Abstract
Multiple system atrophy (MSA) may be difficult to distinguish clinically from other disorders, particularly in the early stages of the disease. An autonomic-only presentation can be indistinguishable from pure autonomic failure. Patients presenting with parkinsonism may be misdiagnosed as having Parkinson disease. Patients presenting with the cerebellar phenotype of MSA can mimic other adult-onset ataxias due to alcohol, chemotherapeutic agents, lead, lithium, and toluene, or vitamin E deficiency, as well as paraneoplastic, autoimmune, or genetic ataxias. A careful medical history and meticulous neurological examination remain the cornerstone for the accurate diagnosis of MSA. Ancillary investigations are helpful to support the diagnosis, rule out potential mimics, and define therapeutic strategies. This review summarizes diagnostic investigations useful in the differential diagnosis of patients with suspected MSA. Currently used techniques include structural and functional brain imaging, cardiac sympathetic imaging, cardiovascular autonomic testing, olfactory testing, sleep study, urological evaluation, and dysphagia and cognitive assessments. Despite advances in the diagnostic tools for MSA in recent years and the availability of consensus criteria for clinical diagnosis, the diagnostic accuracy of MSA remains sub-optimal. As other diagnostic tools emerge, including skin biopsy, retinal biomarkers, blood and cerebrospinal fluid biomarkers, and advanced genetic testing, a more accurate and earlier recognition of MSA should be possible, even in the prodromal stages. This has important implications as misdiagnosis can result in inappropriate treatment, patient and family distress, and erroneous eligibility for clinical trials of disease-modifying drugs.
Keywords: Autonomic testing, Biomarkers, Diagnosis, Orthostatic hypotension, Multiple system atrophy, Neuroimaging
INTRODUCTION
Multiple system atrophy (MSA) is the most rapidly progressive of the synucleinopathies, a group of disorders characterized by the abnormal deposition of the protein α-synuclein (αSyn) in the central and peripheral autonomic nervous system (Roncevic et al., 2014; Wenning et al., 2013). While in patients with Parkinson disease (PD) αSyn predominantly accumulates in neurons forming Lewy bodies and Lewy neurites, in patients with MSA it accumulates mostly in oligodendroglial cells forming glial cytoplasmic inclusions (GCI). A significant percentage of patients with MSA present with genitourinary dysfunction and orthostatic hypotension (OH) due to dysfunction of the autonomic nervous system, frequently combined with a history suggesting rapid eye movement (REM) sleep behavior disorder (RBD). Within a few years patients go on to develop balance, speech and coordination abnormalities that progress fairly rapidly. Depending on their initial predominant motor deficits, MSA is sub-classified into a parkinsonian (MSA-P) and a cerebellar phenotype (MSA-C)(Quinn, 2015). Age at onset, prevalence of cardiovascular autonomic dysfunction, sleep disorders, and retinal abnormalities are similar in both phenotypes (Mendoza-Santiesteban et al., 2015; Palma et al., 2015; Roncevic et al., 2014). Specific neuroimaging markers differ between the cerebellar and parkinsonian phenotypes (Deguchi et al., 2015; Huppertz et al., 2016; Lee et al., 2015), as well as the degree of sudomotor dysfunction which may be more severe in patients with MSA-P (Coon et al., 2016) and urogenital dysfunction which may occur earlier in patients with MSA-C (Zheng et al., 2017).
Patients with MSA have a mean age at onset of 55–60 years, and an average survival from the onset of motor symptoms of 8–9 years, although some pathology-proven cases survived more than 15 years (Fanciulli et al., 2015; Petrovic et al., 2012).
MSA may be difficult to distinguish clinically from other disorders, particularly in patients at the early stages of the disease. An autonomic-only presentation can be indistinguishable from pure autonomic failure (PAF) (Kaufmann et al., 2017b; Muppidi et al., 2017). Patients presenting with parkinsonism may be misdiagnosed as PD. The reverse also occurs; approximately 20% of patients with a clinical diagnosis of MSA turn out to have PD or DLB at autopsy (Koga et al., 2015). Patients presenting with the cerebellar phenotype can mimic other adult-onset ataxias due to alcohol, chemotherapeutic agents, lead, lithium, and toluene, or vitamin E deficiency, as well as paraneoplastic, autoimmune, or genetic ataxias (e.g., spinocerebellar ataxias, fragile X–associated tremor ataxia syndrome, or late-onset Friedreich ataxia) (Klockgether, 2010; Lin et al., 2016). Misdiagnosis can result in inappropriate treatment, patient and family distress, and erroneous eligibility for clinical trials.
The accurate clinical diagnosis of MSA is based on a careful medical history and meticulous neurological examination. Ancillary investigations are helpful to support the diagnosis, rule out potential mimics, and define therapeutic strategies. This review summarizes diagnostic investigations useful in the diagnosis of MSA.
CLINICAL EVALUATION
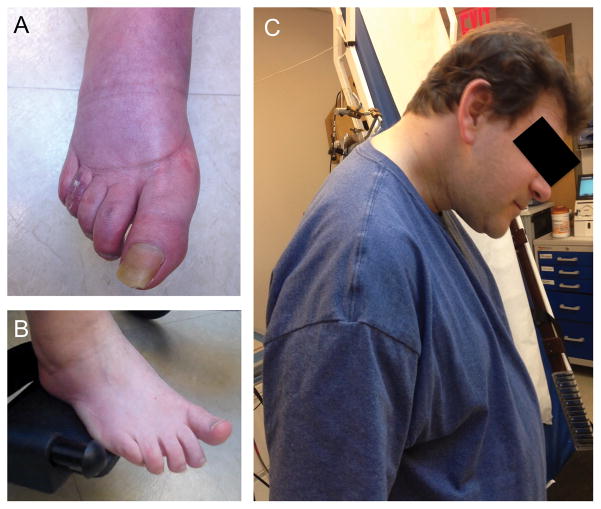
A detailed clinical evaluation, including a medical history (Goldstein et al., 2017b), physical, and neurological examinations with special attention to gait, coordination and muscle tone, is the most important step in the evaluation of a patient with suspected MSA. The medical history should include questions about the onset and progression of motor symptoms as well as non-motor features including symptoms of cardiovascular, gastrointestinal, genitourinary, and sudomotor dysfunction; special attention should be paid to sleep disorders, the presence of cognitive, mood and behavioral problems, dysphagia, and visual abnormalities. Response to anti-parkinsonian medications, particularly levodopa, is usually sub-optimal and often transient (Calandra-Buonaura et al., 2016). Cold hands and feet are a typical feature of the disease (Asahina et al., 2013). A bluish discoloration of the feet is frequently seen in wheelchair-bound patients, probably due to venous stasis (Figure 1A).
Figure 1.
A. Bluish discoloration in the foot of a patient with MSA-C. B. “Striatal toe”, spontaneous extensor toe response in a patient with MSA-C. C. Antecollis in a patient with MSA-P.
Non-motor signs and symptoms
All patients with MSA have gastrointestinal, cardiovascular, urogenital and thermoregulatory abnormalities but the severity of symptoms varies among patients (Fanciulli et al., 2015; Roncevic et al., 2014). Indeed, the diagnosis of probable or possible MSA according to the 2008 consensus criteria (Table 1) relies on either the presence of OH or urinary dysfunction indicating pathological involvement of autonomic neurons (Gilman et al., 2008). Early and severe autonomic failure appears to be associated with poorer prognosis (Coon et al., 2015; Glasmacher et al., 2017). Bladder and sexual dysfunction (erectile dysfunction in men and dyspareunia in women) as well as orthostatic dizziness, lightheadedness or syncope due to neurogenic OH were the earliest signs of the disease in up to 50% of patients, years before the emergence of motor signs (Kaufmann et al., 2017b). Rapid eye movement (REM) sleep behavior disorder (Palma et al., 2015) or stridor (Kaufmann et al., 2017a) may also appear years before the motor symptoms. Cognitive impairment is not a typical presenting feature of patients with MSA, but it may emerge later in the course of the disease (Stankovic et al., 2014). Depressive symptoms can precede the onset of motor signs in around 16% of cases, with the prevalence of depression increasing up to 60% as the disease progresses (Schrag et al., 2010). Pseudobulbar affect with pathological laughter and crying occurs in 36% of patients with MSA-C (Parvizi et al., 2007).
Table 1.
Current consensus criteria for the diagnosis of multiple system atrophy, adapted from (Gilman et al., 2008).
| Criteria for definite MSA include neuropathological findings during postmortem examination of: |
| a) Widespread and abundant cerebral α-synuclein–positive glial cytoplasmic inclusions b) Neurodegenerative changes in striatonigral or olivopontocerebellar region |
| Criteria for probable MSA include a sporadic progressive adult (> 30 years old)–onset disease characterized by: |
| a) Autonomic failure involving urinary incontinence (inability to control the release of urine from the bladder with erectile dysfunction in males) or an orthostatic decrease of blood pressure within 3 min of standing by at least 30 mmHg systolic or 15 mmHg diastolic, and |
| b) Poorly levodopa-responsive parkinsonism (bradykinesia with rigidity, tremor or postural instability), or |
| c) A cerebellar syndrome (gait ataxia with cerebellar dysarthria, limb ataxia or cerebellar oculomotor dysfunction) |
| Criteria for possible MSA include a sporadic progressive adult (> 30 years old)–onset disease characterized by: |
| a) Parkinsonism (bradykinesia with rigidity tremor or postural instability), or |
| b) Cerebellar syndrome (gait ataxia with cerebellar dysarthria limb ataxia or cerebellar oculomotor dysfunction), and |
| c) At least one feature suggesting autonomic dysfunction (otherwise unexplained urinary urgency frequency or incomplete bladder emptying erectile dysfunction in males or significant orthostatic BP decline that does not meet the level required in probable MSA), and |
d) At least one of the following features:
|
Motor signs and symptoms
Virtually all patients with MSA will develop parkinsonism during the course of the disease, regardless of their initial presentation (Gilman et al., 2005; Kollensperger et al., 2010). Most patients have a bilateral rigid-akinetic form but the parkinsonism can occasionally be markedly asymmetric (Batla et al., 2013; Tison et al., 2002). A quivery voice is characteristic. Early falls (i.e., within the first year of the diagnosis) are common in patients with MSA (Tison et al., 2002) although not as frequent as in patients with progressive supranuclear palsy (PSP). Cerebellar dysfunction, particularly a broad-based ataxic gait, eventually develops in up to 60% of patients with MSA, regardless of their initial presentation (Kollensperger et al., 2010). Limb ataxia, scanning (ataxic) dysarthria, and cerebellar oculomotor dysfunction including excessive square wave jerks, mild-moderate hypometric saccades, impaired vestibulo-ocular reflex suppression, spontaneous or positional downbeat nystagmus are also relatively frequent (Anderson et al., 2008; Testa et al., 2001). Corticospinal (pyramidal) signs, including brisk deep tendon reflexes and Babinski sign, are present in 40–50% of patients with MSA; these seem to be more frequent in patients with MSA-C than in those with MSA-P (Kollensperger et al., 2010; Roncevic et al., 2014). Abnormal postures and deformities of the hand and foot, named “striatal” by Charcot who recognized they were caused by lesions in the putamen or caudate (Ashour et al., 2005), are frequent in patients with MSA. Extending the arms typically shows the hands slightly flexed at metacarpophalangeal joints with extension at interphalangeal joints and sometimes ulnar deviation. Patients with MSA-C typically show hyperextended hands with the fingers pointing up (“scooping”). Occasionally, patients with MSA can have an abnormal upward posturing of the big toe, a “striatal toe” that resembles a spontaneous extensor plantar response without the fanning of the toes (Figure 1B). Although some patients with MSA can present with spastic paraparetic gait, this should raise the possibility of other disorders, such as hereditary spastic paraparesis, or adrenoleukodystrophy (Fontes-Villalba et al., 2013). Over the course of the disease, up to 40% of patients with MSA will develop abnormal postures including camptocornia (severe forward trunk flexion, which increases while walking and disappears in the recumbent position), Pisa syndrome (severe lateral tonic bending of the trunk), and cervical dystonia causing disproportionate antecollis (severe forward neck flexion, interfering with eating, speaking and sight) (Figure 1C).
L-dopa response
The motor response to levodopa is variable in patients with MSA (Colosimo, 1998; Kollensperger et al., 2008; Slawek et al., 2006; Tison et al., 2002; van de Warrenburg et al., 2007). Because of the traditional assumption that patients with MSA do not respond to L-dopa therapy as well as those with PD, a L-dopa challenge is frequently used to distinguish between these two disorders. A study investigating the predictive value of an L-dopa challenge in PD versus non-PD patients, however, found that 15% were wrongly classified (Holmberg et al., 2001). Indeed, in series with pathologically confirmed cases, 30–70% of patients with MSA had an initial good therapeutic response to L-dopa (Colosimo et al., 1995; Hughes et al., 1992; Wenning et al., 1995). The beneficial effect is usually short-lived unfortunately and, within 3 years of diagnosis, only a minority of patients still report L-dopa responsiveness. In those with a good L-dopa response, dyskinesia can develop that almost exclusively involve the craniocervical region even after short-term use with a minority of patients showing limb dyskinesias (Boesch et al., 2002; Kollensperger et al., 2010; O’Sullivan et al., 2008).
BRAIN AND CARDIAC NEUROIMAGING
Current consensus guidelines include neuroimaging criteria for the diagnosis of possible MSA (Gilman et al., 2008) (Table 1). These include the presence of atrophy of the putamen, middle cerebellar peduncle, pons or cerebellum on brain magnetic resonance imaging (MRI), and putamen, brainstem or cerebellum hypometabolism on brain fluorodeoxyglucose (FDG) positron emission tomography (PET), as well as dopaminergic denervation on PET or single photon emission computed tomography (SPECT).
Brain magnetic resonance imaging
Brain MRI is the gold standard imaging technique for the evaluation of parkinsonian and cerebellar syndromes, including MSA. A brain MRI including standard sequences and diffusion weighted imaging (DWI) should be included in the initial evaluation of every patient with suspected MSA. Several brain MR modalities are available.
Standard brain magnetic resonance imaging
Brain 1.5-Tesla and 3-Tesla MRI shows several abnormalities including atrophy of the putamen, pons, middle cerebellar peduncles, cerebellum, medulla oblongata, midbrain, a dilated fourth ventricle, as well as various signal intensity alterations in a significant number of patients with MSA (Brooks et al., 2009; Burk et al., 2005; Lee et al., 2004; Lin et al., 2016). In comparison, standard MRI is typically normal in PD. Characteristic MSA brain MRI signal intensity abnormalities include the “hot cross bun” sign, a cruciform hypointensity in the pons that resembles the Easter pastry, and the “putaminal slit” sign, an hyperintense signal in the dorsolateral margin of the putamen, have high positive predictive value for the diagnosis of MSA (Figure 2) (Horimoto et al., 2002; Schrag et al., 2000). Interestingly, patients with early “hot cross bun” sign are more likely to develop severe cerebellar symptoms later in the course of the disease, whereas patients who show early bilateral putaminal slit signs commonly develop the parkinsonian variant of MSA (Horimoto et al., 2002). However, a hyperintense putaminal rim can be a nonspecific “normal” finding on 3-Tesla MR imaging (Lee et al., 2005) and the “hot cross bun” sign can be seen in other disorders including spinocerebellar ataxias (Burk et al., 2001; Lee et al., 2009), leptomeningeal carcinomatosis (Zhang et al., 2013), vasculitis (Muqit et al., 2001) and others. Several MRI algorithms to distinguish MSA-P from PD have been proposed. In general, all have high specificity but low sensitivity (Bhattacharya et al., 2002; Horimoto et al., 2002; Lee et al., 2004; Nair et al., 2013; Schocke et al., 2002; Watanabe et al., 2002). Routine MR imaging has very low specificity to distinguish MSA from other atypical parkinsonian syndromes (Schrag et al., 2000; Yekhlef et al., 2003). Increasing use of 7-Telsa MR imaging, and better sequences to discriminate the brainstem anatomy, should enable the development of more sensitive and specific diagnostic algorithms (Hoch et al., 2016; Kim et al., 2016).
Figure 2.
Conventional brain magnetic resonance imaging. Axial image (A) showing the “hot cross bun” sign and atrophy of cerebellar peduncles, which is shown in detail in C. Sagittal figure (B) showing atrophy in pons, medulla and cerebellum.
Diffusion-weighted imaging and diffusion tensor imaging
Diffusion-weighted imaging (DWI) is a technique used to determine the random movement of water molecules. Quantification of diffusion is performed by calculation of the apparent diffusion coefficient (ADC) in tissue. Movement of water molecules is typically increased in degenerating and ischemic neural tissue. Compared to PD, increased putaminal diffusivity in MSA-P has been consistently reported, even in early disease stages (Barbagallo et al., 2016; Baudrexel et al., 2014; Pellecchia et al., 2009; Schocke et al., 2002; Schocke et al., 2004; Seppi et al., 2004). DWI might be useful in distinguishing MSA-P from PSP (Paviour et al., 2007). Interestingly, a prospective study showed progressive abnormalities of the diffusivity of the putamen over time, thus allowing the possibility of using DWI imaging as a marker of disease progression and an outcome measure in clinical trials for MSA (Pellecchia et al., 2011). Diffusion tensor imaging (DTI), a similar technique to DWI which analyzes the three-dimensional shape of the diffusion and produces 3-D neural tract images (Huisman, 2010), is being increasingly used to distinguish MSA from PD and PSP (Du et al., 2017; Ofori et al., 2017; Planetta et al., 2016; Prodoehl et al., 2013; Worker et al., 2014).
Magnetic resonance volumetry
Quantitative assessment with MR volumetry using region-of-interests (ROI) in patients with MSA showed atrophy of the putamen, caudate, brainstem and cerebellum (Burk et al., 2004; Ghaemi et al., 2002; Huppertz et al., 2016; Sako et al., 2014; Schulz et al., 1999). The MR Parkinsonism Index (MRPI), taking into consideration the volume of the pons, midbrain, and cerebellar peduncles, appears to have high sensitivity and specificity to distinguish between PSP and MSA-P or PD (Hussl et al., 2010; Quattrone et al., 2008). Voxel-based morphometry (VBM), an operator-independent automated method, can detect focal volume differences between 2 or more groups based on study-specific templates (Ashburner et al., 2000). Studies using this approach confirmed previous ROI-based volumetric studies suggesting basal ganglia, infratentorial as well as cortical volume loss in MSA patients (Minnerop et al., 2007; Moller et al., 2017; Specht et al., 2003; Tzarouchi et al., 2010). Moreover, specific longitudinal changes in MSA (early atrophy of basal ganglia followed by late cortical atrophy) have been identified with this technique (Brenneis et al., 2007). Other findings, such as degeneration of the ponto-cerebellar tract and white matter abnormalities in specific areas have been identified using VBM (Minnerop et al., 2007; Yu et al., 2015). Cognitive impairment in MSA is also associated with specific VBM changes (Fiorenzato et al., 2017). Because it requires group comparisons using study-specific templates and sophisticated processing and analysis, VBM is not routinely applied in the clinical diagnostic work-up of patients with MSA (Hotter et al., 2009).
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) acquires signals from carbon-bound, non-exchangeable protons in order to discriminate molecules based on their characteristic chemical changes (Trabesinger et al., 2003). Within the brain, N-acetylaspartate, choline, creatine, and lactate reflect the integrity and function of neurons, glial activity, energy metabolism and anaerobic glycolysis, respectively (Trabesinger et al., 2003). A meta-analysis concluded that MRS of the striatum was not useful in the differential diagnosis of parkinsonian disorders (Clarke et al., 2001). MRS of other brain areas may have better discriminative capacity (Takado et al., 2011; Watanabe et al., 2004).
Functional brain magnetic resonance imaging
Functional MR imaging (fMRI) techniques are able to measure brain activity by detecting changes in regional blood flow. Acquisition of fMRI is usually performed during specific tasks (e.g., motor tasks). Motor control studies using fMRI engage an extensive task-related network including the basal ganglia, cerebellum, and motor cortex in healthy subjects, and defective activation of these structures in PD, MSA and PSP (Planetta et al., 2015). A study assessing longitudinal fMRI changes over the course of 1-year in PD, MSA, and PSP using a hand-grip-force paradigm showed reduced fMRI signal and more widespread and more pronounced changes in basal ganglia, cerebellum, and motor cortex in patients with MSA and PSP compared to PD (Burciu et al., 2016). This technique may be potentially useful as a marker of disease progression and outcome measure in clinical studies.
Brain computerized tomography
Brain computerized tomography (CT) scans are of limited use when evaluating the neuroanatomy of the posterior fossa. For this reason, the usefulness of brain CT in MSA is limited, and should only be used in patients with contraindications to magnetic resonance (MR) imaging (i.e., pacemaker, metal implants). Brain volumetric analysis obtained from 3-dimensional CT scans, however, could be useful to monitor disease progression in MSA (Miyatake et al., 2010).
Brain positron emission tomography
Brain 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) has been extensively used in the differential diagnosis of parkinsonian disorders (Arbizu et al., 2014). In patients with PD, brain 18FDG-PET is normal or shows an increase in uptake in the putamen nucleus whereas in patients with MSA specific hypometabolic patterns have been described. The most characteristic finding in patients with MSA-P is a reduction in 18FDG-PET uptake in both putamen nuclei with a rostrocaudal gradient. This finding has a sensitivity of ~95% and a specificity of 100% to distinguish PD vs. MSA-P (Brooks et al., 2009). Decreased 18FDG-PET uptake can also be detected in the thalamus, brainstem and cortical areas. Thus, current consensus diagnostic criteria for MSA established hypometabolism in the putamen nucleus, mesencephalic region and cerebellum as supportive for MSA-P (Gilman et al., 2008). This pattern has a positive predictive value of 88% in the first 2 years of the disease and of 100% after 5 years (Tang et al., 2010). In patients with MSA-C, hypometabolism of the anterior cerebellar hemispheres and the vermis may be seen 1-year after the onset of motor symptoms, although hypometabolism of the putamen can also be observed (Eckert et al., 2005) and is a supportive feature for the diagnosis of MSA-C (Gilman et al., 2008).
Brain single photon emission computed tomography
It has been reported that single photon emission computed tomography (SPECT) using technetium-99m-ethyl cysteinate dimer perfusion showed different perfusion patterns of the putamen in patient with MSA compared to those with PD; however, the diagnostic accuracy was poor (sensitivity 73.3%, specificity 84%) (Bosman et al., 2003). Combining perfusion SPECT with other imaging modalities may improve diagnostic accuracy (Miyoshi et al., 2016; Van Laere et al., 2006).
Striatal dopaminergic imaging
Pre- and postsynaptic dopaminergic neuronal function as assessed with nuclear imaging techniques has been widely documented in parkinsonian conditions. Multiple tracers are available, including 18F-Dopa (dopamine storage capacity), 11C-dihydrotetrabenazine (DTBZ, vesicular monoamine transporter function) for PET, and 123I-β-CIT and 123I-FPCIT (dopamine transporter binding) for SPECT (Arbizu et al., 2014; Brooks et al., 2009). Because pre-synaptic dopaminergic metabolism is impaired in all basal ganglia disorders, presynaptic dopaminergic markers cannot distinguish between parkinsonian syndromes, although they may be useful to distinguish MSA-C from other adult-onset cerebellar ataxias (Gebus et al., 2017). In contrast, post-synaptic D2/D3 receptor radiomarkers show normal binding in patients with PD but reduced binding in two third of patients with MSA (Brooks et al., 2009).
Cardiac sympathetic neuroimaging
In contrast to patients with PD, in whom cardiac post-ganglionic sympathetic innervation is reduced in virtually all cases, most patients with MSA have preserved post-ganglionic innervation of the heart, as ascertained by normal 18F-dopamine PET or 123I-metaiodobenzylguanidine (MIBG) scintigraphy (Braune, 2001; Goldstein, 2014; Kaufmann et al., 2013; Orimo et al., 2002). A meta-analysis showed that reduced cardiac MIBG scintigraphy has high sensitivity and specificity to distinguish PD from other neurodegenerative disorders (Orimo et al., 2012). Around a third of patients with MSA, however, do have some degree of cardiac sympathetic denervation as shown by reduced MIBG uptake (Nagayama et al., 2010; Nagayama et al., 2008). Interestingly, in addition to GCI in the CNS, such patients also have αSyn deposition within Lewy bodies in sympathetic ganglia or post-ganglionic nerves (Orimo et al., 2007). Therefore, in the differential diagnosis of PD vs. MSA, neuroimaging evidence of intact cardiac sympathetic innervation almost certainly excludes PD, but neuroimaging evidence of cardiac sympathetic denervation does not exclude MSA (Goldstein, 2014; Kaufmann et al., 2013).
AUTONOMIC TESTING
Autonomic dysfunction is a characteristic feature of MSA. Retrospective cohorts reveal that autonomic symptoms were the first manifestation of MSA in 43% of cases with MSA-P and 54% with MSA-C (Roncevic et al., 2014). The most common autonomic complaints of patients with MSA are OH, neurogenic bladder (incontinence or incomplete bladder emptying), and constipation (Kaufmann et al., 2017b; Low et al., 2015; Roncevic et al., 2014).
Cardiovascular autonomic testing
In patients with MSA, αSyn deposition and neurodegeneration occur in the pre-ganglionic neurons within the CNS. This prevents the normal activation of post-ganglionic sympathetic fibers upon standing. Although the post-ganglionic fibers remain mostly intact, neuronal loss in CNS areas involved in the baroreflex prevents the appropriate release of norepinephrine, the sympathetic neurotransmitter, in the upright position. Without adequate vasoconstriction, particularly of the splanchnic vascular bed, blood pressure (BP) falls when standing. Neurogenic OH occurs in ~60% of patients with MSA (Wenning et al., 2013), although this percentage rises to ~70–80% in cohorts from autonomic clinics, perhaps due to selection bias (Low et al., 2015; Roncevic et al., 2014).
The diagnosis of neurogenic OH requires autonomic function testing with continuous BP monitoring to rule out non-neurogenic causes (i.e., OH due to other factors such as volume depletion, anemia, medications side effects). The diagnosis of neurogenic OH in patients with MSA is made by first identifying a sustained fall of at least 30 mmHg in systolic BP or 15 mmHg in diastolic BP within 3 minutes of standing. Once an orthostatic BP fall is detected, it is then necessary to determine whether this fall is due to the chronic failure to increase sympathetic activity to the vasculature when upright (i.e., autonomic failure). Performing a standardized Valsalva maneuver increases intrathoracic pressure and reduces venous return to the heart. Under normal circumstances, the acute reduction in cardiac filling pressure triggers a compensatory baroreflex-mediated reflex increase in sympathetic outflow to the vasculature to maintain BP. When the Valsalva strain is released, venous return is abruptly restored, and cardiac output increases acutely. Normally, BP will “overshoot” from baseline levels for a few heartbeats, owing to the peripheral vasculature being vasoconstricted. This is known as the phase IV overshoot. When the sympathetic nerves cannot be activated, the phase IV BP overshoot is absent and the fall on standing is attributed to a neurogenic cause (Goldstein et al., 2017a). Around 15% of patients with MSA have difficultly performing the Valsalva maneuver with sufficient respiratory strength to raise intrathoracic pressure.
Although cardiovascular autonomic testing provides valuable information, it is often insufficient to reliably distinguish MSA from PD. Patients with MSA have neurogenic OH more frequently than those with PD (~70% vs. ~50%). Despite several reports of abnormalities in the Valsalva maneuver and the heart rate variability during paced deep breathing being worse in patients with MSA than in patients with PD (Baschieri et al., 2015; De Marinis et al., 2000; Deguchi et al., 2006; Schmidt et al., 2009b), the differences are not consistent enough to distinguish between both groups.
In addition to neurogenic OH, patients with autonomic failure frequently have hypertension when supine. The diagnostic gold standard to detect nocturnal hypertension is ambulatory 24-hour BP monitoring (ABPM). Supine hypertension appears to be slightly more severe in MSA compared to PD (Pilleri et al., 2014; Schmidt et al., 2009a; Vichayanrat et al., 2017). In addition, AMBP can be useful to detect hypotensive episodes not found during a regular clinic visit, for example after meals (i.e., postprandial hypotension), and to further define treatment of OH and supine hypertension (Fanciulli et al., 2016; Gibbons et al., 2017; Jordan et al., 2002; Norcliffe-Kaufmann et al., 2014; Palma et al., 2017; Pavelic et al., 2017; Umehara et al., 2016).
Recent prospective studies show that neurogenic OH can be an early feature of MSA (Kaufmann et al., 2017b; Riku et al., 2017). Of the patients first seen at an autonomic clinic with neurogenic OH as the sole clinical feature, around 6% will develop MSA within 4-years. Of those patients that went on to receive a diagnosis of MSA, all had REM sleep behavior disorder first, suggesting that the neurodegenerative process was already present within the CNS. Other cardiovascular autonomic biomarkers associated with an increased future risk of developing MSA in a patient with neurogenic OH include circulating norepinephrine levels >110 pg/ml and a resting heart rate >70 bpm. The full diagnosis of MSA is usually reached within 5 years after the onset of neurogenic OH (Kaufmann et al., 2017b).
Thermoregulatory and sudomotor testing
Up to 80% of patients with MSA have decreased sweating, with global anhidrosis in up to 45% of cases (Iodice et al., 2012). This degree of sweat loss in MSA is greater than in PD and has been classically attributed to a preganglionic lesion (Donadio et al., 2008; Iodice et al., 2012). The preganglionic location of the deficit is based on the findings that thermoregulatory sweat test (TST, which measures integrity of both pre- and post-ganglionic neurons) is abnormal in patients with MSA, whereas quantitative sudomotor axon reflex test (QSART, which measures integrity of post-ganglionic sudomotor function) is relatively preserved. However, recent studies showing abnormal QSART in ~30% of patients with MSA and also histopathology studies showing αSyn deposits in sudomotor nerves, support the involvement of postganglionic sudomotor fibers in MSA (Coon et al., 2016; Doppler et al., 2015; Provitera et al., 2014). Most studies reporting αSyn burden in cutaneous autonomic fibers of MSA patients show less deposition of phosphorylated αSyn aggregates compared to PD (Donadio et al., 2010; Haga et al., 2015; Zange et al., 2015).
OLFACTORY TESTING
Olfactory function has been widely studied in parkinsonian conditions, showing significant hyposmia in the majority of patients with PD (Doty, 2012), with normosmia or mild hyposmia in the majority of patients with MSA (Glass et al., 2012). Olfactory functions tests have very high specificity and moderate sensitivity to distinguish PD from MSA (Krismer et al., 2017) and, therefore, should be included in the routine evaluation of patients with suspected MSA. Interestingly, olfaction is a good biomarker to predict which patients will develop MSA or PD/DLB in patients in the premotor phase of the synucleinopathies (i.e., PAF and/or REM sleep behavior disorder) (Kaufmann et al., 2017b). Due to its reliability and practicality, the University of Pennsylvania Smell Identification Test (UPSIT) is the most widely used and preferred olfactory function test (Doty et al., 1984). In short, the test consists of 40 “scratch and sniff” strips embedded with a microencapsulated odorant. The scent is released using a pencil. The patient then smells the scratched strip and selects the odor from 4 choices. The test is scored out of 40 items. When administering the UPSIT in patients with bradykinesia, patients should be encouraged to inhale deeply and not simply smell the scratch-and-sniff paper. The major limitation of the UPSIT is that some of its odorants might not be familiar to non-US subjects (e.g., pumpkin pie, root beer). An analog 12-item version of the UPSIT, the Brief Smell Identification Test (BSIT), is cross-cultural may be more appropriate for non-US populations (Doty et al., 1996).
SLEEP DISORDERS
Sleep disorders in MSA are common. Virtually all patients with MSA have REM sleep behavior disorder, in many of whom is the presenting feature of the disease (Palma et al., 2015). Although sleep questionnaires appear to have a high false negative and true positive ratio, a videopolysomnography is recommended to confirm RBD and rule out other sleep problems. Obstructive sleep apnea is more frequent than central sleep apnea, occurring in up to 40% of patients with MSA (Ferini-Strambi et al., 2012). Many insurance companies require confirmation of sleep-disordered breathing with polysomnography prior to approving the use of non-invasive ventilation (e.g., CPAP or bilevel positive airway pressure).
A high-pitched sound mainly during inspiration due to upper airway obstruction at the level of the glottic aperture characterizes stridor (Ozawa et al., 2016). The prevalence of stridor in patients with MSA varies from 15% to 40% (Ghorayeb et al., 2002) and can, in some cases, be the presenting feature of the disease (Kaufmann et al., 2017a). Vocal cord paralysis and early stridor in patients with MSA are risk factors for shorter survival (Giannini et al., 2016; Lalich et al., 2014). Indirect laryngoscopy is useful in the diagnosis of stridor, and serial examinations may be required. In patients with stridor, laryngoscopic examination reveals restriction of vocal cords abduction, paradoxical cord movements, and floppy epiglottis (Isozaki et al., 1996). Stridor represents a life-threatening condition as it may lead to respiratory failure (Yamaguchi et al., 2003). It is unclear whether tracheostomy placement prolongs survival (Giannini et al., 2016).
UROLOGICAL EVALUATION
All patients with MSA have urinary dysfunction and this is one of the earliest feature of the disease (Kaufmann et al., 2017b; Kirchhof et al., 2003; Roncevic et al., 2014). The most frequently reported urinary symptom is voiding difficulty, present in 80% of patients followed by nocturia in 74%, urgency in 63%, incontinence in 63%, diurnal frequency in 45%, nocturnal enuresis in 19%, and urinary retention in 8% of patients (Ogawa et al., 2017). Male patients with pre-motor MSA frequently undergo surgery for suspected benign prostate hyperplasia without realizing that MSA is the actual cause of their urinary problems. Urological surgery outcomes are rarely favorable in patients with MSA. Around 40% of patients with MSA have neurogenic detrusor overactivity (i.e., overactive bladder) during the filling phase, and this may be accompanied by uninhibited external sphincter relaxation, which worsens the severity of urge-incontinence (Sakakibara et al., 2001; Stocchi et al., 1997). Weak detrusor contraction during the voiding phase (i.e., detrusor underactivity) is observed in approximately 70% of patients with MSA, resulting in large amount of bladder post-void residual volume (Sakakibara et al., 2001). This can increase the risk of urosepsis and death.
An increased residual urinary volume >100 ml has a positive predictive value of 91.6% and a residual volume < 100 ml has negative predictive value of 67.8% to distinguish MSA vs. PD (Hahn et al., 2005). Thus, evaluation of urinary dysfunction in MSA must include a post-void residual bladder scan to determine whether urinary retention due to underactive bladder is present. This is also useful to define treatment, as patients with a post-void residual bladder volume of > 100 ml benefit from clean intermittent self-catheterization (Fowler et al., 2003). Urodynamic studies can be useful to fully define the nature of the bladder abnormalities and show a different pattern of failure in MSA vs. PD patients (Ogawa et al., 2017). The main abnormalities which lead to chronic urinary retention in patients with MSA are detrusor underactivity, detrusor sphincter dyssinergia, and urethral hypertonia in the voiding phase, while in the filling phase inhibited external sphincter relaxation and bladder neck dysfunction occur. Detrusor hyperreflexia is a relatively common finding in both PD and MSA patients, especially in the early stages of the disease (Ogawa et al., 2017).
DYSPHAGIA EVALUATION
Dysphagia is severe in 32% of patients with MSA (O’Sullivan et al., 2008) and dysphagia was a subjective complaint in 73% of patients with MSA in a small study with post-mortem diagnostic confirmation (Muller et al., 2001). Because dysphagia is associated with a bad prognosis and a short survival time, it should be addressed and treated promptly. A standard dysphagia evaluation should include a modified barium swallow test or videofluoroscopy. If aspiration is mild, dietary (e.g., liquid thickeners) and positional changes may be required. Patients with moderate or severe aspiration may eventually require a gastrostomy tube to ensure nutrition and avoid aspiration into the airway. Whether placement of a gastrostomy tube prolongs survival in MSA is unknown.
COGNITIVE EVALUATION
The current consensus diagnostic criteria for MSA consider dementia as a non-supporting feature of the disease (Gilman et al., 2008). There is increasing evidence, however, showing that cognitive impairment is an integral part of the disease (Koga et al., 2017b; Stankovic et al., 2014). Cognitive disturbances in MSA occur across a wide spectrum ranging from mild single domain deficits to impairments in multiple domains and even to frank dementia in rare cases. Frontal-executive dysfunction is the most common presentation, while memory and visuospatial abilities may also be impaired (Brown et al., 2010; Stankovic et al., 2014). Thus, all patients with MSA should be screened for cognitive impairment with a standardized test, such as the Montreal Cognitive Assessment (MoCA). Operational guidelines for the diagnosis and treatment of cognitive impairment in MSA are lacking.
EMERGING DIAGNOSTIC METHODS
Transcranial sonography
Although transcranial sonography is not possible in 10–20% of subjects due to reduced temporal bone acoustic window, some works suggest that substantia nigra hyperechogenicity may be a potential marker to distinguish PD from atypical parkinsonian syndromes (Bouwmans et al., 2010), either alone or in combination with other diagnostic modalities (Fujita et al., 2016).
Plasma and CSF biomarkers
There is increasing interest in the development of potential biomarkers in plasma and cerebrospinal fluid (CSF) for the diagnosis of MSA (Laurens et al., 2015). The most promising so far include plasma norepinephrine levels (Kaufmann et al., 2017b), plasma catecholaminergic vesicular storage levels (Goldstein et al., 2015), plasma and CSF neurofilament light chain (NfL) protein (Hansson et al., 2017; Hu et al., 2017), and plasma and CSF αSyn levels (Sun et al., 2014). Further research is required to confirm the sensitivity and specificity of these.
Retinal optical coherence tomography
Even though patients with MSA only rarely have visual complaints, recent studies of the retina using optical coherence tomography (OCT) have consistently shown atrophy of the peripapillary retinal nerve fiber layer (RNFL) and to a lesser extent the macular ganglion cell layer (GCL) complex (Mendoza-Santiesteban et al., 2017a). These abnormalities are progressive over time and have been recently confirmed by pathological examination in the retinas from 3 patients with MSA (Mendoza-Santiesteban et al., 2015; Mendoza-Santiesteban et al., 2017b). OCT may be a useful biomarker of disease progression in future clinical trials of patients with MSA.
Skin biopsy
In-vivo studies assessing skin αSyn deposits have reported on the presence of αSyn aggregates in cutaneous autonomic nerves of patients with PD, with variable frequencies ranging from 0% to 100% (Donadio et al., 2014; Navarro-Otano et al., 2015; Wang et al., 2013; Zange et al., 2015). Some of these studies included patients with MSA, in whom no αSyn aggregates were detected. However, another study did find skin phosphorylated αSyn aggregates in 67% of patients with MSA (Doppler et al., 2015). Future studies should define the optimal skin biopsy site, the number of biopsies needed to obtain an optimal result, the tissue fixation method, and the choice of antibody for immunohistochemistry.
PREMOTOR DIAGNOSIS OF MULTIPLE SYSTEM ATROPHY
Current consensus guidelines include a possible, probable and definite diagnosis of MSA but do not include a prodromal or pre-motor category. The ability to make such a diagnosis may be near. In recent years, several prospective studies of at-risk cohorts reported the features of prodromal MSA cases. From these studies, prospective biomarkers have emerged. The accuracy of predictive diagnostic biomarkers will determine the potential eligibility for disease-modifying trials. Follow-up of patients with idiopathic RBD reveals that a small percentage go on to develop MSA (Iranzo et al., 2014; Postuma et al., 2015) rather than PD or DLB, but because of the low phenoconversion rates it is necessary to have additional predictors to diagnose premotor MSA cases. The addition of autonomic biomarkers in a patient with RBD helps further refine future phenoconversion risks (Kaufmann et al., 2017b). Prospective follow-up of patients with isolated autonomic failure and RBD show a combination of autonomic biomarkers and clinical features that provide sensitive prodromal biomarkers of MSA. The challenge remains to apply these in the clinic to recruit patients for future neuroprotective trials in MSA. Undoubtedly, it is this prodromal phase that provides the window of opportunity to intervene early enough to halt the spread of synuclein throughout the CNS and preserve motor function (Muppidi et al., 2017).
CONCLUSIONS
Although ancillary tests (olfactory testing, autonomic testing, neuroimaging, urological evaluation) can assist in the diagnosis and should be performed when possible, the diagnosis of probable or possible MSA is grounded on the clinical history and the neurological exam and can only be confirmed pathologically after the patient’s death. Recent reports revealed that only 62% of patients clinically diagnosed with MSA by community neurologists have the correct diagnosis at autopsy (Koga et al., 2015). The most common diseases misdiagnosed as MSA included PD, DLB and PSP. On the other hand, autopsy studies show that ~5% of patients clinically diagnosed with PD in general neurology clinics turn out to have MSA at autopsy (Joutsa et al., 2014). Thus, considering that, in the U.S., the estimated point prevalence of PD is ~100 per 100,000 inhabitants between the ages of 50 and 60 (Pringsheim et al., 2014), and that the estimated point prevalence of MSA is 7.8 per 100,000 inhabitants in the same age group (Fanciulli et al., 2015), there might be at least 5 additional subjects with MSA misdiagnosed as PD per 100,000 inhabitants. Despite the availability of consensus criteria for clinical diagnosis and recent advances in diagnostic techniques, the diagnostic accuracy of MSA remains sub-optimal. Recent neuropathology studies raise the possibility of additional phenotypes, including a “minimal change” variant, and a frontotemporal lobar degeneration-synuclein variant (Koga et al., 2017a). As other factors emerge, including the possibility of specific genomic traits (Federoff et al., 2015), better recognition of all the phenotypic variants of MSA should be possible. This has important implications when planning clinical trials of potential neuroprotective agents in these patients.
Acknowledgments
Funding sources: National Institutes of Health (U54 NS065736) and Dysautonomia Foundation, Inc.
Footnotes
Conflict of interests: All authors report no conflict of interests related to this article.
References
- Anderson T, Luxon L, Quinn N, Daniel S, David Marsden C, Bronstein A. Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Movement disorders : official journal of the Movement Disorder Society. 2008;23:977–984. doi: 10.1002/mds.21999. [DOI] [PubMed] [Google Scholar]
- Arbizu J, Luquin MR, Abella J, de la Fuente-Fernandez R, Fernandez-Torron R, Garcia-Solis D, Garrastachu P, Jimenez-Hoyuela JM, Llaneza M, Lomena F, Lorenzo-Bosquet C, Marti MJ, Martinez-Castrillo JC, Mir P, Mitjavila M, Ruiz-Martinez J, Vela L. Functional neuroimaging in the diagnosis of patients with Parkinsonism: Update and recommendations for clinical use. Rev Esp Med Nucl Imagen Mol. 2014;33:215–226. doi: 10.1016/j.remn.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Asahina M, Low DA, Mathias CJ, Fujinuma Y, Katagiri A, Yamanaka Y, Shimada J, Poudel A, Kuwabara S. Skin temperature of the hand in multiple system atrophy and Parkinson’s disease. Parkinsonism & related disorders. 2013;19:560–562. doi: 10.1016/j.parkreldis.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashour R, Tintner R, Jankovic J. Striatal deformities of the hand and foot in Parkinson’s disease. Lancet neurology. 2005;4:423–431. doi: 10.1016/S1474-4422(05)70119-8. [DOI] [PubMed] [Google Scholar]
- Barbagallo G, Sierra-Pena M, Nemmi F, Traon AP, Meissner WG, Rascol O, Peran P. Multimodal MRI assessment of nigro-striatal pathway in multiple system atrophy and Parkinson disease. Movement disorders : official journal of the Movement Disorder Society. 2016;31:325–334. doi: 10.1002/mds.26471. [DOI] [PubMed] [Google Scholar]
- Baschieri F, Calandra-Buonaura G, Doria A, Mastrolilli F, Palareti A, Barletta G, Solieri L, Guaraldi P, Martinelli P, Cortelli P. Cardiovascular autonomic testing performed with a new integrated instrumental approach is useful in differentiating MSA-P from PD at an early stage. Parkinsonism & related disorders. 2015;21:477–482. doi: 10.1016/j.parkreldis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Batla A, Stamelou M, Mensikova K, Kaiserova M, Tuckova L, Kanovsky P, Quinn N, Bhatia KP. Markedly asymmetric presentation in multiple system atrophy. Parkinsonism & related disorders. 2013;19:901–905. doi: 10.1016/j.parkreldis.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Baudrexel S, Seifried C, Penndorf B, Klein JC, Middendorp M, Steinmetz H, Grunwald F, Hilker R. The value of putaminal diffusion imaging versus 18-fluorodeoxyglucose positron emission tomography for the differential diagnosis of the Parkinson variant of multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2014;29:380–387. doi: 10.1002/mds.25749. [DOI] [PubMed] [Google Scholar]
- Bhattacharya K, Saadia D, Eisenkraft B, Yahr M, Olanow W, Drayer B, Kaufmann H. Brain magnetic resonance imaging in multiple-system atrophy and Parkinson disease: a diagnostic algorithm. Archives of neurology. 2002;59:835–842. doi: 10.1001/archneur.59.5.835. [DOI] [PubMed] [Google Scholar]
- Boesch SM, Wenning GK, Ransmayr G, Poewe W. Dystonia in multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 2002;72:300–303. doi: 10.1136/jnnp.72.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman T, Van Laere K, Santens P. Anatomically standardised 99mTc-ECD brain perfusion SPET allows accurate differentiation between healthy volunteers, multiple system atrophy and idiopathic Parkinson’s disease. European journal of nuclear medicine and molecular imaging. 2003;30:16–24. doi: 10.1007/s00259-002-1009-9. [DOI] [PubMed] [Google Scholar]
- Bouwmans AE, Vlaar AM, Srulijes K, Mess WH, Weber WE. Transcranial sonography for the discrimination of idiopathic Parkinson’s disease from the atypical parkinsonian syndromes. Int Rev Neurobiol. 2010;90:121–146. doi: 10.1016/S0074-7742(10)90009-3. [DOI] [PubMed] [Google Scholar]
- Braune S. The role of cardiac metaiodobenzylguanidine uptake in the differential diagnosis of parkinsonian syndromes. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2001;11:351–355. doi: 10.1007/BF02292766. [DOI] [PubMed] [Google Scholar]
- Brenneis C, Egger K, Scherfler C, Seppi K, Schocke M, Poewe W, Wenning GK. Progression of brain atrophy in multiple system atrophy. A longitudinal VBM study. Journal of neurology. 2007;254:191–196. doi: 10.1007/s00415-006-0325-6. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Seppi K Neuroimaging Working Group on, M.S.A. Proposed neuroimaging criteria for the diagnosis of multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2009;24:949–964. doi: 10.1002/mds.22413. [DOI] [PubMed] [Google Scholar]
- Brown RG, Lacomblez L, Landwehrmeyer BG, Bak T, Uttner I, Dubois B, Agid Y, Ludolph A, Bensimon G, Payan C, Leigh NP, Group NS. Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain. 2010;133:2382–2393. doi: 10.1093/brain/awq158. [DOI] [PubMed] [Google Scholar]
- Burciu RG, Chung JW, Shukla P, Ofori E, Li H, McFarland NR, Okun MS, Vaillancourt DE. Functional MRI of disease progression in Parkinson disease and atypical parkinsonian syndromes. Neurology. 2016;87:709–717. doi: 10.1212/WNL.0000000000002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk K, Buhring U, Schulz JB, Zuhlke C, Hellenbroich Y, Dichgans J. Clinical and magnetic resonance imaging characteristics of sporadic cerebellar ataxia. Archives of neurology. 2005;62:981–985. doi: 10.1001/archneur.62.6.981. [DOI] [PubMed] [Google Scholar]
- Burk K, Globas C, Wahl T, Buhring U, Dietz K, Zuhlke C, Luft A, Schulz JB, Voigt K, Dichgans J. MRI-based volumetric differentiation of sporadic cerebellar ataxia. Brain. 2004;127:175–181. doi: 10.1093/brain/awh013. [DOI] [PubMed] [Google Scholar]
- Burk K, Skalej M, Dichgans J. Pontine MRI hyperintensities (“the cross sign”) are not pathognomonic for multiple system atrophy (MSA) Movement disorders : official journal of the Movement Disorder Society. 2001;16:535. doi: 10.1002/mds.1107. [DOI] [PubMed] [Google Scholar]
- Calandra-Buonaura G, Doria A, Lopane G, Guaraldi P, Capellari S, Martinelli P, Cortelli P, Contin M. Pharmacodynamics of a low subacute levodopa dose helps distinguish between multiple system atrophy with predominant Parkinsonism and Parkinson’s disease. Journal of neurology. 2016;263:250–256. doi: 10.1007/s00415-015-7961-7. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Lowry M. Systematic review of proton magnetic resonance spectroscopy of the striatum in parkinsonian syndromes. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2001;8:573–577. doi: 10.1046/j.1468-1331.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- Colosimo C. Pisa syndrome in a patient with multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 1998;13:607–609. doi: 10.1002/mds.870130342. [DOI] [PubMed] [Google Scholar]
- Colosimo C, Albanese A, Hughes AJ, de Bruin VM, Lees AJ. Some specific clinical features differentiate multiple system atrophy (striatonigral variety) from Parkinson’s disease. Archives of neurology. 1995;52:294–298. doi: 10.1001/archneur.1995.00540270090024. [DOI] [PubMed] [Google Scholar]
- Coon EA, Fealey RD, Sletten DM, Mandrekar JN, Benarroch EE, Sandroni P, Low PA, Singer W. Anhidrosis in multiple system atrophy involves pre- and postganglionic sudomotor dysfunction. Movement disorders : official journal of the Movement Disorder Society. 2016 doi: 10.1002/mds.26864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon EA, Sletten DM, Suarez MD, Mandrekar JN, Ahlskog JE, Bower JH, Matsumoto JY, Silber MH, Benarroch EE, Fealey RD, Sandroni P, Low PA, Singer W. Clinical features and autonomic testing predict survival in multiple system atrophy. Brain. 2015;138:3623–3631. doi: 10.1093/brain/awv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marinis M, Stocchi F, Gregori B, Accornero N. Sympathetic skin response and cardiovascular autonomic function tests in Parkinson’s disease and multiple system atrophy with autonomic failure. Movement disorders : official journal of the Movement Disorder Society. 2000;15:1215–1220. doi: 10.1002/1531-8257(200011)15:6<1215::aid-mds1023>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Ikeda K, Kume K, Takata T, Kokudo Y, Kamada M, Touge T, Honjo N, Masaki T. Significance of the hot-cross bun sign on T2*-weighted MRI for the diagnosis of multiple system atrophy. Journal of neurology. 2015;262:1433–1439. doi: 10.1007/s00415-015-7728-1. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Sasaki I, Ikeda K, Shimamura M, Urai Y, Tsukaguchi M, Touge T, Takeuchi H, Kuriyama S. The validity of a hyperventilation test for an investigation of autonomic failure: assessment in patients with multiple system atrophy and Parkinson’s disease. International journal of clinical practice. 2006;60:1542–1547. doi: 10.1111/j.1742-1241.2005.00802.x. [DOI] [PubMed] [Google Scholar]
- Donadio V, Cortelli P, Elam M, Di Stasi V, Montagna P, Holmberg B, Giannoccaro MP, Bugiardini E, Avoni P, Baruzzi A, Liguori R. Autonomic innervation in multiple system atrophy and pure autonomic failure. Journal of neurology, neurosurgery, and psychiatry. 2010;81:1327–1335. doi: 10.1136/jnnp.2009.198135. [DOI] [PubMed] [Google Scholar]
- Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P, Capellari S, Avoni P, Baruzzi A, Liguori R. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology. 2014;82:1362–1369. doi: 10.1212/WNL.0000000000000316. [DOI] [PubMed] [Google Scholar]
- Donadio V, Nolano M, Elam M, Montagna P, Provitera V, Bugiardini E, Baruzzi A, Santoro L, Liguori R. Anhidrosis in multiple system atrophy: a preganglionic sudomotor dysfunction? Movement disorders : official journal of the Movement Disorder Society. 2008;23:885–888. doi: 10.1002/mds.21972. [DOI] [PubMed] [Google Scholar]
- Doppler K, Weis J, Karl K, Ebert S, Ebentheuer J, Trenkwalder C, Klebe S, Volkmann J, Sommer C. Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2015;30:1688–1692. doi: 10.1002/mds.26293. [DOI] [PubMed] [Google Scholar]
- Doty RL. Olfactory dysfunction in Parkinson disease. Nature reviews Neurology. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiology & behavior. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Du G, Lewis MM, Kanekar S, Sterling NW, He L, Kong L, Li R, Huang X. Combined Diffusion Tensor Imaging and Apparent Transverse Relaxation Rate Differentiate Parkinson Disease and Atypical Parkinsonism. AJNR American journal of neuroradiology. 2017;38:966–972. doi: 10.3174/ajnr.A5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, Feigin AS, Eidelberg D. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage. 2005;26:912–921. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Fanciulli A, Gobel G, Ndayisaba JP, Granata R, Duerr S, Strano S, Colosimo C, Poewe W, Pontieri FE, Wenning GK. Supine hypertension in Parkinson’s disease and multiple system atrophy. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2016;26:97–105. doi: 10.1007/s10286-015-0336-4. [DOI] [PubMed] [Google Scholar]
- Fanciulli A, Wenning GK. Multiple-system atrophy. The New England journal of medicine. 2015;372:249–263. doi: 10.1056/NEJMra1311488. [DOI] [PubMed] [Google Scholar]
- Federoff M, Schottlaender LV, Houlden H, Singleton A. Multiple system atrophy: the application of genetics in understanding etiology. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2015;25:19–36. doi: 10.1007/s10286-014-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferini-Strambi L, Marelli S. Sleep dysfunction in multiple system atrophy. Current treatment options in neurology. 2012;14:464–473. doi: 10.1007/s11940-012-0189-2. [DOI] [PubMed] [Google Scholar]
- Fiorenzato E, Weis L, Seppi K, Onofrj M, Cortelli P, Zanigni S, Tonon C, Kaufmann H, Shepherd TM, Poewe W, Krismer F, Wenning G, Antonini A, Biundo R Movement Disorders Society M.S.A.N, Imaging Study, G. Brain structural profile of multiple system atrophy patients with cognitive impairment. J Neural Transm (Vienna) 2017;124:293–302. doi: 10.1007/s00702-016-1636-0. [DOI] [PubMed] [Google Scholar]
- Fontes-Villalba A, Palma JA, Fernandez-Seara MA, Pastor MA, de Castro P. Clinical reasoning: a 47-year-old man with progressive gait disturbance and stiffness in his legs. Neurology. 2013;80:e223–227. doi: 10.1212/WNL.0b013e318293e136. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, O’Malley KJ. Investigation and management of neurogenic bladder dysfunction. Journal of neurology, neurosurgery, and psychiatry. 2003;74(Suppl 4):iv27–iv31. doi: 10.1136/jnnp.74.suppl_4.iv27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Suzuki K, Numao A, Watanabe Y, Uchiyama T, Miyamoto T, Miyamoto M, Hirata K. Usefulness of Cardiac MIBG Scintigraphy, Olfactory Testing and Substantia Nigra Hyperechogenicity as Additional Diagnostic Markers for Distinguishing between Parkinson’s Disease and Atypical Parkinsonian Syndromes. PloS one. 2016;11:e0165869. doi: 10.1371/journal.pone.0165869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebus O, Montaut S, Monga B, Wirth T, Cheraud C, Alves Do Rego C, Zinchenko I, Carre G, Hamdaoui M, Hautecloque G, Nguyen-Them L, Lannes B, Chanson JB, Lagha-Boukbiza O, Fleury MC, Devys D, Nicolas G, Rudolf G, Bereau M, Mallaret M, Renaud M, Acquaviva C, Koenig M, Koob M, Kremer S, Namer IJ, Cazeneuve C, Echaniz-Laguna A, Tranchant C, Anheim M. Deciphering the causes of sporadic late-onset cerebellar ataxias: a prospective study with implications for diagnostic work. Journal of neurology. 2017;264:1118–1126. doi: 10.1007/s00415-017-8500-5. [DOI] [PubMed] [Google Scholar]
- Ghaemi M, Hilker R, Rudolf J, Sobesky J, Heiss WD. Differentiating multiple system atrophy from Parkinson’s disease: contribution of striatal and midbrain MRI volumetry and multi-tracer PET imaging. Journal of neurology, neurosurgery, and psychiatry. 2002;73:517–523. doi: 10.1136/jnnp.73.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F. Sleep disorders and their determinants in multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 2002;72:798–800. doi: 10.1136/jnnp.72.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini G, Calandra-Buonaura G, Mastrolilli F, Righini M, Bacchi-Reggiani ML, Cecere A, Barletta G, Guaraldi P, Provini F, Cortelli P. Early stridor onset and stridor treatment predict survival in 136 patients with MSA. Neurology. 2016;87:1375–1383. doi: 10.1212/WNL.0000000000003156. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S, Kaufmann H. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. Journal of neurology. 2017 doi: 10.1007/s00415-016-8375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, May SJ, Shults CW, Tanner CM, Kukull W, Lee VM, Masliah E, Low P, Sandroni P, Trojanowski JQ, Ozelius L, Foroud T North American Multiple System Atrophy Study, G. The North American Multiple System Atrophy Study Group. J Neural Transm (Vienna) 2005;112:1687–1694. doi: 10.1007/s00702-005-0381-6. [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasmacher SA, Leigh PN, Saha RA. Predictors of survival in progressive supranuclear palsy and multiple system atrophy: a systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2017 doi: 10.1136/jnnp-2016-314956. [DOI] [PubMed] [Google Scholar]
- Glass PG, Lees AJ, Mathias C, Mason L, Best C, Williams DR, Katzenschlager R, Silveira-Moriyama L. Olfaction in pathologically proven patients with multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2012;27:327–328. doi: 10.1002/mds.23972. [DOI] [PubMed] [Google Scholar]
- Goldstein DS. Dysautonomia in Parkinson disease. Comprehensive Physiology. 2014;4:805–826. doi: 10.1002/cphy.c130026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Cheshire WP. Beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver. Clinical Autonomic Research. 2017a doi: 10.1007/s10286-017-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Cheshire WP., Jr The autonomic medical history. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017b doi: 10.1007/s10286-017-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, Sharabi Y, Holmes C. Plasma biomarkers of decreased vesicular storage distinguish Parkinson disease with orthostatic hypotension from the parkinsonian form of multiple system atrophy. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2015;25:61–67. doi: 10.1007/s10286-015-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga R, Sugimoto K, Nishijima H, Miki Y, Suzuki C, Wakabayashi K, Baba M, Yagihashi S, Tomiyama M. Clinical Utility of Skin Biopsy in Differentiating between Parkinson’s Disease and Multiple System Atrophy. Parkinson’s disease. 2015;2015:167038. doi: 10.1155/2015/167038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn K, Ebersbach G. Sonographic assessment of urinary retention in multiple system atrophy and idiopathic Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2005;20:1499–1502. doi: 10.1002/mds.20586. [DOI] [PubMed] [Google Scholar]
- Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K Swedish Bio, F.s. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88:930–937. doi: 10.1212/WNL.0000000000003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch MJ, Chung S, Ben-Eliezer N, Bruno MT, Fatterpekar GM, Shepherd TM. New Clinically Feasible 3T MRI Protocol to Discriminate Internal Brain Stem Anatomy. AJNR American journal of neuroradiology. 2016;37:1058–1065. doi: 10.3174/ajnr.A4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg B, Johnels B, Ingvarsson P, Eriksson B, Rosengren L. CSF-neurofilament and levodopa tests combined with discriminant analysis may contribute to the differential diagnosis of Parkinsonian syndromes. Parkinsonism & related disorders. 2001;8:23–31. doi: 10.1016/s1353-8020(00)00083-3. [DOI] [PubMed] [Google Scholar]
- Horimoto Y, Aiba I, Yasuda T, Ohkawa Y, Katayama T, Yokokawa Y, Goto A, Ito Y. Longitudinal MRI study of multiple system atrophy - when do the findings appear, and what is the course? Journal of neurology. 2002;249:847–854. doi: 10.1007/s00415-002-0734-0. [DOI] [PubMed] [Google Scholar]
- Hotter A, Esterhammer R, Schocke MF, Seppi K. Potential of advanced MR imaging techniques in the differential diagnosis of parkinsonism. Movement disorders : official journal of the Movement Disorder Society. 2009;24(Suppl 2):S711–720. doi: 10.1002/mds.22648. [DOI] [PubMed] [Google Scholar]
- Hu X, Yang Y, Gong D. Cerebrospinal fluid levels of neurofilament light chain in multiple system atrophy relative to Parkinson’s disease: a meta-analysis. Neurol Sci. 2017;38:407–414. doi: 10.1007/s10072-016-2783-7. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Colosimo C, Kleedorfer B, Daniel SE, Lees AJ. The dopaminergic response in multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 1992;55:1009–1013. doi: 10.1136/jnnp.55.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman TA. Diffusion-weighted and diffusion tensor imaging of the brain, made easy. Cancer Imaging. 2010;10(Spec no A):S163–171. doi: 10.1102/1470-7330.2010.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz HJ, Moller L, Sudmeyer M, Hilker R, Hattingen E, Egger K, Amtage F, Respondek G, Stamelou M, Schnitzler A, Pinkhardt EH, Oertel WH, Knake S, Kassubek J, Hoglinger GU. Differentiation of neurodegenerative parkinsonian syndromes by volumetric magnetic resonance imaging analysis and support vector machine classification. Movement disorders : official journal of the Movement Disorder Society. 2016;31:1506–1517. doi: 10.1002/mds.26715. [DOI] [PubMed] [Google Scholar]
- Hussl A, Mahlknecht P, Scherfler C, Esterhammer R, Schocke M, Poewe W, Seppi K. Diagnostic accuracy of the magnetic resonance Parkinsonism index and the midbrain-to-pontine area ratio to differentiate progressive supranuclear palsy from Parkinson’s disease and the Parkinson variant of multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2010;25:2444–2449. doi: 10.1002/mds.23351. [DOI] [PubMed] [Google Scholar]
- Iodice V, Lipp A, Ahlskog JE, Sandroni P, Fealey RD, Parisi JE, Matsumoto JY, Benarroch EE, Kimpinski K, Singer W, Gehrking TL, Gehrking JA, Sletten DM, Schmeichel AM, Bower JH, Gilman S, Figueroa J, Low PA. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. Journal of neurology, neurosurgery, and psychiatry. 2012;83:453–459. doi: 10.1136/jnnp-2011-301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranzo A, Fernandez-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, Gelpi E, Vilaseca I, Sanchez-Valle R, Llado A, Gaig C, Santamaria J. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PloS one. 2014;9:e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isozaki E, Naito A, Horiguchi S, Kawamura R, Hayashida T, Tanabe H. Early diagnosis and stage classification of vocal cord abductor paralysis in patients with multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 1996;60:399–402. doi: 10.1136/jnnp.60.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J, Biaggioni I. Diagnosis and treatment of supine hypertension in autonomic failure patients with orthostatic hypotension. J Clin Hypertens (Greenwich) 2002;4:139–145. doi: 10.1111/j.1524-6175.2001.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Gardberg M, Roytta M, Kaasinen V. Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism & related disorders. 2014;20:840–844. doi: 10.1016/j.parkreldis.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Goldstein DS. Autonomic dysfunction in Parkinson disease. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 117. 2013. pp. 259–278. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Norcliffe-Kaufmann L, Palma JA Autonomic Disorders, C. Reply to “Pure autonomic failure vs. Manifest CNS synucleinopathy: Relevance of stridor and autonomic biomarkers”. Annals of neurology. 2017a;81:910–911. doi: 10.1002/ana.24949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann H, Norcliffe-Kaufmann L, Palma JA, Biaggioni I, Low PA, Singer W, Goldstein DS, Peltier AC, Shibao CA, Gibbons CH, Freeman R, Robertson D Autonomic Disorders, C. Natural history of pure autonomic failure: A United States prospective cohort. Annals of neurology. 2017b;81:287–297. doi: 10.1002/ana.24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Jeong HJ, Bae YJ, Park SY, Kim E, Kang SY, Oh ES, Kim KJ, Jeon B, Kim SE, Cho ZH, Kim YB. Loss of substantia nigra hyperintensity on 7 Tesla MRI of Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Parkinsonism & related disorders. 2016;26:47–54. doi: 10.1016/j.parkreldis.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Kirchhof K, Apostolidis AN, Mathias CJ, Fowler CJ. Erectile and urinary dysfunction may be the presenting features in patients with multiple system atrophy: a retrospective study. Int J Impot Res. 2003;15:293–298. doi: 10.1038/sj.ijir.3901014. [DOI] [PubMed] [Google Scholar]
- Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet neurology. 2010;9:94–104. doi: 10.1016/S1474-4422(09)70305-9. [DOI] [PubMed] [Google Scholar]
- Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85:404–412. doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S, Dickson DW. Recent advances in neuropathology, biomarkers and therapeutic approach of multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 2017a doi: 10.1136/jnnp-2017-315813. [DOI] [PubMed] [Google Scholar]
- Koga S, Parks A, Uitti RJ, van Gerpen JA, Cheshire WP, Wszolek ZK, Dickson DW. Profile of cognitive impairment and underlying pathology in multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2017b;32:405–413. doi: 10.1002/mds.26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollensperger M, Geser F, Ndayisaba JP, Boesch S, Seppi K, Ostergaard K, Dupont E, Cardozo A, Tolosa E, Abele M, Klockgether T, Yekhlef F, Tison F, Daniels C, Deuschl G, Coelho M, Sampaio C, Bozi M, Quinn N, Schrag A, Mathias CJ, Fowler C, Nilsson CF, Widner H, Schimke N, Oertel W, Del Sorbo F, Albanese A, Pellecchia MT, Barone P, Djaldetti R, Colosimo C, Meco G, Gonzalez-Mandly A, Berciano J, Gurevich T, Giladi N, Galitzky M, Rascol O, Kamm C, Gasser T, Siebert U, Poewe W, Wenning GK, Emsa SG. Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Movement disorders : official journal of the Movement Disorder Society. 2010;25:2604–2612. doi: 10.1002/mds.23192. [DOI] [PubMed] [Google Scholar]
- Kollensperger M, Geser F, Seppi K, Stampfer-Kountchev M, Sawires M, Scherfler C, Boesch S, Mueller J, Koukouni V, Quinn N, Pellecchia MT, Barone P, Schimke N, Dodel R, Oertel W, Dupont E, Ostergaard K, Daniels C, Deuschl G, Gurevich T, Giladi N, Coelho M, Sampaio C, Nilsson C, Widner H, Sorbo FD, Albanese A, Cardozo A, Tolosa E, Abele M, Klockgether T, Kamm C, Gasser T, Djaldetti R, Colosimo C, Meco G, Schrag A, Poewe W, Wenning GK, European MSASG. Red flags for multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2008;23:1093–1099. doi: 10.1002/mds.21992. [DOI] [PubMed] [Google Scholar]
- Krismer F, Pinter B, Mueller C, Mahlknecht P, Nocker M, Reiter E, Djamshidian-Tehrani A, Boesch SM, Wenning GK, Scherfler C, Poewe W, Seppi K. Sniffing the diagnosis: Olfactory testing in neurodegenerative parkinsonism. Parkinsonism & related disorders. 2017;35:36–41. doi: 10.1016/j.parkreldis.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Lalich IJ, Ekbom DC, Starkman SJ, Orbelo DM, Morgenthaler TI. Vocal fold motion impairment in multiple system atrophy. Laryngoscope. 2014;124:730–735. doi: 10.1002/lary.24402. [DOI] [PubMed] [Google Scholar]
- Laurens B, Constantinescu R, Freeman R, Gerhard A, Jellinger K, Jeromin A, Krismer F, Mollenhauer B, Schlossmacher MG, Shaw LM, Verbeek MM, Wenning GK, Winge K, Zhang J, Meissner WG. Fluid biomarkers in multiple system atrophy: A review of the MSA Biomarker Initiative. Neurobiology of disease. 2015;80:29–41. doi: 10.1016/j.nbd.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Lee EA, Cho HI, Kim SS, Lee WY. Comparison of magnetic resonance imaging in subtypes of multiple system atrophy. Parkinsonism & related disorders. 2004;10:363–368. doi: 10.1016/j.parkreldis.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim TH, Mun CW, Kim TH, Han YH. Progression of subcortical atrophy and iron deposition in multiple system atrophy: a comparison between clinical subtypes. Journal of neurology. 2015;262:1876–1882. doi: 10.1007/s00415-015-7785-5. [DOI] [PubMed] [Google Scholar]
- Lee WH, Lee CC, Shyu WC, Chong PN, Lin SZ. Hyperintense putaminal rim sign is not a hallmark of multiple system atrophy at 3T. AJNR American journal of neuroradiology. 2005;26:2238–2242. [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Liu CS, Wu HM, Wang PS, Chang MH, Soong BW. The ‘hot cross bun’ sign in the patients with spinocerebellar ataxia. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2009;16:513–516. doi: 10.1111/j.1468-1331.2008.02524.x. [DOI] [PubMed] [Google Scholar]
- Lin DJ, Hermann KL, Schmahmann JD. The Diagnosis and Natural History of Multiple System Atrophy, Cerebellar Type. Cerebellum. 2016;15:663–679. doi: 10.1007/s12311-015-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PA, Reich SG, Jankovic J, Shults CW, Stern MB, Novak P, Tanner CM, Gilman S, Marshall FJ, Wooten F, Racette B, Chelimsky T, Singer W, Sletten DM, Sandroni P, Mandrekar J. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet neurology. 2015;14:710–719. doi: 10.1016/S1474-4422(15)00058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Santiesteban CE, Gabilondo I, Palma JA, Norcliffe-Kaufmann L, Kaufmann H. The Retina in Multiple System Atrophy: Systematic Review and Meta-Analysis. Frontiers in neurology. 2017a;8:206. doi: 10.3389/fneur.2017.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Santiesteban CE, Palma JA, Martinez J, Norcliffe-Kaufmann L, Hedges TR, 3rd, Kaufmann H. Progressive retinal structure abnormalities in multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2015;30:1944–1953. doi: 10.1002/mds.26360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Santiesteban CE, Palma JA, Ortuno-Lizaran I, Cuenca N, Kaufmann H. Pathologic confirmation of retinal ganglion cell loss in multiple system atrophy. Neurology. 2017b;88:2233–2235. doi: 10.1212/WNL.0000000000004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnerop M, Specht K, Ruhlmann J, Schimke N, Abele M, Weyer A, Wullner U, Klockgether T. Voxel-based morphometry and voxel-based relaxometry in multiple system atrophy-a comparison between clinical subtypes and correlations with clinical parameters. Neuroimage. 2007;36:1086–1095. doi: 10.1016/j.neuroimage.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Miyatake S, Mochizuki H, Naka T, Ugawa Y, Tanabe H, Kuzume D, Suzuki M, Ogata K, Kawai M. Brain volume analyses and somatosensory evoked potentials in multiple system atrophy. Journal of neurology. 2010;257:419–425. doi: 10.1007/s00415-009-5338-5. [DOI] [PubMed] [Google Scholar]
- Miyoshi F, Kanasaki Y, Shinohara Y, Fujii S, Kaminou T, Tanabe Y, Ogawa T. Significance of combined use of MRI and perfusion SPECT for evaluation of multiple system atrophy, cerebellar type. Acta Radiol. 2016;57:742–749. doi: 10.1177/0284185115598810. [DOI] [PubMed] [Google Scholar]
- Moller L, Kassubek J, Sudmeyer M, Hilker R, Hattingen E, Egger K, Amtage F, Pinkhardt EH, Respondek G, Stamelou M, Moller F, Schnitzler A, Oertel WH, Knake S, Huppertz HJ, Hoglinger GU. Manual MRI morphometry in Parkinsonian syndromes. Movement disorders : official journal of the Movement Disorder Society. 2017;32:778–782. doi: 10.1002/mds.26921. [DOI] [PubMed] [Google Scholar]
- Muller J, Wenning GK, Verny M, McKee A, Chaudhuri KR, Jellinger K, Poewe W, Litvan I. Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Archives of neurology. 2001;58:259–264. doi: 10.1001/archneur.58.2.259. [DOI] [PubMed] [Google Scholar]
- Muppidi S, Miglis MG. Is pure autonomic failure an early marker for Parkinson disease, dementia with Lewy bodies, and multiple system atrophy? And other updates on recent autonomic research. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017;27:71–73. doi: 10.1007/s10286-017-0408-8. [DOI] [PubMed] [Google Scholar]
- Muqit MM, Mort D, Miskiel KA, Shakir RA. “Hot cross bun” sign in a patient with parkinsonism secondary to presumed vasculitis. Journal of neurology, neurosurgery, and psychiatry. 2001;71:565–566. doi: 10.1136/jnnp.71.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama H, Ueda M, Yamazaki M, Nishiyama Y, Hamamoto M, Katayama Y. Abnormal cardiac [(123)I]-meta-iodobenzylguanidine uptake in multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2010;25:1744–1747. doi: 10.1002/mds.23338. [DOI] [PubMed] [Google Scholar]
- Nagayama H, Yamazaki M, Ueda M, Nishiyama Y, Hamamoto M, Katayama Y, Mori O. Low myocardial MIBG uptake in multiple system atrophy with incidental Lewy body pathology: an autopsy case report. Movement disorders : official journal of the Movement Disorder Society. 2008;23:1055–1057. doi: 10.1002/mds.22031. [DOI] [PubMed] [Google Scholar]
- Nair SR, Tan LK, Mohd Ramli N, Lim SY, Rahmat K, Mohd Nor H. A decision tree for differentiating multiple system atrophy from Parkinson’s disease using 3-T MR imaging. Eur Radiol. 2013;23:1459–1466. doi: 10.1007/s00330-012-2759-9. [DOI] [PubMed] [Google Scholar]
- Navarro-Otano J, Casanova-Molla J, Morales M, Valls-Sole J, Tolosa E. Cutaneous autonomic denervation in Parkinson’s disease. J Neural Transm (Vienna) 2015;122:1149–1155. doi: 10.1007/s00702-014-1355-3. [DOI] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L, Kaufmann H. Is ambulatory blood pressure monitoring useful in patients with chronic autonomic failure? Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2014;24:189–192. doi: 10.1007/s10286-014-0229-y. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131:1362–1372. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- Ofori E, Krismer F, Burciu RG, Pasternak O, McCracken JL, Lewis MM, Du G, McFarland NR, Okun MS, Poewe W, Mueller C, Gizewski ER, Schocke M, Kremser C, Li H, Huang X, Seppi K, Vaillancourt DE. Free water improves detection of changes in the substantia nigra in parkinsonism: A multisite study. Movement disorders : official journal of the Movement Disorder Society. 2017 doi: 10.1002/mds.27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Sakakibara R, Kuno S, Ishizuka O, Kitta T, Yoshimura N. Prevalence and treatment of LUTS in patients with Parkinson disease or multiple system atrophy. Nat Rev Urol. 2017;14:79–89. doi: 10.1038/nrurol.2016.254. [DOI] [PubMed] [Google Scholar]
- Orimo S, Kanazawa T, Nakamura A, Uchihara T, Mori F, Kakita A, Wakabayashi K, Takahashi H. Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta neuropathologica. 2007;113:81–86. doi: 10.1007/s00401-006-0160-y. [DOI] [PubMed] [Google Scholar]
- Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K, Nagao T, Yokochi M. Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 2002;73:776–777. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo S, Suzuki M, Inaba A, Mizusawa H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism & related disorders. 2012;18:494–500. doi: 10.1016/j.parkreldis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Sekiya K, Aizawa N, Terajima K, Nishizawa M. Laryngeal stridor in multiple system atrophy: Clinicopathological features and causal hypotheses. Journal of the neurological sciences. 2016;361:243–249. doi: 10.1016/j.jns.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Palma JA, Fernandez-Cordon C, Coon EA, Low PA, Miglis MG, Jaradeh S, Bhaumik AK, Dayalu P, Urrestarazu E, Iriarte J, Biaggioni I, Kaufmann H. Prevalence of REM sleep behavior disorder in multiple system atrophy: a multicenter study and meta-analysis. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2015;25:69–75. doi: 10.1007/s10286-015-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma JA, Kaufmann H. Epidemiology, Diagnosis, and Management of Neurogenic Orthostatic Hypotension. Mov Disord Clin Pract. 2017;4:298–308. doi: 10.1002/mdc3.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Joseph J, Press DZ, Schmahmann JD. Pathological laughter and crying in patients with multiple system atrophy-cerebellar type. Movement disorders : official journal of the Movement Disorder Society. 2007;22:798–803. doi: 10.1002/mds.21348. [DOI] [PubMed] [Google Scholar]
- Pavelic A, Krbot Skoric M, Crnosija L, Habek M. Postprandial hypotension in neurological disorders: systematic review and meta-analysis. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017;27:263–271. doi: 10.1007/s10286-017-0440-8. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Thornton JS, Lees AJ, Jager HR. Diffusion-weighted magnetic resonance imaging differentiates Parkinsonian variant of multiple-system atrophy from progressive supranuclear palsy. Movement disorders : official journal of the Movement Disorder Society. 2007;22:68–74. doi: 10.1002/mds.21204. [DOI] [PubMed] [Google Scholar]
- Pellecchia MT, Barone P, Mollica C, Salvatore E, Ianniciello M, Longo K, Varrone A, Vicidomini C, Picillo M, De Michele G, Filla A, Salvatore M, Pappata S. Diffusion-weighted imaging in multiple system atrophy: a comparison between clinical subtypes. Movement disorders : official journal of the Movement Disorder Society. 2009;24:689–696. doi: 10.1002/mds.22440. [DOI] [PubMed] [Google Scholar]
- Pellecchia MT, Barone P, Vicidomini C, Mollica C, Salvatore E, Ianniciello M, Liuzzi R, Longo K, Picillo M, De Michele G, Filla A, Brunetti A, Salvatore M, Pappata S. Progression of striatal and extrastriatal degeneration in multiple system atrophy: a longitudinal diffusion-weighted MR study. Movement disorders : official journal of the Movement Disorder Society. 2011;26:1303–1309. doi: 10.1002/mds.23601. [DOI] [PubMed] [Google Scholar]
- Petrovic IN, Ling H, Asi Y, Ahmed Z, Kukkle PL, Hazrati LN, Lang AE, Revesz T, Holton JL, Lees AJ. Multiple system atrophy-parkinsonism with slow progression and prolonged survival: a diagnostic catch. Movement disorders : official journal of the Movement Disorder Society. 2012;27:1186–1190. doi: 10.1002/mds.25115. [DOI] [PubMed] [Google Scholar]
- Pilleri M, Levedianos G, Weis L, Gasparoli E, Facchini S, Biundo R, Formento-Dojot P, Antonini A. Heart rate circadian profile in the differential diagnosis between Parkinson disease and multiple system atrophy. Parkinsonism & related disorders. 2014;20:217–221. doi: 10.1016/j.parkreldis.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Planetta PJ, Kurani AS, Shukla P, Prodoehl J, Corcos DM, Comella CL, McFarland NR, Okun MS, Vaillancourt DE. Distinct functional and macrostructural brain changes in Parkinson’s disease and multiple system atrophy. Hum Brain Mapp. 2015;36:1165–1179. doi: 10.1002/hbm.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planetta PJ, Ofori E, Pasternak O, Burciu RG, Shukla P, DeSimone JC, Okun MS, McFarland NR, Vaillancourt DE. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain. 2016;139:495–508. doi: 10.1093/brain/awv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology. 2015;84:1104–1113. doi: 10.1212/WNL.0000000000001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Movement disorders : official journal of the Movement Disorder Society. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Li H, Planetta PJ, Goetz CG, Shannon KM, Tangonan R, Comella CL, Simuni T, Zhou XJ, Leurgans S, Corcos DM, Vaillancourt DE. Diffusion tensor imaging of Parkinson’s disease, atypical parkinsonism, and essential tremor. Movement disorders : official journal of the Movement Disorder Society. 2013;28:1816–1822. doi: 10.1002/mds.25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provitera V, Nolano M, Caporaso G, Stancanelli A, Manganelli F, Iodice R, Selim MM, De Rosa A, Lanzillo B, Pellecchia MT, De Michele G, Santoro L. Postganglionic sudomotor denervation in patients with multiple system atrophy. Neurology. 2014;82:2223–2229. doi: 10.1212/WNL.0000000000000518. [DOI] [PubMed] [Google Scholar]
- Quattrone A, Nicoletti G, Messina D, Fera F, Condino F, Pugliese P, Lanza P, Barone P, Morgante L, Zappia M, Aguglia U, Gallo O. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology. 2008;246:214–221. doi: 10.1148/radiol.2453061703. [DOI] [PubMed] [Google Scholar]
- Quinn N. A short clinical history of multiple system atrophy. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2015;25:3–7. doi: 10.1007/s10286-014-0265-7. [DOI] [PubMed] [Google Scholar]
- Riku Y, Watanabe H, Mimuro M, Iwasaki Y, Ito M, Katsuno M, Sobue G, Yoshida M. Non-motor multiple system atrophy associated with sudden death: pathological observations of autonomic nuclei. Journal of neurology. 2017 doi: 10.1007/s00415-017-8604-y. [DOI] [PubMed] [Google Scholar]
- Roncevic D, Palma JA, Martinez J, Goulding N, Norcliffe-Kaufmann L, Kaufmann H. Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm (Vienna) 2014;121:507–512. doi: 10.1007/s00702-013-1133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Videourodynamic and sphincter motor unit potential analyses in Parkinson’s disease and multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 2001;71:600–606. doi: 10.1136/jnnp.71.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako W, Murakami N, Izumi Y, Kaji R. The difference in putamen volume between MSA and PD: evidence from a meta-analysis. Parkinsonism & related disorders. 2014;20:873–877. doi: 10.1016/j.parkreldis.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Berg D, Herting, Prieur S, Junghanns S, Schweitzer K, Globas C, Schols L, Reichmann H, Ziemssen T. Loss of nocturnal blood pressure fall in various extrapyramidal syndromes. Movement disorders : official journal of the Movement Disorder Society. 2009a;24:2136–2142. doi: 10.1002/mds.22767. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Herting B, Prieur S, Junghanns S, Schweitzer K, Globas C, Schols L, Reichmann H, Berg D, Ziemssen T. Valsalva manoeuvre in patients with different Parkinsonian disorders. J Neural Transm (Vienna) 2009b;116:875–880. doi: 10.1007/s00702-009-0239-4. [DOI] [PubMed] [Google Scholar]
- Schocke MF, Seppi K, Esterhammer R, Kremser C, Jaschke W, Poewe W, Wenning GK. Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology. 2002;58:575–580. doi: 10.1212/wnl.58.4.575. [DOI] [PubMed] [Google Scholar]
- Schocke MF, Seppi K, Esterhammer R, Kremser C, Mair KJ, Czermak BV, Jaschke W, Poewe W, Wenning GK. Trace of diffusion tensor differentiates the Parkinson variant of multiple system atrophy and Parkinson’s disease. Neuroimage. 2004;21:1443–1451. doi: 10.1016/j.neuroimage.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Schrag A, Good CD, Miszkiel K, Morris HR, Mathias CJ, Lees AJ, Quinn NP. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology. 2000;54:697–702. doi: 10.1212/wnl.54.3.697. [DOI] [PubMed] [Google Scholar]
- Schrag A, Sheikh S, Quinn NP, Lees AJ, Selai C, Mathias C, Litvan I, Lang AE, Bower JH, Burn DJ, Low P, Jahanshahi M. A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2010;25:1077–1081. doi: 10.1002/mds.22794. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Skalej M, Wedekind D, Luft AR, Abele M, Voigt K, Dichgans J, Klockgether T. Magnetic resonance imaging-based volumetry differentiates idiopathic Parkinson’s syndrome from multiple system atrophy and progressive supranuclear palsy. Annals of neurology. 1999;45:65–74. [PubMed] [Google Scholar]
- Seppi K, Schocke MF, Donnemiller E, Esterhammer R, Kremser C, Scherfler C, Diem A, Jaschke W, Wenning GK, Poewe W. Comparison of diffusion-weighted imaging and [123I]IBZM-SPECT for the differentiation of patients with the Parkinson variant of multiple system atrophy from those with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2004;19:1438–1445. doi: 10.1002/mds.20229. [DOI] [PubMed] [Google Scholar]
- Slawek J, Derejko M, Lass P, Dubaniewicz M. Camptocormia or Pisa syndrome in multiple system atrophy. Clin Neurol Neurosurg. 2006;108:699–704. doi: 10.1016/j.clineuro.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Specht K, Minnerop M, Abele M, Reul J, Wullner U, Klockgether T. In vivo voxel-based morphometry in multiple system atrophy of the cerebellar type. Archives of neurology. 2003;60:1431–1435. doi: 10.1001/archneur.60.10.1431. [DOI] [PubMed] [Google Scholar]
- Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG, Burn DJ, Holton JL, Kaufmann H, Kostic VS, Ling H, Meissner WG, Poewe W, Semnic M, Seppi K, Takeda A, Weintraub D, Wenning GK Movement Disorders Society, M.S.A.S.G. Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Movement disorders : official journal of the Movement Disorder Society. 2014;29:857–867. doi: 10.1002/mds.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchi F, Carbone A, Inghilleri M, Monge A, Ruggieri S, Berardelli A, Manfredi M. Urodynamic and neurophysiological evaluation in Parkinson’s disease and multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 1997;62:507–511. doi: 10.1136/jnnp.62.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZF, Xiang XS, Chen Z, Zhang L, Tang BS, Xia K, Jiang H. Increase of the plasma alpha-synuclein levels in patients with multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2014;29:375–379. doi: 10.1002/mds.25688. [DOI] [PubMed] [Google Scholar]
- Takado Y, Igarashi H, Terajima K, Shimohata T, Ozawa T, Okamoto K, Nishizawa M, Nakada T. Brainstem metabolites in multiple system atrophy of cerebellar type: 3.0-T magnetic resonance spectroscopy study. Movement disorders : official journal of the Movement Disorder Society. 2011;26:1297–1302. doi: 10.1002/mds.23550. [DOI] [PubMed] [Google Scholar]
- Tang CC, Poston KL, Eckert T, Feigin A, Frucht S, Gudesblatt M, Dhawan V, Lesser M, Vonsattel JP, Fahn S, Eidelberg D. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet neurology. 2010;9:149–158. doi: 10.1016/S1474-4422(10)70002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D, Monza D, Ferrarini M, Soliveri P, Girotti F, Filippini G. Comparison of natural histories of progressive supranuclear palsy and multiple system atrophy. Neurol Sci. 2001;22:247–251. doi: 10.1007/s100720100021. [DOI] [PubMed] [Google Scholar]
- Tison F, Yekhlef F, Chrysostome V, Balestre E, Quinn NP, Poewe W, Wenning GK. Parkinsonism in multiple system atrophy: natural history, severity (UPDRS-III), and disability assessment compared with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2002;17:701–709. doi: 10.1002/mds.10171. [DOI] [PubMed] [Google Scholar]
- Trabesinger AH, Meier D, Boesiger P. In vivo 1H NMR spectroscopy of individual human brain metabolites at moderate field strengths. Magn Reson Imaging. 2003;21:1295–1302. doi: 10.1016/j.mri.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Tzarouchi LC, Astrakas LG, Konitsiotis S, Tsouli S, Margariti P, Zikou A, Argyropoulou MI. Voxel-based morphometry and Voxel-based relaxometry in parkinsonian variant of multiple system atrophy. J Neuroimaging. 2010;20:260–266. doi: 10.1111/j.1552-6569.2008.00343.x. [DOI] [PubMed] [Google Scholar]
- Umehara T, Matsuno H, Toyoda C, Oka H. Clinical characteristics of supine hypertension in de novo Parkinson disease. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2016;26:15–21. doi: 10.1007/s10286-015-0324-8. [DOI] [PubMed] [Google Scholar]
- van de Warrenburg BP, Cordivari C, Ryan AM, Phadke R, Holton JL, Bhatia KP, Hanna MG, Quinn NP. The phenomenon of disproportionate antecollis in Parkinson’s disease and multiple system atrophy. Movement disorders : official journal of the Movement Disorder Society. 2007;22:2325–2331. doi: 10.1002/mds.21634. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Casteels C, De Ceuninck L, Vanbilloen B, Maes A, Mortelmans L, Vandenberghe W, Verbruggen A, Dom R. Dual-tracer dopamine transporter and perfusion SPECT in differential diagnosis of parkinsonism using template-based discriminant analysis. J Nucl Med. 2006;47:384–392. [PubMed] [Google Scholar]
- Vichayanrat E, Low DA, Iodice V, Stuebner E, Hagen EM, Mathias CJ. Twenty-four-hour ambulatory blood pressure and heart rate profiles in diagnosing orthostatic hypotension in Parkinson’s disease and multiple system atrophy. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2017;24:90–97. doi: 10.1111/ene.13135. [DOI] [PubMed] [Google Scholar]
- Wang N, Gibbons CH, Lafo J, Freeman R. alpha-Synuclein in cutaneous autonomic nerves. Neurology. 2013;81:1604–1610. doi: 10.1212/WNL.0b013e3182a9f449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Fukatsu H, Katsuno M, Sugiura M, Hamada K, Okada Y, Hirayama M, Ishigaki T, Sobue G. Multiple regional 1H-MR spectroscopy in multiple system atrophy: NAA/Cr reduction in pontine base as a valuable diagnostic marker. Journal of neurology, neurosurgery, and psychiatry. 2004;75:103–109. [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, Hirayama M, Sobue G. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125:1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Ben-Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinicopathological study of 35 cases of multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 1995;58:160–166. doi: 10.1136/jnnp.58.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Kollensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W European Multiple System Atrophy Study, G. The natural history of multiple system atrophy: a prospective European cohort study. Lancet neurology. 2013;12:264–274. doi: 10.1016/S1474-4422(12)70327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worker A, Blain C, Jarosz J, Chaudhuri KR, Barker GJ, Williams SC, Brown RG, Leigh PN, Dell’Acqua F, Simmons A. Diffusion tensor imaging of Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PloS one. 2014;9:e112638. doi: 10.1371/journal.pone.0112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Arai K, Asahina M, Hattori T. Laryngeal stridor in multiple system atrophy. European neurology. 2003;49:154–159. doi: 10.1159/000069077. [DOI] [PubMed] [Google Scholar]
- Yekhlef F, Ballan G, Macia F, Delmer O, Sourgen C, Tison F. Routine MRI for the differential diagnosis of Parkinson’s disease, MSA, PSP, and CBD. J Neural Transm (Vienna) 2003;110:151–169. doi: 10.1007/s00702-002-0785-5. [DOI] [PubMed] [Google Scholar]
- Yu F, Barron DS, Tantiwongkosi B, Fox P. Patterns of gray matter atrophy in atypical parkinsonism syndromes: a VBM meta-analysis. Brain Behav. 2015;5:e00329. doi: 10.1002/brb3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zange L, Noack C, Hahn K, Stenzel W, Lipp A. Phosphorylated alpha-synuclein in skin nerve fibres differentiates Parkinson’s disease from multiple system atrophy. Brain. 2015;138:2310–2321. doi: 10.1093/brain/awv138. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tian Y, Jin T, Zhang H, Sun L. The “hot cross bun” sign in leptomeningeal carcinomatosis. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2013;40:597–598. [PubMed] [Google Scholar]
- Zheng J, Yang X, Chen Y, Zhao Q, Tian S, Huang H, Xu Y. Onset of bladder and motor symptoms in multiple system atrophy: differences according to phenotype. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2017 doi: 10.1007/s10286-017-0405-y. [DOI] [PubMed] [Google Scholar]