Abstract
Objective
HIV patients have increased atherosclerotic coronary vascular disease (ASCVD), thought to be mediated through inflammatory mechanisms. We hypothesized that among asymptomatic HIV+ patients with subclinical coronary plaque, statin therapy would modulate unique inflammatory and cardiovascular proteins in relation to change in subclinical coronary plaque volume. We tested this hypothesis using a novel proteomics approach.
Design
40 HIV+ participants were randomized to atorvastatin (40 mg/day) vs. placebo, and underwent CT coronary angiography to quantify plaque volume at baseline and 1 year.
Methods
We used Olink Cardiovascular III and Cardiometabolic panels based on dual antibody epitope recognition with linked DNA amplification to compare change over time in 184 proteins in treatment vs. placebo and in relation to change in coronary plaque volume.
Results
Six proteins (TFPI, CCL24, NT-Pro BNP, MBL2, LTBR, PCOLCE) changed significantly in the atorvastatin vs. placebo group, many in innate immune and other novel inflammatory pathways. Twenty-six proteins changed significantly in relationship to total coronary plaque volume over one year. Notably, many of these proteins changed only weakly in relationship to change in LDL. Overlapping these 2 broad discovery approaches, proteins involved in myocardial fibrosis/collagen formation and monocyte chemoattraction changed with statin treatment, in relationship to plaque volume, but not LDL.
Conclusions
This proof-of-concept study employing a proteomic discovery platform offers insight into statin effects on novel immune pathways relevant to ASCVD progression in HIV. Novel biomarker discovery may enhance precision medicine strategies to estimate the efficacy of targeted therapies to reduce ASCVD progression and events in HIV.
Keywords: HIV, cardiovascular disease, statin plaque proteomics
Introduction
Patients living with HIV (PLHIV) have an increased risk of myocardial infarction and heart failure, related to increased traditional risk factors, heightened systemic immune activation and generalized inflammation.1–4 The nature of this signal is not clear and may be changing with more focus on CVD risk by HIV clinicians,5 fewer co-infections, changing patterns of drug use and other factors. It is clear however, that antiretroviral therapy alone may not completely eliminate immune activation and arterial inflammation.6 More aggressive therapies with statins may reduce CVD in HIV, and specific studies are now underway to assess the effects of statins to prevent CVD in PLHIV. Importantly, statins have pleotropic effects and strategies are needed to identify the multiple potential pathways by which statins might reduce plaque in HIV. Serial measurement of soluble protein biomarkers reflecting cardiac specific and/or vascular pathophysiology offer an attractive methodology to provide unique insights into both the rapid progression and potential early efficacy of treatments of subclinical cardiovascular disease (CVD) in PLHIV. To this end, we have previously shown that PLHIV have higher soluble ST2 (sST2), galectin-3 and high sensitivity troponin T (hs-cTnT) levels than non-HIV controls.7 In PLHIV, sST2 is associated with evidence of diastolic dysfunction and both sST2 and Growth differentiation factor-15 (GDF-15) are strongly associated with all-cause mortality.8 Biomarkers of inflammation and thrombosis have also been associated with increased risk of mortality in PLHIV.9 Moreover, markers of immune activation have been shown to relate uniquely to plaque volume and arterial inflammation in cross sectional studies of HIV patients.10, 11
Statins are a potentially useful treatment strategy for CVD in HIV, given their effects on multiple inflammatory and immune pathways, including pathways relevant to monocyte activation (sCD14), arterial inflammation (LP-PLA2), and lipid oxidation (oxidized LDL), beyond effects on LDL-cholesterol concentrations.12–14 In longitudinal studies, we recently demonstrated that one-year of “high-intensity” atorvastatin therapy resulted in a significant reduction in sST2 versus placebo independent of LDL lowering.15 Others have shown that another high potency statin, rosuvastatin, is associated with a reduction in amino-terminal B-type natriuretic peptide levels.16 There is increased recognition that even well suppressed HIV infection is associated with multiple novel mechanisms that rapidly accelerate CVD compared to non-HIV positive patients with similar traditional CVD risk-factors.2, 6 A traditional “knowledge” based approach to biomarker selection seeks relevant soluble proteomic biomarkers, typically based on insights from non-HIV infected populations, to provide mechanistic insights for subclinical disease progression and to prevention of ASCVD events in PLHIV. This methodology is both slow and expensive. Recent introduction of a hybrid antibody-aptamer technology now provides a high precision “discovery” approach to measuring large panels of soluble protein based biomarkers, overcoming the methodological limitations of earlier multiplex technologies, to provide new insights into the traditional and novel mechanisms underlying the rapid progression of ASCVD in PLHIV.17–19 This is also an opportunity to develop a panel of biomarkers that provide early signals to the efficacy of specific preventive therapies in PLHIV; in other words a “precision medicine” approach.
In this proof-of-concept study, we took advantage of a recently completed randomized statin study in PLHIV in which changes in coronary plaque were measured using CCTA, hypothesizing that we could identity from a large panel of novel soluble proteins those that respond, unique from LDL lowering, to high-intensity statin therapy versus placebo. Furthermore, we sought to discover soluble proteins whose change in level in response to statin therapy was related significantly to changes in coronary plaque volume over the course of a year.
Methods
Study Design and Patient Population
This study was a single center 12-month randomized, double-blind, placebo-controlled clinical trial with enrollment starting in 2009 and completion of the last study visit in 2013. The study design and primary results have been previously reported.20 Primary results of the study demonstrated significant effects of statin treatment on reducing coronary plaque volume. In summary, men and women with HIV disease were screened. Participants enrolled were 18–60 years old, HIV+, on stable antiretroviral therapy (ART) without history of CVD, with evidence of subclinical plaque on CCTA. LDL-cholesterol was between 70–130 mg/dL, and participants did not meet guidelines at the time of the study for statin therapy. 20 Participants were randomized in a 1:1 ratio to either atorvastatin (starting at dose of 20 mg per day and escalating to 40 mg per day at the three-month visit) or placebo groups. All subjects received standardized lifestyle counseling based on NCEP guidelines. All participants provided written informed consent and the study was approved by the MGH institutional IRB. The trial is registered on ClinicalTrials.gov (NCT00965185).
Proteomic biomarker assessment
Plasma EDTA samples collected from study participants at baseline and at 12-months and stored at −80° C, were sent to Olink Proteomics (Watertown, MA, USA) for analysis of 184 unique proteins. Analyses were performed using a high-throughput technique using the Olink Cardiovascular III (CVD III, n=92 proteins, http://www.olink.com/products/cvd-iii-panel/) and Olink Cardiometabolic (n=92 proteins, http://www.olink.com/products/cardiometabolic-panel/) panels. Each panel measures 92 selected proteins simultaneously in plasma samples. The kits use a proximity extension assay (PEA) technology, where 92 oligonucleotide-labeled antibody probe pairs are allowed to bind to their respective target present in the sample.18, 19 The PEA technique has a major advantage over prior multiplex immunoassay technologies in that only correctly matched antibody pairs give rise to a signal, providing high specificity and allowing for the accurate assessment of change in levels over time with repeated measures. Individual proteins and the coefficient of variation with repeated measures are shown for the Cardiovascular III and the Cardiometabolic panels in Supplemental Tables 1 and 2 respectively. More comprehensive characteristics of the PEA technique, performance characteristics of the panels and the individual protein assays within each panel can be found at http://www.olink.com/products/document-download-center/.
CCTA Protocol and Analysis
CCTA was performed as previously described on a Somatom Definition Flash 128-slice dual source CT (Siemens Medical Solutions, Forchheim, Germany) according to the guidelines of the Society of Cardiac Computed Tomography (SCCT) at enrollment and one year follow-up.20 For assessment of total plaque in CCTA data sets, cross sectional and multiplanar reconstructed images were assessed for the presence and qualitative composition of atherosclerotic plaque for each coronary artery segment defined by the SCCT model.21
Statistical Methods
Overall Approach
In this analysis, we took 2 distinct discovery approaches to identify relevant proteins representing potentially important novel immune and inflammatory cardiac pathways in HIV that: 1) responded to statins relative to placebo in a controlled trial, and 2) simultaneously changed in relationship to total coronary plaque. With each approach, we identified markers in exploratory, hypothesis generating analyses, to determine novel areas for future investigation and to narrow down and exclude pathways of lesser relevance to statin effects on plaque in HIV. Moreover, we overlapped each approach to determine a novel set of proteins that both changed in relationship to statin and related to change in plaque but not LDL. For each exploratory analysis, we cast a broad net, without correcting for multiple comparisons in this hypothesis-generating study in which we aimed to show for the first time the potential power of “discovery” proteomics to advance our understanding of statin biology in HIV, with the expectation that this approach could be recapitulated in larger studies with expanded endpoints.
Analysis of Statin Effects on Individual Proteins
We compared the means of the within-patient changes in these proteins in the atorvastatin vs. placebo groups using the independent samples t-test for each protein. The t-statistics for the treatment effects as well as corresponding P values were determined. In order to plot the treatment differences using a common descriptor, we used the t-statistic, creating a waterfall plot, taking into account directionality of change relative to placebo. For proteins with relatively large treatment effects, e.g., an absolute value of t-statistic > 2.0, or P value < 0.05, we determined absolute changes in the levels of the proteins in each arm, to further determine directionality and magnitude of the change, relative to baseline.
Analysis of Relationship of Change in Proteins with Change in Plaque, LDL, CRP, and sCD14
We next conducted correlation analysis to estimate Pearson’s linear correlation coefficient, r, of the change in each protein with the change in total plaque volume, LDL, hs-CRP, a marker of generalized inflammation, and sCD-14, a marker of immune activation and innate immunity among all the subjects, in both groups combined (atorvastatin and placebo). In this analysis r values ≥ 0.32 corresponded to P < 0.05. We created a waterfall plot of these r values for each protein in relationship to change in coronary plaque volume to show the strength and directionality of the relationship with change in plaque volume.
Examination of Overlapping Proteins in the Two Discovery Approaches
We examined the outputs of each approach to identify overlapping proteins meeting these pre-specified conditions, e.g. changing in response to statin treatment, in relationship to change in plaque volume.
Results
Characteristics of the Participants at Baseline
Eighty-one HIV-infected participants underwent screening for this study. 40 participants were randomized to atorvastatin or placebo. Demographic, clinical characteristics, lipids, immunologic and systemic inflammatory markers of these 40 study participants have previously been described 14, 20 and are also shown in Supplemental Table 3. Participants were all on antiretroviral therapy and most participants had undetectable viremia, with similar immunological and virological indices between groups. All participants had started ART more than 2 years prior to initiating the study and all were on a stable regimen for at least 6 months prior to study initiation. After randomization, 1 out of 21 participants in the placebo group and 2 out of 19 in the atorvastatin group discontinued and did not have a 12-month evaluation, resulting in a similar discontinuation rate between groups.
Changes in protein biomarkers in the atorvastatin group vs. placebo
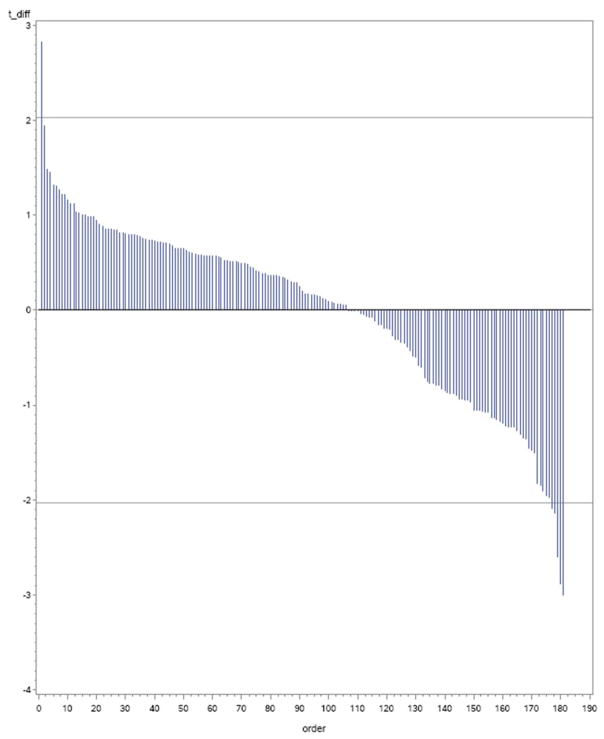
Compared to placebo, atorvastatin treatment was associated with decreases in 5 proteins (TFPI, CCL24, MBL2, LTBR, PCOLCE) and an increase in 1 protein (NT-Pro BNP) that achieved an absolute t-statistic > 2.0 (P<0.05) of the 184 proteins tested. The distribution of the mean differences in levels over time for atorvastatin vs. placebo for all 184 proteins are standardized and shown as a function of the t-statistic in the waterfall plot shown in Figure 1. An additional 6 proteins demonstrated a P value of <0.1 for change over time in the atorvastatin vs. placebo groups. In Table 1, a summary is provided for selected proteins with placebo vs. atorvastatin between group longitudinal mean changes associated with P values < 0.05 and < 0.10.
Figure 1. Proteomics Measurements with T-statistics Shown for Mean Difference Between Baseline and 12-months for Atorvastatin vs. Placebo Groups.
Waterfall plot showing T-statistic for differences over time shown on Y-axis and ordering of proteins on x axis.
Table 1.
Baseline Levels and Change Over Time by Treatment Group in Selected Discovery Proteins¶.
| Biomarker† | Baseline level Atorvastatin (N=17) | Change in level Atorvastatin | Baseline level Placebo (N=20) | Change in Level Placebo | t-statistic for treatment effect | P value for treatment effect |
|---|---|---|---|---|---|---|
| TFPI | 379.91±52.51 | −57.25±16.13 | 387.43±69.89 | 25.40± 21.34 | −3.00 | 0.004 |
| CCL24 | 48.60±40.67 | −5.77±3.01 | 62.03±39.80 | 10.14±4.39 | −2.88 | 0.005 |
| NT-proBNP | 1.41±0.51 | 0.52±0.24 | 2.27±2.52 | −0.62±0.31 | 2.83 | 0.006 |
| MBL2 | 10.32±1.25 | −0.36±0.16 | 11.17±1.16 | 0.17±0.13 | −2.60 | 0.01 |
| LTBR | 6.97±3.08 | −0.47±0.40 | 7.61±2.37 | 0.64±0.35 | −2.09 | 0.046 |
| PCOLCE | 6.28±0.56 | 0.55±0.10 | 6.78±1.51 | −0.19±0.34 | 1.95 | 0.048 |
| TR | 18.28±14.72 | −4.50±2.99 | 16.16±7.91 | 2.33±1.47 | −2.14 | 0.052 |
| SELE | 12.92±7.88 | −1.56±1.11 | 11.14±5.10 | 1.00±0.76 | −1.95 | 0.07 |
| TNF-R1 | 60.45±27.97 | −5.18±4.75 | 58.04±19.11 | 5.44±2.90 | −1.97 | 0.07 |
| CCL16 | 108.91±58.26 | −8.51±6.65 | 120.92±63.94 | 7.00±5.44 | −1.82 | 0.08 |
| CHIT1 | 57.03±37.52 | −1.97±4.63 | 48.47±35.12 | 7.31±2.20 | −1.90 | 0.08 |
| RETN | 82.63±45.87 | −13.24±9.90 | 75.23±23.07 | 7.95±6.43 | −1.85 | 0.08 |
Results for baseline are Mean ± SD and for change results are Mean ± SEM
For full list of abbreviations, see Supplemental Tables 1 and 2.
Association of biomarker level changes with change in coronary plaque volume
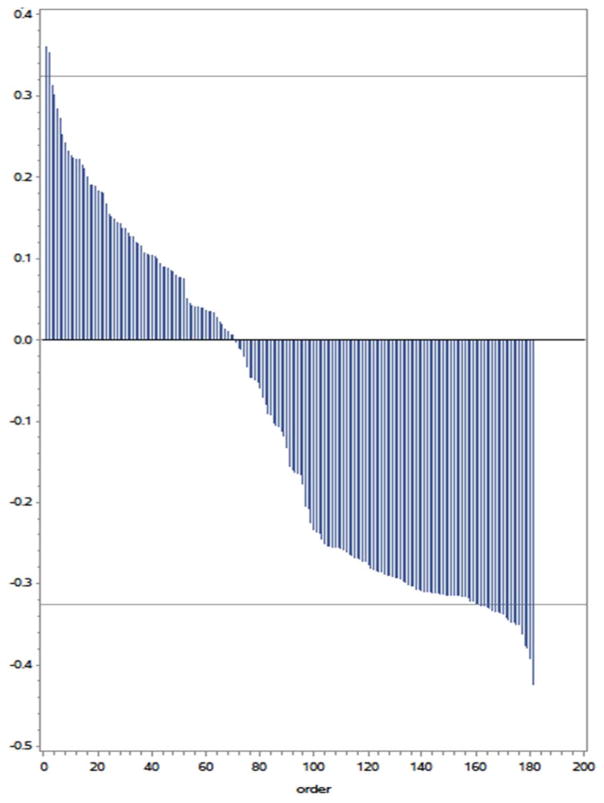
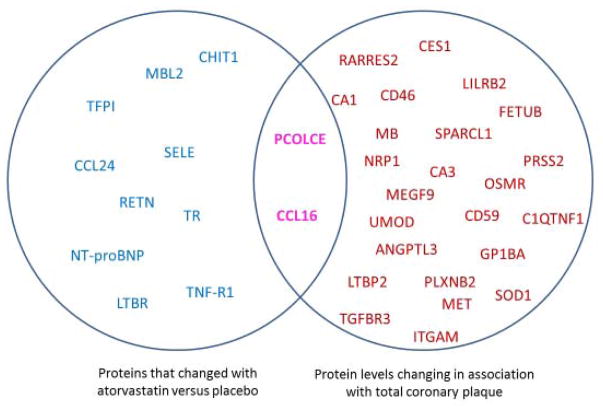
As previously reported, subjects receiving atorvastatin had a decrease of −4.7% (−25.4, 15.9) in total coronary plaque volume in contrast to subjects taking placebo who had a 18.2% (1.5, 59.9) increase in total plaque volume (P=0.01). Mean change in all 184 protein levels, among the combined group of patients in the atorvastatin and placebo groups, were individually correlated with change in total coronary plaque volume over 1-year. The correlation coefficients (r values) across the 184 proteins are shown in the waterfall plot in Figure 2. Using a threshold of an r value ≥ 0.32, corresponding to a P value < 0.05, we identified 2 proteins whose mean change in levels positively correlated with change in overall plaque volume and 24 whose mean change in levels were negatively correlated to change in plaque volume (i.e. the levels rose as plaque volume decreased). We had previously reported that atorvastatin therapy was associated with not only a reduction in overall plaque volume, but also reductions in markers of innate immunity, e.g. sCD14 in this HIV cohort. We sought to explore the association of these novel 26 proteins with changes in LDL cholesterol, hs-CRP and sCD14 levels (Table 2). Generally, the change in the levels of these 26 proteins that were associated with change in total plaque volume had minimal association with changes in LDL cholesterol and generalized inflammation. Using these strategies, we found two proteins, PCOLCE and CCL16 that changed with atorvastatin therapy and simultaneously associated with change in total coronary plaque volume (Figure 3).
Figure 2. Correlation Coefficients for Change in Total Coronary Plaque Volume and Change in Proteomics Measurements.
Waterfall plot showing correlation coefficients for the relationships shown on Y-axis and ordering of proteins on x axis.
Table 2.
Correlation between Change in Discovery Proteins and Change in Total Plaque Volume, Direct LDL, Biomarkers of General Inflammation (hs-CRP), and Innate Immunity (sCD14).
| Biomarker name† | Correlation with total Plaque | Correlation with direct LDL | Correlation with hs-CRP change | Correlation with sCD14 change |
|---|---|---|---|---|
| delta_87_RARRES2 | 0.36062 | 0.07010 | −0.35502 | −0.03756 |
| delta_96_CCL16 | 0.35349 | 0.18122 | −0.13628 | 0.14318 |
| delta_41_CD46 | −0.32117 | −0.09735 | −0.00018 | −0.10307 |
| delta_81_LILRB2 | −0.32149 | −0.09212 | 0.05382 | 0.00478 |
| delta_48_SPARCL1 | −0.32392 | −0.07221 | −0.05827 | −0.06988 |
| delta_58_MB | −0.32454 | −0.06682 | 0.17209 | 0.07181 |
| delta_57_FETUB | −0.32646 | 0.01070 | 0.03738 | −0.00925 |
| delta_58_CES1 | −0.32664 | 0.02490 | 0.15254 | 0.12728 |
| delta_50_MEGF9 | −0.32870 | −0.05149 | 0.04539 | −0.02618 |
| delta_78_CD59 | −0.32988 | −0.02881 | 0.02881 | −0.02275 |
| delta_38_ANGPTL3 | −0.33259 | 0.04340 | 0.08772 | −0.01895 |
| delta_80_OSMR | −0.33358 | −0.02648 | 0.09852 | 0.00970 |
| delta_66_NRP1 | −0.33411 | −0.06587 | 0.09126 | −0.01045 |
| delta_82_UMOD | −0.33499 | −0.10297 | 0.03703 | −0.06022 |
| delta_28_LTBP2 | −0.33720 | −0.09109 | 0.00382 | −0.05292 |
| delta_25_PCOLCE | −0.34104 | −0.17712 | −0.09962 | −0.13054 |
| delta_27_MET | −0.34388 | −0.04730 | 0.05865 | 0.00780 |
| delta_49_PLXNB2 | −0.34700 | −0.04257 | 0.03681 | −0.03846 |
| delta_73_GP1BA | −0.34735 | −0.02186 | 0.10834 | −0.05493 |
| delta_76_TGFBR3 | −0.34972 | −0.10180 | −0.01165 | −0.07101 |
| delta_42_ITGAM | −0.35019 | −0.11810 | −0.05927 | −0.05171 |
| delta_87_C1QTNF1 | −0.36165 | −0.01269 | 0.22708 | −0.04508 |
| delta_02_CA1 | −0.37562 | −0.07583 | 0.09945 | 0.10987 |
| delta_61_PRSS2 | −0.37835 | −0.04826 | 0.07503 | −0.05772 |
| delta_40_SOD1 | −0.39095 | −0.14320 | −0.03208 | −0.13884 |
| delta_75_CA3 | −0.42357 | −0.16448 | −0.00004 | −0.06935 |
For full list of abbreviations, see Supplemental Tables 1 and 2
Figure 3. Intersection of Discovery Approaches.
The potential intersection of proteomic biomarkers that are influenced by atorvastatin therapy and those associated with total coronary plaque volume. Proteins shown on the left in blue change in response to statin vs. placebo. Proteins shown on the right in red are those with changes significantly associated with change in total plaque. Protein levels that change in response to atorvastatin and whose change is associated with change in total coronary plaque are shown at the intersection of the circles in purple. For details on change with statin vs. placebo and degree and direction of change with plaque, see Tables 1 and Table 2. For full list of abbreviations, see Supplemental Tables 1 and 2.
Discussion
To our knowledge this is the first application of a large proteomic discovery platform applied to PLHIV to investigate novel mechanisms associated with non-LDL lowering effects of statin therapy and plaque regression. Our data suggest novel mechanisms associated with effects of statin therapy to reduce coronary atherosclerosis plaque volume in HIV using panels of proteins identified in advance for their role in cardiovascular physiology. We recognize that this is a pilot study for feasibility suggesting trends for specific biomarkers that would need to be reconciled in a larger cohort. PLHIV are potentially an ideal population to evaluate a larger discovery based panel of cardiac related biomarkers. Inflammation remains a driving factor for premature atherosclerosis in PLHIV despite undetectable viral loads.2, 6 Imaging has shown not only increased total coronary plaque volume vs. demographic and traditional risk factor matched controls, but a selective increase in noncalcified plaque, which is most vulnerable to sudden rupture, acute coronary syndrome or sudden death.22, 23 Epidemiologic data confirms PLHIV are at higher risk of MI events with an overall 50% higher risk after adjustment for demographics and this differential of risk continues to increase.24 Furthermore, imaging studies with PET have identified vascular inflammation in the aorta that is present to the same extent as patients with known coronary vascular disease.10 Recently, investigators using cell and animal based models have identified novel mechanisms of inflammation associated with HIV that may drive premature atherosclerosis.2 Some PLHIV have also been identified as having lipodystrophy associated with an increase in visceral fat with and increased activation of the renin-angiotensin-aldosterone system.25 Therefore, PLHIV are exposed to the direct chronic inflammatory effects of the virus, as well as indirect effects such as lipodystrophy and other mechanisms such as translocation of bacteria through the impaired immunologic defense of the intestinal wall.26, 27
We and others have previously shown that statin therapy modulates unique cardiovascular proteins that are associated with a poor cardiovascular prognosis in patients with and without HIV infection.7, 8, 16, 28 Specific to PLHIV we have shown that while statin therapy remains a potent therapy to reduce LDL levels it is also associated with reduction in measures of innate immunity, such as sCD14.12, 29 Early and accelerated progression of atherosclerosis in PLHIV compared to age, gender and traditional cardiovascular risk factor matched controls likely reflects pathophysiology unique to HIV infection, even when well suppressed.2 These mechanisms are incompletely understood, but include immune cells; with more rapid induction of HIV infected macrophages to foam cells through the HIV Nef protein and single-stranded RNA inhibition of cholesterol efflux; as well as chronic T-cell activation with subsequent endothelial cell activation and athrogenesis.30, 31 PLHIV also have greater oxidative stress that can lead to increased endothelial dysfunction and oxidation of LDL.32–34 HIV can inhibit autophagy and this may play an important role in HIV specific plaque progression and instability.35 Additional mechanisms have also been proposed to account for the early and vulnerable nature of inflammation driven atherosclerosis in PLHIV.2
Evaluation of proteomics allows assessment of not only the novel influence of the HIV infection, but the interaction with traditional risk factors and environmental factors on atherosclerosis. Furthermore, assays with adequate precision allow for serial measures to provide critical longitudinal information that can reflect the natural history of the disease and the influence of a therapeutic intervention. For example, we previously showed using a high sensitive cardiac troponin assay, reflecting myocyte injury, in asymptomatic older adults without HIV that a modest increase in level over time identified subjects at highest risk for cardiovascular events and a simple intervention such as moderate exercise could mitigate this rise in level.36, 37 Others have shown that a moderate dose statin can show a similar effect to blunt the rise in cardiac troponin.28 Such findings are examples of how a biomarker can reflect the progression of subclinical cardiovascular disease and could potentially be used as indicators of early efficacy of a therapy. The complexity of HIV infection on the development and progression of atherosclerosis likely defies the ability of a single soluble protein biomarker to longitudinally track progression of subclinical atherosclerosis and measure the efficacy of a pleotropic therapy, such as atorvastatin in PLHIV. Single protein ELISA type assays have been used by us and others to provide mechanistic insights and prognostic information, demonstrating that statin therapy reduces immune activation12 and reduces the level of a cardiovascular prognostic marker sST2, potentially reflecting improvement in endothelial function and cardiac fibrosis.15
The PEA methodology by Olink Proteomics provides for the first time the ability to measure large panels of soluble proteins and follow their longitudinal change over time with adequate precision such that these changes in levels can be associated with a therapeutic intervention (atorvastatin) and the disease progression of interest, coronary atherosclerosis. This work suggests there are multiple novel protein levels that change as the result of high-intensity statin therapy and are associated with change in the volume of coronary atherosclerotic plaque that appear unrelated to changes in LDL cholesterol or general inflammation markers. Identification of these novel proteins would be challenging using single assay proteomics and a “knowledge” based approach to choose assays in a field where many novel mechanisms remain to be discovered. We suggest a technique where one can look at the intersection of proteins that change over one-year as a result of high-intensity statin therapy and are also associated with change in coronary plaque volume potentially providing novel insights into the non-LDL cholesterol related mechanisms of coronary atherosclerosis and the pleotropic mechanisms of statin therapy in PLHIV. Our findings of non LDL lowering associations with statin treatment and coronary plaque progression related to inflammation has precedence in the general population. In the REVERSAL study, change in hs-CRP levels, a measure of generalized inflammation were found to be only weakly associated with change in LDL levels (r=0.13), but both change in CRP and LDL cholesterol levels were independently associated with change in coronary plaque volume.38 Our findings suggest similar overlap with two proteins lowered by high intensity statin, not associated with LDL lowering, and in this study also not associated with change in CRP level. These two proteins include: Chemokine ligand 16 (CCL16) and Procollagen C-endopeptidase enhancer (PCOLCE). CCL16 is a chemokine when activated that promotes monocyte recruitment.39 CCL16 may also have a role in vascular endothelial cell migration and potentially angiogenesis to form new capillaries in some models.40 PCOLCE1 and 2 are expressed within the myocardium and play an important role in myocardial fibrosis through the conversion of procollagen to collagen so that it may be incorporated into insoluble fibrils. In a pressure overload mouse model PCOLCE2-null versus wild type mice exhibited less myocardial collagen content and less muscle stiffness.41 PCOLCE is also upregulated in response to aldosterone.42 These findings are relevant to PLHIV who have both increased aldosterone production and evidence of asymptomatic cardiac fibrosis.25, 43
Though a focus of this study has been to demonstrate a methodology to identify novel proteins that intersect in the domains of change with atorvastatin therapy relative to placebo and being associated with change in total coronary plaque volume, the pleotropic effects of atorvastatin may also influence proteins levels that could reflect important prognostic information without being associated with change in coronary plaque volume. One such protein is tissue factor inhibitor (TFPI) which was recently shown to be elevated in PLHIV.44 Tissue factor is produced by activated monocytes, prevalent in PLHIV, and represents an extrinsic mechanism for thrombosis important to conversion of stable atherosclerosis to an acute coronary syndrome. Reduction in TFPI levels, a counter regulatory protein to tissue factor, as suggested for the first time in HIV in this study, likely reflects a reduction of tissue factor (which has a shorter half-life challenging in-vitro diagnostic measures).
The limitations of this proteomics discovery feasibility study are two-fold. First, based on the number of biomarkers studied and the relatively small number of study subjects, false discovery would be anticipated. In contrast, we purposely sought to identify proteins through two broad but differing approaches and then view the intersection of these approaches in a prespecified fashion to obtain a limited number of highly relevant proteins. An advantage in this regard is that we used data from a highly informative randomized, placebo-controlled longitudinal intervention trial with deep phenotyping to assess plaque changes. Nonetheless, definitive conclusions cannot be made whether changes in these specific biomarker levels with respect to atorvastatin therapy could be used to develop a precision medicine strategy to identify the efficacy of high-intensity statin therapy to reduce ASCVD events or induce coronary plaque regression. Rather we aim to demonstrate the potential utility of this approach to identify novel proteins, as we have done in this study. Ultimately, whether a discovery approach to proteomics, now with the methodological precision to evaluate change over time, can be utilized to identify PLHIV who will have a reduction in ASCVD events from long-term statin therapy, and whether these findings are associated with significant regression of coronary atherosclerosis, will need to be answered in subsequent larger studies. Importantly, it will be interesting to contrast the results obtained here among HIV patients in similarly designed statin studies among non-HIV patients to further identify HIV-specific proteins that may be mechanistically involved in mediating nontraditional statin effects on plaque in HIV. If further studies bear out an important set of proteins, these could ultimately be assessed in large-scale, events driven trials such as the 6500 subject NIH sponsored randomized trial to prevent vascular events in HIV (REPRIEVE, NCT02344290). Finally, as with any proteomics approach, we cannot determine causality per se, and further studies will need to assess for example how the fibrosis or monocyte chemoattraction pathways identified in this study mechanistically relate to statin effects on plaque in HIV.
In conclusion, observations from our randomized trial suggest for the first time that statin therapy may reduce several novel proteins in PLHIV implicated in cardiovascular disease. Furthermore, the use of large panels of protein based precision assays to measure longitudinal changes could provide both mechanistic insights into the unique nature of HIV accelerated atherosclerosis and therapies targeting more than just lowering of LDL cholesterol. Further studies are now needed to confirm and extend these findings.
Supplementary Material
Acknowledgments
C.D. Conceived, wrote and edited the manuscript.
J.L. Recruited the participants and edited the manuscript.
R.C. Analyzed the data and edited the manuscript.
I.G. Assessed the methodology and edited the manuscript.
L.S. Edited the manuscript.
M.V.Z. Edited the manuscript.
H.L. Analyzed the data and edited the manuscript.
S.G. Conceived, wrote and edited the manuscript.
This study was supported by Olink Proteomics and NIH P30DK040561.
Conflicts of Interest and Source of Funding: Dr. deFilippi has received research support from Roche Diagnostics and consulted for Roche Diagnostics, Alere, Ortho Diagnostics, Metanomics, and Siemens healthcare diagnostics; serves on an endpoint committee for Radiometer and Quintiles; and receives royalties from UpToDate all unrelated to this manuscript. Dr. Lo has served as consultant for Gilead Sciences unrelated to this project. Dr. Grundberg is an employee of Olink Proteomics. Dr. Zanni has participated in a Scientific Advisory Board meeting for Roche Diagnostics and has received research funding from Gilead, both unrelated to this project. Dr. Christenson is a consultant for Roche Diagnostics and Siemens Healthcare Diagnostics unrelated to this project. Dr. Grinspoon has received consulting fees from Theratechnologies and Navidea and research funding from Gilead, Theratechnologies and KOWA, all unrelated to this project. For the remaining authors nothing was declared.
References
- 1.Freiberg MS, Chang CH, Kuller LH, et al. Hiv infection and the risk of acute myocardial infarction. JAMA Internal Medicine. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearns A, Gordon J, Burdo TH, Qin X. Hiv-1-associated atherosclerosis: Unraveling the missing link. J Am Coll Cardiol. 2017;69:3084–3098. doi: 10.1016/j.jacc.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo J, Plutzky J. The biology of atherosclerosis: General paradigms and distinct pathogenic mechanisms among hiv-infected patients. J Infect Dis. 2012;205:S368–S374. doi: 10.1093/infdis/jis201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann JM, Huang S, Gupta DK, Lipworth L, Mumma MT, Blot WJ, Akwo EA, Kripalani S, Whooley MA, Wang TJ, Freiberg MS. Association of neighborhood socioeconomic context with participation in cardiac rehabilitation. J Am Heart Assoc. 2017;6:006260. doi: 10.1161/JAHA.117.006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, Hurley LB, Marcus JL, Quesenberry JCP, Silverberg MJ. Declining relative risk for myocardial infarction among hiv-positive compared with hiv-negative individuals with access to care. Clinical Infectious Diseases. 2015;60:1278–1280. doi: 10.1093/cid/civ014. [DOI] [PubMed] [Google Scholar]
- 6.Zanni MV, Toribio M, Robbins GK, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiology. 2016;1:474–480. doi: 10.1001/jamacardio.2016.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitch KV, DeFilippi C, Christenson R, Srinivasa S, Lee H, Lo J, Lu MT, Wong K, Petrow E, Sanchez L, Looby SE, Hoffmann U, Zanni M, Grinspoon SK. Subclinical myocyte injury, fibrosis and strain in relationship to coronary plaque in asymptomatic hiv-infected individuals. AIDS. 2016;30:2205–2214. doi: 10.1097/QAD.0000000000001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Secemsky EA, Scherzer R, Nitta E, Wu AH, Lange DC, Deeks SG, Martin JN, Snider J, Ganz P, Hsue PY. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in hiv-infected individuals. JACC Heart Fail. 2015;3:591–599. doi: 10.1016/j.jchf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, Cohen CJ, Phillips A, Lundgren JD, Neaton JD. Relevance of interleukin-6 and d-dimer for serious non-aids morbidity and death among hiv-positive adults on suppressive antiretroviral therapy. PLoS One. 2016;11:e0155100. doi: 10.1371/journal.pone.0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with hiv. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with hiv infection. Nat Rev Cardiol. 2014;11:728–741. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 12.Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, Pate MM, Aberg JA, Zanni MV, Grinspoon SK. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in intrepid: A randomized trial in hiv. AIDS. 2017;31:797–806. doi: 10.1097/QAD.0000000000001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin reduces vascular inflammation and t-cell and monocyte activation in hiv-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nou E, Lu MT, Looby SE, Fitch KV, Kim EA, Lee H, Hoffmann U, Grinspoon SK, Lo J. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with hiv. AIDS. 2016;30:583–590. doi: 10.1097/QAD.0000000000000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo J, Defilippi C, Christenson R, Fitch K, Looby S, Kim E, Lu M, Hoffmann U, Grinspoon S. Statin therapy lowers myocardial fibrosis marker sst2 in patients living with hiv. AHA Scientific Sessions. 2016 [Google Scholar]
- 16.Dirajlal-Fargo S, Kinley B, Jiang Y, Longenecker CT, Hileman CO, Debanne S, McComsey GA. Statin therapy decreases n-terminal pro-b-type natriuretic peptide in hiv: Randomized placebo-controlled trial. AIDS. 2015;29:313–321. doi: 10.1097/QAD.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S. Homogenous 96-plex pea immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39:e102. doi: 10.1093/nar/gkr424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind L, Arnlov J, Lindahl B, Siegbahn A, Sundstrom J, Ingelsson E. Use of a proximity extension assay proteomics chip to discover new biomarkers for human atherosclerosis. Atherosclerosis. 2015;242:205–210. doi: 10.1016/j.atherosclerosis.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in hiv-infected patients with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2:e52–63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifarth H, Schlett CL, Lehman SJ, Bamberg F, Donnelly P, Januzzi JL, Koenig W, Truong QA, Hoffmann U. Correlation of concentrations of high-sensitivity troponin t and high-sensitivity c-reactive protein with plaque progression as measured by ct coronary angiography. J Cardiovasc Comput Tomogr. 2014;8:452–458. doi: 10.1016/j.jcct.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, Lo J, Grinspoon SK. Noncalcified coronary atherosclerotic plaque and immune activation in hiv-infected women. Journal of Infectious Diseases. 2013;208:1737–1746. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble cd163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in hiv-infected patients. Journal of Infectious Diseases. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, Freiberg MS, Lloyd-Jones DM. Patterns of cardiovascular mortality for hiv-infected adults in the united states: 1999 to 2013. Am J Cardiol. 2016;117:214–220. doi: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasa S, Fitch KV, Wong K, Torriani M, Mayhew C, Stanley T, Lo J, Adler GK, Grinspoon SK. Raas activation is associated with visceral adiposity and insulin resistance among hiv-infected patients. J Clin Endocrinol Metab. 2015;100:2873–2882. doi: 10.1210/jc.2015-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasa S, Fitch KV, Lo J, Kadar H, Knight R, Wong K, Abbara S, Gauguier D, Capeau J, Boccara F, Grinspoon SK. Plaque burden in hiv-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS. 2015;29:443–452. doi: 10.1097/QAD.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated hiv infection. J Infect Dis. 2014;210:1228–1238. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford I, Shah ASV, Zhang R, McAllister DA, Strachan FE, Caslake M, Newby DE, Packard CJ, Mills NL. High-sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. Journal of the American College of Cardiology. 2016;68:2719–2728. doi: 10.1016/j.jacc.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin treatment reduces markers of monocyte activation in hiv-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–595. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pushkarsky T, Shilov E, Kruglova N, Naumann R, Brichacek B, Jennelle L, Sviridov D, Kruglov A, Nedospasov SA, Bukrinsky M. Short communication: Accumulation of neutral lipids in liver and aorta of nef-transgenic mice. AIDS Res Hum Retroviruses. 2017;33:57–60. doi: 10.1089/aid.2016.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernard MA, Han X, Inderbitzin S, Agbim I, Zhao H, Koziel H, Tachado SD. Hiv-derived ssrna binds to tlr8 to induce inflammation-driven macrophage foam cell formation. PLoS One. 2014;9:e104039. doi: 10.1371/journal.pone.0104039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy P, Wang X, Lin PH, Yao Q, Chen C. Hiv nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Surg Res. 2009;156:257–264. doi: 10.1016/j.jss.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Liang H, Fan X, Zhu L, Shen T. Liver damage in patients with hcv/hiv coinfection is linked to hiv-related oxidative stress. Oxid Med Cell Longev. 2016;2016:8142431. doi: 10.1155/2016/8142431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulgan T, Morrow J, D’Aquila RT, Raffanti S, Morgan M, Rebeiro P, Haas DW. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis. 2003;37:1711–1717. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- 35.Dinkins C, Pilli M, Kehrl JH. Roles of autophagy in hiv infection. Immunol Cell Biol. 2015;93:11–17. doi: 10.1038/icb.2014.88. [DOI] [PubMed] [Google Scholar]
- 36.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin t using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.deFilippi CR, de Lemos JA, Newman AB, Guralnik JM, Christenson RH, Pahor M, Church T, Espeland M, Krithevsky SB, Stafford R, Seliger SL. Impact of moderate physical activity on the longitudinal trajectory of a cardiac specific biomarker of injury: Results from a randomized pilot study of exercise intervention. Am Heart J. 2016;179:151–156. doi: 10.1016/j.ahj.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O’Shaughnessy C, Ganz P. Statin therapy, ldl cholesterol, c-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 39.Starr AE, Dufour A, Maier J, Overall CM. Biochemical analysis of matrix metalloproteinase activation of chemokines ccl15 and ccl23 and increased glycosaminoglycan binding of ccl16. J Biol Chem. 2012;287:5848–5860. doi: 10.1074/jbc.M111.314609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strasly M, Doronzo G, Cappello P, Valdembri D, Arese M, Mitola S, Moore P, Alessandri G, Giovarelli M, Bussolino F. Ccl16 activates an angiogenic program in vascular endothelial cells. Blood. 2004;103:40–49. doi: 10.1182/blood-2003-05-1387. [DOI] [PubMed] [Google Scholar]
- 41.Baicu CF, Zhang Y, Van Laer AO, Renaud L, Zile MR, Bradshaw AD. Effects of the absence of procollagen c-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am J Physiol Heart Circ Physiol. 2012;303:H234–240. doi: 10.1152/ajpheart.00227.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessler-Icekson G, Schlesinger H, Freimann S, Kessler E. Expression of procollagen c-proteinase enhancer-1 in the remodeling rat heart is stimulated by aldosterone. Int J Biochem Cell Biol. 2006;38:358–365. doi: 10.1016/j.biocel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Hsue PY, Tawakol A. Inflammation and fibrosis in hiv: Getting to the heart of the matter. Circ Cardiovasc Imaging. 2016;9:e004427. doi: 10.1161/CIRCIMAGING.116.004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barska K, Kwiatkowska W, Knysz B, Arczynska K, Karczewski M, Witkiewicz W. The role of the tissue factor and its inhibitor in the development of subclinical atherosclerosis in people living with hiv. PLoS One. 2017;12:e0181533. doi: 10.1371/journal.pone.0181533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.