Abstract
Objective
This study sought to examin effects of age and tongue exercise on the posterior digastric (opener) and the temporalis (closer). We hypothesized 1) age would result in differing morphological (cross sectional area) and biochemical (myosin heavy chain isoform) components of these muscles; 2) tongue exercise would result in coactivation of these muscles inducing a decrease in age-related differences between age groups.
Design
Young adult (9 months) and old (32 months) Fischer 344 Brown Norway rats were randomized into a tongue exercise or control group. Post-training, posterior digastric and temporalis muscles were harvested and analyzed using: 1) Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) to assess percent myosin heavy chain (MyHC) content; 2) Immunohistochemical staining to determine cross sectional area (CSA).
Results
A larger proportion of slowly contracting MyHC isoforms in the posterior digastric and temporalis muscles were found in old. No significant main effects for age or exercise in fiber size were found in posterior digastric muscle. An interaction between age and exercise for temporalis cross sectional area indicated the old exercise group had smaller average cross sectional area than all other groups.
Conclusions
Findings suggest that: 1) Increasing age induces biochemical changes in muscles of the jaw, specifically showing an increase the proportion of slower contracting MyHC isoforms; 2) Increasing age and tongue exercise induce a reduction in muscle fiber cross sectional area in the temporalis muscle only. However, continued study of these cranial muscle systems is warranted to better understand these changes that occur with age and exercise.
Keywords: digastric, temporalis, muscle physiology, tongue, ageing
Introduction
Aging results in progressive decrements in muscle function and can have a negative impact on deglutition. (J. P. Newton, Abel, Robertson, & Yemm, 1987; Nicosia et al., 2000; Peyron, Blanc, Lund, & Woda, 2004; Roy, Stemple, Merrill, & Thomas, 2007; Schindler & Kelly, 2002) Age-related dysphagia is associated with reductions in quality of life and potentially serious complications such as aspiration pneumonia.(Eisenstadt, 2010; Roy et al., 2007; Thein et al., 2009) Swallowing requires the coordination of many muscle groups, including those of the jaw and the tongue, which can be affected by sarcopenia (Rosenberg, 1997) and associated reductions in muscle strength,(Porter, Vandervoort, & Lexell, 1995; Saitoh et al., 2007) muscle size, (Rosenberg, 1997) fiber number,(Faulkner, Larkin, Claflin, & Brooks, 2007) and an increase in muscle fatigue.(Thein et al., 2009) Within the tongue, human intrinsic muscles critical to bolus propulsion have been shown to decrease in size.(Nakayama, 1991) Animal studies have further demonstrated that changes in lingual muscle contractile and fiber properties(Schaser, Wang, Volz, & Connor, 2011) result in slower fiber contraction.(Nadine P Connor, Ota, Nagai, Russell, & Leverson, 2008)
Muscles of mastication also show reductions in relative cross-sectional area with age.(Galo, Vitti, Santos, Hallak, & Regalo, 2006; J. Newton, Yemm, & Menhinick, 1993) Mastication involves a series of complex movements in coordination with the tongue that allows for bolus formation, saliva mixing, and ultimately initiation of the swallow.(Palmer, Rudin, Lara, & Crompton, 1992; Prinz & Lucas, 1997; Saitoh et al., 2007; Schindler & Kelly, 2002; Woda, Mishellany, & Peyron, 2006) Specifically, muscles of mastication, including the masseter, temporalis, pterygoid, and digastric(McLoon & Andrade, 2012) must act in concert with the intrinsic and extrinsic tongue muscles for successful bolus formation and swallowing. (Schindler & Kelly, 2002)
Older individuals often swallow more slowly, and for some people there may be a reduction in tongue (Machida et al., 2016) and palatal pressure reserves that result in inadequate bolus formation and propulsion,(Steele & Cichero, 2014) increasing risk of aspiration.(J. Robbins, Levine, Wood, Roecker, & Luschei, 1995) Physiological changes in mastication with aging include an increased number of masticatory cycles,(Mioche, Bourdiol, Monier, Martin, & Cormier, 2004; Peyron et al., 2004) decreased accuracy of bites,(Ballard, Robin, Woodworth, & Zimba, 2001) reduced bite force,(Bakke, Holm, Jensen, Michler, & Møller, 1990) and decreased jaw opening force.(Iida et al., 2013) These changes may produce less efficient particalization of food, resulting in boli that are less comminuted.(Mioche et al., 2004) The muscles of the tongue and the jaw work together simultaneously to effectively prepare and propel a bolus.(K. M. Hiiemae, Hayenga, & Reese, 1995; Hori, Ono, & Nokubi, 2006; Kakizaki, Uchida, Yamamura, & Yamada, 2002; Naganuma, Inoue, Yamamura, Hanada, & Yamada, 2001) The structure and function of the tongue and jaw muscles are mutually impacted by age related decline. (Galo et al., 2006; J. Newton et al., 1993; J. P. Newton et al., 1987; Porter et al., 1995)
Treatments for presbyphagia include tongue exercise with the goal of strengthening muscles of the tongue and improving palatal pressure generation and bolus propulsion during swallowing.(J. Robbins et al., 1995) This treatment has been modeled in our laboratory in the rat, which allows study of biochemical and physiological changes that are not possible in humans.(N. P. Connor et al., 2009; German, Crompton, Gould, & Thexton, 2017; Kletzien, Russell, Leverson, & Connor, 2013; Krekeler & Connor, 2016) We have demonstrated that tongue exercise increases generative force capacity in the tongue,(N. P. Connor et al., 2009; Kletzien et al., 2013; Krekeler & Connor, 2016) and induces changes in intrinsic tongue muscle fiber type. Although muscles of the jaw have been shown to co-activate with muscles of the tongue, (Kayalioglu, Shcherbatyy, Seifi, & Liu, 2007; Yamamoto, Matsuo, Fujiwara, & Kawamura, 1982) our previous work demonstrated that tongue exercise did not have a significant impact on masticatory patterns in rats.(Krekeler & Connor, 2016) However, It is not known if tongue exercise induces biochemical changes in the muscles of the jaw that were not detected using behavioral measures of mastication. The hypothesis that increased tongue strength could impact masticatory function has been supported in the literature. Specifically the tongue and jaw coordinate during masticatory functions in bolus preparation and transit.(K. M. Hiiemae et al., 1995; Hori et al., 2006; Kakizaki et al., 2002; Naganuma et al., 2001) Thus, increased tongue strength could provide better lingual support for masticatory functions and improve timing sequelae in both chewing and swallowing.
This gap in knowledge is important to explore because tongue exercise in our model approximates tongue exercise treatments used to treat dysphagia in humans. Therefore, to gain a full understanding of the potential biological effects of this treatment, we must study exercise effects on the cellular level.(German et al., 2017) Because feeding and mastication are both critical components of the oropharyngeal swallow, it is necessary to understand the implications of tongue exercise on supporting systems in the mascitatory musculature.
In a previous (see: Krekeler & Connor, 2016), we examined behavioral effects of age and tongue exercise on masticatory function. The purpose of this follow up study is to examine the effects of age and tongue exercise on biochemical and morphologic properties of two muscles of mastication, the posterior digastric and the temporalis. The digastric muscle is involved in opening of the jaw and the temporalis is involved in jaw closure; together these muscles work along with others to form a complete masticatory cycle. We hypothesized that: (1) muscle morphology (cross sectional area) and biochemistry (myosin heavy chain isoform proportions) would differ in young adult and the old rat groups; specifically, that age would be associated with a decrease in cross sectional area and a shift from fast-contracting isoforms to more slowly contracting isoforms; and, (2) tongue exercise would induce morphometric and biochemical changes in the posterior digastric muscle and the temporalis muscle; specifically, that tongue exercise would be associated with a decrease in age-related differences between the young adult and old rat groups.(De-Ary-Pires, Ary-Pires, & Pires-Neto, 2003; K. Hiiemae & Houston, 1971)
Methods
Animal Exercise and Mastication Measure
The University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee approved all procedures. Animal subjects were 34 male Fischer 344 Brown Norway rats (17 young adult rats [9-months] and 17 old rats [32-months]). Animal training and exercise procedures are reported in detail elsewhere.(Krekeler & Connor, 2016) Briefly, rats were randomized into control and exercise groups with 9 tongue exercise animals and 8 control animals per age group. Mastication testing was performed at two time points: before exercise training was initiated and at the end of the 8-week training period. Mastication testing was performed using a pasta-biting task previously described.(Allred et al., 2008; Kane et al., 2011; Plowman et al., 2013; Tennant et al., 2010) The pasta biting task involved quantifying number of bites, time to eat, and interbite interval using acoustic signals emitted from the rats biting uncooked pasta pieces. After pre-training data were collected, rats underwent the tongue exercise program over 8 weeks that involved progressively increasing the tongue force required to receive a water reward. Rats underwent a water-restriction protocol to provide motivation for pressing the tongue on the disc to receive a water reward. Tongue force targets were progressively increased force targets over 8 weeks. This progressive increase of force targets in an 8 week training period mirrors the human tongue training intervention.(JoAnne Robbins et al., 2005) Post-training pasta biting data were collected at completion of 8 weeks of tongue exercise.
Tissue Preparation
Following post-training pasta biting testing, rats were euthanized following anesthesia with isoflurane using Beuthanasia via intraperitoneal injection (0.2 cc). The paired posterior digastric and paired temporalis muscles were harvested. Each of the paired sides of the temporalis muscle were randomized to the myosin heavy chain (MyHC) assay or the muscle fiber cross sectional area (CSA) assay. The two paired bellies of the posterior digastric lie very closely together and therefore are difficult to separate without tearing. Thus the digastric muscle bellies were analyzed together, and were prepared first for CSA analysis, then homogenized for MyHC analysis. Tissues to be used for CSA analysis were frozen in an optimal cutting temperature (OCT) compound, while tissues bound for MyHC analysis were snap frozen in liquid nitrogen. All prepared tissues were kept frozen at −80°C until analyzed. All muscles were processed using standard lab protocols for CSA and MyHC analysis using the extensor digitorum longus (EDL) and soleus (SO) muscles as control tissues.(N. P. Connor et al., 2009)
Myosin Heavy Chain Analysis with SDS-Page
Myosin heavy chain (MyHC) analysis was performed using a Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) assay to illuminate the distribution of MyHC isoforms in the muscle. Because MyHC composition is highly linked to muscle fiber type,(Scott, Stevens, & Binder–Macleod, 2001) this analysis allowed us to infer the types of muscle fibers present and their associated contraction speeds and force generation capacities.(Pette & Staron, 2000) MyHC isoforms studied in muscles of the rat include MyHC type I, IIb, IIx, and IIa and separate in this order in a gel.(Larsson & Ansved, 1995) First, muscle tissue fibers were homogenized, muscle proteins were extracted and analyzed for protein concentration (Bradford Protein Assay) using 0.4 μg of protein per well. Then, protein aliquots of 4 μL (0.4 μg total concentration) were processed using SDS-PAGE (0.75-mm-thick 6% acrylamide-30% glycerol separating gel, 18 × 16 cm, and a 4% acrylamide-30% glycerol stacking gel) and stained using a silver staining kit (SilverQuest Silver Staining Kit, Invitrogen, Carlsbad, CA). Gel bands were then scanned and analyzed using an optical density measurement program as shown in Figure 1 (UN-Scan-IT gel version 6.1; Silk Scientific, Orem, UT). A percentage of each MyHC type per animal was determined by first subtracting the background difference from a blank space on the gel within a lane, and then producing a ratio for an individual band to the total density of all the bands within a lane.(Kletzien et al., 2013; Schaser et al., 2011) MyHC analysis was performed on all excised tissues for both the anterior digastric muscle and a randomized right or left temporalis muscle. As discussed previously, due to the close proximity of the digastric bellies, homogenization of both bellies were included in MyHC analysis.
Figure 1.
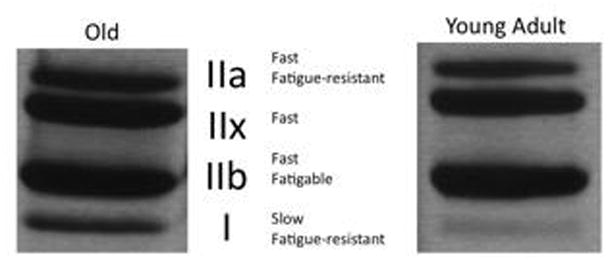
Scanned Gel Bands for Analysis. After SDS-PAGE assay, dried gel was scanned and processed using UN-Scan-IT to determine relative percentage of MHC isoform composition.
Please see JPEG image attached.
Cross Sectional Area Analysis with IHC
Tissue frozen in OCT was sectioned at −20 °C into 8–10 μm cross-sections using a cryostat (Leica CM 1850; Meyer Instruments, Houston, TX). Sections were taken through the medial belly of the digastric, and through both the anterior and posterior temporais as described in Sano et al. (2007) and Tanaka et al. (2008).(Sano et al., 2007; Tanaka et al., 2008) Between 3–4 sections per animal were taken, and muscle sections were mounted on slides, air-dried, and stored at −20 °C until immunohistochemical (IHC) staining. As previously described,(N. P. Connor et al., 2009) slides were washed in phosphate buffered saline (PBS; Sigma, St. Louis, MO) and blocker was added to slides for one hour (10% normal goat serum [NGS], Invitrogen, Carlsbad, CA; with 0.1% triton Acros, New Jersey; and PBS). Primary antibody (anti-laminin rabbit; Sigma L9393; St. Louis, MO) was then added (1:2K) to diluent (1% NGS, 0.1% triton and PBS) for 2 hours. Slides were then washed again in PBS before the addition of secondary (Anti-goat rabbit—Alexa fluor 488; Invitrogen Life Technologies, Eugene, OR) 1:800 with PBS to slides for 1 hour. After staining, slides were washed a final time in PBS, cover slipped using ProLong anti-fade (Invitrogen Life Technologies, Eugene, OR). Slides were dried overnight at room temperature and were photographed within one week of staining using a Keyence-Biorevo Scope (Keyence, Itasca, IL). Using visual determination, the most intact muscle slice per slide was chosen for further analysis. Digastric muscle slides were photographed at 40x magnification (resolution 1360 × 1024) with approximately 16 photos per animal. Temporalis muscle slides were photographed at 20x magnification (resolution 2720 × 2048) with approximately 14 photos per animal. Semi-automatic Muscle Analysis using Segmentation of Histology (SMASH),(Smith & Barton, 2014) a MATLAB application (MathWorks, Natick, MA), was used to determine cross sectional area (CSA) and Ferets diameter, (Tsubaki & Jimbo, 1979) a safeguarded measurement of fiber size that accounts for sectioning errors (digastric= 0.13321 μm/pixel; temporalis= 0.22642 μm/pixel). Some tissue was freezer-damaged, potentially resulting in altered cell size and structure. In consequence, these tissues were excluded from analysis and are reflected in the degrees of freedom for each analysis in the results section.
Statistical Analyses
Statistical Package for the Social Sciences (SPSS; IBM Corporation, Armonk, NY) statistical software was used for all analyses. A two-way analysis of variance (ANOVA) was used (age × exercise) to discern differences in MyHC isoform composition with a critical α-level of ≤ 0.05 using a Fisher’s protected least significant difference approach for multiple comparisons. Levene’s Test of Homogeneity of Variance was used to determine if parametric assumptions were met; when homogeneity was violated, a rank ordered non-parametric substitute was used. For analysis of fiber cross-sectional area (μm2) and Ferets diameter (μm), a two-way analysis of variance (age × exercise) with a covariate (ANCOVA) was used to account for muscle size differences between the young and the old rat groups. That is, because animal weights differed significantly between the two age groups, it was necessary to use animal weight as a covariate in the ANCOVA (t(32)=8.708, p= <0.0001; old rats M=468.2, SD= 47.6; young adult rats M=356.9, SD=22.6). Coefficient of variance was also calculated for CSA and Ferets diameter and then compared using a two-way ANCOVA (age × exercise) to determine differences in variation of fiber size among groups. A second researcher (JW) performed SMASH analysis on 10% of the cross-sectional images and an intra-class correlation coefficient (ICC) was calculated to confirm a high level of reliability in the measurements analyzed in SMASH (ICC= 0.98).
Results
Myosin Heavy Chain Isoform Composition
Significant main effects for age were found in the posterior digastric MyHC isoform composition in type I, IIx, and IIb, but not type IIa (Table 1). In the posterior digastric, the old rat group had a higher percentage of MyHC isoform type I than young adult group (F1,29= 38.82, p<0.0001; Figure 2), a lower percentage of type IIx (F1,29= 49.56, p<0.0001; Figure 2), and a lower percentage of type IIb (F1,29= 49.75, p=<0.0001; Figure 2). There was no difference between age groups in percentage of MyHC isoform type IIa (F1,29= 0.051, p=0.82; Figure 2). Exercise did not have a significant effect on MyHC isoform type.
Table 1.
Posterior Digastric Myosin Heavy Chain Isoform Percentages per Age Group × Condition (Exercise or Control). There were no significant interaction effects between age and exercise in percentage of MHC isoform.
| MHC Isoform Type | Young Adult Control (%) | Young Adult Exercise (%) | Old Control (%) | Old Exercise (%) |
|---|---|---|---|---|
| Type I | 6.16 | 7.07 | 14.31 | 13.61 |
| Type IIa | 24.77 | 25.77 | 24.64 | 25.64 |
| Type IIx | 33.35 | 32.35 | 30.50 | 30.29 |
| Type IIb | 35.72 | 34.81 | 30.56 | 30.46 |
Figure 2.
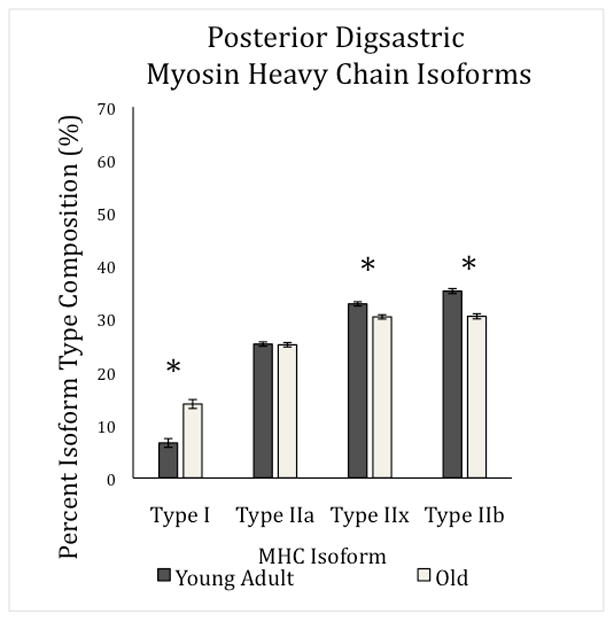
Posterior Digastric Myosin Heavy Chain Isoform Percentages per Age Group. There was a main effect for age in percentage of MHC isoform composition in type I, IIx, and IIb (F1,29= 38.82, p<0.0001; F1,29= 49.56, p<0.0001; F1,29= 49.75, p=<0.0001 respectively).This demonstrates a general shift from faster contracting fiber types to slower contracting types. Standard error is denoted in error bars.
In the temporalis muscle, main effects for age in type IIx and IIb were found; the old rat group had a larger percentage of MyHC isoform type IIx (F1,30= 12.85, p=0.001; Figure 3) and a smaller percentage of isoform type IIb (F1,30= 6.83, p=0.014; Figure 3) than the young adult group. There was no difference between the age groups in percentage of MyHC isoform type I (F1,30= 0.30, p=0.59; Figure 3) or type IIa (F1,30= 1.498, p=0.23; Figure 3). Similar to the posterior digastric, exercise was not found to have an effect on MyHC isoform type in the temporalis muscle (Figure 3, Table 2).
Figure 3.
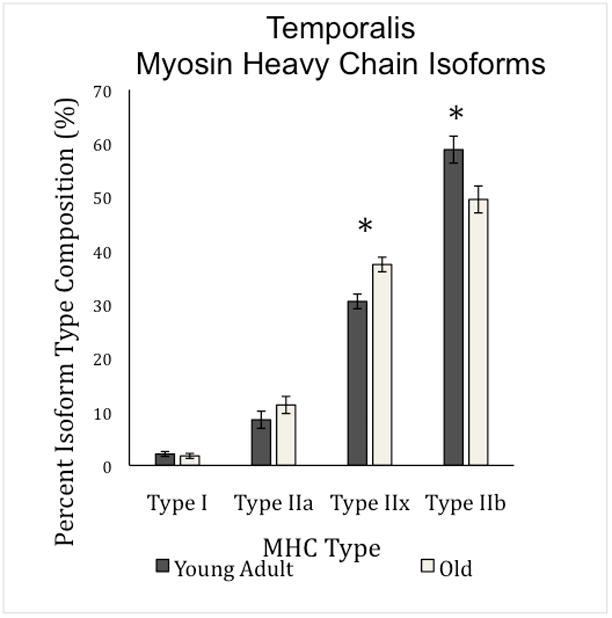
Temporalis Myosin Heavy Chain Isoform Percentages per Age Group. There was a main effect for age in percentage of MHC isoform composition in type IIx, and IIb only (F1,29= 49.56, p<0.0001; F1,29= 49.75, p=<0.0001 respectively).This demonstrates a general shift from faster contracting fiber types to slower contracting types. Standard error is denoted in error bars.
Table 2.
Temporalis Myosin Heavy Chain Isoform Percentages per Age Group × Condition (Exercise or Control). There were no significant interaction effects between age and exercise in percentage of MHC isoform.
| MHC Isoform Type | Young Adult Control (%) | Young Adult Exercise (%) | Old Control (%) | Old Exercise (%) |
|---|---|---|---|---|
| Type I | 1.89 | 2.36 | 1.41 | 2.14 |
| Type IIa | 8.35 | 8.65 | 11.32 | 11.19 |
| Type IIx | 30.04 | 31.06 | 39.22 | 35.64 |
| Type IIb | 59.72 | 57.93 | 48.06 | 51.04 |
Cross Sectional Area
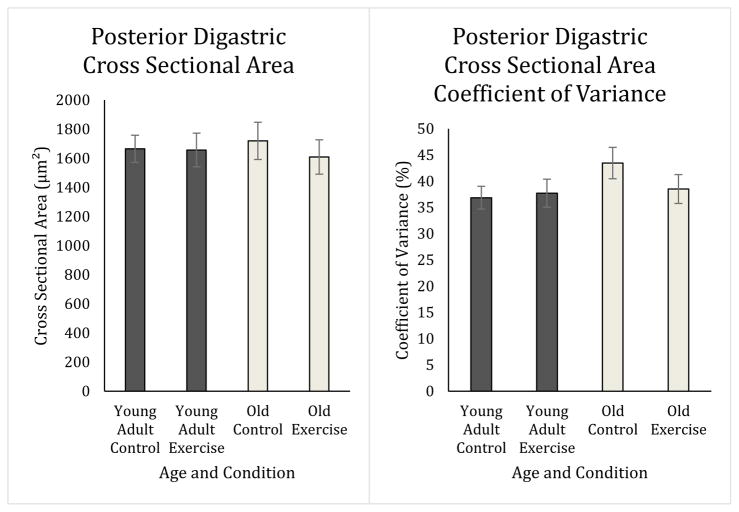
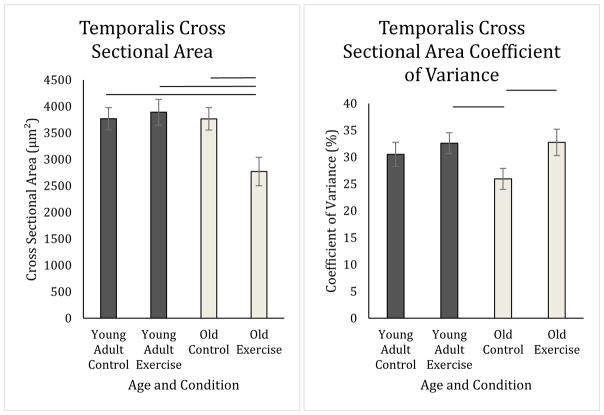
There were no significant main effects found for either age or exercise in the posterior digastric for average cross sectional area (Age: F1,22= 0.00, p=0.98; Exercise: F1,22= .47, p=0.50), Feret’s diameter (Age: F1,22= 0.029, p=0.87; Exercise: F1,22= 2.98, p=0.099), or coefficient of variance (Age: F1,22= 0.86, p=0.36; Exercise: F1,22= 1.03, p=0.32) (Figure 4). However, there was an interaction between age and exercise in the temporalis muscle for cross sectional area (F1,24= 8.00, p=0.009; Figure 5), Feret’s diameter (F1,24= 8.65, p=0.007), and coefficient of variance of cross sectional area (F1,29= 6.05, p=0.021; Figure 5). Feret’s diameter is an additional measure meant to rule out oblique sectioning of the tissue. Because Feret’s diameter and cross sectional area data were highly correlated, Feret’s diameter data are presented in Table 3, but not discussed further. As shown in Figure 5, the old exercise group on average had smaller average fiber size than old control, young adult control, and young adult exercise groups (CSA: p=0.002, p=0.021, p=0.018 respectively). In Figure 5, the old control rat group had a lower variability in average fiber size as determined by the coefficient of variance than the old exercise group (CSA: p= 0.015) and young adult exercise group (CSA: p= 0.040), but not the young adult control group (CSA: p=0.187).
Figure 4.
Posterior Digastric Cross Sectional Area and Coefficient of Variance. There was no effect of age or condition on fiber size (Age: p=0.984; Exercise: p=0.50) or coefficient of variance (Age: p=0.36; Exercise: p=0.32) in the posterior digastric muscle.
Figure 5.
Temporalis Cross Sectional Area and Coefficient of Variance. Old exercise rats on average had smaller cross sectional area of fibers than did all other groups (Old control: p=0.002, Young adult control: p=0.021, Young adult exercise: p=0.018). Old control animals had a smaller coefficient of variance of fiber cross sectional area as compared to old exercise rats (p= 0.015) and young exercise rats (p= 0.040) only.
Table 3.
Feret’s Diameter in the Posterior Digastric and the Temporalis. No significant main effects were found for age or exercise condition in the posterior digastric muscle Feret’s diameter, indicating age and exercise did not have an effect on fiber size in the posterior digastric. In the temporalis muscle the old exercise group had on average a smaller measure of Feret’s diameter than the old control group (p=0.001), young adult control group (p=0.0.025), and young adult exercise group (p=0.024) suggesting the exercise condition resulted in a reduced fiber cross sectional area in the old animal group.
| Muscle | Age × Condition | Feret’s Diameter | Standard Error |
|---|---|---|---|
| Temporalis | Old Control | 59.563 | 1.652 |
| Old Exercise | 51.242* | 2.099 | |
| Young Adult Control | 58.763 | 1.645 | |
| Young Adult Exercise | 59.504 | 1.910 | |
| Posterior Digastric | Old Control | 39.434 | 1.31 |
| Old Exercise | 36.813 | 1.21 | |
| Young Adult Control | 38.639 | 0.954 | |
| Young Adult Exercise | 38.208 | 1.178 |
Feret’s diameter values were covaried by weight of the animal at the end of the study.
Significance of p<0.05
Discussion
Our first hypothesis was that age would impact biochemistry of rat jaw muscles as quantified by alterations in MyHC isoform from faster-contracting MyHC isoforms to more slowly contracting MyHC isoforms. Second, we hypothesized that tongue exercise would alter MyHC isoform composition in the old rat group to resolve pretraining differences between the young adult and old rat group. Our data supported these hypotheses in part. We found that in the posterior digastric and the temporalis muscles, increasing age resulted in a larger proportion of more slowly contracting MyHC isoforms. Specifically, in the posterior digastric muscle, there were smaller percentages of fast contracting IIb and IIx MyHC isoforms in the old group than in the young adult group and a larger percentage of type I (Figure 2). In the temporalis muscle, there was a lower percentage of the type IIb MyHC isoform (Figure 3). Our findings are in agreement with literature examining limb musculature(Larsson & Ansved, 1995; Lexell, Taylor, & Sjöström, 1988; Pette & Staron, 2000; Porter et al., 1995; Scott et al., 2001) and in two studies(Monemi, Liu, Thornell, & Eriksson, 2000; Monemi, Thornell, & Eriksson, 1999) of human jaw muscles. However, we did not find that tongue exercise had a significant impact on biochemistry of MyHC isoforms in either the posterior digastric or temporalis muscles. Although it has been demonstrated that the muscles of the jaw are active during tongue movements for licking in pigs(Kayalioglu et al., 2007) and rats,(Yamamoto et al., 1982) tongue exercise may not have been specific enough to induce biochemical changes in these muscles of the jaw. The specificity argument is reinforced by prior findings of significant alterations in MyHC isoform composition within the extrinsic tongue muscles following tongue exercise in rats.(Kletzien et al., 2013)
Second, we hypothesized that age and exercise would affect cross sectional area of masticatory muscle fibers. That is, we hypothesized that these muscles would be smaller in the old rat group and that muscle fiber size differences between the old rat group and the young adult rat group would be attenuated with exercise. In the posterior digastric muscle, there were no significant changes in muscle fiber size with age or exercise. This finding is supported by another study(Monemi, Thornell, et al., 1999) examining human digastric muscle where cross sectional area was examined stratified by fiber type and there was not a significant difference between old and young adult in relative cross-sectional area in all but one fiber type. Further, in our study, the coefficient of variance among posterior digastric fibers was not significantly different between groups, indicating that variation in muscle fiber size was not affected by exercise or age. This absence of significant change in CSA with age in posterior digastric suggests that effects of age may be dependent on muscle function and regional location, and therefore have different rates of change as compared to other muscles of the jaw.(McLoon & Andrade, 2012; Monemi, Thornell, et al., 1999) Other authors have suggested that some cranial muscle systems could be spared from typical aging, which could be related to the high motoneuron innervation ratios or functional attributes of these muscles.(N. P. Connor et al., 2009; McLoon & Andrade, 2012; Nakayama, 1991) These mechanisms have yet to be definitively determined and should be explored in future studies.
For the temporalis muscle, we found a significant age by exercise interaction for both cross sectional area and coefficient of variation. The old control group had less variation in muscle fiber size than the young adult and old exercise groups, suggesting that age may be associated with stability in temporalis muscle fiber size when a tongue exercise intervention is not provided. Further, the old exercise group had on average smaller CSA in the temporalis than all other groups. Muscle fiber size is influenced by muscle fiber type as demonstrated in one study of the rat temporalis muscle where type IIb fibers had more than double the CSA than IIa fibers.(Tanaka et al., 2008) Although we did not detect differences between exercise and control groups in MyHC composition of the temporalis, these changes in muscle fiber size and variability in muscle fiber size might be indicative that tongue exercise did have an effect on the temporalis. Yet, another alternative explanation has been proposed: rather than increasing CSA with exercise, CSA may remain the same or decrease with a concordant increase in the number of muscle fibers.(Gonyea, Sale, Gonyea, & Mikesky, 1986; Tesch & Larsson, 1982) Further work should be conducted to explore how fiber size and MyHC composition could interact with tongue exercise-effects in the temporalis.
As discussed, deglutition is a complex process initiated by the coordination of jaw muscles opening and closing to masticate and form a bolus that may be safely swallowed without entering the airway. In interpreting findings from our current study, it is important to examine these data along with the behavioral measures of our previous work in a rat model of mastication.(Krekeler & Connor, 2016) Using our aging rat model, we discovered significant differences in patterns of mastication in the old rat group when compared to young adult rats: 1) older rats used a greater number of bites during mastication, 2) took longer to eat, and 3) had shorter intervals between bites.(Krekeler & Connor, 2016) In our current study, we demonstrated that age had a significant impact on myosin heavy chain isoform proportions in the posterior digastric and temporalis muscles, with larger percentages of more slowly contracting isoforms. Our behavioral data demonstrated that the old rat group took more time to eat and required more bites than did the young adult rats. However, it is unclear if the shift in MyHC isoforms, thus indicating a shift in fiber type toward more slowly contracting fibers, is impacting the speed of mastication or if the speed of mastication is causing an adaptation towards a more slowly contracting fiber type. More work must be completed to determine pathophysiology of aging effects on these cranial muscles.
In our prior study, tongue exercise did not have a significant impact on any of our behavioral measures of mastication.(Krekeler & Connor, 2016) This current study sought to determine if underlying changes in biochemistry and morphology in muscles of the jaw with coactivation during tongue protrusion(Kayalioglu et al., 2007; Yamamoto et al., 1982) that were not detectable by our behavioral measures. The digastric showed no significant alterations of fiber size or fiber size variability with exercise or age. Yet, tongue exercise was associated with smaller muscle fibers in the temporalis in old exercise rats. An explanation for the directionality of this effect is unclear. Myosin heavy chain isoform proportions were not significantly impacted by exercise, however, the change in muscle fiber size in the old exercise and variability of fiber size in old control rats suggest that changes occurring at the muscle level may not have been detected by whole muscle homogenization in our SDS-PAGE protocol. In future studies, using a multi-staining protocol for specific fiber type of these muscles in cross section to identify which fiber types are changing in size could bring clarity to these findings.
Limitations
One of the inherent limitations of using an animal model are the various differences in anatomy and physiology between rats and humans. However, our rat model of deglutition has been well established and used to study many aspects of swallowing behavior with age.(Cartee, 1995; Nadine P Connor et al., 2008; N. P. Connor et al., 2009; Kletzien et al., 2013; Russell, Ciucci, Hammer, & Connor, 2013; Schaser et al., 2011) Another limitation in this study was the sole use of SDS-PAGE to quantify fiber type. While this methodology has been well established in analysis of muscle fiber type (Pette & Staron, 2000; Scott et al., 2001), only a gross estimate of discrete fiber categories can be assessed and hybrid fibers are unidentifiable using this method. Further, using laminin staining only for cross sectional area measurements did not allow us to categorize fiber size changes by fiber type. In future studies, use of a multi-staining technique to illuminate multiple fiber types per slice will be used to further analyze these differences.
Conclusion
The findings in our study emphasize the importance and need for further study of the cranial muscles, as their biochemistry, composition, and functions differ greatly from the frequently studied limb muscles.(Monemi, Kadi, Liu, Thornell, & Eriksson, 1999) The composition of muscle fibers is not easily studied, as the variances in fiber type exist in a continuum rather than distinct categories.(Ingalls, 2004) Further, muscle fiber type transitions are complex and are influenced by a variety of factors including age(Porter et al., 1995) and atrophy,(Lexell et al., 1988) neuromuscular innervation, hormones, and exercise.(Larsson & Ansved, 1995; Pette & Staron, 2000) Conclusions from the limited studies of muscles in the head and neck suggest changes in MyHC and CSA are region-dependent and related to innervation.(McLoon & Andrade, 2012) A majority of the published literature, including articles cited in this manuscript, are examining these changes in the limb. There is a frank call for more research examining these effects on muscles in the head and neck. There are few animal models of mastication, thus the work presented here greatly contributes towards unveiling the mechanisms underlying age-related changed in deglutition.(McLoon & Andrade, 2012) The findings in our study suggest that aging and exercise can influence biochemical and morphological changes in relative proportions of MyHC isoforms and cross sectional area, but it is not yet clear how muscle fibers types are changing or what other factors influence this change. More high quality work in this area must be examined to develop a broad understanding of how age and exercise influence muscles of the jaw involved in deglutition.
Highlights.
Increased age resulted in more slowly contracting fibers in posterior digastric and temporalis
Age/tongue exercise did not affect fiber size in posterior digastric.
Tongue exercise training resulted in reduced CSA in old temporalis muscles
Acknowledgments
Funding
This study was funded by the National Institute on Deafness and Other Communication Disorders (R01DC005935, R01DC008149, R01DC014358, T32-DC009401).
The authors would like to acknowledge the constant support and guidance from the members of the Connor Lab. Special thanks to John Russell, Heidi Kletzien, Jared Cullen, Kellie Bowen, and Jacqueline Weycker.
Footnotes
Presentations: Paper presented in part in poter format at the American Speech and Hearing Association Annual Meeting in Denver, CO. November 13, 2015.
Conflict of Interest: There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, et al. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. 2008;170(2):229–244. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke M, Holm B, Jensen BL, Michler L, Møller E. Unilateral, isometric bite force in 8–68-year-old women and men related to occlusal factors. European Journal of Oral Sciences. 1990;98(2):149–158. doi: 10.1111/j.1600-0722.1990.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Robin DA, Woodworth G, Zimba LD. Age-related changes in motor control during articulator visuomotor tracking. J Speech Lang Hear Res. 2001;44(4):763–777. doi: 10.1044/1092-4388(2001/060). [DOI] [PubMed] [Google Scholar]
- Cartee GD. What insights into age-related changes in skeletal muscle are provided by animal models? J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):137–141. doi: 10.1093/gerona/50a.special_issue.137. [DOI] [PubMed] [Google Scholar]
- Connor NP, Ota F, Nagai H, Russell JA, Leverson G. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. Journal of Speech, Language, and Hearing Research. 2008;51(4):818–827. doi: 10.1044/1092-4388(2008/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52(3):732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Ary-Pires B, Ary-Pires R, Pires-Neto M. The human digastric muscle: patterns and variations with clinical and surgical correlations. Annals of Anatomy-Anatomischer Anzeiger. 2003;185(5):471–479. doi: 10.1016/S0940-9602(03)80110-3. [DOI] [PubMed] [Google Scholar]
- Eisenstadt S. Dysphagia and aspiration pneumonia in older adults. J Am Acad Nurse Pract. 2010;22(1):17–22. doi: 10.1111/j.1745-7599.2009.00470.x. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clinical and Experimental Pharmacology and Physiology. 2007;34(11):1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- Galo R, Vitti M, Santos CM, Hallak JE, Regalo SC. The effect of age on the function of the masticatory system--an electromyographical analysis. Gerodontology. 2006;23(3):177–182. doi: 10.1111/j.1741-2358.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton A, Gould FD, Thexton AJ. Animal Models for Dysphagia Studies: What Have We Learnt So Far. Dysphagia. 2017:1–5. doi: 10.1007/s00455-016-9778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonyea WJ, Sale DG, Gonyea FB, Mikesky A. Exercise induced increases in muscle fiber number. [journal article] European Journal of Applied Physiology and Occupational Physiology. 1986;55(2):137–141. doi: 10.1007/BF00714995. [DOI] [PubMed] [Google Scholar]
- Hiiemae K, Houston WJB. The structure and function of the jaw muscles in the rat (Rattus norvegicus L.) Zoological Journal of the Linnean Society. 1971;50:76–99. [Google Scholar]
- Hiiemae KM, Hayenga SM, Reese A. Patterns of tongue and jaw movement in a cinefluorographic study of feeding in the macaque. Arch Oral Biol. 1995;40(3):229–246. doi: 10.1016/0003-9969(95)98812-d. [DOI] [PubMed] [Google Scholar]
- Hori K, Ono T, Nokubi T. Coordination of tongue pressure and jaw movement in mastication. J Dent Res. 2006;85(2):187–191. doi: 10.1177/154405910608500214. [DOI] [PubMed] [Google Scholar]
- Iida T, Tohara H, Wada S, Nakane A, Sanpei R, Ueda K. Aging decreases the strength of suprahyoid muscles involved in swallowing movements. Tohoku J Exp Med. 2013;231(3):223–228. doi: 10.1620/tjem.231.223. [DOI] [PubMed] [Google Scholar]
- Ingalls CP. Nature vs. nurture: can exercise really alter fiber type composition in human skeletal muscle? Journal of Applied Physiology. 2004;97(5):1591–1592. doi: 10.1152/classicessays.00010.2004. [DOI] [PubMed] [Google Scholar]
- Kakizaki Y, Uchida K, Yamamura K, Yamada Y. Coordination between the masticatory and tongue muscles as seen with different foods in consistency and in reflex activities during natural chewing. Brain Res. 2002;929(2):210–217. doi: 10.1016/s0006-8993(01)03392-3. [DOI] [PubMed] [Google Scholar]
- Kane JR, Ciucci MR, Jacobs AN, Tews N, Russell JA, Ahrens AM, et al. Assessing the role of dopamine in limb and cranial-oromotor control in a rat model of Parkinson’s disease. J Commun Disord. 2011;44(5):529–537. doi: 10.1016/j.jcomdis.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayalioglu M, Shcherbatyy V, Seifi A, Liu ZJ. Arch Oral Biol. Vol. 52. England: 2007. Roles of intrinsic and extrinsic tongue muscles in feeding: electromyographic study in pigs; pp. 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H, Russell JA, Leverson GE, Connor NP. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J Appl Physiol (1985) 2013;114(4):472–481. doi: 10.1152/japplphysiol.01370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekeler BN, Connor NP. Age-related changes in mastication are not improved by tongue exercise in a rat model. The Laryngoscope. 2016 doi: 10.1002/lary.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of ageing on the motor unit. Progress in neurobiology. 1995;45(5):397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy?: Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. Journal of the neurological sciences. 1988;84(2):275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Machida N, Tohara H, Hara K, Kumakura A, Wakasugi Y, Nakane A, et al. Effects of aging and sarcopenia on tongue pressure and jaw-opening force. Geriatr Gerontol Int. 2016 doi: 10.1111/ggi.12715. [DOI] [PubMed] [Google Scholar]
- McLoon LK, Andrade F. Craniofacial muscles: A new framework for understanding the effector side of craniofacial muscle control. Springer Science & Business Media; 2012. [Google Scholar]
- Mioche L, Bourdiol P, Monier S, Martin JF, Cormier D. Changes in jaw muscles activity with age: effects on food bolus properties. Physiology & behavior. 2004;82(4):621–627. doi: 10.1016/j.physbeh.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Monemi M, Kadi F, Liu JX, Thornell L, Eriksson P. Adverse changes in fibre type and myosin heavy chain compositions of human jaw muscle vs. limb muscle during ageing. Acta physiologica scandinavica. 1999;167(4):339–346. doi: 10.1046/j.1365-201x.1999.00624.x. [DOI] [PubMed] [Google Scholar]
- Monemi M, Liu JX, Thornell LE, Eriksson PO. Myosin heavy chain composition of the human lateral pterygoid and digastric muscles in young adults and elderly. Journal of Muscle Research & Cell Motility. 2000;21(4):303–312. doi: 10.1023/a:1005632624826. [DOI] [PubMed] [Google Scholar]
- Monemi M, Thornell LE, Eriksson PO. Diverse changes in fibre type composition of the human lateral pterygoid and digastric muscles during aging. Journal of the neurological sciences. 1999;171(1):38–48. doi: 10.1016/s0022-510x(99)00244-0. [DOI] [PubMed] [Google Scholar]
- Naganuma K, Inoue M, Yamamura K, Hanada K, Yamada Y. Tongue and jaw muscle activities during chewing and swallowing in freely behaving rabbits. Brain Res. 2001;915(2):185–194. doi: 10.1016/s0006-8993(01)02848-7. [DOI] [PubMed] [Google Scholar]
- Nakayama M. Histological study on aging changes in the human tongue. Nihon Jibiinkoka Gakkai Kaiho. 1991;94(4):541–555. doi: 10.3950/jibiinkoka.94.541. [DOI] [PubMed] [Google Scholar]
- Newton J, Yemm RA, Menhinick S. Changes in human jaw muscles with age and dental state. 1993;10(1):16–22. doi: 10.1111/j.1741-2358.1993.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Newton JP, Abel EW, Robertson EM, Yemm R. Changes in human masseter and medial pterygoid muscles with age: a study by computed tomography. Gerodontics. 1987;3(4):151–154. [PubMed] [Google Scholar]
- Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55(11):M634–640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- Palmer JB, Rudin NJ, Lara G, Crompton AW. Coordination of mastication and swallowing. Dysphagia. 1992;7(4):187–200. doi: 10.1007/BF02493469. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microscopy research and technique. 2000;50(6):500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Peyron MA, Blanc O, Lund JP, Woda A. Influence of age on adaptability of human mastication. J Neurophysiol. 2004;92(2):773–779. doi: 10.1152/jn.01122.2003. [DOI] [PubMed] [Google Scholar]
- Plowman EK, Maling N, Rivera BJ, Larson K, Thomas NJ, Fowler SC, et al. Differential sensitivity of cranial and limb motor function to nigrostriatal dopamine depletion. Behav Brain Res. 2013;237:157–163. doi: 10.1016/j.bbr.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5(3):129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Prinz JF, Lucas PW. An optimization model for mastication and swallowing in mammals. Proc Biol Sci. 1997;264(1389):1715–1721. doi: 10.1098/rspb.1997.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society. 2005;53(9):1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50(5):M257–262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–991s. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia. 2013;28(1):95–104. doi: 10.1007/s00455-012-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh E, Shibata S, Matsuo K, Baba M, Fujii W, Palmer JB. Chewing and food consistency: effects on bolus transport and swallow initiation. Dysphagia. 2007;22(2):100–107. doi: 10.1007/s00455-006-9060-5. [DOI] [PubMed] [Google Scholar]
- Sano R, Tanaka E, Korfage J, Langenbach G, Kawai N, Van Eijden T, et al. Heterogeneity of fiber characteristics in the rat masseter and digastric muscles. Journal of Anatomy. 2007;211(4):464–470. doi: 10.1111/j.1469-7580.2007.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia. 2011;26(3):256–263. doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler JS, Kelly JH. Swallowing disorders in the elderly. Laryngoscope. 2002;112(4):589–602. doi: 10.1097/00005537-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Scott W, Stevens J, Binder–Macleod SA. Human skeletal muscle fiber type classifications. Physical therapy. 2001;81(11):1810–1816. [PubMed] [Google Scholar]
- Smith LR, Barton ER. SMASH–semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skeletal muscle. 2014;4(1):1. doi: 10.1186/2044-5040-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CM, Cichero JA. Physiological Factors Related to Aspiration Risk: A Systematic Review. Dysphagia. 2014 doi: 10.1007/s00455-014-9516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Sano R, Kawai N, Korfage J, Nakamura S, Izawa T, et al. Regional differences in fiber characteristics in the rat temporalis muscle. Journal of anatomy. 2008;213(6):743–748. doi: 10.1111/j.1469-7580.2008.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Asay AL, Allred RP, Ozburn AR, Kleim JA, Jones TA. The vermicelli and capellini handling tests: simple quantitative measures of dexterous forepaw function in rats and mice. J Vis Exp. 2010;(41) doi: 10.3791/2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch PA, Larsson L. Muscle hypertrophy in bodybuilders. [journal article] European Journal of Applied Physiology and Occupational Physiology. 1982;49(3):301–306. doi: 10.1007/BF00441291. [DOI] [PubMed] [Google Scholar]
- Thein M, Ershler WB, Artz AS, Tecson J, Robinson BE, Rothstein G, et al. Diminished quality of life and physical function in community-dwelling elderly with anemia. Medicine (Baltimore) 2009;88(2):107–114. doi: 10.1097/MD.0b013e31819d89d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubaki J, Jimbo G. A proposed new characterization of particle shape and its application. Powder Technology. 1979;22(2):161–169. [Google Scholar]
- Woda A, Mishellany A, Peyron MA. The regulation of masticatory function and food bolus formation. J Oral Rehabil. 2006;33(11):840–849. doi: 10.1111/j.1365-2842.2006.01626.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Fujiwara T, Kawamura Y. EMG activities of masticatory muscles during licking in rats. Physiol Behav. 1982;29(5):905–913. doi: 10.1016/0031-9384(82)90342-0. [DOI] [PubMed] [Google Scholar]