Summary
Long non-coding RNAs (lncRNAs), which are encoded by a vast less explored region of the human genome may hold missing drivers of cancer and have gained attention recently as a potentially crucial layer of cancer cell regulation. LncRNAs are aberrantly expressed in a broad spectrum of cancers, and they play key roles in promoting and maintaining tumor initiation and progression, demonstrating their clinical potential as biomarkers and therapeutic targets. Recent discoveries have revealed that lncRNAs act as key signal transduction mediators in cancer signaling pathways by interacting with proteins, RNA and lipids. Here we review the mechanisms by which lncRNAs regulate cellular responses to extracellular signals and discuss their clinical potential as diagnostic indicators, stratification markers, and therapeutic targets of combinatorial treatments.
Keywords: Long non-coding RNAs, Cancer, Biomarker, Metastasis, Signaling Pathway, Ligand, Receptor, Signal Transducer, Kinase, Effector, Anti-sense Oligonucleotides, Locked nucleic acid, Nanoparticle-delivered siRNAs
LncRNAs as diagnostic and prognostic markers for human cancer
It is being recognized that certain single-nucleotide polymorphisms (SNPs) are associated with cancer risk. Large-scale data analysis from cancer genome-wide association studies (GWAS) indicate that the majority of SNPs associate with non-coding genes [9, 10]. The majority of recurrent somatic mutations, copy number alterations and cancer related SNPs are related to non-coding RNAs [11–13] (recently reviewed [14]), and the presence of risk SNPs may modulate the expression of corresponding non-coding RNAs. Among those non-coding genes, long non-coding RNAs (lncRNAs) are emerging as a new class of indispensable players involved in the development and progression of cancer [3–5]. Indeed, SNPs of lncRNAs have been shown to be associated with risk for prostate cancer [15, 16], lung cancer [17], breast cancer [18–20], among other cancer types [21, 22]. Moreover, the dysregulation of a number of lncRNA targets has correlated with the stage and prognosis of several tumor types [21, 22] including prostate cancer [15, 16], lung cancer [17], and breast cancer [18–20] as well as being linked to resistance against chemotherapy and targeted therapy [26–29]. Correlation analyses indicate that numerous lncRNAs are upregulated in cancer cells that are resistant to DNA damage inducers [30–33], anti-hormone therapies [34–36], targeted therapies [37], and signaling pathway inhibitors [18] (Figure 1). Indeed the expression of HOTAIR activates estrogen receptor (ER) target transcription program and contributes to the resistance of tamoxifen [36]. Loss-of-function studies using small hairpin RNA-based knockdown and CRISPR/cas9-mediated genetic depletion indicate that lncRNAs facilitate cancer cell growth, apoptosis resistance, and cell mobility [30, 38, 39]. Gain-of-function studies suggest that increased expression of lncRNAs may enhance cell viability during drug treatment [32, 36, 40].
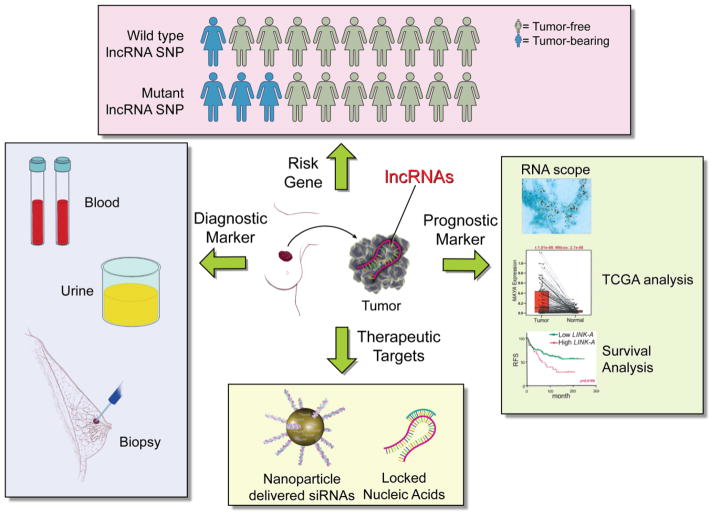
Figure 1. The value of lncRNAs as breast cancer risk genes, diagnostic markers, prognostic markers and therapeutic targets.
LncRNAs as cancer risk genes: the search for genomic mutations related to lncRNAs suggests that disease-related SNP of lncRNAs may situate lncRNAs as cancer risk genes. Populations with the wild type allele may exhibit low incidence of cancer; mutant allele carriers may show elevated incidence of cancer development. LncRNAs as diagnostic markers: the detection of lncRNAs in human blood, urine or biopsy samples could be beneficial for risk detection in a gene carrier for early diagnosis of human cancer. LncRNAs as prognostic markers: the expression status of lncRNAs in human cancer tissues could be correlated with cancer stage, metastatic potential, resistance to target therapy and patient outcome. The expression level of lncRNA could stratify cancer patients with target therapy. LncRNAs as therapeutic targets: anti-sense oligonucleotides-based strategies are under development. siRNAs targeting lncRNAs could be encapsulated in nanoparticles for improved tissue distribution and pharmacodynamics. LNAs could also be the cargo of nanoparticles. Without delivery vehicles, LNAs exhibits adequate half-life in serum and tolerable toxicology.
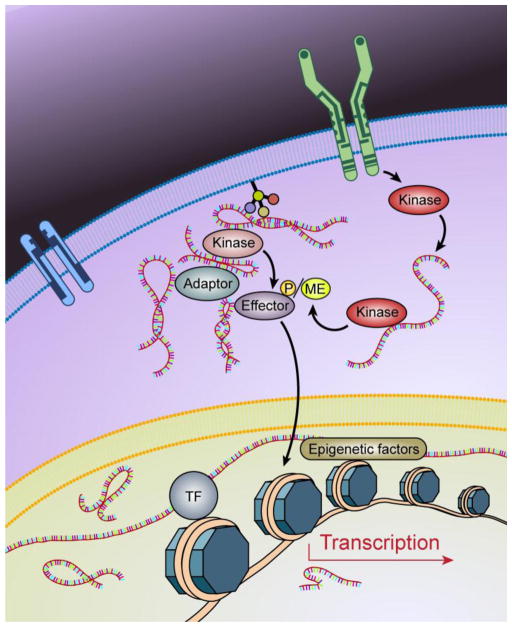
LncRNA derives from a number of sources including intergenic regions, the anti-sense strand of the protein coding sequence, intronic transcription, and alternative splicing (reviewed in [1, 2]). A considerable percentage of known lncRNAs either reside within the cytosol or shuttle between the nucleus and the cytoplasm [41]. Current studies indicate that these cytoplasmic lncRNAs play important functional roles in modulating messenger RNA translation and decay in a base-pairing dependent manner [42–44] or by competing with a protein or miRNA-mediated mRNA decoy [45]. In addition, cytoplasmic lncRNAs have been shown to regulate cytoplasmic protein trafficking from the cytosol to the nucleus for transcriptional activation [46]. Recent studies have also indicated that lncRNAs may associate with proteins, cellular lipids, and metabolic intermediates (Figure 2).
Figure 2. LncRNAs are key regulators of cancer signaling.
In response to extra- or intra-cellular signals, complicated signaling cascades are strictly regulated to achieve coordinate cellular activities. LncRNAs could be involved in all of the steps of signaling cascades: lncRNAs may associate with cellular receptors to modulate the hetero- and homo-dimers of the receptors; lncRNAs mediates the recruitment of kinases to receptors for activation/inactivation; lncRNAs intercedes second messenger-mediated signaling events; lncRNAs interacts with protein kinases to modulate the kinase-dependent post-translational modifications of effectors; lncRNAs facilitate the subcellular relocations of kinases/effectors, lncRNAs regulate epigenetics modifications and assemble/dissemble of transcriptional machinery.
Although still largely unexplored, it has been suggested that lncRNAs are essential mediators of intracellular signaling pathways. Here we describe emerging insights into the role of lncRNAs in regulating signaling pathways in cancer. New insights into the regulatory roles of lncRNAs in cancer for governing novel mechanisms and pathways by which cancer cells acquire their invasiveness and metastatic properties serves as the basis of a new approach in the fight against cancer. This understanding of lncRNAs in cancer signaling should stimulate new directions for future research endeavors and therapeutic options that focus on lncRNAs as novel cancer prognostic markers and therapeutic targets.
LncRNA directs the outcome of cell signaling pathways
In addressing the functional role of lncRNAs, one of the possible explanations is to identify the binding proteins of a given lncRNA (Box 1). Accumulating research has demonstrated that key signaling mediators, such as receptors, protein kinases and transcription factors, directly associate with lncRNAs and that their enzymatic activities are regulated by those lncRNAs (Figure 2). For example, Lnc-DC associates with STAT3 to prevent SHP1-dependent dephosphorylation of STAT3, which leads to dendritic cell differentiation [47]. NKILA, the lncRNA that associates with IkappaB (IκB), inhibits IKK-mediated IκB phosphorylation at Ser32 and Ser36, via direct association with the IκB N-terminus. Consequently, NF-kappa B (NF-kB) signaling is negatively regulated by NKILA to suppress cancer metastasis [46]. The lncRNA NBR2 (neighbor of BRCA1 gene 2) has been demonstrated to associate with AMPK upon energy stress and to promote the kinase activity of AMPK [48]. Cardiac and apoptosis-related lncRNA (Carlr) associates with p65 NF-kB in macrophages cells, and knockdown of Carlr impairs the expression of NF-kB target genes [49]. A PI3K p85 subunit-interacting lncRNA, AK023948, positively regulates the AKT pathway in breast cancer [50]. In addition, LINK-A directly associates with the non-receptor tyrosine kinase BRK and promotes the recruitment of BRK to the liganded receptor EGFR, leading to hyper-activation of the EGF/BRK/HIF signaling pathway [51]. Moreover, MAYA, via interaction with the scaffold protein LLGL2 and the methyltransferase NSUN6, forms an RNA-protein complex for methylation of MST1, a master regulator of the Hippo-YAP pathway [52].
Box 1. Biochemical Approaches to identify lncRNA-protein Interactions.
LncRNA Pulldown Combined with Mass Spectrometry
This is an unbiased and open-ended lncRNA pulldown assay for better understanding of the lncRNA-associated protein partners. Typically, the pulldown assay uses a RNA probe labeled with a high-affinity biotin tag which allows the probe to be recovered. After incubation, the biotinylated RNA probe can bind with a protein/protein complex in a cell lysate and then the complex is purified using magnetic streptavidin beads. The proteins are then eluted from the RNA and detected by Western blot or mass spectrometry. This assay has the advantages of enrichment of low-abundance protein targets, isolation of intact protein complexes, and being compatible with immunoblotting and mass spectrometry analysis. This methodology will drive the advances of studying lncRNA functions and the related mechanisms [18, 35, 51, 52, 129]
Capture Hybridization Analysis of RNA Targets (CHART)
This method has been used to identify RNA-bound DNA, RNA or protein partners, mainly focusing on the lncRNA molecules within nucleus [130, 131]. Briefly, the formaldehyde-cross-linked chromatin extracts will be subjected to target RNA capture using biotinylated anti-sense oligonucleotides. The DNA-RNA hybrids will be digested by RNase H and enriched by streptavidin beads. The CHART-enriched proteins could be subjected to mass spectrometry to identify proteins that associate with target RNA molecules [132].
Chromatin isolation by RNA purification (ChIRP)
Similar to the Chromatin IP (ChIP) assay and CHART assay, the ChIRP method is based on the biotinylated oligonucleotides to immunopreciate endogenous lncRNAs from cell or tissues to identify the lncRNA-associated DNA or proteins [133]. The probe design requires pre-screen and validation for maximum hybridization efficiency. Elutes from the ChIRP method using magnetic streptavidin beads could be subjected to mass spectrometry or immunoblotting for protein analysis [134].
RNA Antisense Purification with Mass Spectrometry (RAP-MS)
Similar to the CHART and ChIRP methods, RAP techniques applies anti-sense oligonucleotides as probe for RNA capture [135]. Distinct from the two previous methods, RAP method using 90mer oligonucleotides to create stable DNA-RNA hybrids; applies UV-crosslinking to capture transient RNA-protein interaction by introducing covalent bonds; purify RNA-protein complex by stringent denaturing condition to limit unspecific binding; and quantitative determine RNA-binding proteins using Stable isotope labeling with amino acids in cell culture (SILAC) [135]. The RAP-MS method has been used to identify RNA-associated proteins of nuclear lncRNA or mitochondria lncRNAs [80].
Crosslinking immunoprecipitation (CLIP)-coupled with high-throughput sequencing (HTS)
In brief, after UV cross-linking, RNA are labeled with 32P-γ-ATP and partially digested. The RNA-protein complex is immunoprecipitated using an antibody. The bound RNAs are ligated with an RNA linker (20 nucleotides) and the RNA-protein complex is subjected to SDS-PAGE and autoradiography. Under the +++ RNase condition, the bound RNAs are overdigested, which exhibits a ~7kD molecular weight shift (roughly the molecular weight of the RNA linker). Under the + RNase condition, the ~15–20kD molecular weight shift corresponds to the 26–41 nucleotide RNA fragments (minus the RNA linker). Therefore, the RNase over-digestion (+++ RNase condition) is considered the negative control, and the ~15–20kD molecular shift band is excised for the subsequent steps. The CLIP assay will yield a mixture of RNA fragments of 25–55 nucleotides, plus 2–20 nucleotide RNA linkers, which will be subjected to sequencing. HITS-CLIP has been used to demonstrate the binding RNA motif of a given protein [136].
In vitro RNA pulldown followed by dot-blot assay
The biotinylated RNA and recombinant proteins are incubated to promote binding. The bound RNA is subjected to partial RNase digestion (+ RNase condition), and the remaining bound RNA will be subjected to protease K digestion and RNA extraction. The RNA, in fragments, will be hybridized to a dot-blot. The dot-blot is a nylon membrane spotted with 54–60mer antisense DNA oligonucleotides tiling along the lncRNA targets. After stringent washing, the protein-bound RNA sequence is visualized by detection of Streptavidin-HRP signals. Depending on the different sequence motifs (regions) bound and protected by interested proteins, these RNA sequence motifs are hybridized to different positions on the dot-blot. Using this method, it has been demonstrated that two distinct positions, corresponding to nt. 235–288 and nt. 991–1044 of BCAR4, directly bind to SNIP1 and PNUTS respectively [35]. Similarly, two regions of LINK-A, nucleotides 481–540 and nucleotides 781–840, associate with the two domains of BRK at the SH3 domain and the C-terminal tail [51]. Another example is that the nucleotides 241–300 and 841–900 of MAYA are responsible for LLGL2 and NSUN6 binding respectively [52].
Recent observations indicate that certain lncRNAs associate with a variety of phospholipids [18]. Genome wide identification of lncRNAs in cell lipid fraction indicated that about 1.6% lncRNAs may associate with lipid directly or indirectly. By an open-ended screening, a cohort of lncRNAs, including XLOC-002384, SNHG6, SNHG9, RP11-383G10.5, and LINKC00607 exhibited particularly strong and specific interaction with Lysophosphatidic acid (LPA), Lysobisphosphatidic acids (LBPAs), Phosphatidic acid (PA), cardiolipin, and PE respectively, suggesting that lncRNAs may play important roles in regulating lipid metabolism, lipid signaling, mitochondrial function, cholesterol transportation, or even the formation of multivesicular bodies [53–56].
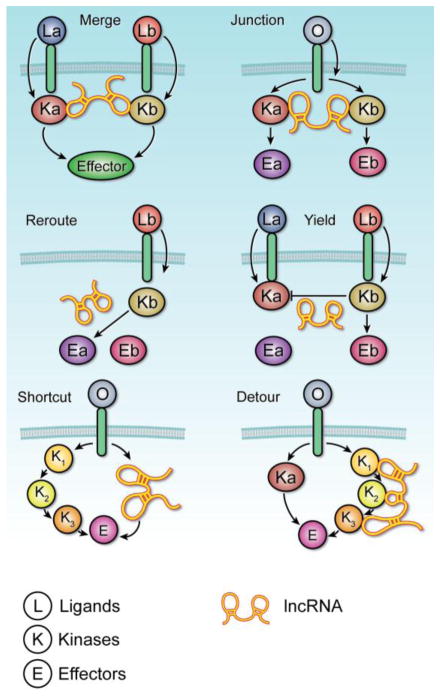
In summary, the proposed functional roles of lncRNAs in regulating signaling cascades and crosstalk between pathways can be classified into six categories (Figure 3): (i) the “Merge” category refers to when an lncRNA could conjoin the kinases of neighboring pathways, allowing the pathways to work in parallel to mediate a common cellular effect. Thus, the ligand of either receptor is able to activate the effector, accelerating the consequent cellular response; (ii) in the “Switch” group, lncRNA may couple one receptor to multiple kinases, creating a junction in the pathway. When the appropriate ligand binds to the receptor, the linked kinases are each able to activate their respective effectors in response to that one signal protein; (iii) In response to the cellular environment and specific ligand, a lncRNA could ‘reroute’ and form a bridge between a kinase and one of many possible effectors. In this scenario, one receptor and its associated kinase are able to achieve an array of cellular effects in accordance to the cellular context; (iv) lncRNAs may also be categorized by their ability to “yield.” In the situation of antagonistic pathways, a lncRNA is able to mediate interaction between two kinases so that only one pathway is activated at a given time. Therefore, activation of a pathway is blocked when the other is already activated; (v) lncRNAs can also be classified into a “shortcut” group. In the presence of the lncRNA, the conventional kinase cascade could be bypassed in favor of a shortcut that enables a more direct route to activating the downstream effector. Consequently, the lncRNA allows for more rapid induction of the desired cellular response; and (vi) lncRNAs may “detour.” In instances where the conventional pathway is disrupted or blocked, lncRNAs may facilitate the formation of an alternative pathway by way of recruiting substitute pathway components or mediators. In response to poisons or inhibitors, a cellular effect may still be achieved by the more circuitous route enabled by the lncRNA.
Figure 3. LncRNAs direct the outcome of a signaling network.
The functional role of lncRNAs in regulating cancer signaling can be classified as following. Merge – The lncRNA conjoins the kinases of neighboring pathways, allowing the pathways to work in parallel to mediate a common cellular effect. Thus, the ligand of either receptor is able to activate the effector, accelerating the consequent cellular response. Switch – The lncRNA couples one receptor to multiple kinases, creating a junction in the pathway. When the appropriate ligand binds to the receptor, the linked kinases are each able to activate their respective effectors in response to that one signal protein. Reroute – In response to the cellular environment and specific ligand, the lncRNA will form a bridge between the kinase and one of many possible effectors. In this scenario, one receptor and its associated kinase are able to achieve an array of cellular effects in accordance to the cellular context. Yield – In the situation of antagonistic pathways, the lncRNA is able to mediate interaction between two kinases so that only one pathway is activated at a given time. Effectively, activation of a pathway is blocked when the other is already activated. Shortcut – In the presence of the lncRNA, the conventional kinase cascade is bypassed in favor of a shortcut that enables a more direct route to activating the downstream effector. Consequently, the lncRNA allows for more rapid induction of the desired cellular response. Detour – In instances where the conventional pathway is disrupted or blocked, lncRNAs can facilitate the formation of an alternative pathway by way of recruiting substitute pathway components or mediators. In response to poisons or inhibitors, a cellular effect may still be achieved by the more circuitous route enabled by the lncRNA.
LncRNA-protein interactions: the building blocks of cancer signaling cascades
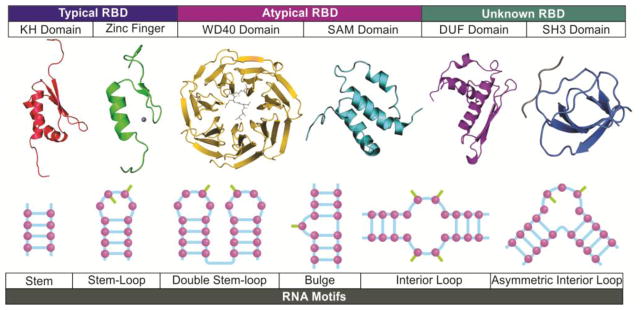
The interactions between lncRNAs and binding proteins act as one of the major mechanisms for lncRNA-mediated signaling pathways. In addition to typical RNA binding domains, atypical RNA binding domains are less recognized, but they play important roles in mediating lncRNA-protein interaction. Indeed, recent evidence has indicated that classical RNA-binding domains, non-classical RNA binding domains and domains with unknown RNA-binding domains all play critical roles in mediating RNA-protein interactions in given signaling pathways (Figure 4).
Figure 4. The protein domains and RNA motifs involved in lnc-RNA-protein interactions.
In addition to typical RNA binding domains (RBD), atypical RBDs and domain with unknown RNA binding mediate the interactions between lncRNAs and the key mediators of cancer signaling pathways. Non-canonical RNA-binding domains, such as the WD40 and SAM domains, are required for the RNA-protein complex formation upon ligand binding. Domains with unknown RNA-binding properties, including the DUF and SH3 domains, facilitate the recruitment of SNIP1 to lncRNA BCAR4 and LINK-A-BRK interaction. The RNA binding domains may recognize nucleotide sequences or structures of RNA motifs, resulting in specific lncRNA-protein interactions. The single-stranded lncRNA molecule forms complex secondary and 3-dimentional structures. Stem, base-paring formed between two complimentary strands of the RNA molecule. The double helices are essential for RNA folding, and also provide docking sites for sequence-mediated interaction. Stem-loop, the RNA sequence forms a complimentary base-paring with an unpaired loop. The nitrogenous bases within the single-stranded loop could face inside or outside of the loop to form additional hydrogen bonds with binding partners. Double stem-loop: two or more stem-loop structures side by side. The nitrogenous bases from both loops may coordinate the lncRNA-protein interaction. Bulge: the unpaired residues flanking with base-pairs may be packed toward the helix or extrude to outside of the stem, which are all important for protein binding. Symmetric and asymmetric interior loops: the structure of separate RNA strands flanked with stacking base-pairs can be found in the middle of RNA strands. The loops provide free nitrogenous bases for stacking interaction or hydrophobic interactions.
Open-ended proteomic studies using oligo-dT capture to identify the interactome of mRNAs and potentially polyadenylated non-coding RNAs have indicated that classical RNA-binding domains, non-classical RNA binding domains and domains with unknown RNA-binding all exhibit RNA-binding possibility [57]. Proteins harbor non-canonical RNA-binding domains, such as the zinc finger, WD40 and SAM domains, which all have demonstrable RNA-binding [57]. Structural analyses have demonstrated that WD40 repeats may form an RNA-binding surface that can interact with snRNAs via base-stacking interactions, which is required for the ribonucleic particle biogenesis [58, 59]. In cancer signaling pathways, the WD40 domains of LLGL2 mediate LLGL2-MAYA interaction, which is required for the RNA-protein complex formation [52]. Another example of WD40 domain mediation of RNA-protein interaction is LRRK2, in which the WD40 domain of this protein serine/threonine kinase is required for the association between LRRK2 and LINK-A [51].
Domains with unknown RNA-binding properties, including the DUF and SH3 domains, have also been suggested to be involved in RNA binding [57]. The DUF domain of Dicer has been shown to interact with single-stranded or double-stranded RNA [60, 61]. Characterization of the DUF domain of SNIP1 has revealed that it is essential for interaction with the lncRNA BCAR4 [35]. The SH3 domain of BRK contributes to the BRK-LINK-A interaction and the consequent hyperactivation of BRK [51]. Evidence has also been reported that the kinase domain of protein kinases may be involved in RNA-kinase interactions. Characterization of the NBR2-AMPK interaction indicated that deletion of the C-terminal half of AMPK abolished the RNA-protein interaction [48]. Global characterization of unknown RNA binding domains indicated that unstructured domains exhibited potential RNA-binding, suggesting the diversity of lncRNA-protein interactions [62].
Unlike proteins, which contain domain structures that can provide pilot information on the potential function, cellular location, and regulation involved in RNA-protein interaction, RNA motifs involved in RNA-protein interaction are more challenging to define. Using cross-linking based technologies (Box 1), the RNA motifs responsible for protein binding could be identified. It has been demonstrated that two distinct positions, corresponding to nt. 235–288 and nt. 991–1044 of BCAR4, directly bind to SNIP1 and PNUTS respectively [35]. Similarly, two regions of LINK-A, nucleotides 481–540 and nucleotides 781–840, associate with the two domains of BRK at the SH3 domain and the C-terminal tail [51]. Another example is that the nucleotides 241–300 and 841–900 of MAYA are responsible for LLGL2 and NSUN6 binding respectively [52]. The requisition of the identified RNA motifs can be validated by deletion mutants and CRISPR/cas9-mediated genomic editing [18]. Using CRISPR/cas9 technology, the specific RNA motif could be engineered without affecting the flanking sequence and unlikely to affect the intrinsic expression status of the lncRNAs, which provide advantages in studying the cellular consequence of lncRNAs. The secondary and 3-dimensional structures of RNAs are likely to play important roles in RNA-protein interaction (Figure 4). Within our limited knowledge, the nucleotides located in the loop region of the RNA motif are critical for RNA-protein binding, and the hairpin loop is one of the more common structures found in RNA molecules [63]. Crystallographic dissection of RNA-protein interactions is urgently needed to pinpoint the underlying molecular mechanisms.
LncRNAs directly associate with phospholipids and other small molecules
The observations that RNA association with the cell membrane is involved in the formation of the Escherichia coli signal recognition particle [64] and regulation of Saccharomyces cerevisiae cell membrane permeability [65] support the notion that RNA-lipid interactions are important to life. The association of polynucleotides with charged lipid membranes, including phosphatidylcholines (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS), have been observed in the cell nucleus [66] and RNA virus [67]. Biophysical studies have suggested that the phospholipid bilayer can absorb single-stranded DNA and RNA [68, 69]. Membranes composed of cationic or zwitterionic phospholipids with bivalent cations associate with RNA [68, 70]. It is intriguing that lncRNAs have been shown to interact with phospholipids with strong binding affinity, which are comparable to lipid-binding proteins [18, 71–73]. However, RNA-lipid interactions in their appropriate biological context need to be further demonstrated.
RNA nucleotides contain a negatively charged backbone and nitrogenous bases that possess a weak positive charge. The interaction between RNAs and phospholipids may be explained by the 3-dimensional structure of RNA. Structural analysis has shown that the loop structures of RNA molecules may be important for such binding [74]. It has been indicated that an 18 nucleotide stem-loop structure is required for LINK-A-PIP3 interaction. Deletion of the loop region, or mutations of either 4′C to A or 6′C to A abolished RNA-PIP3 interaction [18]. It is likely that the loop formed by the RNA molecules arranges the negatively charged backbone into a circle. It is possible that the cytosine bases at the 4′ and 6′ positions are arranged toward the center of the loop, allowing for potential hydrogen bonding with the three phospho-groups of PIP3. In addition to this particular stem-loop structure, other parts of LINK-A may also contribute to the LINK-A-PIP3 interaction. Ultimately, to understand the 3-dimensional interaction between LINK-A and PIP3 will require structural analysis using crystallography or nuclear magnetic resonance (NMR).
In addition to macromolecules, RNAs have been demonstrated to associate with small molecules, including mental complexes, amino acids, and other organic or inorganic complexes [75]. Structural studies indicate that RNA molecules associates with arginine, which triggers a conformational change in the secondary structure of the RNA [76]. Hence, RNA molecules can likely serve as sensors for the regulation of metabolic pathways.
LncRNAs modulate enzyme activity
The functional roles of lncRNA-protein interactions are diverse. One of the surprising, yet frequent, observations is that once a protein binds with an lncRNA, the enzymatic activity of the binding protein may be modulated. For instance, the lncRNA NBR2 has been indicated as an AMPK activator upon energy stress [48]. It has been demonstrated that lncRNAs can modulate the activity of various enzymes (e.g., kinases) in the following ways (Figure 5).
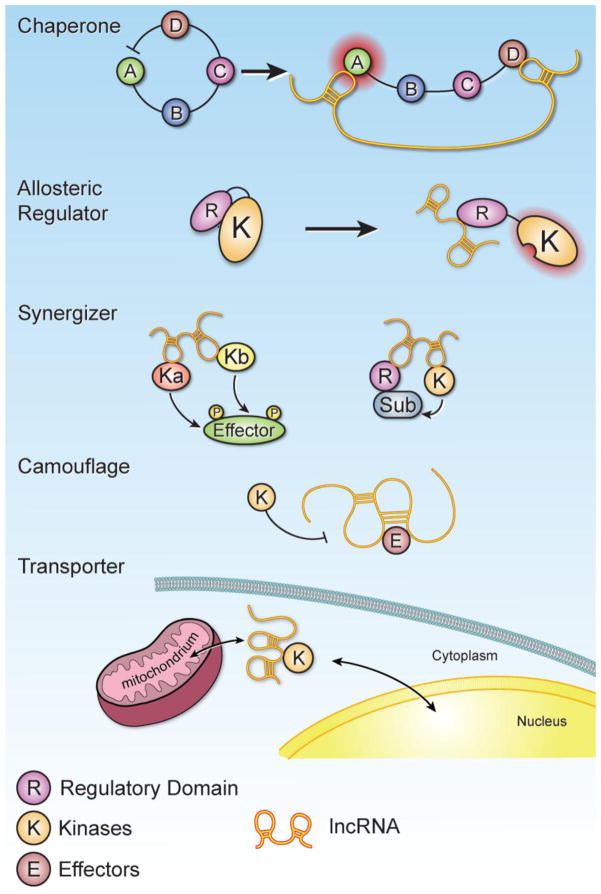
Figure 5. LncRNA governs the enzymatic activities of protein kinases in signaling circuitry.
Upon lncRNA-kinase interaction, the enzymatic activities of protein kinase could be modulated. Chaperones – The lncRNA interaction with subunits of an enzyme can induce a conformational change, altering its enzymatic activity. A–D illustrate the domains of the protein kinases, by which the inhibitory effect of domain D is repressed upon lncRNA binding, leading to enzymatic activation of domain A. Allosteric Regulators – When the lncRNA binds to the allosteric site of the enzyme, modifying the conformation of the regulatory domain (R) and kinase domain (K), the enzyme could be either inhibited or activated. Synergizers – The lncRNA conjoins multiple kinases and/or regulators together, mediating cooperative action on its effectors or substrates. Left panel illustrate that two kinases are orchestrated by lncRNA phosphorylation of effector. Right panel illustrate the scenario that the regulatory unit (R) and kinase (K) are bridged by lncRNAs as a mega complex for substrate modification. Camouflage – The presence of the lncRNA disguises the surface of target proteins, preventing the reorganization and post-translational modification of target proteins by upstream signaling events. Transporter – the presence of the lncRNA facilities the relocation of binding proteins from cytosol to mitochondria or shuttling between cytosol, nucleus and other subcellular compartments.
LncRNAs can modulate the activity of various enzymes (e.g., kinases) by acting as “Chaperones,” whereby lncRNA interacts with subunits of an enzyme and can induce a conformational change, altering its enzymatic activity. BRK is negatively regulated by its C-terminus, which harbors a phosphorylated tyrosine [77]. Protease digestion assay suggest a potential conformational change in BRK upon LINK-A binding on both the SH3 domain and C-terminus of BRK, which attenuates its self-inhibition, leading to enhanced enzymatic activity of BRK [51].
LncRNAs can modulate enzyme activity by acting as “Allosteric Regulators,” whereby lncRNA binds to the allosteric site of the enzyme, modifying the conformation of the active site. One example is that the PH domain of AKT serves as docking site to associate with PIP3, which modulate the kinase activity of AKT [78]. The presence of LINK-A enhances the interaction between PH domain and PIP3, leading to hyper-activation of AKT [18]. Another example is that long non-coding RNAs that are upregulated in breast cancer brain metastasis associates with the negative regulatory domain of JAK2, leading to alleviation of the inhibitory role of JH2 domain, and enzymatic activation [79].
LncRNAs may act as “Synergizers”, by which lncRNA conjoins multiple enzymes and/or regulators together, mediating cooperative action on its effectors or substrates. Recent studies indicate that the lncRNA AK023948 associates with both DHX9 and p85 and that the presence of AK023948 is required for the DHX9-p85 interaction, consequently resulting in enhanced PI3K activity and AKT phosphorylation [50]. Another example is that upon ligand binding, the signaling events trigger RNA-protein complex formation containing LLGL2-MAYA-NSUN6. LLGL2 recruits the substrate MST1 to the complex; NSUN6 acts as the methyltransferase; and MAYA, the lncRNA, serves as a scaffold that bridges the interaction between LLGL2 and NSUN6 upon extracellular stimulation, leading to MST1 methylation [52].
Alternatively, the lncRNA can also “Camouflage” or disguise the surface of target proteins, preventing the reorganization and post-translational modification of target proteins by upstream signaling events. Lnc-DC interacts with STAT3 in the cytoplasm, preventing SHP1-dependent dephosphorylation of STAT3 (Tyr705). These events facilitate the differentiation of dendritic cells [47]. NKILA is upregulated in breast cancer and associates with IkB, preventing IKK-dependent IkB phosphorylation at Ser32/36 [46]. Hence, it is clear that signaling pathways in cancer and other cellular activities are tightly regulated by lncRNAs.
The diverse roles of lncRNAs may also contribute to the relocalization of binding proteins as “Transporters”. Recent studies indicate that the lncRNA SAMMSON associates with p32, a mitochondria surface protein. The SAMMSON-p32 interaction facilitates relocalization of p32 to mitochondria, resulting in enhanced mitochondrial metabolism [80, 81]. The relocation of the lncRNA component of the RNA processing endoribonuclease (RMRP) from the nucleus to the mitochondria facilitates the accumulation of RMRP in the mitochondria, which is required for mitochondrial respiration [82]. Hence, lncRNA may communicate between nucleus and mitochondria for fine-tuning the cellular and mitochondrial activities [81].
Targeting lncRNAs in cancer therapy
From a clinical perspective, lncRNA serves as promising therapeutic targets. Multiple therapeutic strategies have been developed to target lncRNAs. Anti-sense oligonucleotide (ASO)-based strategies that downregulate the transcripts of lncRNAs via RNase H-dependent degradation are under active investigation (recently reviewed by Matsui and Corey [83]). Alternatively, liposome/nanoparticle delivered siRNAs have been developed to knockdown lncRNAs in vivo via Dicer- and Agonaute-depednent RNA silencing [84–86], which have been evaluated in xenograft models and have been found to inhibit tumorigenesis and long-distance metastasis [85, 87]. Small molecule inhibitors to block lncRNA-protein interactions or interfere with lncRNA-protein complexes formation are also on the rise [83]. Cancers frequently become resistant to administered chemotherapeutic agents. In these chemotherapy-resistant tumors, dysregulated lncRNAs can contribute significantly to the development of this resistance. Given the failure of certain pathway inhibitors during clinical trials, combinations of pathway-specific inhibitors integrated with a lncRNA-directed strategy may provide maximum efficacy in treating human cancer, which is under active investigation. Targeting lncRNAs using a variety of technologies, including anti-sense oligonucleotides-based strategies, siRNAs and small molecular inhibitors should be evaluated for their effects on cancer initiation, progression, metastasis and response to therapy.
Anti-sense oligonucleotide
Anti-sense oligonucleotide (ASO), including ASO gapmers [88], duplex RNA [89] and locked nucleic acids (LNAs) [90] binds to lncRNA transcripts via base-pairing. The RNA-DNA duplex triggers RNase-H-dependent cleavage [91]. The newer generation of ASOs incorporates chemical modification of the sugar backbone to improve binding affinity and in vivo stability [92, 93]. The Locked nucleic acids (LNAs) [90, 94, 95] and S-constrained ethyl (cEt) modifications [96] have been advanced to clinical trials [97–99]. LNAs are synthesized using modified RNA nucleotides, which contain an extra covalent bond between the 2′-O and 4′-C of the ribofuranose ring [100, 101]. The mixed LNA-DNA-LNA gapmers can base-pair with RNA targets, which can be used to silence RNA targets in cell line-based experiments and animal models. The incorporation of bridged nucleic acid (BNA) monomers can serve a similar purpose as LNAs [102]. The application of ASOs to knockdown lncRNAs in vivo has been tested in a variety of cancer models, with noteworthy inhibitory effects on tumor growth and progression [103, 104]. LNAs targeting PVT1 have been shown to sensitize ovarian cancer cells to cisplatin, substantiating the effectiveness of combinatorial treatment [105]. Additionally, lncARSR promotes cancer cell resistance to Sunitinib, but cancer cell sensitivity to Sunitinib could be improved by targeting lncARSR using LNAs [106]. For lncRNAs that act as tumor suppressors, LNAs mimicking GAS5 binding sequence to the hormone receptors is sufficient to induce cancer cell apoptosis [107]. Clinical trial using LNAs targeting Bcl-2 oncoprotein [97], HIF1α (NCT00466583), and AR [108] have shown promise and are under evaluation. Beyond its uses in cancer, LNAs have also been proposed to benefit patients with cardiovascular diseases [109], kidney diseases [110], neuronal disorders [111], and other human pathological conditions.
Nanoparticle-delivered siRNAs
Targeting lncRNAs using a siRNA-based strategy has been successfully applied in several preclinical models [112]. Recently, dioleoyl phosphatidylcholine (DOPC) based nanoliposomes have been developed for the delivery of nucleotide based-therapeutics (siRNA, microRNA, lncRNA and antisense oligos, etc.) for in vivo and clinical use [113–121]. Studies indicate that a single injection of DOPC-nanoliposomal siRNAs can inhibit the expression of target proteins for around 3–5 days in mouse tumors [119, 121]. DOPC nanoliposomes, with an average size of 50 nm, can be administered via a single intravenous or intraperitoneal injection to deliver select siRNAs and anti-miRs into tumor cells in vivo. This single administration produces a significant repression in the expression levels of the gene targets (e.g., Bcl2, eEF2K, FoxM1 and Kras or miRs155, mıR34a, and JAK2) and in tumor size in mouse models and preclinical models of human cancer, including subcutaneous xenografts and orthotopic tumor models [79, 113–115, 117, 119, 121–123].
Small molecule inhibitors of lncRNAs
LncRNAs form complicated tertiary structures. Whether the secondary or 3-dimentional structure of lncRNAs molecules are conserved between species remain elusive [124]. One explanation is that a portion of the secondary or tertiary structures of lncRNA molecules, for example, key stem-loop structures for protein binding, are conserved; similar to the protein domain conservation that linker regions are variable. RNA molecules are potential targets for small molecule inhibitors. High-throughput screening has been employed to identify small molecule compounds that could potentially inhibit RNAs [125–127]. Efforts to establish platforms to aid the design and identification of small molecule inhibitors for oncogenic non-coding RNAs are under development [128], which will facilitate the large scale development of pharmaceutical agents that target lncRNAs.
Concluding Remarks
The interwoven signaling pathways present in many human cancer types complicate the development of targeted therapies or chemotherapeutic agents. LncRNAs play a pivotal role in mediating the crosstalk between the various cellular components, including proteins, RNAs, and lipids, that are involved in cancer cell signal transduction. Due to the prevalence of lncRNA involvement in cancer pathways, numerous studies have elucidated the importance of lncRNAs in various disease processes of cancer, including initiation, progression, invasion, and metastasis. Identification of the specific lncRNAs that function in various human cancer types has led to pioneering efforts to develop lncRNA-based clinical applications such as biomarkers for diagnosis, prognostic indicators, stratification markers, drug sensitizers, and therapeutic targets. The lncRNA profile of each human cancer type should be systematically investigated to improve clinical outcomes for cancer patients by engendering a personalized approach to medicine.
Future studies on the regulatory and biological roles of lncRNAs in cancer signaling will define the future of the field (see Outstanding Questions). Although a huge list of lncRNAs have been identified thus far, it has been a strenuous task to demonstrate the functional relevance of lncRNAs in cancer. To answer this problem, thorough examinations of lncRNA candidates involved in cancer signaling pathway need to be conducted to reveal the physiological relevance of lncRNAs in cell apoptosis, survival, metastasis, and metabolism. Cellular and xenograft models have been the common means of studying the roles that lncRNAs play in cancer and are useful tools in cursory evaluations of their functions. However, conclusions that are more definitive will require representative in vivo models of cancer, such as genetic models that better recapitulate the tumor microenvironment. It will be crucial to determine if tissue-specific expression of lncRNAs, by genetic knock-in, can induce spontaneous tumor formation, which can then be blocked by targeting the lncRNAs.
Outstanding Questions.
Can lncRNAs serve as regulatory components of cancer signaling pathways?
Do lncRNAs have bona fide biological functional roles in cancer?
Can effective therapeutic strategies be developed by targeting either lncRNAs alone or in combination with chemotherapeutic and targeted therapy agents?
Highlights.
Trends Box
Somatic mutations or disease-associated SNPs of lncRNAs implicate lncRNAs as cancer risk genes
Through RNA-protein, RNA-RNA, RNA-lipid interactions, lncRNAs modulate cancer signaling pathways and consequences
Dysregulation of lncRNAs lead to hyper- or hypo-activation of cellular pathways, leading to resistance to current targeted therapies
Targeting lncRNA alone or a co-targeting strategy may help overcome the targeted therapy resistance
Acknowledgments
We apologize to colleagues whose work was not able to be included in this review due to space limitations. We thank Mr. D. Aten for assistance with figure presentation. We are grateful to Mr. Peter K. Park and Sergey Egranov for assistance with manuscript drafting. Research in the C. L. and L. Y. laboratories are funded by NIH R00 award (R00DK094981), R01 award (1R01 CA218025-01), and CPRIT (150094) to C.Lin., and the NIH R00 award (R00CA166527), R01 award (1R01 CA218036-01), CPRIT award (R1218), and DOD breakthrough (BC151465) grants to L.Y.
Footnotes
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.St Laurent G, et al. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31(5):239–51. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eddy SR. Non-coding RNA genes and the modern RNA world. Nat Rev Genet. 2001;2(12):919–29. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- 3.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 5.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 6.Ye N, et al. Functional roles of long non-coding RNA in human breast cancer. Asian Pac J Cancer Prev. 2014;15(15):5993–7. doi: 10.7314/apjcp.2014.15.15.5993. [DOI] [PubMed] [Google Scholar]
- 7.Meng J, et al. A four-long non-coding RNA signature in predicting breast cancer survival. J Exp Clin Cancer Res. 2014;33:84. doi: 10.1186/s13046-014-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19(R2):R152–61. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrow J, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22(9):1760–74. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, et al. Genome-wide analysis of human SNPs at long intergenic noncoding RNAs. Hum Mutat. 2013;34(2):338–44. doi: 10.1002/humu.22239. [DOI] [PubMed] [Google Scholar]
- 11.Melton C, et al. Recurrent somatic mutations in regulatory regions of human cancer genomes. Nat Genet. 2015;47(7):710–6. doi: 10.1038/ng.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheetham SW, et al. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108(12):2419–25. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khurana E, et al. Role of non-coding sequence variants in cancer. Nat Rev Genet. 2016;17(2):93–108. doi: 10.1038/nrg.2015.17. [DOI] [PubMed] [Google Scholar]
- 15.Guo H, et al. Modulation of long noncoding RNAs by risk SNPs underlying genetic predispositions to prostate cancer. Nat Genet. 2016;48(10):1142–50. doi: 10.1038/ng.3637. [DOI] [PubMed] [Google Scholar]
- 16.Jin G, et al. Human polymorphisms at long non-coding RNAs (lncRNAs) and association with prostate cancer risk. Carcinogenesis. 2011;32(11):1655–9. doi: 10.1093/carcin/bgr187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan H, et al. A Novel Genetic Variant in Long Non-coding RNA Gene NEXN-AS1 is Associated with Risk of Lung Cancer. Sci Rep. 2016;6:34234. doi: 10.1038/srep34234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin A, et al. The LINK-A lncRNA interacts with PtdIns(3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat Cell Biol. 2017;19(3):238–251. doi: 10.1038/ncb3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu T, et al. Association between SNPs in Long Non-coding RNAs and the Risk of Female Breast Cancer in a Chinese Population. J Cancer. 2017;8(7):1162–1169. doi: 10.7150/jca.18055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Z, et al. Genetic Polymorphisms in Long Noncoding RNA H19 Are Associated With Susceptibility to Breast Cancer in Chinese Population. Medicine (Baltimore) 2016;95(7):e2771. [Google Scholar]
- 21.Zhao X, et al. The rs6983267 SNP and long non-coding RNA CARLo-5 are associated with endometrial carcinoma. Environ Mol Mutagen. 2016;57(7):508–15. doi: 10.1002/em.22031. [DOI] [PubMed] [Google Scholar]
- 22.Jendrzejewski J, et al. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci U S A. 2012;109(22):8646–51. doi: 10.1073/pnas.1205654109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, et al. Polymorphisms in Long Noncoding RNA H19 Contribute to the Protective Effects of Coal Workers’ Pneumoconiosis in a Chinese Population. Int J Environ Res Public Health. 2016;13(9) doi: 10.3390/ijerph13090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millis MP, et al. Variants in the plasmacytoma variant translocation gene (PVT1) are associated with end-stage renal disease attributed to type 1 diabetes. Diabetes. 2007;56(12):3027–32. doi: 10.2337/db07-0675. [DOI] [PubMed] [Google Scholar]
- 25.Ingle JN, et al. Genetic Polymorphisms in the Long Noncoding RNA MIR2052HG Offer a Pharmacogenomic Basis for the Response of Breast Cancer Patients to Aromatase Inhibitor Therapy. Cancer Res. 2016;76(23):7012–7023. doi: 10.1158/0008-5472.CAN-16-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majidinia M, Yousefi B. Long non-coding RNAs in cancer drug resistance development. DNA Repair (Amst) 2016;45:25–33. doi: 10.1016/j.dnarep.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Pan JJ, et al. Long Non-coding RNAs and Drug Resistance. Asian Pac J Cancer Prev. 2015;16(18):8067–73. doi: 10.7314/apjcp.2015.16.18.8067. [DOI] [PubMed] [Google Scholar]
- 28.Askarian-Amiri ME, et al. The Regulatory Role of Long Noncoding RNAs in Cancer Drug Resistance. Methods Mol Biol. 2016;1395:207–27. doi: 10.1007/978-1-4939-3347-1_12. [DOI] [PubMed] [Google Scholar]
- 29.Chen QN, et al. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget. 2017;8(1):1925–1936. doi: 10.18632/oncotarget.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang CL, et al. A long non-coding RNA contributes to doxorubicin resistance of osteosarcoma. Tumour Biol. 2016;37(2):2737–48. doi: 10.1007/s13277-015-4130-7. [DOI] [PubMed] [Google Scholar]
- 31.Tsang WP, Kwok TT. Riboregulator H19 induction of MDR1-associated drug resistance in human hepatocellular carcinoma cells. Oncogene. 2007;26(33):4877–81. doi: 10.1038/sj.onc.1210266. [DOI] [PubMed] [Google Scholar]
- 32.Tsang WP, et al. Induction of drug resistance and transformation in human cancer cells by the noncoding RNA CUDR. RNA. 2007;13(6):890–8. doi: 10.1261/rna.359007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou Z, et al. Long noncoding RNAs expression patterns associated with chemo response to cisplatin based chemotherapy in lung squamous cell carcinoma patients. PLoS One. 2014;9(9):e108133. doi: 10.1371/journal.pone.0108133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijer D, et al. Functional screen for genes responsible for tamoxifen resistance in human breast cancer cells. Mol Cancer Res. 2006;4(6):379–86. doi: 10.1158/1541-7786.MCR-05-0156. [DOI] [PubMed] [Google Scholar]
- 35.Xing Z, et al. lncRNA Directs Cooperative Epigenetic Regulation Downstream of Chemokine Signals. Cell. 2014;159(5):1110–25. doi: 10.1016/j.cell.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue X, et al. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene. 2016;35(21):2746–55. doi: 10.1038/onc.2015.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silveira RA, et al. Protein-coding genes and long noncoding RNAs are differentially expressed in dasatinib-treated chronic myeloid leukemia patients with resistance to imatinib. Hematology. 2014;19(1):31–41. doi: 10.1179/1607845413Y.0000000094. [DOI] [PubMed] [Google Scholar]
- 38.Hung T, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43(7):621–9. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, et al. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34(17):3182–93. doi: 10.1128/MCB.01580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014;281(7):1750–8. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 41.Rashid F, et al. Long Non-coding RNAs in the Cytoplasm. Genomics Proteomics Bioinformatics. 2016;14(2):73–80. doi: 10.1016/j.gpb.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YK, et al. Staufen1 regulates diverse classes of mammalian transcripts. EMBO J. 2007;26(11):2670–81. doi: 10.1038/sj.emboj.7601712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature. 2011;470(7333):284–8. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beltran M, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008;22(6):756–69. doi: 10.1101/gad.455708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cesana M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–69. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, et al. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27(3):370–81. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Wang P, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344(6181):310–3. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat Cell Biol. 2016;18(4):431–42. doi: 10.1038/ncb3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castellanos-Rubio A, et al. Cytoplasmic Form of Carlr lncRNA Facilitates Inflammatory Gene Expression upon NF-kappaB Activation. J Immunol. 2017;199(2):581–588. doi: 10.4049/jimmunol.1700023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koirala P, et al. LncRNA AK023948 is a positive regulator of AKT. Nat Commun. 2017;8:14422. doi: 10.1038/ncomms14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin A, et al. The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18(2):213–24. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, et al. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19(2):106–119. doi: 10.1038/ncb3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin ME, et al. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91(3–4):130–8. doi: 10.1016/j.prostaglandins.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zechner R, et al. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15(3):279–91. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paradies G, et al. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta. 2014;1837(4):408–17. doi: 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Patel D, Witt SN. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxid Med Cell Longev. 2017;2017:4829180. doi: 10.1155/2017/4829180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castello A, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149(6):1393–406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 58.Jin W, et al. Structural basis for snRNA recognition by the double-WD40 repeat domain of Gemin5. Genes Dev. 2016;30(21):2391–2403. doi: 10.1101/gad.291377.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertram K, et al. Cryo-EM structure of a human spliceosome activated for step 2 of splicing. Nature. 2017;542(7641):318–323. doi: 10.1038/nature21079. [DOI] [PubMed] [Google Scholar]
- 60.Dlakic M. DUF283 domain of Dicer proteins has a double-stranded RNA-binding fold. Bioinformatics. 2006;22(22):2711–4. doi: 10.1093/bioinformatics/btl468. [DOI] [PubMed] [Google Scholar]
- 61.Kurzynska-Kokorniak A, et al. Revealing a new activity of the human Dicer DUF283 domain in vitro. Sci Rep. 2016;6:23989. doi: 10.1038/srep23989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castello A, et al. Comprehensive Identification of RNA-Binding Domains in Human Cells. Mol Cell. 2016;63(4):696–710. doi: 10.1016/j.molcel.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Svoboda P, Di Cara A. Hairpin RNA: a secondary structure of primary importance. Cell Mol Life Sci. 2006;63(7–8):901–8. doi: 10.1007/s00018-005-5558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batey RT, et al. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science. 2000;287(5456):1232–9. doi: 10.1126/science.287.5456.1232. [DOI] [PubMed] [Google Scholar]
- 65.MacIntosh GC, et al. Characterization of Rny1, the Saccharomyces cerevisiae member of the T2 RNase family of RNases: unexpected functions for ancient enzymes? Proc Natl Acad Sci U S A. 2001;98(3):1018–23. doi: 10.1073/pnas.98.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu D, Rhodes DG. Binding of phosphorothioate oligonucleotides to zwitterionic liposomes. Biochim Biophys Acta. 2002;1563(1–2):45–52. doi: 10.1016/s0005-2736(02)00384-x. [DOI] [PubMed] [Google Scholar]
- 67.Tuma R, et al. Structure, Interactions and Dynamics ofPRD1Virus II. Organization of the Viral Membrane and DNA. Journal of Molecular Biology. 1996;257(1):102–115. doi: 10.1006/jmbi.1996.0150. [DOI] [PubMed] [Google Scholar]
- 68.Kõiv A, et al. Differential scanning calorimetry study on the binding of nucleic acids to dimyristoylphosphatidylcholine-sphingosine liposomes. Chemistry and Physics of Lipids. 1994;70(1):1–10. doi: 10.1016/0009-3084(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 69.Michanek A, et al. RNA and DNA interactions with zwitterionic and charged lipid membranes—A DSC and QCM-D study. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2010;1798(4):829–838. doi: 10.1016/j.bbamem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Khvorova A, et al. RNAs that bind and change the permeability of phospholipid membranes. Proc Natl Acad Sci U S A. 1999;96(19):10649–54. doi: 10.1073/pnas.96.19.10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frech M, et al. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272(13):8474–81. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 72.Ching TT, et al. Specific binding of the C-terminal Src homology 2 domain of the p85alpha subunit of phosphoinositide 3-kinase to phosphatidylinositol 3,4,5-trisphosphate. Localization and engineering of the phosphoinositide-binding motif. J Biol Chem. 2001;276(47):43932–8. doi: 10.1074/jbc.M105159200. [DOI] [PubMed] [Google Scholar]
- 73.Yates LA, et al. Structural and functional characterization of the kindlin-1 pleckstrin homology domain. J Biol Chem. 2012;287(52):43246–61. doi: 10.1074/jbc.M112.422089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Childs-Disney JL, Disney MD. Approaches to Validate and Manipulate RNA Targets with Small Molecules in Cells. Annu Rev Pharmacol Toxicol. 2016;56:123–40. [Google Scholar]
- 75.Chow CS, Bogdan FM. A Structural Basis for RNAminus signLigand Interactions. Chem Rev. 1997;97(5):1489–1514. doi: 10.1021/cr960415w. [DOI] [PubMed] [Google Scholar]
- 76.Puglisi JD, et al. Conformation of the TAR RNA-arginine complex by NMR spectroscopy. Science. 1992;257(5066):76–80. doi: 10.1126/science.1621097. [DOI] [PubMed] [Google Scholar]
- 77.Qiu H, Miller WT. Regulation of the nonreceptor tyrosine kinase Brk by autophosphorylation and by autoinhibition. J Biol Chem. 2002;277(37):34634–41. doi: 10.1074/jbc.M203877200. [DOI] [PubMed] [Google Scholar]
- 78.Bellacosa A, et al. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17(3):313–25. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 79.Wang S, et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J Clin Invest. 2017 doi: 10.1172/JCI91553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leucci E, et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature. 2016;531(7595):518–22. doi: 10.1038/nature17161. [DOI] [PubMed] [Google Scholar]
- 81.Vendramin R, et al. Non-coding RNAs: the dark side of nuclear-mitochondrial communication. EMBO J. 2017;36(9):1123–1133. doi: 10.15252/embj.201695546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noh JH, et al. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev. 2016;30(10):1224–39. doi: 10.1101/gad.276022.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16(3):167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buyens K, et al. Liposome based systems for systemic siRNA delivery: stability in blood sets the requirements for optimal carrier design. J Control Release. 2012;158(3):362–70. doi: 10.1016/j.jconrel.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Lee JM, et al. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed Res Int. 2013;2013:782041. doi: 10.1155/2013/782041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–55. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Young SW, et al. Nanoparticle-siRNA: A potential cancer therapy? Crit Rev Oncol Hematol. 2016;98:159–69. doi: 10.1016/j.critrevonc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 88.Kole R, et al. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov. 2012;11(2):125–40. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams T, Fried M. A mouse locus at which transcription from both DNA strands produces mRNAs complementary at their 3′ ends. Nature. 1986;322(6076):275–9. doi: 10.1038/322275a0. [DOI] [PubMed] [Google Scholar]
- 90.Vester B, Wengel J. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry. 2004;43(42):13233–41. doi: 10.1021/bi0485732. [DOI] [PubMed] [Google Scholar]
- 91.Chan JH, et al. Antisense oligonucleotides: from design to therapeutic application. Clin Exp Pharmacol Physiol. 2006;33(5–6):533–40. doi: 10.1111/j.1440-1681.2006.04403.x. [DOI] [PubMed] [Google Scholar]
- 92.Geary RS, et al. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 93.Moreno PM, Pego AP. Therapeutic antisense oligonucleotides against cancer: hurdling to the clinic. Front Chem. 2014;2:87. doi: 10.3389/fchem.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sarma K, et al. Locked nucleic acids (LNAs) reveal sequence requirements and kinetics of Xist RNA localization to the X chromosome. Proc Natl Acad Sci U S A. 2010;107(51):22196–201. doi: 10.1073/pnas.1009785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.You Y, et al. Design of LNA probes that improve mismatch discrimination. Nucleic Acids Res. 2006;34(8):e60. doi: 10.1093/nar/gkl175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seth PP, et al. Design, synthesis and evaluation of constrained methoxyethyl (cMOE) and constrained ethyl (cEt) nucleoside analogs. Nucleic Acids Symp Ser (Oxf) 2008;(52):553–4. doi: 10.1093/nass/nrn280. [DOI] [PubMed] [Google Scholar]
- 97.Durig J, et al. The novel antisense Bcl-2 inhibitor SPC2996 causes rapid leukemic cell clearance and immune activation in chronic lymphocytic leukemia. Leukemia. 2011;25(4):638–47. doi: 10.1038/leu.2010.322. [DOI] [PubMed] [Google Scholar]
- 98.Hong D, et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med. 2015;7(314):314ra185. doi: 10.1126/scitranslmed.aac5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pandey SK, et al. Identification and characterization of modified antisense oligonucleotides targeting DMPK in mice and nonhuman primates for the treatment of myotonic dystrophy type 1. J Pharmacol Exp Ther. 2015;355(2):329–40. doi: 10.1124/jpet.115.226969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tereshko V, et al. Crystal structures of the catalytic domain of human protein kinase associated with apoptosis and tumor suppression. Nat Struct Biol. 2001;8(10):899–907. doi: 10.1038/nsb1001-899. [DOI] [PubMed] [Google Scholar]
- 101.Kumar R, et al. The first analogues of LNA (locked nucleic acids): phosphorothioate-LNA and 2′-thio-LNA. Bioorg Med Chem Lett. 1998;8(16):2219–22. doi: 10.1016/s0960-894x(98)00366-7. [DOI] [PubMed] [Google Scholar]
- 102.Sekiguchi M, et al. Synthesis and properties of a novel bridged nucleic acid analogue, 5′-amino-3′,5′-BNA. Nucleosides Nucleotides Nucleic Acids. 2005;24(5–7):1097–100. doi: 10.1081/ncn-200061836. [DOI] [PubMed] [Google Scholar]
- 103.Gutschner T, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73(3):1180–9. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dean NM, Bennett CF. Antisense oligonucleotide-based therapeutics for cancer. Oncogene. 2003;22(56):9087–96. doi: 10.1038/sj.onc.1207231. [DOI] [PubMed] [Google Scholar]
- 105.Iden M, et al. The lncRNA PVT1 Contributes to the Cervical Cancer Phenotype and Associates with Poor Patient Prognosis. PLoS One. 2016;11(5):e0156274. doi: 10.1371/journal.pone.0156274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qu L, et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29(5):653–68. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Pickard MR, Williams GT. The hormone response element mimic sequence of GAS5 lncRNA is sufficient to induce apoptosis in breast cancer cells. Oncotarget. 2016;7(9):10104–16. doi: 10.18632/oncotarget.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bianchini D, et al. First-in-human Phase I study of EZN-4176, a locked nucleic acid antisense oligonucleotide to exon 4 of the androgen receptor mRNA in patients with castration-resistant prostate cancer. Br J Cancer. 2013;109(10):2579–86. doi: 10.1038/bjc.2013.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ottaviani L, da Costa Martins PA. Non-coding RNAs in cardiac hypertrophy. J Physiol. 2017;595(12):4037–4050. doi: 10.1113/JP273129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang C, et al. Fighting against kidney diseases with small interfering RNA: opportunities and challenges. J Transl Med. 2015;13:39. doi: 10.1186/s12967-015-0387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khorkova O, Wahlestedt C. Oligonucleotide therapies for disorders of the nervous system. Nat Biotechnol. 2017;35(3):249–263. doi: 10.1038/nbt.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rupaimoole R, et al. Long Noncoding RNA Ceruloplasmin Promotes Cancer Growth by Altering Glycolysis. Cell Rep. 2015;13(11):2395–402. doi: 10.1016/j.celrep.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ozpolat B, et al. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–6. doi: 10.1016/j.addr.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tekedereli I, et al. Therapeutic Silencing of Bcl-2 by Systemically Administered siRNA Nanotherapeutics Inhibits Tumor Growth by Autophagy and Apoptosis and Enhances the Efficacy of Chemotherapy in Orthotopic Xenograft Models of ER (−) and ER (+) Breast Cancer. Mol Ther Nucleic Acids. 2013;2:e121. doi: 10.1038/mtna.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nick AM, et al. Silencing of p130cas in ovarian carcinoma: a novel mechanism for tumor cell death. J Natl Cancer Inst. 2011;103(21):1596–612. doi: 10.1093/jnci/djr372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Naina HV, Harris S. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(19):1840. doi: 10.1056/NEJMc1203095. author reply 1840. [DOI] [PubMed] [Google Scholar]
- 117.Nishimura M, et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013;3(11):1302–15. doi: 10.1158/2159-8290.CD-13-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tekedereli I, et al. Targeted silencing of elongation factor 2 kinase suppresses growth and sensitizes tumors to doxorubicin in an orthotopic model of breast cancer. PLoS One. 2012;7(7):e41171. doi: 10.1371/journal.pone.0041171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aslan B, et al. The ZNF304-integrin axis protects against anoikis in cancer. Nat Commun. 2015;6:7351. doi: 10.1038/ncomms8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pradeep S, et al. Erythropoietin Stimulates Tumor Growth via EphB4. Cancer Cell. 2015;28(5):610–22. doi: 10.1016/j.ccell.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Akar U, et al. Targeting p70S6K prevented lung metastasis in a breast cancer xenograft model. Mol Cancer Ther. 2010;9(5):1180–7. doi: 10.1158/1535-7163.MCT-09-1025. [DOI] [PubMed] [Google Scholar]
- 122.Kretz M, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012;26(4):338–43. doi: 10.1101/gad.182121.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ozpolat B, et al. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med. 2010;267(1):44–53. doi: 10.1111/j.1365-2796.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 124.Rivas E, et al. A statistical test for conserved RNA structure shows lack of evidence for structure in lncRNAs. Nat Methods. 2017;14(1):45–48. doi: 10.1038/nmeth.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Howe JA, et al. Selective small-molecule inhibition of an RNA structural element. Nature. 2015;526(7575):672–7. doi: 10.1038/nature15542. [DOI] [PubMed] [Google Scholar]
- 126.Velagapudi SP, et al. Design of a small molecule against an oncogenic noncoding RNA. Proc Natl Acad Sci U S A. 2016;113(21):5898–903. doi: 10.1073/pnas.1523975113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Connelly CM, et al. The Emerging Role of RNA as a Therapeutic Target for Small Molecules. Cell Chem Biol. 2016;23(9):1077–90. doi: 10.1016/j.chembiol.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Velagapudi SP, et al. Defining RNA-Small Molecule Affinity Landscapes Enables Design of a Small Molecule Inhibitor of an Oncogenic Noncoding RNA. ACS Cent Sci. 2017;3(3):205–216. doi: 10.1021/acscentsci.7b00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jain AK, et al. LncPRESS1 Is a p53-Regulated LncRNA that Safeguards Pluripotency by Disrupting SIRT6-Mediated De-acetylation of Histone H3K56. Mol Cell. 2016;64(5):967–981. doi: 10.1016/j.molcel.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simon MD. Capture hybridization analysis of RNA targets (CHART) Curr Protoc Mol Biol. 2013;Chapter 21(Unit 21):25. doi: 10.1002/0471142727.mb2125s101. [DOI] [PubMed] [Google Scholar]
- 131.Simon MD, et al. The genomic binding sites of a noncoding RNA. Proc Natl Acad Sci U S A. 2011;108(51):20497–502. doi: 10.1073/pnas.1113536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang Y, et al. Unveiling the hidden function of long non-coding RNA by identifying its major partner-protein. Cell Biosci. 2015;5:59. doi: 10.1186/s13578-015-0050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chu C, et al. Chromatin isolation by RNA purification (ChIRP) J Vis Exp. 2012;(61) doi: 10.3791/3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chu C, et al. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161(2):404–16. doi: 10.1016/j.cell.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McHugh CA, et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature. 2015;521(7551):232–6. doi: 10.1038/nature14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moore MJ, et al. Mapping Argonaute and conventional RNA-binding protein interactions with RNA at single-nucleotide resolution using HITS-CLIP and CIMS analysis. Nat Protoc. 2014;9(2):263–93. doi: 10.1038/nprot.2014.012. [DOI] [PMC free article] [PubMed] [Google Scholar]