Abstract
The association of hand preference (left, mixed, and right) with cognitive, academic, motor, and behavioral function was evaluated in 864 extremely preterm children at 10 years of age. Left-handed and right-handed children performed similarly but mixed-handed children had greater odds of functional deficits across domains than right-handed children.
Non-right handedness (mixed and left-handedness) occurs in approximately 22% of preterm children, a rate almost twice that in term-born children (12%).1 Those born extremely preterm (EP; <28 weeks) are also at high risk of neonatal brain injury and altered brain development and structure,2, 3 as well as cognitive, emotional/behavioral, and motor difficulties.4–6 One theory for the increased prevalence of non-right handedness in some preterm children is that it may be caused by early brain injury, disrupting the normal neuroanatomical distribution of motor and potentially other functions (see7 for review). Therefore, handedness may be an easily assessed correlate of adverse functional outcomes in preterm survivors, but the associations of mixed as opposed to left-hand preference with outcomes remain unclear.
Studies of preterm children have combined left- and mixed-handed groups to increase statistical power, with equivocal evidence for disadvantage in non-right-handed preterm children.8–10 Stronger left-handedness has been associated with poorer reading, spelling, mathematics, and working memory,11 and greater right-handedness has been positively associated with general intellectual ability.12 In the general population, mixed hand preference is infrequent but has been linked with childhood mental health difficulties including autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD).13–15 However, data examining mixed handedness in preterm children are limited and conflicting.12, 16 The purpose of this study was to examine associations of hand preference (left, mixed, and right) with cognitive, academic, and motor skills as well as behavior and mental health in a large cohort of 10 year-old extremely preterm children. We hypothesized that left- and mixed-handed children would both have poorer outcomes than right-handed children.
Methods
The ELGAN (Extremely Low Gestational Age Newborn) study is a multi-center prospective, observational study of the risk of structural and functional neurologic disorders in extremely preterm infants born 2002–04.17 At 2 years of age, 1102 of 1200 survivors underwent developmental assessment.18 At 10 years of age, 966 children were recruited for follow-up and 889 were enrolled. Institutional review boards of all participating institutions approved this follow up study.
A modified 12-item version of the Dean Laterality Preference Schedule was used to assess “laterality preference.”19, 20 Parents rated their child’s hand preference for various manual tasks on a 5-point scale (“always left” [−2] to “always right” [+2]). Total scores were defined as right-handed (>10), left-handed (<−10), or mixed-handed (−10 to 10). Primary outcomes were measures of cognitive functioning: 1) general cognitive ability (Differential Ability Scales II (DAS-II) Verbal and Nonverbal Reasoning scales);21 2) attention, inhibitory control, and cognitive flexibility (DAS-II: Recall of Digits Backward, Recall of Sequential Order; Developmental NEuroPSYchological Assessment-II (NEPSY-II):22 Auditory Attention and Response Set, Inhibition and Inhibition-Switching, Animal Sorting); 3) processing speed (NEPSY-II Inhibition-Naming); 4) language (Oral and Written Language Scales [OWLS]);23 5) visual-spatial processing (NEPSY-II Geometric Puzzles). Academic achievement was assessed with the Wechsler Individual Achievement Test-III24 (WIAT-III [C]: Word Reading, Pseudoword Decoding, Spelling, and Numerical Operations). Motor function was measured with the NEPSY-II Visuomotor Precision subtest, the Manual Ability Classification System (MACS),25 and the Gross Motor Function Classification System (GMFCS).26 We converted continuous scores to age-standardized z-scores against the normative mean to allow comparison across measures. Behavior and mental health measures included diagnosis of ASD (for methods see27), and positive screens on the parent-rated Child Symptom Inventory-4 Parent Checklist (CSI-4)28 for ADHD, oppositional defiant disorder, conduct disorder, generalized anxiety disorder, motor tics, vocal tics, major depression, dysthymic disorder, social phobia, separation anxiety, enuresis, and encopresis.
In our sample, scores on most tests had a larger number of children at the low end of the distribution (so-called “fat tail”). Therefore, we focused on scores of z ≤ −1. Multivariable logistic regression models assessed the risk of very low scores (z ≤ −2) or positive screens for behavior problems associated with mixed- and left-handedness compared with right-handed children. Models included potential confounders (mother’s age at birth, child’s sex, gestational age category, and birth weight z-score <−2). As motor deficits may confound performance on cognitive and motor tests, we conducted sensitivity analyses excluding children with MACS scores ≥ level 3, to determine whether findings were similar in those without manual impairment. Given the large number of comparisons, our interpretation focused on the direction and magnitude of effects.
Results
Handedness data were available for 864 of the 966 children recruited, with a mean age of 10 years (SD: 0.7). Most children (n=670, 78%) were classified as right-preference, 17% as left-preference (n=145), and 6% (n=49) had mixed preference (Table I; available at www.jpeds.com). Compared with others, children with mixed preference were more likely to have mothers with no formal education past high school and who were not married, and less likely to have a small head circumference at birth. Boys were over-represented in the left- and mixed-handed groups.
Cognitive, Academic, and Motor Outcomes (Table 2 [available at www.jpeds.com] and Figure 1, A)
Figure 1.
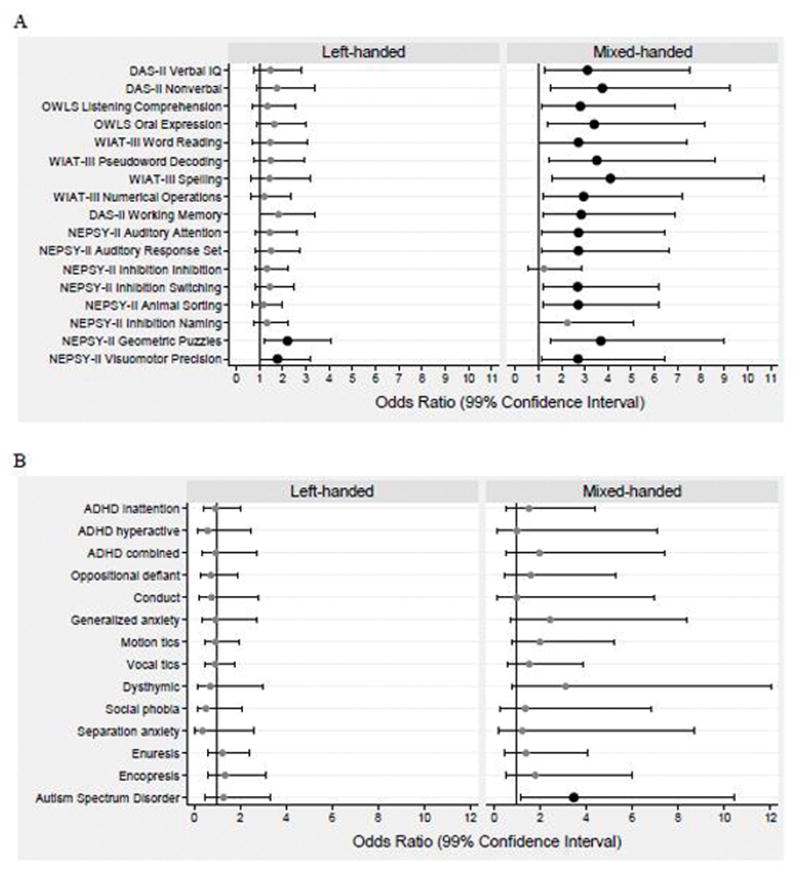
Forest plots of odds ratios and 99% confidence intervals for risk of outcomes of interest at age 10 years associated with left-hand preference (left panels) and mixed hand preference (right panels) relative to children with right-hand preference.
Part A: Odds of z scores ≤ −2 on each DAS-II, OWLS, WIAT-III, and NEPSY-II assessment.
Part B: Odds of positive screens for behavior problems on the CSI-4 and for an ASD diagnosis at age 10 years.
Analyses were adjusted for mother’s age < 21 years at delivery, gestational age category 23–24 weeks, birth weight Z-score < −2, and male sex. Large black dots indicate that the odds ratios are significantly >1 (p < 0.01), as does the lower bound of the confidence interval to the right of the vertical line.
Children with left-handed preference generally had similar rates to right-handed children of very low scores on cognitive, academic, and motor measures, except for greater difficulties in visual processing and fine motor skill (Geometric Puzzles and Visuomotor Precision). In contrast, mixed-handed children more often had very low scores in cognitive and academic domains other than simple inhibition, speed of processing, and spelling. Rates of milder deficits (−2 < z ≤ −1) were similar across groups. Most children in each group had low MACS scores but mixed-handed children were more likely than others to have fine motor/manual difficulties (very low Visuomotor Precision or MACS ≥level 3), and gross motor difficulties (GMFCS ≥level 3). Sensitivity analyses excluding children with manual handling difficulties yielded similar patterns as the primary analysis, although the magnitude of some relationships weakened (Figure 2; available at www.jpeds.com).
Behavior and Mental Health (Table 3 [available at www.jpeds.com] and Figure 1, B)
Children in the mixed handedness group were more likely than others to be diagnosed with an ASD. Compared with their left- and right-handed peers, those in the mixed group more often had parent-reported symptoms of ADHD (inattentive and hyperactive/impulsive), generalized anxiety, motion tics, vocal tics, and dysthymic disorder, although these odds ratios included a value of 1 after adjustment for covariates (Figure 1, B).
Discussion
In this study, we investigated associations of inconsistency and direction of hand preference with a wide range of functional outcomes at school-age. Contrary to our hypothesis, left-handed preference was generally not associated with poorer cognitive, academic, motor, or behavioral functioning than right-handedness at age 10 years. As expected, however, children with mixed hand preference did less well than their right-handed peers on most measures.
In contrast to some previous reports,11, 12 left-handed extremely preterm children had similar rates of cognitive, academic, and motor difficulties to their right-handed peers. The exception to this was poorer fine motor skill and visual-spatial perception/mental rotation in the left-handed group compared with right-handers. Previous studies with smaller cohorts have shown that handedness was related to dexterity in preterm children but the direction of reported effects has varied.8, 9 Our results broadly concurred with evidence that children with right hand preference in the general population outperform those with left hand preference, on at least some visual-spatial skills, such as mental rotation.29 However, our left-handed children showed similar performances to right-handed children for more complex visual-spatial reasoning tasks. Our study did not include familial handedness as a covariate that may have been useful in identifying “non-pathological” left-handedness.11
We found mixed-handed children were at increased risk, relative to right-handed children, of difficulties in verbal and non-verbal ability, attention and cognitive flexibility, academic achievement, motor abilities, and parent-rated behavior. The cognitive and academic difficulties identified were considerable, suggesting that mixed handedness may arise in the context of altered brain development or injury sufficiently severe to also produce major cognitive and academic deficits. Despite the loss in power, this pattern was similar after excluding children with manual handling difficulties, suggesting that the observed deficits were not attributable to physical limitations in performing the tasks and therefore that cerebral palsy is an unlikely explanation for the observed associations. In the only other report of a contemporary extremely preterm sample, mixed-handedness was not related to cognitive or motor difficulties in 180 6-year-old extremely preterm children.12 Although the direction of hand preference may emerge relatively early, the degree of hand preference may strengthen over childhood.7, 30, 31 Consequently, differences in assessment of handedness (questionnaire versus performance),32 sample size, and age at assessment may have contributed to the different patterns of findings.
The results of our study indicated an association between adverse behavioral and mental health outcomes and mixed handedness in extremely preterm children, including some evidence for increased attention, mood, anxiety, and autism spectrum features. These findings extend earlier work reporting similar risks for mixed-handed individuals in the general population to the EP population.13, 14 Although previous smaller studies have not found increased rates of behavioral problems in non-right handed preterm children,11 differences in methods and sample size in the current study may explain discrepant findings. Our behavioral findings reflect parental ratings of behavior (for outcomes other than ASD), and as such do not necessarily indicate the presence of clinically diagnosable disorders. The association of mixed handedness with ASD was among children who also had cognitive impairment, and not among ASD children who were not cognitively limited. One interpretation of our overall findings is that neurological injury manifests both as mixed handedness and functional difficulties in cognitive, motor, and behavioral domains. Hence, the clustering of mixed handedness, behavior difficulties, and cognitive difficulties merits further exploration.
Our study has several strengths. The high follow-up rate in this large, well-characterized cohort gave us the power to examine wide-ranging functional correlates of mixed handedness as distinct from left-handedness in the most biologically vulnerable preterm survivors. Limitations included the absence of a control group and the need to rely on normative data, but we selected well-validated cognitive measures with recent United States’ population norms. We could not control for familial handedness, making it difficult to identify “pathological” left-handedness. Although our sample size was large, the study was not powered to directly compare the smaller numbers of left-handed children with mixed-handed children. We chose a 99% confidence interval in an effort to balance the risks of type 1 and type 2 errors with multiple comparisons. We might not have achieved both of these goals. Handedness measurement is challenging, with a wide variety of methods used to assess handedness in the literature and no universal definition of mixed handedness. The measures and cut-points used to define hand-preference may influence results. We used a questionnaire rather than actual performance of actions, and these two facets of handedness may not always align. However, our approach identified similar proportions of children with non-right handedness as previous reports,1 and used symmetrical cut-points. Future studies should measure both reported hand preference and performance to determine the robustness of these associations. Finally, the capacity of handedness to predict later outcomes could not be addressed in this cross-sectional study.
Supplementary Material
Acknowledgments
Supported by the National Institute of Neurologic Disorders and Stroke (5U01NS040069-05, 2R01NS040069-06A2), National Institute of Health Office of Director (1UG3OD023348-01), and the National Institute of Child Health and Human Development (5P30HD018655-28).
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- ASD
autism spectrum disorder
- CSI-4
Child Symptom Inventory-4 Parent Checklist
- DAS-II
Differential Ability Scales II
- ELGAN
Extremely Low Gestational Age Newborn
- EP
extremely preterm
- MACS
Manual Ability Classification System
- OWLS
Oral and Written Language Scales
- VLBW
very low birth weight
- WIAT-III
Wechsler Individual Achievement Test-III
Appendix
Additional members of the Extremely Low Gestational Age Newborns (ELGAN) Study Investigators include:
-
Baystate Medical Center, Springfield, MA
Bhavesh Shah, MD; Rachana Singh, MD, MS; Anne Smith, PhD; Deborah Klein, BSM, RN; Susan McQuiston, PhD
-
Boston Medical Center
Julie Rollins, MA; Laurie Douglass, MD
-
Boston Children’s Hospital, Boston, MA
Janice Ware, PhD; Taryn Coster, BA; Brandi Henson, PsyD; Rachel Wilson, PhD; Kirsten McGhee, PhD; Patricia Lee, PhD; Aimee Asgarian, PhD; Anjali Sadhwani, PhD
-
Tufts Medical Center, Boston, MA
Ellen Perrin, MD; Emily Neger, MA; Kathryn Mattern, BA; Jenifer Walkowiak, PhD; Susan Barron, PhD
-
University of Massachusetts Medical School, Worcester, MA
Jean Frazier, MD; Lauren Venuti, BA; Beth Powers, RN; Ann Foley, EdM; Brian Dessureau, PhD; Molly Wood, PhD; Jill Damon-Minow, PsyD
-
Yale University School of Medicine, New Haven, CT
Richard Ehrenkranz, MD; Jennifer Benjamin, MD; Elaine Romano, APRN; Kathy Tsatsanis, PhD; Katarzyna Chawarska, PhD; Sophy Kim, PhD; Susan Dieterich, PhD; Karen Bearrs, PhD
-
Wake Forest University Baptist Medical Center, Winston-Salem, NC
Nancy Peters, RN; Patricia Brown, RN; Emily Ansusinha, BA; Ellen Waldrep, MA; Jackie Friedman, PhD; Gail Hounshell, PhD; Debbie Allred, PhD
-
University Health Systems of Eastern Carolina, Greenville, NC
Stephen C. Engelke, MD; Nancy Darden-Saad, BS, RN; Gary Stainback, PhD
-
North Carolina Children’s Hospital, Chapel Hill, NC
Diane Warner, MD, MPH; Janice Wereszczak, MSN; Janice Bernhardt, MS, RN; Joni McKeeman, PhD; Echo Meyer, PhD
-
Helen DeVos Children’s Hospital, Grand Rapids, MI
Steve Pastyrnak, PhD; Wendy Burdo-Hartman, MD; Julie Rathbun, BSW, BSN, RN; Sarah Nota, BS; Teri Crumb, BSN, RN
-
Sparrow Hospital, Lansing, MI
Madeleine Lenski, MPH; Deborah Weiland, MSN; Megan Lloyd, MA, EdS
-
University of Chicago Medical Center, Chicago, IL
Scott Hunter, PhD; Michael Msall, MD; Rugile Ramoskaite, BA; Suzanne Wiggins, MA; Krissy Washington, MA; Ryan Martin, MA; Barbara Prendergast, BSN, RN; Megan Scott, PhD
-
William Beaumont Hospital, Royal Oak, MI
Judith Klarr, MD; Beth Kring, RN; Jennifer DeRidder, RN; Kelly Vogt, PhD
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Domellöf E, Johansson AM, Ronnqvist L. Handedness in preterm born children: a systematic review and a meta-analysis. Neuropsychologia. 2011;49:2299–310. doi: 10.1016/j.neuropsychologia.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheong JLY, Anderson PJ, Roberts G, Burnett AC, Lee KJ, Thompson DK, et al. Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PLoS ONE. 2013;8:e77475. doi: 10.1371/journal.pone.0077475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med. 2014;19:90–6. doi: 10.1016/j.siny.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Holsti A, Adamsson M, Hagglof B, Farooqi A, Serenius F. Chronic Conditions and Health Care Needs of Adolescents Born at 23 to 25 Weeks’ Gestation. Pediatrics. 2017:139. doi: 10.1542/peds.2016-2215. [DOI] [PubMed] [Google Scholar]
- 6.Lundequist A, Bohm B, Lagercrantz H, Forssberg H, Smedler AC. Cognitive outcome varies in adolescents born preterm, depending on gestational age, intrauterine growth and neonatal complications. Acta Paediatr. 2015;104:292–9. doi: 10.1111/apa.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop DVM. Handedness and Developmental Disorder. London: Mac Keith Press; 1990. [Google Scholar]
- 8.Luciana M, Lindeke L, Georgieff M, Mills M, Nelson CA. Neurobehavioral evidence for working-memory deficits in school-aged children with histories of prematurity. Dev Med Child Neurol. 1999;41:521–33. doi: 10.1017/s0012162299001140. [DOI] [PubMed] [Google Scholar]
- 9.Powls A, Botting N, Cooke RW, Marlow N. Handedness in very-low-birthweight (VLBW) children at 12 years of age: relation to perinatal and outcome variables. Dev Med Child Neurol. 1996;38:594–602. doi: 10.1111/j.1469-8749.1996.tb12124.x. [DOI] [PubMed] [Google Scholar]
- 10.Saigal S, Rosenbaum P, Szatmari P, Hoult L. Non-right handedness among ELBW and term children at eight years in relation to cognitive function and school performance. Dev Med Child Neurol. 1992;34:425–33. doi: 10.1111/j.1469-8749.1992.tb11455.x. [DOI] [PubMed] [Google Scholar]
- 11.Pascoe L, Scratch SE, Burnett AC, Thompson DK, Lee KJ, Doyle LW, et al. Neurodevelopmental Outcomes and Neural Mechanisms Associated with Non-right Handedness in Children Born Very Preterm. J Int Neuropsychol Soc. 2015;21:610–21. doi: 10.1017/S1355617715000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marlow N, Hennessy EM, Bracewell MA, Wolke D. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez A, Kaakinen M, Moilanen I, Taanila A, McGough JJ, Loo S, et al. Mixed-handedness is linked to mental health problems in children and adolescents. Pediatrics. 2010;125:e340–8. doi: 10.1542/peds.2009-1165. [DOI] [PubMed] [Google Scholar]
- 14.Lindell AK, Hudry K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol Rev. 2013;23:257–70. doi: 10.1007/s11065-013-9234-5. [DOI] [PubMed] [Google Scholar]
- 15.Markou P, Ahtam B, Papadatou-Pastou M. Elevated Levels of Atypical Handedness in Autism: Meta-Analyses. Neuropsychol Rev. 2017 doi: 10.1007/s11065-017-9354-4. [DOI] [PubMed] [Google Scholar]
- 16.Ross G, Lipper E, Auld PA. Hand preference, prematurity and developmental outcome at school age. Neuropsychologia. 1992;30:483–94. doi: 10.1016/0028-3932(92)90095-4. [DOI] [PubMed] [Google Scholar]
- 17.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helderman JB, O’Shea TM, Kuban KC, Allred EN, Hecht JL, Dammann O, et al. Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 2012;129:494–502. doi: 10.1542/peds.2011-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean RS. Reliability and predictive validity of the Dean Laterality Preference Schedule with preadolescents. Percept Mot Skills. 1978;47:1345–6. doi: 10.2466/pms.1978.47.3f.1345. [DOI] [PubMed] [Google Scholar]
- 20.Piro JM. Handedness and intelligence: Patterns of hand preference in gifted and nongifted children. Dev Neuropsychol. 1998;14:619–30. [Google Scholar]
- 21.Elliott CD. Differential Ability Scales. 2. San Antonio, TX: Pearson; 2007. [Google Scholar]
- 22.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. New York: The Psychological Corporation; 1998. [Google Scholar]
- 23.Carrow-Woolfolk E. Oral and Written Language Scales: Written Expression Scale Manual. Circle Pines, MN: American Guidance Service; 1996. [Google Scholar]
- 24.Wechsler D. Wechsler Individual Achievement Test-Third Edition. Oxford, UK: Pearson Assessment; 2009. [Google Scholar]
- 25.Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–54. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 26.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–50. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 27.Joseph RM, O’Shea TM, Allred EN, Heeren T, Hirtz D, Paneth N. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. doi: 10.1002/aur.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadow KD, JS . Child Symptom Inventory-4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- 29.Somers M, Shields LS, Boks MP, Kahn RS, Sommer IE. Cognitive benefits of right-handedness: a meta-analysis. Neurosci Biobehav Rev. 2015;51:48–63. doi: 10.1016/j.neubiorev.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 30.McManus IC, Sik G, Cole DR, Mellon AF, Wong J, Kloss J. The development of handedness in children. British Journal of Developmental Psychology. 1988;6:257–73. [Google Scholar]
- 31.Johansson AM, Domellof E, Ronnqvist L. Long-term influences of a preterm birth on movement organization and side specialization in children at 4–8 years of age. Dev Psychobiol. 2014;56:1263–77. doi: 10.1002/dev.21206. [DOI] [PubMed] [Google Scholar]
- 32.Steenhuis RE. The relation between hand preference and hand performance: what you get depends on what you measure. Laterality. 1999;4:3–26. doi: 10.1080/713754324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


