 | 4.28 | 1.40 – 13.1 | 28 | 32 | 1.57 | 0.64 – 3.86 |
|
| ||||||||||||
| MCP1 pg/mL | 200 | 200 | 1.60 | 1.05 – 2.42 | 103 | 103 | 1.80 | 0.95 – 3.41 | 97 | 97 | 1.39 | 0.81 – 2.39 |
|
| ||||||||||||
| < 356 | 50 | 39 | 1 | 24 | 18 | 1 | 26 | 21 | 1 | |||
| 356–501 | 50 | 54 | 1.25 | 0.68 – 2.31 | 19 | 31 | 1.85 | 0.74 – 4.62 | 31 | 23 | 0.68 | 0.27 – 1.72 |
| 502–601 | 50 | 36 | 0.96 | 0.50 – 1.84 | 33 | 13 | 0.58 | 0.21 – 1.55 | 17 | 23 | 2.34 | 0.83 – 6.62 |
| > 601 | 50 | 71 | 1.74 | 0.98 – 3.09 | 27 | 41 | 1.78 | 0.78 – 4.06 | 23 | 30 | 1.94 | 0.79 – 4.77 |
|
| ||||||||||||
| sCD163 ng/mL | 200 | 200 | 1.39 | 0.92 – 2.09 | 103 | 103 | 1.48 | 0.77 – 2.83 | 97 | 97 | 1.39 | 0.80 – 2.44 |
|
| ||||||||||||
| < 715 | 50 | 37 | 1 | 21 | 19 | 1 | 29 | 18 | 1 | |||
| 715–933 | 50 | 34 | 0.88 | 0.46 – 1.68 | 26 | 14 | 0.46 | 0.16 – 1.32 | 24 | 20 | 1.35 | 0.54 – 3.37 |
| 933–1271 | 50 | 59 | 1.67 | 0.91 – 3.05 | 31 | 32 | 1.17 | 0.51 – 2.67 | 19 | 27 | 2.77 | 1.02 – 7.50 |
| > 1271 | 50 | 70 | 1.94 | 1.03 – 3.66 | 25 | 38 | 1.82 | 0.72 – 4.63 | 25 | 32 | 2.15 | 0.84 – 5.52 |
|
| ||||||||||||
| TRAIL pg/mL | 200 | 200 | 1.25 | 0.93 – 1.68 | 103 | 103 | 0.85 | 0.50 – 1.44 | 97 | 97 | 1.51 | 0.99 – 2.30 |
|
| ||||||||||||
| < 46 | 51 | 41 | 1 | 21 | 23 | 1 | 30 | 18 | 1 | |||
| 46–63 | 49 | 45 | 1.14 | 0.61 – 2.13 | 22 | 20 | 0.95 | 0.38 – 2.35 | 27 | 25 | 1.38 | 0.54 – 3.56 |
| 64–79 | 52 | 42 | 0.88 | 0.48 – 1.62 | 30 | 20 | 0.43 | 0.17 – 1.10 | 22 | 22 | 1.40 | 0.53 – 3.68 |
| > 79 | 48 | 72 | 1.71 | 1.00 – 2.91 | 30 | 40 | 1.08 | 0.50 – 2.32 | 18 | 32 | 2.34 | 1.03 – 5.32 |
N, number; OR, odds ratio; CI, confidence interval. ORs were adjusted for age at serum sample date (continuous), race/ethnicity (categorical), and HCV infection status at serum sample date.
B cell activation markers
There was a significant association between B cell activation markers with AIDS-NHL risk, with ORs ranging from 1.23 to 4.91 for each unit increase on the natural log scale (Supplemental Table 3, Figure 1). These associations were observed when markers were measured close to diagnosis (< 4 years), and persisted when markers were measured ≥ 4 years prior to diagnosis. These biomarkers were significantly associated with both systemic lymphoma and PCNSL, although CXCL13 appeared to be more strongly associated with systemic lymphoma while sCD23 was more strongly associated with PCNSL (Supplemental Table 2, Figure 1).
We observed strong correlations between several markers of microbial translocation (sCD14), macrophage activation (sCD163, ICAM, BAFF, and IL18), and B cell activation (IP10 and sCD27), with Pearson’s pairwise correlations ranging from 0.3 to 0.6.
Discussion
In this prospective study, we defined the association between serum levels of molecules associated with microbial translocation and immune activation/inflammation with subsequent AIDS-NHL diagnosis. Key novel observations were that markers of bacterial exposure and host response to bacteria (sCD14, LBP, EndoCAb), intestinal barrier dysfunction (FABP2 and Haptoglobin), and macrophage activation (BAFF, CCL17, ICAM, IL18, MCP1, and sCD163,) were highly predictive of subsequent AIDS-NHL diagnosis in men with HIV infection. Importantly, these associations were observed after careful adjustment of HIV disease status, immune suppression, and antiretroviral drug therapy.
sCD14 displayed the strongest association with AIDS-NHL of all the microbial translocation markers examined. sCD14 is the soluble form of the LPS co-receptor and is produced by macrophages on exposure to LPS and other inflammatory activators.[21, 22] Furthermore, sCD14 was more strongly associated with AIDS-NHL when measured close to the diagnosis date (< 4 years), and was more strongly associated with PCNSL than systemic lymphoma. PCNSL is an aggressive presentation of AIDS-NHL believed to arise principally due to the oncogenic properties of uncontrolled EBV reactivation in severely immunocompromised individuals. These observations are consistent with the hypothesis that microbial translocation is a consequence of compromised gut immunity (lower CD4+ T cell count), which occurs close to the time of AIDS-NHL diagnosis,[23] and is more strongly associated with PCNSL in comparison to systemic lymphoma development.[24] In a prior study, serum levels of sCD14 greater than or equal to 1.76 ×106 pg/ml (median value in controls) were associated with a 2.7-fold increased risk of AIDS-NHL, in samples collected a median of 1 year prior to AIDS-NHL diagnosis.[25] Our results build on this prior observation in a larger study sample with a longer lag time from specimen collection to AIDS-NHL diagnosis.
LBP was another biomarker found to be strongly associated with subsequent AIDS-NHL diagnosis, particularly when it was measured close to NHL diagnosis date (< 4 years); however, in contrast to sCD14, LBP was more strongly associated with systemic lymphoma when compared to PCNSL. LBP is an LPS binding protein which promotes binding of LPS to its receptor. Additionally, we observed that levels of EndoCAb, a neutralizing antibody against LPS, were significantly reduced prior to AIDS-NHL, with consistent associations across lag times from blood collection to AIDS-NHL diagnosis, and between systemic lymphoma and PCNSL. In prior studies, LBP and EndoCAb have been described as indirect markers of LPS: LBP is positively assocaited while EndoCAb is inversely associated with LPS levels [16]. Prior research suggests that HIV-infection impairs EndoCAb response.[26] Thus, the observed inverse association between EndoCAb levels and AIDS-NHL risk is consistent with the notion that LPS in circulation among HIV-infected persons at risk for NHL depletes circulating EndoCAb, further supporting the hypothesized role of microbial translocation in AIDS-NHL.
Structural damage to the gastrointestinal tract in chronic HIV infection has been well-described,[18] and the integrity of the intestinal barrier is an important factor in HIV-related systemic immune activation that may also influence lymphomagenesis.[17] FABP2 (a lipid transport protein and marker of enterocyte damage) and haptoglobin (a physiological modulator of intercellular tight junctions) are markers of intestinal barrier integrity. For both of these markers, a significantly increased risk for AIDS-NHL risk was observed only among those men with the highest levels (4th quartile), particularly for systemic lymphoma. In prior studies, higher levels of FABP2 were seen to be associated with lower CD4+ T cell counts, liver disease progression, and mortality in HIV infection.[27–29] To our knowledge, no prior studies have examined serum haptoglobin in HIV infection or lymphoma.
We previously showed that pre-NHL diagnosis serum levels of markers related to B-cell activation (sCD23, sCD27, CXCL13, IL6, IL10, IP10, and TNFα) were associated with risk for AIDS-NHL.[11, 13, 15, 30] In this study, we found that other cytokines and chemokines that are mainly secreted by activated macrophages (BAFF, CCL17, ICAM, IL18, MCP1, and sCD163) were associated with risk for AIDS-NHL.[31–35] Macrophages have numerous functions related to inflammation, immunity, and tumor growth, and can be phenotypically polarized by the microenvironment to become M1 or M2. M1 typically secrete pro-inflammatory cytokines (TNFα, IL-6), while M2 mainly promote Th2 responses, immunosuppression, and tumor promotion.[31, 36] It is interesting to note that many of the cytokines or chemokines associated with AIDS-NHL risk are typically produced by M1 polarized macrophages, including IP10, TNFα, IL18, IL6 and BAFF, [31, 32, 36] which may be reflecting microbial translocation and activation of macrophages by LPS. Some notable M2 markers, including CCL17 and CD163, [31, 36] were also associated with AIDS-NHL risk, possibly reflecting the presence of tumor-associated, tumor growth-promoting, macrophages. However additional studies are needed to elucidate these associations.
Interestingly, we found an association between pre-diagnosis serum levels of CCL17 (TARC) and the risk for AIDS-NHL. CCL17 was previously shown to be associated with the development of Hodgkin lymphoma (HL), suggesting that there may be common mechanisms contributing to the development of these lymphatic system cancers as they relate to CCL17.[37] High levels of sCD163 (4th quartile) were associated with increased AIDS-NHL risk, particularly PCNSL (which tend to be EBV-assocaited). In prior work, sCD163 correlated with plasma EBV-DNA in patients with HL [38]. Thus, we hypothesize that CCL17 and sCD163 may be associated with EBV reactivation in AIDS-NHL and HL.
We also found that pre-diagnosis levels of BAFF were associated with risk for AIDS-NHL. BAFF is a member of the TNF family of cytokines and plays an important role in normal B cell proliferation and activation. High levels of soluble BAFF may be contributing to prolonged B cell survival and increased susceptibility to mutation and transformation.[39] Prior studies have found that BAFF was elevated in patients with NHL and correlated with aggressive disease.[40]
The question of whether microbial translocation and macrophage activation markers are etiologically associated with AIDS-NHL development is impossible to determine in the context of an observational study. Interestingly, sCD14 was highly correlated with B cell activation markers (sCD27 and IP10), providing a link between microbial translocation and the B cell activation that precedes AIDS-NHL. However, pre-clinical disease in the cases may be responsible for the observed associations. Interestingly, we found that risk estimates for many of our markers were comparable when analyses were stratified by short (< 4 years) or long (≥ years) lag time from specimen collection to AIDS-NHL diagnosis, suggesting that the associations are less likely to be due to undiagnosed disease. Some notable exceptions were sCD14 and LBP, both of which were more strongly associated with AIDS-NHL when measured closer to NHL diagnosis, suggesting a role of undiagnosed AIDS-NHL in the observed associations.
Systemic immune activation occurs early in the course of HIV infection.[16, 17] Therefore, it is reasonable to speculate that a failure to control immune activation may contribute to lymphomagenesis. In accordance with this, a recent study showed that plasma levels of LBP, FABP2 and IL6 were significantly elevated in patients from whom antiretroviral drug treatment was introduced during chronic HIV infection. In contrast, patients who started treatment with a potent combination of antiretroviral drugs at the time of HIV diagnosis were able to normalize their levels of LBP, FABP2 and IL6.[41] This finding supports early introduction of therapy to limit systemic immune activation and thereby reduce the incidence of lymphomagenesis. Even though this is an interesting hypothesis, we were not able to test it in this study, as only a small percentage of cases and controls (6%) were treated with potent combinations of antiretroviral drugs.
The main strength of this study is the inclusion of a large sample of known AIDS-NHL cases with detailed longitudinal covariate data and specimen availability before AIDS-NHL. Compared to prior studies, we examined a larger number of biomarkers indicative of microbial translocation and related immune activation, with longer lag times from biomarker measurement to AIDS-NHL diagnosis. Also, our larger study population allowed us to examine differences in biomarker associations by lag time and site (CNS versus systemic). In addition to the markers presented in this paper, this study population has been characterized with respect to a number of important biomarkers related to AIDS-NHL, permitting us to evaluate our findings in this context. Although we controlled for CD4+ T-cell numbers and date of HIV infection among seroconverters, our observed associations may still be influenced by residual confounding by HIV progression. Also, our population consisted largely of white men who have sex with men, who developed lymphoma prior to initiation of HAART, potentially limiting the generalizability of the findings. We did not correct for type 1 error, thus we cannot exclude the possibility of chance findings. However, our goal of this study was to examine several biomarkers in a related pathway to demonstrate strength of evidence (not necessarily statistical significance for a single biomarker) for a role of microbial translocation in AIDS-NHL, thus type 1 error correction may not be appropriate.
Here we show that systemic immune activation in chronic HIV infection plays a prominent role in the development of AIDS-NHL. Additionally, the cytokine profile seen to precede AIDS-NHL diagnosis is consistent with an M1 macrophage activation, which in part may driven by endotoxin exposure due to microbial translocation. Future research is needed to better understand their biologic basis. For example, studies are needed to better define how LPS drives chronic B-cell activation and subsequent AIDS-NHL development. If these results are replicated, attenuation of microbial translocation-induced immune activation could be an effective therapeutic strategy to reduce AIDS-NHL risk.
Supplementary Material
Acknowledgments
Sources of support: This work was supported by grants from the National Institute of Health (NIH) (P30-AI-028697, R01-CA-168482, R01-CA-168482-S) and from the UCLA AIDS Institute, UCLA Center for AIDS Research (AI28697) and the UCLA Jonsson Comprehensive Cancer Center (CA016042). Data/specimens utilized for the work reported in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels, Otoniel Martinez-Maza), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson, Gypsyamber D’Souza), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Cancer incidence data were provided by the following state agencies: 1) Maryland Cancer Registry, Center for Cancer Prevention and Control, Department of Health and Mental Hygiene, Baltimore, MD 21201; 2) Illinois Department of Public Health, Illinois State Cancer Registry; 3) Bureau of Health Statistics & Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania; 4) Ohio Cancer Incidence Surveillance System (OCISS), Ohio Department of Health (ODH), a cancer registry partially supported in the National Program of Cancer Registries at the Centers for Disease Control and Prevention (CDC) through Cooperative Agreement # 5U58DP000795-05; and 5) California Department of Public Health pursuant to California Health and Safety Code Section 103885; CDC’s National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute.
Authorship contributions
Conception and design: Marta Epeldegui, Otoniel Martínez-Maza, Shehnaz K. Hussain
Acquisition of data: Marta Epeldegui, Larry Magpantay, Yu Guo, Gordana Halec, Bernard Macatangay, Joseph Margolick, Anne F. Rositch, Steven Wolinsky, Otoniel Martínez-Maza, Shehnaz K. Hussain
Analysis and interpretation of data: Marta Epeldegui, William G. Cumberland, Priscilla K. Yen, Otoniel Martínez-Maza, Shehnaz K. Hussain
Writing, review, and/or revision of the manuscript: Marta Epeldegui, Larry Magpantay, Yu Guo, Gordana Halec, William G. Cumberland, Priscilla K. Yen, Bernard Macatangay, Joseph Margolick, Anne F. Rositch, Steven Wolinsky, Otoniel Martínez-Maza, Shehnaz K. Hussain
Study supervision: Marta Epeldegui, Otoniel Martínez-Maza, Shehnaz K. Hussain.
Footnotes
Conflict-of-interest disclosure: All authors declare no competing financial interests.
References
- 1.Hessol NA, Seaberg EC, Preston-Martin S, Massad LS, Sacks HS, Silver S, et al. Cancer risk among participants in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2004;36(4):978–985. doi: 10.1097/00126334-200408010-00013. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 3.Seaberg EC, Benning L, Sharrett AR, Lazar JM, Hodis HN, Mack WJ, et al. Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke. 2010;41(10):2163–2170. doi: 10.1161/STROKEAHA.110.583856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews GV, Moyle GJ, Mandalia S, Bower M, Nelson M, Gazzard BG. Absence of association between individual thymidine analogues or nonnucleoside analogues and lipid abnormalities in HIV-1-infected persons on initial therapy. J Acquir Immune Defic Syndr. 2000;24(4):310–315. doi: 10.1097/00126334-200008010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet F, Balestre E, Thiebaut R, Morlat P, Pellegrin JL, Neau D, et al. Factors associated with the occurrence of AIDS-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: Aquitaine Cohort, France. Clin Infect Dis. 2006;42(3):411–417. doi: 10.1086/499054. [DOI] [PubMed] [Google Scholar]
- 6.Lewden C, Salmon D, Morlat P, Bevilacqua S, Jougla E, Bonnet F, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34(1):121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 7.Grulich AE, Hendry O, Clark E, Kippax S, Kaldor JM. Circumcision and male-to-male sexual transmission of HIV. AIDS. 2001;15(9):1188–1189. doi: 10.1097/00002030-200106150-00020. [DOI] [PubMed] [Google Scholar]
- 8.Gaidano G, Capello D, Carbone A. The molecular basis of acquired immunodeficiency syndrome-related lymphomagenesis. Semin Oncol. 2000;27(4):431–441. [PubMed] [Google Scholar]
- 9.Gaidano G, Pastore C, Capello D, Cilli V, Saglio G. Molecular pathways in low grade B-cell lymphoma. Leuk Lymphoma. 1997;26(Suppl 1):107–113. doi: 10.3109/10428199709058607. [DOI] [PubMed] [Google Scholar]
- 10.Carbone DJ. Under lock and key: youth under the influence of HIV. Body Posit. 2001;14(5):28–29. [PubMed] [Google Scholar]
- 11.Vendrame E, Hussain SK, Breen EC, Magpantay LI, Widney DP, Jacobson LP, et al. Serum levels of cytokines and biomarkers for inflammation and immune activation, and HIV-associated non-Hodgkin B-cell lymphoma risk. Cancer Epidemiol Biomarkers Prev. 2014;23(2):343–349. doi: 10.1158/1055-9965.EPI-13-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epeldegui M, Widney DP, Martinez-Maza O. Pathogenesis of AIDS lymphoma: role of oncogenic viruses and B cell activation-associated molecular lesions. Curr Opin Oncol. 2006;18(5):444–448. doi: 10.1097/01.cco.0000239882.23839.e5. [DOI] [PubMed] [Google Scholar]
- 13.Epeldegui M, Vendrame E, Martinez-Maza O. HIV-associated immune dysfunction and viral infection: role in the pathogenesis of AIDS-related lymphoma. Immunol Res. 2010;48(1–3):72–83. doi: 10.1007/s12026-010-8168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Maza O, Breen EC. B-cell activation and lymphoma in patients with HIV. Curr Opin Oncol. 2002;14(5):528–532. doi: 10.1097/00001622-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, et al. B-Cell Stimulatory Cytokines and Markers of Immune Activation Are Elevated Several Years Prior to the Diagnosis of Systemic AIDS-Associated Non-Hodgkin B-Cell Lymphoma. Cancer Epidemiology Biomarkers & Prevention. 2011;20(7):1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 17.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 19.Detels R, Jacobson L, Margolick J, Martinez-Maza O, Munoz A, Phair J, et al. The multicenter AIDS Cohort Study, 1983 to. Public Health. 2012;126(3):196–198. doi: 10.1016/j.puhe.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo C. The Multicenter AIDS Cohort Study: Rationale, organization and selected characteristics of the participants. American Journal of Epidemiology. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 21.Hailman E, Vasselon T, Kelley M, Busse LA, Hu MC, Lichenstein HS, et al. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156(11):4384–4390. [PubMed] [Google Scholar]
- 22.Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS. 2015;29(10):1263–1265. doi: 10.1097/QAD.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bower M, Fisher M, Hill T, Reeves I, Walsh J, Orkin C, et al. CD4 counts and the risk of systemic non-Hodgkin’s lymphoma in individuals with HIV in the UK. Haematologica. 2009;94(6):875–880. doi: 10.3324/haematol.2008.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambinder RF, Bhatia K, Martinez-Maza O, Mitsuyasu R. Cancer biomarkers in HIV patients. Curr Opin HIV AIDS. 2010;5(6):531–537. doi: 10.1097/COH.0b013e32833f327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks MA, Rabkin CS, Engels EA, Busch E, Kopp W, Rager H, et al. Markers of microbial translocation and risk of AIDS-related lymphoma. AIDS. 2013;27(3):469–474. doi: 10.1097/QAD.0b013e32835c1333. [DOI] [PubMed] [Google Scholar]
- 26.Haynes BF. Gut microbes out of control in HIV infection. Nat Med. 2006;12(12):1351–1352. doi: 10.1038/nm1206-1351. [DOI] [PubMed] [Google Scholar]
- 27.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.French AL, Evans CT, Agniel DM, Cohen MH, Peters M, Landay AL, et al. Microbial translocation and liver disease progression in women coinfected with HIV and hepatitis C virus. J Infect Dis. 2013;208(4):679–689. doi: 10.1093/infdis/jit225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17(13):1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 30.Hussain SK, Widney D, Jacobson LP, Breen EC, Levine a, Detels R, et al. Elevated serum levels of CXCL13 precede HIV-associated non Hodgki’s lymphoma. 12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI); Bethesda, MD. 2010. [Google Scholar]
- 31.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez AM, Ouellet M, Deshiere A, Breton Y, Tremblay MJ. HIV-1-Mediated BAFF Secretion in Macrophages Does Not Require Endosomal TLRs, Type-I IFN, and Nef, but Depends on the Cellular Phenotype Status. J Immunol. 2016;196(9):3806–3817. doi: 10.4049/jimmunol.1501249. [DOI] [PubMed] [Google Scholar]
- 33.Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195(2):245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulahian TH, Hogger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12(9):1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 36.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niens M, Visser L, Nolte IM, van der Steege G, Diepstra A, Cordano P, et al. Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22. Br J Haematol. 2008;140(5):527–536. doi: 10.1111/j.1365-2141.2007.06964.x. [DOI] [PubMed] [Google Scholar]
- 38.Jones K, Vari F, Keane C, Crooks P, Nourse JP, Seymour LA, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res. 2013;19(3):731–742. doi: 10.1158/1078-0432.CCR-12-2693. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, Li JY, Xu W. Role of BAFF/BAFF-R axis in B-cell non-Hodgkin lymphoma. Crit Rev Oncol Hematol. 2014;91(2):113–122. doi: 10.1016/j.critrevonc.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Novak AJ, Grote DM, Stenson M, Ziesmer SC, Witzig TE, Habermann TM, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104(8):2247–2253. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 41.Allers K, Puyskens A, Epple HJ, Schurmann D, Hofmann J, Moos V, et al. The effect of timing of antiretroviral therapy on CD4+ T-cell reconstitution in the intestine of HIV-infected patients. Mucosal Immunol. 2016;9(1):265–274. doi: 10.1038/mi.2015.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.