Abstract
Background and rationale
Obesity, an independent risk factor for the development of myocardial diseases is a growing healthcare problem worldwide. It’s well established that GSK-3β is critical to cardiac pathophysiology. However, the role cardiomyocyte (CM) GSK-3β in diet-induced cardiac dysfunction is unknown.
Methods
CM-specific GSK-3β knockout (CM-GSK-3β-KO) and littermate controls (WT) mice were fed either a control diet (CD) or high-fat diet (HFD) for 55 weeks. Cardiac function was assessed by transthoracic echocardiography.
Results
At baseline, body weights and cardiac function were comparable between the WT and CM-GSK-3β-KOs. However, HFD-fed CM-GSK-3β-KO mice developed severe cardiac dysfunction. Consistently, both heart weight/tibia length and lung weight/tibia length were significantly elevated in the HFD-fed CM-GSK-3β-KO mice. The impaired cardiac function and adverse ventricular remodeling in the CM-GSK-3β-KOs were independent of body weight or the lean/fat mass composition as HFD-fed CM-GSK-3β-KO and controls demonstrated comparable body weight and body masses. At the molecular level, on a CD, CM-GSK-3α compensated for the loss of CM-GSK-3β, as evident by significantly reduced GSK-3αs21 phosphorylation (activation) resulting in a preserved canonical β-catenin ubiquitination pathway and cardiac function. However, this protective compensatory mechanism is lost with HFD, leading to excessive accumulation of β-catenin in HFD-fed CM-GSK-3β-KO hearts, resulting in adverse ventricular remodeling and cardiac dysfunction.
Conclusion
In summary, these results suggest that cardiac GSK-3β is crucial to protect against obesity-induced adverse ventricular remodeling and cardiac dysfunction.
Keywords: High Fat Diet, Obesity, GSK-3β, Cardiac Function
Introduction
Obesity has reached epidemic proportions worldwide and is associated with an increased risk of heart failure (HF) [1]. Importantly, saturated fatty acids found in HFDs have been demonstrated to alter cardiac cell function and signaling leading to cardiac dysfunction and HF. Currently, available therapies for the treatment of myocardial diseases are inadequate and new molecular targets are needed. Thus, there is a need to identify specific molecular targets within the heart that are dysregulated by HFD and leads to cardiac dysfunction.
GSK-3, a ubiquitously expressed serine-threonine kinase, was originally identified and named based on its ability to regulate the rate-limiting enzyme of glycogen deposition, glycogen synthase. GSK-3 has two isoforms: alpha (α) and beta (β), which share 98% sequence homology in their kinase domains but differ in their N and C terminals. Overall, the two isoforms are 85% identical and hence it is not surprising that a number of studies have demonstrated overlapping effects in various pathologies. Apart from their numerous overlapping functions, both isoforms also have unique roles and one typically cannot compensate for the loss of the other. In fact, expression of GSK-3α did not rescue embryonic lethality in GSK-3β KO mice [2] and GSK-3β could not render protection from progressive heart failure and aging phenotype in GSK-3α germline KO mouse [3, 4].
While initially GSK-3 was shown to be important for regulating the critical step of glycogen synthesis, it is now well established that GSK-3 is essential for the regulation of many other signaling cascades, including those critical for cardiac homeostasis [4–11]. The role of GSK-3s in myocardial biology has been extensively studied [4–9, 12–14]. Using inducible CM-specific GSK-3β knockouts, we demonstrated a critical role of GSK-3β in myocardial ischemic injury. Cardiac GSK-3β inhibition markedly protected against MI-induced adverse remodeling. Based on these findings, we anticipated that CM-GSK-3β KO hearts would also be protected from transverse aortic constriction (TAC)-induced pathological hypertrophy and cardiac dysfunction. However, to our surprise, we found that cardiac GSK-3β has no role in basal or TAC-induced pathological cardiac hypertrophy [5]. These results suggest that the phenotypic outcome of GSK-3β modulation in hearts is highly stress-dependent. Another high profile study with genetically engineered mouse models of GSK-3β inhibition (Tg-DnGSK-3β and GSK-3β+/−) and activation (β knock-in) also demonstrated completely opposing roles of GSK-3β in two different pathologies, i.e ischemic injury and ischemic/reperfusion (I/R) injury [13]. Taken together, the available literature suggests that the role of GSK-3β in myocardial biology is complex and context-dependent, and sustained activation or chronic systemic inhibition can lead to serious consequences. Indeed, CM-specific inducible deletion of both GSK-3 isoforms (α and β) in fully mature adult hearts leads to fatal cardiomyopathy [9].
While numerous studies conducted so far indicate an important role of GSK-3 in cardiac function in various cardiovascular pathologies, the role of cardiomyocyte GSK-3β in regulating cardiac function in a diet-induced obesity model is unknown. Given the data that chronic obesity induced by HFD is a risk factor for cardiovascular disease and GSK-3 activity is increased with obesity [15, 16], in this study we sought to determine the specific role of CM-GSK-3β in HFD-induced cardiac dysfunction. Herein, for the first time, we employ an inducible CM-specific KO mouse to investigate the role of cardiac GSK-3β in cardiac function with chronic obesity. Results from this study demonstrate a critical role of GSK-3β in cardiac function. On a CD, cardiac GSK-3α compensates for the loss of GSK-3β and protects the heart against cardiac dysfunction. However, this compensatory protection is lost with chronic HFD, leading to cardiac β-catenin accumulation, resulting in cardiac dysfunction in CM-GSK-3β-KO mice.
Methods and Materials
An expanded Materials and Methods section is available in the Online Data Supplement.
Mice
CM-specific GSK-3β-KO mice were generated by crossing GSK-3βfl/fl mice with mice carrying the Mer-Cre-Mer transgene driven by the α-myosin heavy chain promoter (gift from Dr. J. Molkentin, Cincinnati Children’s Hospital, Cincinnati, Ohio) [17]. Mice were crossed for two generations in order to generate GSK-3βfl/flCre+/− mice. Both mouse strains were on the C57BL/6 background. At 10 weeks of age, when physiological development is largely complete, all male mice were placed on a tamoxifen chow diet (400mg/kg; TD.130860 from Envigo) for 15 days followed by regular chow for an additional 30 days (to allow the clearance of tamoxifen from the mice). GSK-3βfl/fl/Cre+/−/Tam mice were conditional knockout (KO), whereas littermates GSK-3βfl/fl/Cre−/−Tam represented controls (WT). The Institutional Animal Care and Use Committee of Vanderbilt University Medical Center approved all animal procedures and treatments (protocol # M1700133-00). All animals were housed in a temperature controlled room with a 12:12h light-dark cycle and received humane care.
Statistical Analysis
Data are expressed as mean±SEM. Differences between two groups were analyzed using unpaired t-test (Graph Pad Prism Software Inc., San Diego, CA). To analyze differences between the four groups (effect of diet and genotype) we used 2-way ANOVA followed by Tukey test for post hoc analysis using SigmaStat (Version 11, 2008, Systat Software, Inc., Chicago, IL). Significance was accepted at P<0.05.
Results
CM-Specific Deletion of GSK-3β
Global deletion of GSK-3β is embryonically lethal [2]. To investigate the role of cardiac GSK-3β in obesity-induced cardiac dysfunction, we employed the αMHC-Mer-Cre-Mer system to create CM-specific inducible GSK-3β-KOs as previously reported [5]. Specifically, ten-week-old WT (GSK-3βfl/flCre−/−) and KO (GSK-3βfl/fl Cre+/−) mice were subjected to tamoxifen diet for two weeks (Fig. 1A). After a four-week washout period, GSK-3β protein deletion in the heart was quantified by western blotting. Tamoxifen treatment reduced the expression of CM GSK-3β by 85% in the KO hearts compared to littermate WT hearts. Importantly, GSK-3α expression was comparable between the two groups after tamoxifen treatment (Fig. 1B, C).
Fig. 1.
Characterization of CM-specific inducible GSK-3β KO mouse model: (a) Experimental design. Ten-week-old male WT and KO mice were fed tamoxifen diet for two weeks. After a four week wash-out period, WT and KOs were fed either a CD or HFD for 55 weeks (b) Immunoblot and (c) Quantification of GSK-3α/β expression from WT and KO hearts shows significant reduction in GSK-3β (~85%) expression in KO LV lysates compared to WT. As expected, GSK-3α expression was comparable between the WT and CM-GSK-3β KO hearts. (d) Baseline body weights (e) Fat mass (f) Lean mass (g) Left ventricular ejection fraction (LV EF%) (h) Left ventricular fractional shortening (LV FS%) (i) Left ventricular end-diastolic interior dimension (LVIDd) (j) Left ventricular end-systolic interior dimension (LVIDs) in WT and KO animals (D–J, n=12–14 per group). * p < 0.05 CD vs. HFD; # p < 0.05 WT vs KO
Baseline Characteristics of WT and CM-GSK-3β-KO mice
At baseline, we did not see any differences in body weight (WT: 28.9±0.8, KO: 27.5±0.5 g), fat mass (WT: 0.13±0.0, KO: 0.11±0.0 g), and lean mass (WT: 0.70±0.0, KO: 0.70±0.0 g) between the WT and KO animals (Fig. 1D, E, F). Importantly, cardiac function measured using two-dimensional motion mode-echocardiography revealed no differences in the cardiac parameters between the lean WT and KO animals (LV EF; WT: 61.2±1.7, KO: 61.9±3.1; LV FS; WT: 32.4±1.1, KO: 33.0±2.3) (Fig. 1G, H, I, J), findings that are consistent with our previous report [5].
CM-GSK-3β Deletion has No Effect on Body Weights and Masses after 55 weeks of CD or HF-feeding
After the baseline analysis of cardiac function and body mass compositions (lean vs fat), WT and KO animals were subjected to a CD or HFD for 55 weeks and body weights were recorded weekly. In the present study, we chose a chronic HFD protocol as its recapitulates human diabetic cardiomyopathy [16, 18]. At the end of 55 weeks, we did not see any difference in the body weights (WT: 34.2±0.6, KO: 32.7±0.8 g), fat mass (WT: 0.31±0.02, KO: 0.30±0.03 g) and lean mass (WT: 0.63±0.01, KO: 0.65±0.01 g) between WT and KO mice on CD. As expected, HF-feeding significantly increased body weights (WT: 54.0±0.0, KO: 54.0±1.4 g), fat mass (WT: 0.70±0.02, KO: 0.70±0.03 g) and lean mass (WT: 0.51±0.01, KO: 0.52±0.01 g) in both WT and KO mice compared to their littermate controls on CD. Importantly, similar to the baseline data, even after 55 weeks, there was no effect of GSK-3β deletion on body weight, fat mass, or lean mass in either CD or HFD groups (Fig. 2A, B, C). As anticipated, high fat feeding increased the levels of total cholesterol, insulin and fasting blood glucose compared to their littermates on CD (Suppl. Fig 1). However, GSK-3β deletion does not lead to any effect on the plasma cholesterol, insulin and fasting blood glucose in both, the control or HFD groups.
Fig. 2.
CM-specific GSK-3β deletion leads to cardiac dysfunction in HF-fed KO mice (a) Body weights (b) Fat mass (c) Lean mass (d) Left ventricular ejection fraction (LV EF%) (e) Left ventricular fractional shortening (LV FS%) (f) Lung weight/tibia length ratio (LW/TL) (g) Left ventricular end-diastolic interior dimension (LVIDd) (h) Left ventricular end-systolic interior dimension (LVIDs) in WT and KO animals after 55 weeks on CD or HFD (n=12–14 per group).
* p < 0.05 CD vs. HFD; # p < 0.05 WT vs KO
CM-Specific Inducible Deletion Of GSK-3β Leads To Cardiac Dysfunction In an HFD-Induced Obesity Model
To determine the effect of GSK-3β deletion and chronic high fat feeding on cardiac function, we performed two-dimensional echocardiography in WT and KO animals fed either a CD or HFD (Fig. 2D, E). The cardiac function of WT and KO animals fed a CD was comparable. These results suggest that GSK-3β deletion does not lead to cardiac dysfunction on a CD. Surprisingly, in spite of marked obesity, cardiac function (LV EF and LV FS) of WT mice fed a HFD was similar to their littermates on CD (LV EF, WT CD: 55.1±3.6, WT HFD: 53.0±2.0; LV FS, WT CD: 28.8±2.3, WT HFD: 27.0±1.3). In contrast, LV EF and LV FS were significantly reduced in KO mice fed a HFD (LV EF, KO CD: 48.5±2.5, KO HFD: 42.7±4.0 (P<0.05); LV FS, KO CD: 28.8±2.3, KO HFD: 21.0±2.0 (P<0.05)). Consistently, the ratio of Lung Weight (LW)/Tibia Length (TL), which is an index of heart failure, was significantly increased in the KO animals fed HFD (Fig. 2F). However, left ventricular internal dimensions at end-systole (LVIDs) and end diastole (LVIDd) were unaltered in both genotypes on either diet (Fig. 2G, H). These results suggest that while the left ventricular function is preserved in the KO mice on CD, chronic HFD led to cardiac dysfunction and heart failure in obese KO animals.
Effect of HFD on Cardiac Hypertrophy and Fibrosis
Given the role of GSK-3β and HFD in cardiac hypertrophy, we performed morphometric analysis in the whole organ as well as at the cellular level (Fig. 3A, B). At the organ level, we calculated the ratio of heart weight (HW) to TL in WT and KO animals. On CD, we saw a significant increase in the HW/TL in KO animals compared to WT (Fig. 3B). These results suggest that chronic deletion of GSK-β in CMs induces cardiac hypertrophy in CD-fed aged KO hearts. Importantly, observed hypertrophy on CD diet was not maladaptive, as the cardiac function of KOs and littermate controls were comparable. As previously reported, HF feeding led to a further increase in the HW/TL ratio in both WT and KO hearts compared to their littermate controls (Fig. 3B). Interestingly, at 55 weeks, we see an additive effect of GSK-3β deletion and HFD at an organ level on cardiac hypertrophy. Compared to the WT, the KO’s had a significantly higher HW/TL ratio (Fig. 3B, P<0.05). To examine hypertrophy at the cellular level, we measured CM cross-sectional area using hematoxylin/eosin-stained heart sections (Fig 3C, D). Compared to the WT hearts, CM cross-sectional area was increased in KO hearts on CD (WT: 427.0±8, KO: 531.0±9 μm2, P <0.05). HFD increased CM cross-sectional area in WT hearts compared to their littermate controls on CD (P<0.05). HFD increased CM area to a similar extent in WT and KO hearts (WT: 559.0±9, KO: 543.0±10 μm2). The expression of fetal gene, brain natriuretic peptide (BNP), was also comparable between the groups (Suppl. Fig 2A). Previous studies have shown that chronic HFD induces not only cardiac hypertrophy but also cardiac fibrosis [16]. To determine the contribution of fibrotic remodeling in the observed cardiac phenotype, WT and KO hearts were analyzed for cardiac fibrosis using Masson trichrome staining. As expected, irrespective of the genotype, HFD treatment led to increased fibrosis in both groups. We observed a trend of increased fibrosis in HF-fed KO hearts; however, this difference was not statistically significant (Suppl. Fig. 2B, C). Next, we performed Q-PCR analysis for the fibrosis marker collagen-1. Consistent with our immunohistochemical data, expression of collagen-1 was significantly increased in HF-fed KO hearts, suggesting adverse fibrotic remodeling (Suppl. Fig 2D). Taken together, these results suggest an accelerated adverse cardiac remodeling in HF-fed KO hearts.
Fig. 3.
CM-GSK-3β deletion leads to adverse cardiac remodeling in HF-fed CM-GSK-3β-KO mice. (a) Representative hematoxylin-eosin stained sections of whole heart isolated from WT and KO animals at 55 weeks post CD or HFD. (b) Increased cardiac hypertrophy in aged KO mice on control diet (CD) compared to WT as seen with increased heart weight (HW)/tibia length (TL). HF- feeding further increased HW/TL in KO animals compared to WT-HF (n=10–13 per group). (c) Representative images of LV myocardium stained with hematoxylin-eosin and (d) Quantification of CM cross-sectional area at 55 weeks post CD or HFD from WT and KO animals (n=6 per group).
*, p < 0.05 CD vs. HFD; # p < 0.05 WT vs KO
Mechanism of HFD-Induced Cardiac Dysfunction and Adverse Ventricular Remodeling in CM-GSK-3β KO mice
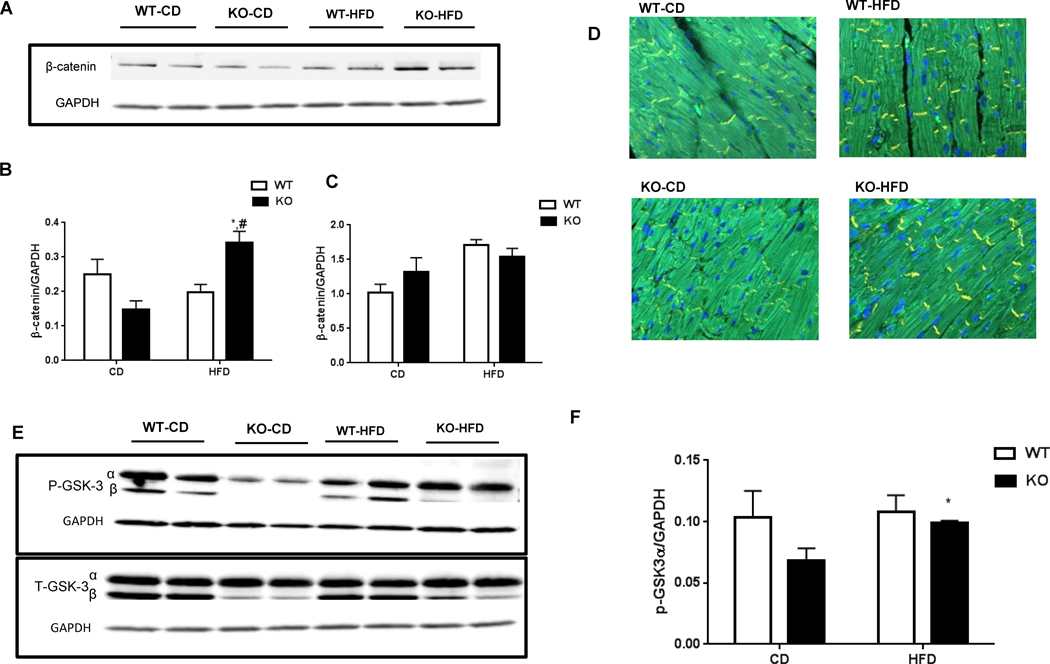
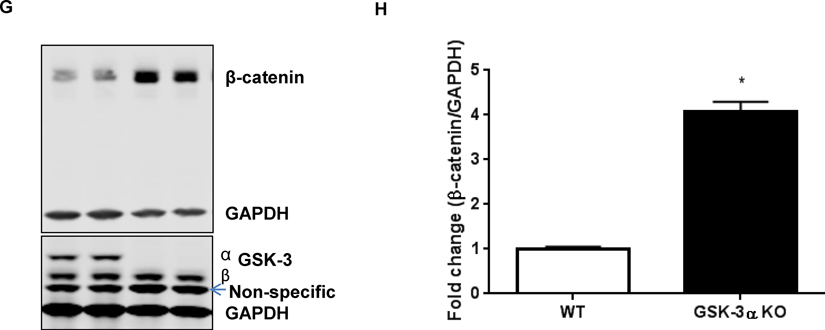
Cardiac function of WT and KO animals were comparable on CD, eliminating the effect of genotype alone for the cardiac dysfunction in obese CM-GSK-3β-KO mice. Additionally, HFD by itself did not lead to cardiac dysfunction. However, when there is dual stress, such as both HFD and GSK-3β deletion, the left ventricular function is compromised. To determine the possible mechanisms that could have led to cardiac dysfunction in HF-fed KO mice, we examined pathways implicated in CM biology and heart function. Given the critical role of MAP kinases such as p38, JNKs, and ERKs in cardiac hypertrophy and remodeling, we examined MAPK signaling in the LV lysates from all four groups. Interestingly, the ERK1/2 pathway, a well-known mediator of cardiac hypertrophy, was activated in CD-KO hearts (Suppl. Fig 3A). The activated ERK1/2 signaling might be responsible for the adaptive hypertrophy in KOs on CD [19]. However, we did not see activation of any other kinases specifically in the HFD-KO animals that exhibited reduced cardiac function (Suppl. Fig 3B, C). Saturated fatty acids, which make up for the bulk of fatty acids found in HFD, are known to induce cardiac apoptosis, which over time can lead to cardiac dysfunction and ultimately failure [20]. To determine if the cardiac dysfunction seen in the HF-fed KO animals was a result of CM apoptosis, we investigated cell death in WT and KO hearts. As anticipated, irrespective of the genotype, HF feeding led to increased TUNEL positive CMs in both WT as well as KO hearts. However, CM viability was comparable between the HF-fed WT and KO animals (data not shown). Consistently, we did not see any alterations in BAX, a marker for apoptosis or Bcl-xl, an anti-apoptotic marker (Suppl. Fig 3D, E). These results suggest that CM apoptosis was not the cause of the cardiac dysfunction observed in the KO mice. Previously, we have shown that cardiac GSK-3α is a potent regulator of cardiomyocyte proliferation in the post-infarct myocardium [12]. To determine the proliferative role of cardiac GSK-3β in diet-induced obesity model, we stained heart sections from all the four groups with Ki67, a marker for DNA synthesis. HF-feeding increased cardiomyocyte proliferation in both WT as well as the KO hearts (Suppl Fig 4). Cardiac lipid deposition resulting from chronic obesity induced by HFD can lead to cardiac dysfunction. To rule out the differences in lipid deposition, we stained heart sections with perilipin-1, one of the most abundant lipid droplet protein. We did not observe any difference in the perilipin-1 levels between the WT or KO animals fed a HFD (Suppl. Fig. 5). A previous study showed that chronic HFD feeding increased β-catenin expression and reduced GSK-3β in hearts that were fed an HFD for eleven months [16]. A more recent study demonstrated a critical role of activated β-catenin in mediating the diabetic cardiomyopathy [21]. Furthermore, activation of β-catenin is strongly linked to various cardiac diseases [22, 23]. Hence, we sought to examine the role of the canonical GSK-3β-β-catenin pathway in our study. Indeed, we observed an impaired β-catenin ubiquitination and degradation signaling in the HFD-KO hearts, which resulted in significantly increased β-catenin protein accumulation (Fig. 4A, B, C, D). Importantly, the transcript level of β-catenin was comparable between HF-fed WT and KO hearts, suggesting the post-translational changes in β-catenin levels between HF-fed WT and KO animals (Fig. 4C). Since HFD itself has been shown to reduce GSK-3β activity, we examined p-GSK-3α/β in the hearts of WT and KO animals fed either diet. To our surprise, the phosphorylation of GSK-3α was significantly decreased, leading to increased total GSK-3 activity in the CD-KO hearts. This compensatory mechanism of GSK-3α activation in order to maintain the total GSK-3 activity in GSK-3β deficient CMs was completely lost with HF feeding. Thus, in lean KO mice, GSK-3α compensates for the loss of GSK-3β to preserve the physiological β-catenin ubiquitination and degradation. However, this compensatory mechanism is impaired in HFD fed KO hearts, resulting in excessive β-catenin accumulation, which is known to induce cardiac dysfunction. To further confirm the role of GSK-3α in β-catenin ubiquitination pathway, we use mouse embryonic fibroblasts (MEFs) from WT and GSK-3α KO animals. Indeed, we saw a significant increase in β-catenin accumulation in GSK-3α KO MEFs compared to WT (Fig 4G, H). These results further confirm a role of GSK-3α in β-catenin ubiquitination pathway. Interestingly, a recent study using embryonic stem cells shows that either GSK-3 isoform can phosphorylate β-catenin when the other isoform is absent [24].
Fig. 4.
Mechanism of HFD-induced cardiac dysfunction in CM-GSK-3β KO mice. β-catenin expression (a) Immunoblotting (b) Quantification (c) m RNA expression (d) Representative images of β-catenin expression in WT and KO hearts fed CD or HFD for 55 weeks. (e) Immnublot and (f) Quantification of total and phospho-GSK-3α in LV lysates from WT and KO animals. (g) Immunoblot and (h) Quantification of β-catenin expression in mouse embryonic fibroblasts from WT and GSK-3α KO animals.
* p < 0.05 CD vs. HFD; # p < 0.05 WT vs KO
Discussion
Numerous studies, including those from our group, have demonstrated a critical role of CM GSK-3β in various myocardial pathologies [5, 7–9, 11, 13, 25]. However, the role of CM GSK-3β in obesity-associated cardiac dysfunction is unknown. Herein, we specifically deleted GSK-3β from fully mature CMs and examined its role in cardiac function with chronic HF-feeding. The key findings are: 1) CM-GSK-3β does not regulate body weight in HFD-induced obesity, 2) in lean mice, CM GSK-3α makes up for the loss of GSK-3β, as seen with increased GSK-3α activity in CM-GSK-3β-KO hearts, and protects the heart against cardiac dysfunction, 3) this compensatory mechanism is lost with chronic obesity, resulting in reduced GSK-3 activity in obese CM-GSK-3β-KO hearts, and 4) reduced GSK-3 activity in HF-fed CM-GSK-3β-KO hearts results in excessive β-catenin accumulation, ultimately leading to cardiac dysfunction.
A number of studies deleting GSK-3β specifically in liver, skeletal muscle and β-cells reported no differences in body weights at baseline or with chronic high fat feeding [17, 26]. Consistent with previous studies, we report no alterations in body weights or composition in CM-GSK-3β-KO mice fed either a CD or HFD. These results suggest that obesity alone was not sufficient to cause cardiac dysfunction observed in the present study.
Previously, we have shown that deleting GSK-3β acutely from CMs does not lead to any cardiac phenotype at baseline (without stress) [5]. In contrast to our previous reports with acute models, herein, we observed an increase in CM size and HW/TL ratio in CM-GSK-3β-KO animals fed a CD, which was increased further with HF-feeding. These findings suggest that long-term inhibition of GSK-3β is required to develop the hypertrophic phenotype. Importantly, similar observations were reported by Flepsi et. al. [27] and Huisamen et. al. [28] who investigated the effect of chronic GSK-3β inhibition (using chemical inhibitors) on cardiac hypertrophy in pre-diabetic rats. In both studies, GSK-3β inhibition led to cardiac hypertrophy in chow-fed Wistar rats, which was further elevated by HFD. The implications of these findings are broad since several ongoing trials are testing GSK-3 inhibitors as potential therapeutics for chronic conditions such as neurologic and metabolic diseases. However, our current data provides a cautionary note for the potential adverse consequences of chronic pharmacological GSK-3 inhibition in the heart. To our surprise, cardiomyocyte area was smaller in HF-fed GSK-3β-KO hearts compared to the HF-fed WT. We believe that excessive β-catenin accumulation in the HFD fed CM-GSK-3β KOs hearts is responsible for the smaller cardiomyocytes. This line of thought is strongly supported by studies with a mouse model of cardiac-specific β-catenin stabilization [29]. Baurand et. al. [29] reported an 82% reduction in the cross-sectional area of CMs isolated from mouse hearts genetically manipulated for CM-specific β-catenin stabilization. β-catenin, an armadillo-related protein is known to induce hypertrophic genes, and is normally phosphorylated by GSK-3s to maintain constitutive β-catenin degradation and ubiquitination [16].
A variety of MAPK signaling pathways are activated in response to cardiac stress, including ERK1/2, p38 and c-Jun N-terminal kinase (JNK) [30–33]. In the present study, we observed an increase in cardiac ERK1/2 signaling, specifically in aged GSK-3β-KO animals on a CD. Accumulating evidence suggests that ERK1/2 signaling promotes compensated cardiac hypertrophy and protects myocardium from cardiac stress [34–38]. Activation of ERK1/2 signaling in CM-GSK-3β KO hearts may account for the observed compensated hypertrophy in KOs on CD.
We have previously demonstrated that deletion of GSK-3α from cardiomyocytes significantly lowered CM death and apoptosis in ischemic hearts by inducing anti-apoptotic protein Bcl-2 and reducing the expression of the pro-apoptotic protein, Bax [12]. While chronic HF-feeding itself increased cardiomyocyte apoptosis, there was no effect of GSK-3β inhibition on CM viability between the two HF-fed groups. Furthermore, cardiomyocyte proliferation or ectopic lipid accumulation was also altered by CM GSK-3β deletion. Hence, the observed cardiac dysfunction was not due to alterations in CM viability, proliferation or lipid accumulation in HF-fed CM-GSK-3β KO animals.
To our complete surprise, we did not observe any alterations in cardiac function between the WT-CD and WT-HFD fed mice in spite of marked chronic obesity. Numerous studies using chronic HF feeding to investigate metabolic pathologies have reported an improvement, no change, or even deterioration in cardiac function with chronic HF feeding [39–42]. While the precise cause for these differences is unknown, we can speculate that a number of factors such as the age of the animal when HFD is started, duration of HF feeding or variation in cardiac function assessment could have led to these differences.
GSK-3α and GSK-3β are ubiquitously expressed in various tissues and have been shown to perform a redundant function with one isoform compensating for the loss of other [10]. A very interesting finding in this study was an alteration in the cardiac GSK-3α activity in the CM-GSK-3β-KO hearts. In the absence of cardiac GSK-3β, we observed a significant increase in GSK-3α activity (reduced phosphorylation) in CD-fed CM-GSK-3β-KO hearts. However, this effect was abolished in HF-fed CM-GSK-3β-KO hearts, leading to an impaired canonical GSK-3β-β-catenin degradation pathway. We further confirmed the role of GSK-3α in β-catenin ubiquitination and proteasomal degradation using WT and GSK-3α KO MEFs. Thus, an impaired β-catenin degradation system leads to significant accumulation of β-catenin, which is known to cause cardiac dysfunction and heart failure [21, 22, 29]. These results suggest a compensatory mechanism in the heart wherein GSK-3α compensates for the loss of GSK-3β and thus protects the CM-GSK-3β-KO hearts against dysfunction. This compensatory ability is lost with chronic obesity, rendering the GSK-3β deleted hearts susceptible to dysfunction.
Supplementary Material
Fig. 5.
Schematic representation of perturbations in cardiac β-catenin degradation in CM-GSK3β-KO hearts in diet-induced obesity model. On control diet, in the absence of cardiac GSK-3β, cardiac GSK-3α compensates for the loss (increased activity) and maintains cardiac β-catenin proteasomal degradation. This compensatory mechanism is lost with chronic obesity induced by high-fat diet resulting in cardiac β-catenin accumulation in myocytes ultimately leading to cardiac dysfunction.
Acknowledgments
This work was supported by research grants to HL from the NHLBI (R01HL133290, R01HL119234) and American Heart Association (13SDG16930103). MG was supported by Training Grant in Cardiovascular Research (T32 HL007411) from the NHLBI.
Footnotes
Disclosures
The authors have no conflicting interests to disclose in relation to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Kerkela R, Kockeritz L, Macaulay K, Zhou J, Doble BW, Beahm C, et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest. 2008;118:3609–18. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, et al. GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123:1821–32. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Lal H, Chen X, Shang X, Song J, Li Y, et al. GSK-3alpha directly regulates beta-adrenergic signaling and the response of the heart to hemodynamic stress in mice. J Clin Invest. 2010;120:2280–91. doi: 10.1172/JCI41407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woulfe KC, Gao E, Lal H, Harris D, Fan Q, Vagnozzi R, et al. Glycogen synthase kinase-3beta regulates post-myocardial infarction remodeling and stress-induced cardiomyocyte proliferation in vivo. Circ Res. 2010;106:1635–45. doi: 10.1161/CIRCRESAHA.109.211482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lal H, Zhou J, Ahmad F, Zaka R, Vagnozzi RJ, Decaul M, et al. Glycogen synthase kinase-3alpha limits ischemic injury, cardiac rupture, post-myocardial infarction remodeling and death. Circulation. 2012;125:65–75. doi: 10.1161/CIRCULATIONAHA.111.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lal H, Ahmad F, Zhou J, Yu JE, Vagnozzi RJ, Guo Y, et al. Cardiac Fibroblast GSK-3beta Regulates Ventricular Remodeling and Dysfunction in Ischemic Heart. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lal H, Ahmad F, Woodgett J, Force T. The GSK-3 family as therapeutic target for myocardial diseases. Circ Res. 2015;116:138–49. doi: 10.1161/CIRCRESAHA.116.303613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Ahmad F, Parikh S, Hoffman NE, Rajan S, Verma VK, et al. Loss of Adult Cardiac Myocyte GSK-3 Leads to Mitotic Catastrophe Resulting in Fatal Dilated Cardiomyopathy. Circ Res. 2016;118:1208–22. doi: 10.1161/CIRCRESAHA.116.308544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel P, Woodgett JR. Glycogen Synthase Kinase 3: A Kinase for All Pathways? Curr Top Dev Biol. 2017;123:277–302. doi: 10.1016/bs.ctdb.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Gupte M, Umbarkar P, Singh AP, Sui JY, Force T, et al. Entanglement of GSK-3beta, beta-catenin and TGF-beta1 signaling network to regulate myocardial fibrosis. J Mol Cell Cardiol. 2017;110:109–20. doi: 10.1016/j.yjmcc.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad F, Lal H, Zhou J, Vagnozzi RJ, Yu JE, Shang X, et al. Cardiomyocyte-specific deletion of Gsk3alpha mitigates post-myocardial infarction remodeling, contractile dysfunction, and heart failure. J Am Coll Cardiol. 2014;64:696–706. doi: 10.1016/j.jacc.2014.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109:502–11. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, et al. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci U S A. 2008;105:20900–5. doi: 10.1073/pnas.0808315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi XH, Wang Y, Li J, Wang FW, Tian GH, Yin MS, et al. Activation of Wnt/beta-catenin/GSK3beta signaling during the development of diabetic cardiomyopathy. Cardiovasc Pathol. 2015;24:179–86. doi: 10.1016/j.carpath.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Li L, Zhao H, Peng S, Zuo Z. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism. 2015;64:917–25. doi: 10.1016/j.metabol.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28:6314–28. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calligaris SD, Lecanda M, Solis F, Ezquer M, Gutierrez J, Brandan E, et al. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One. 2013;8:e60931. doi: 10.1371/journal.pone.0060931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Chen Z, Yang H, Luo F, Chen L, Cai H, et al. Selumetinib, an Oral Anti-Neoplastic Drug, May Attenuate Cardiac Hypertrophy via Targeting the ERK Pathway. PLoS One. 2016;11:e0159079. doi: 10.1371/journal.pone.0159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller TA, Icli B, Cote GM, Lebrasseur NK, Borkan SC, Pimentel DR, et al. Palmitate alters neuregulin signaling and biology in cardiac myocytes. Biochem Biophys Res Commun. 2009;379:32–7. doi: 10.1016/j.bbrc.2008.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P, Su J, Song X, Wang S. Activation of nuclear beta-catenin/c-Myc axis promotes oxidative stress injury in streptozotocin-induced diabetic cardiomyopathy. Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Hirschy A, Croquelois A, Perriard E, Schoenauer R, Agarkova I, Hoerstrup SP, et al. Stabilised beta-catenin in postnatal ventricular myocardium leads to dilated cardiomyopathy and premature death. Basic Res Cardiol. 2010;105:597–608. doi: 10.1007/s00395-010-0101-8. [DOI] [PubMed] [Google Scholar]
- 23.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4:469–83. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Wang R, Liu X, Wu Y, Zhou T, Yang Y, et al. A Chemical-Genetic Approach Reveals the Distinct Roles of GSK3alpha and GSK3beta in Regulating Embryonic Stem Cell Fate. Dev Cell. 2017;43:563–76. e4. doi: 10.1016/j.devcel.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho J, Zhai P, Maejima Y, Sadoshima J. Myocardial injection with GSK-3beta-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res. 2011;108:478–89. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, Cras-Meneur C, et al. Conditional ablation of Gsk-3beta in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 2010;53:2600–10. doi: 10.1007/s00125-010-1882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flepisi TB, Lochner A, Huisamen B. The consequences of long-term glycogen synthase kinase-3 inhibition on normal and insulin resistant rat hearts. Cardiovasc Drugs Ther. 2013;27:381–92. doi: 10.1007/s10557-013-6467-8. [DOI] [PubMed] [Google Scholar]
- 28.Huisamen B, Hafver TL, Lumkwana D, Lochner A. The Impact of Chronic Glycogen Synthase Kinase-3 Inhibition on Remodeling of Normal and Pre-Diabetic Rat Hearts. Cardiovasc Drugs Ther. 2016;30:237–46. doi: 10.1007/s10557-016-6665-2. [DOI] [PubMed] [Google Scholar]
- 29.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, et al. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res. 2007;100:1353–62. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- 30.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–46. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Molkentin JD. Regulation of cardiac hypertrophy and remodeling through the dual-specificity MAPK phosphatases (DUSPs) J Mol Cell Cardiol. 2016;101:44–9. doi: 10.1016/j.yjmcc.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, et al. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J Mol Cell Cardiol. 2007;43:137–47. doi: 10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lal H, Verma SK, Golden HB, Foster DM, Smith M, Dostal DE. Stretch-induced regulation of angiotensinogen gene expression in cardiac myocytes and fibroblasts: opposing roles of JNK1/2 and p38alpha MAP kinases. J Mol Cell Cardiol. 2008;45:770–8. doi: 10.1016/j.yjmcc.2008.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–83. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maejima Y, Galeotti J, Molkentin JD, Sadoshima J, Zhai P. Constitutively active MEK1 rescues cardiac dysfunction caused by overexpressed GSK-3alpha during aging and hemodynamic pressure overload. Am J Physiol Heart Circ Physiol. 2012;303:H979–88. doi: 10.1152/ajpheart.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–41. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 38.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–9. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daltro PS, Barreto BC, Silva PG, Neto PC, Sousa Filho PHF, Santana Neta D, et al. Therapy with mesenchymal stromal cells or conditioned medium reverse cardiac alterations in a high-fat diet-induced obesity model. Cytotherapy. 2017;19:1176–88. doi: 10.1016/j.jcyt.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Brainard RE, Watson LJ, Demartino AM, Brittian KR, Readnower RD, Boakye AA, et al. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS One. 2013;8:e83174. doi: 10.1371/journal.pone.0083174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung CL, Pan SH, Han CL, Chang CW, Hsu YL, Su CH, et al. Membrane Proteomics of Impaired Energetics and Cytoskeletal Disorganization in Elderly Diet-Induced Diabetic Mice. J Proteome Res. 2017;16:3504–13. doi: 10.1021/acs.jproteome.7b00148. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Ruiz-Velasco A, Wang S, Khan S, Zi M, Jungmann A, et al. Metabolic stress-induced cardiomyopathy is caused by mitochondrial dysfunction due to attenuated Erk5 signaling. Nat Commun. 2017;8:494. doi: 10.1038/s41467-017-00664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.