Abstract
Background
The prevalence and time trends of food allergy change during childhood depending on the age of the child and the type of food.
Objective
To study prevalence and longitudinal trends in food allergy from birth to 18 years in an unselected birth cohort in the Isle of Wight.
Method
Information on food allergy was collected at ages 1, 2, 4, 10 and 18 years from the Isle of Wight Birth Cohort (n = 1456). Skin prick testing (SPT) was performed at the age of 1 and 2 years in symptomatic children. At 4, 10 and 18 years of age, participants were tested to a panel of food and aero-allergens. Food allergy was diagnosed based on the criteria: symptoms suggestive of a typical IgE mediated reaction and reaction <4 hours following exposure to a known food allergen. McNemar’s test was used to determine significance of changes in prevalence over time.
Results
The prevalence of food allergy remained relatively constant in early childhood (5.3%, 4.4% and 5.0% at 1, 2 and 4 years respectively), with significant decline at 10 years (2.3%, p<0.001 versus 4 years) followed by significant rise at 18 years (4%, p=0.02 versus 10 years). Cow’s milk (1.6–3.5 %) and egg (1.1–1.4%) were the most common allergens in the first 10 years with peanut (1%) and tree-nuts (0.5%) becoming more prevalent beyond 10 years. Fruit and wheat allergy were less common at 10 years, and shellfish and kiwi emerged during adolescence. The prevalence of food allergy plus positive SPT were 1.3%, 0.8%, 0.8%, 0.9% and 2.2% at 1, 2, 4, 10 and 18 years respectively.
Conclusion
Food allergy is highly prevalent in infancy with partial resolution during late childhood. However, a number of children acquire new food allergy during adolescence resulting in a relatively higher prevalence at 18 years.
INTRODUCTION
Food allergy (FA) is increasingly recognised as a public health problem, and along with other atopic conditions is a major cause of chronic morbidity in the developed world. FA is defined as an immunologically mediated adverse reaction to food, which can be both immunoglobulin E (IgE) and non-IgE mediated. Allergic sensitisation is the development of specific IgE to food or aeroallergens and can occur in the absence of food allergy symptoms. FA is diagnosed based on an allergy focussed history and/or oral challenges. Allergic sensitisation to the suspected food confirms the presence of IgE mediated FA.
There is huge variability in the prevalence of FA worldwide; however, a meta-analysis in 2014 showed overall prevalence rates of 17% for self-reported FA and 0.9% for challenge proven FA. Clinically defined FA prevalence varies widely (1–13%) depending on the specific criteria used (3, 4). Food allergy in infancy can be a transient phenomenon or may persist into adulthood. The prevalence and trends in food allergy and sensitisation patterns in childhood have been previously explored in birth cohorts (4–7). However, there is limited knowledge about prevalence, time trends and overall transitions, i.e. change in status from food allergic to non-food allergic (acquired tolerance) and from non-food allergic to food allergic (acquired food allergy) from one assessment to the next. The Isle of Wight Birth Cohort was followed up from early childhood, into later childhood and on into young adult life, providing a life course perspective between childhood and adulthood.
There is evidence of rising prevalence and severity of FA and anaphylaxis in several studies (8–11). The reasons for reported variation in prevalence include differences in study methodology, the diagnostic criteria used for defining FA (e.g. self- reported food allergy, skin prick testing, open (OFC) or double-blind, placebo-controlled food challenges (DBPCFC)), geography and local dietary habits (12). We were in the unique position of being able to analyse 18 years of longitudinal prevalence data on FA which was collected using similar methodology from the same, relatively static, population in the Isle of Wight (IOW), UK. Our study aimed to determine the prevalence and longitudinal trends of FA and sensitisation to food allergens over the first 18 years of life. This will add valuable information to the current knowledge of the natural history of food sensitisation and allergy.
METHODS
The IOW birth cohort was established in 1989, and has been followed up prospectively for 18 years (13, 14). This unselected birth cohort was established with the aim of studying the natural history of allergic diseases and the influence of genetic and environmental factors on the development and progression of allergy. The study was approved by the Isle of Wight research ethics committee (06/Q1701/34). All children born on the IOW between 1 January 1989 and 28 February 1990 (n=1536), were enrolled in the study and 1456 were available for further follow-up (15). Detailed questionnaires were completed at each follow-up. Allergic symptoms of FA, eczema, asthma and rhinitis, atopic family history and home and environmental factors were explored. Prospectively collected food reaction and sensitisation data was analysed to study food allergy trends.
Definition of FA
The diagnostic criteria used for food allergy have been published previously (15, 16). In summary, we collected detailed information on food related allergic reactions and applied an a priori definition of food allergy based on the following criteria:
A report involving a recognised food allergen as defined by the Committee on Toxicity of Chemicals in Foods, Consumer Products and the Environment (17).
-
Recognised IgE-mediated symptoms (18) such as:
localised symptoms: itching, sting/ burning of the lips/ mouth or throat, urticaria/ hives, angioedema
abdominal: nausea, vomiting, crampy/ colicky abdominal pain, diarrhoea
respiratory: wheeze, stridor, watery rhinitis, redness of eyes/ nose
skin: urticaria, itching, flushed skin, worsening eczema
systemic reaction: anaphylaxis
Temporal relationship of a reaction with symptoms developing within 4 hours of food ingestion.
Only if all 3 criteria were fulfilled, were children designated as having FA.
Skin prick test (SPT)
SPT results were available for a subgroup of the dataset. SPT was performed, using a standardised method, at 1 and 2 years in symptomatic children only, and at 4, 10, and 18 years in all consenting participants attending the Centre (14, 15). The SPT panel included common aero- and food allergens: house dust mite, grass pollen mix, tree pollen mix, cat and dog epithelia, Alternaria alternata, Cladosporium herbarum (aero-allergen panel) plus cow’s milk, hen’s egg, wheat, soya, cod fish and peanut (food panel). Further SPT was also performed if symptoms were reported to other allergens. Histamine and physiological saline acted as positive and negative control (Alk-Abello, Horsholm, Denmark). A positive skin prick test was defined as a mean wheal diameter ≥ 3mm larger than the negative control at 15 minutes.
Statistical analysis
SPSS statistical package Version 17 was used to analyse data (IBM SPSS Statistics, Version 17, USA). Frequency tables of FA, sensitisation and combined FA and sensitisation at each time point (1, 2, 4, 10 and 18 years) was used to determine prevalence of FA and sensitisation pattern for allergens over time. Cross tab analysis was used for paired data in FA over time. Significance of changes in FA prevalence rates over time (1, 4, 10 and 18 years) was tested using McNemar’s test for paired data and Chi square/ Fishers exact test for independent data.
RESULTS
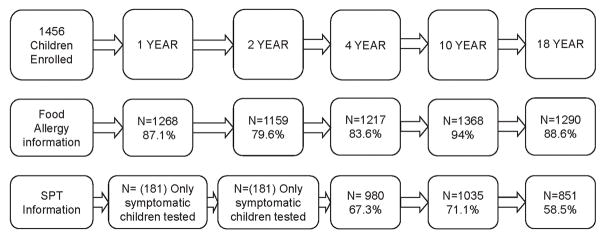
Of the 1536 children enrolled at birth in 1989, 1456 were available for further follow-up. Most children had more than one assessment but not all were seen at every time point. Figure 1 graphically depicts the availability of information regarding FA and SPT data at various stages of the study. Demographic information and allergic comorbidity in the cohort participants at each assessment, stratified for sex, is provided in the supplementary table S1 and S2.
Figure 1.
Participation data/ availability of information at ages 1, 2, 4, 10 and 18 years in the Isle of Wight birth cohort (Consort diagram)
Prevalence of food allergy
The prevalence of FA by self-report, and FA diagnosed based on study criteria at ages 1, 2, 4, 10 and 18 years are reported in Table 1. The rates based on study criteria are significantly lower (p<0.05) as compared to the self-reported rates. The longitudinal trend suggests FA rates remain relatively constant in early childhood, at 5.3%, 4.4% and 5.0% at ages 1, 2, and 4 years respectively. There is a significant decline at 10 years (2.3%, p<0.001 versus 4 years) associated with the resolution of pre-existing childhood allergies, followed by a significant rise at 18 years (4%, p=0.02 versus 10 years) due to an increase in the prevalence of new food allergies between 10 and 18 years. FA data was further stratified based on SPT results (Table S1). Among children with reported food allergic symptoms, SPT information was available in 32.7% to 85.1% of the allergic population at 5 time points. The prevalence of those with defined FA plus skin prick test ranged from 0.8% to 2.2%, which equated to 16%–54% of those diagnosed with FA.
Table 1.
Prevalence of food allergy, based on self report and study criteria and food allergen sensitisation at varying ages
| FA Status | I year | 2 years | 4 years | 10 years | 18 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | % | 95% CI | No | % | 95% CI | No | % | 95% CI | No | % | 95% CI | No | % | 95% CI | |
| Reported food allergy | 108 | 8.5% | 7–10% | 106 | 9.1% | 7.4–10.8% | 111 | 9.1% | 7.5–10.7 | 117 | 8.6% | 7.1–10.1% | 103 | 8.0% | 6.5–9.5% |
| Food allergy (study criteria) | 67 | 5.3% | 4.2–6.7% | 51 | 4.4% | 3.4–5.7% | 61 | 5.0% | 3.9–6.4% | 35 | 2.3% | 1.7–3.3% | 52 | 4.1% | 3.2–5.4% |
| Availability of food allergy information | 1268 (87.1%) | 1159 (79.6%) | 1217 (83.6%) | 1368 (94%) | 1290 (88.6%) | ||||||||||
| Food sensitisation* | - | - | - | - | - | - | 31 | 3.4% | 2.2–4.4% | 46 | 4.4% | 3.4–5.9% | 182 | 21.4% | 18.8–24.3% |
| FA and SPT positive $ | 16 | 1.3% | 0.8–2.1% | 9 | 0.8% | 0.4–1.5% | 8 | 0.8% | 0.4–1.6% | 9 | 0.9% | 0.4–1.6% | 19 | 2.2% | 1.4–3.4% |
| Availability of food sensitisation Information | - | - | 980 (67.3%) | 1035 (71.1%) | 851 (58.5%) | ||||||||||
Note:
Skin prick tests at one and two years were carried out in only symptomatic children and hence it was not possible to calculate cohort prevalence
Total number of children with “food allergy information” was used as denominator as skin tests were carried out in only symptomatic children
Rates of individual food allergies
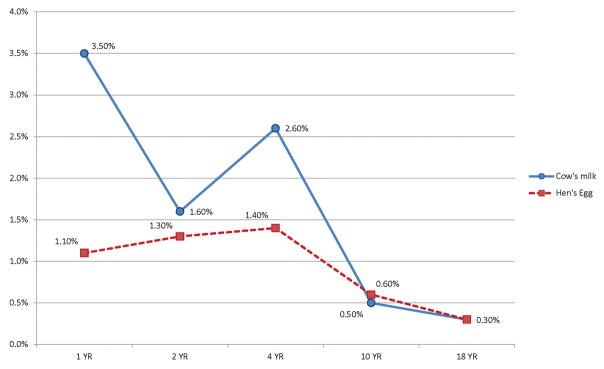
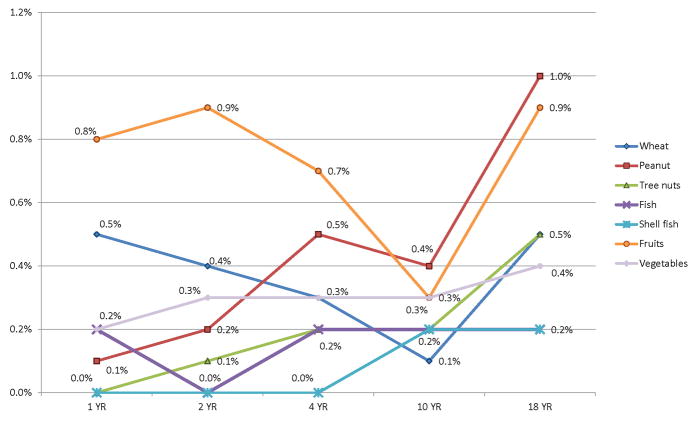
The FA rates of individual food groups are provided in Table 2. Cow’s milk and egg allergy were most prevalent at ages 1, 2, 4 and 10 years. Their prevalence declined with time and by 18 years, the prevalence of both milk and egg allergy had significantly dropped to 0.3% (Figure 2). FA to peanuts, fruits, tree nuts and wheat were most prevalent at 18 years (Figure 3). Vegetables, mushrooms and mustard were new emerging allergies at 10 and 18 years. Allergies to fruits range from 0.3 to 0.9% at different ages (Figure 3), and included reports of a variety of fruits (eg strawberry, kiwi, orange, bananas, apples Table S3).
Table 2.
Prevalence of food allergies based on food groups and age
| Food group | 1 year | 2 years | 4 years | 10 years | 18 years |
|---|---|---|---|---|---|
| N=1268 | N=1159 | N=1217 | N=1368 | N=1290 | |
| All FA | 67 (5.3%) | 51 (4.4%) | 61 (5.0%) | 32 (2.3%) | 52 (4.0%) |
| Cow’s milk | 44 (3.5%) | 19 (1.6%) | 32 (2.6%) | 7 (0.5%) | 4 (0.3%) |
| Hen’s egg | 14 (1.1%) | 15 (1.3%) | 17 (1.4%) | 8 (0.6%) | 4 (0.3%) |
| Wheat | 6 (0.5%) | 5 (0.4%) | 3 (0.3%) | 1 (0.1%) | 6 (0.5%) |
| Soya | - | - | 4 (0.3%) | - | - |
| Fish | 2 (0.2%) | - | 2 (0.2%) | 2 (0.2%) | 2 (0.2%) |
| Shellfish | - | - | - | 2 (0.2%) | 3 (0.2%) |
| Peanut | 1 (0.1%) | 2 (0.2%) | 6 (0.5%) | 6 (0.4%) | 12 (1.0%) |
| Tree nuts | - | 1 (0.1%) | 2 (0.2%) | 2 (0.2%) | 7 (0.5%) |
| Fruits | 10 (0.8%) | 11 (0.9%) | 9 (0.7%) | 4 (0.3%) | 11 (0.9%) |
| Kiwi | - | - | - | 1 (0.1%) | 3 (0.2%) |
| Vegetables | 2 (tomatoes) (0.2%) | 3 (tomatoes) (0.3%) | 4 (tomatoes/ brussel sprouts) (0.3%) | 4 (tomatoes) (0.3%) | 5 (tomatoes/ leek/cucumber (0.4%) |
| Miscellaneous | - | 2 (0.2%) | 2 (0.2%) | 2 (0.2%) | 3 (0.3%) |
(FA based on study criteria: Reaction to known food allergen, typical IgE reaction symptoms, and reaction timing < 4 hrs.)
Miscellaneous included; corn, chocolate (1 year), red meat and chocolate (2 years), chocolate and oats (10 years), cinnamon, mustard and linseed (18 years).
Figure 2.
Prevalence of cow’s milk and hen’s egg allergy based on study criteria (known food allergen, typical immediate allergy symptoms, and timing <4 hrs) at 1, 2, 4, 10 and 18 years.
Figure 3.
Prevalence of other food allergies (wheat, peanut, tree nuts, fish, shellfish, fruits and vegetables) at different ages in the Isle of Wight Birth Cohort
The prevalence of multiple food allergy (i.e. 2 or more food groups) remained in the range of 0.7% to 1.3% over 18 years, with no significant increase in prevalence over time. The majority of children with multiple allergies were allergic to 2 food groups, and rarely to 3 food groups. Therefore, the subtotal of individual FA percentages exceeds the overall prevalence at 1, 2, 4, 10 and 18 years. Cow’s milk and egg were the most common multiple food allergies at 1, 2 and 4 years (4 cases each), while at 10 years these were peanut and egg (n=3) and at 18 years, multiple nuts (4 cases).
Sensitisation to food and aeroallergens
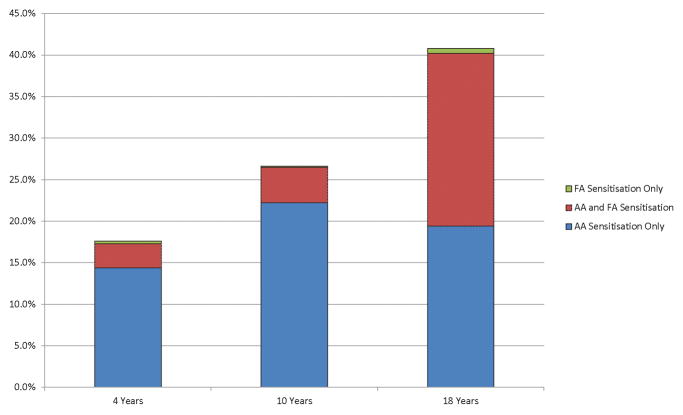
The sensitisation rates to food and aeroallergens increased with age. For aeroallergens the sensitisation rates were 17.3%, 26.7% and 40.3% at 4, 10 and 18 years respectively, compared to sensitisation rates to food allergens of 3.4%, 4.4% and 21.4% at the same time points (Table 1). The majority of food allergen sensitised children were also sensitised to aeroallergens (Figure 4).
Figure 4.
Sensitisation patterns to aeroallergens and food allergens at 4, 10 and 18 years, based on SPT results. Aeroallergen sensitisation increases with age and reaches a maximum of 40.2% by 18 years and food allergen sensitisation reaches a maximum of 21.4% by 18 years of age. The majority of the food allergen sensitised population are also sensitised to aeroallergens, while there were few who were sensitised only to food allergens.
FA: food allergens
AA: aeroallergens
AA+FA: aero- plus food allergens
Milk, peanut, wheat and cod were the most common food allergens causing sensitisation. Cow’s milk sensitisation rates remained similar at 4 (1.1%) and 18 years (1.0%) with a dip to 0.3% at 10 years (Figure 2). Peanut sensitisation rates were higher than peanut FA rates and increased between 4, 10 and 18 years from 1.0%, to 1.7%, to 6.3% respectively (Figure 3). Peanut allergy rates did not mirror this trend, and remained at 0.5, 0.4 and 1% respectively. Wheat sensitisation rates also did not reflect observed food allergy rates. They gradually increased from 0.1% to 6.3%, whilst wheat allergy rates remained <0.5% at all time points. Similarly, cod sensitisation remained low at 4 and 10 years but increased at 18 years to 1.8% with no corresponding increase in prevalence of cod allergy.
DISCUSSION
This is the first study reporting prevalence and natural history of both food allergy (FA) and sensitisation from birth to 18 years in an unselected population, using strict criteria and capturing time trends in individual food allergies. As the IOW cohort is an unselected and longitudinal birth cohort which has been followed up to the age of 18 years, it was possible to follow trends of FA throughout childhood. We showed an expected decline in FA prevalence after early childhood which we associate with the resolution of transient food allergies, but there was an unexpected rise in overall prevalence during teenage years, which was more prominent for food allergen sensitisation than for FA.
The prevalence of FA in childhood has been previously reported using methods which ranged from self-reported questionnaire surveys to double-blind, placebo-controlled food challenges (DBPCFC) (19). DBPCFC is considered the ‘gold standard’ but has only been used in a minority of research studies, often with biased populations due to difficulties in administration. In our study, FA was diagnosed using symptom based criteria, reflecting clinical practice, and included the characteristics of the reaction, timing from exposure and exposure to common food allergens. The results have been published previously (15). We did not use reproducibility of allergic reaction as a diagnostic criterion. Although useful when present, it may not be present in individuals who have had a severe or anaphylactic reaction and would not be further exposed. Other studies have used similar criteria to define food allergy (20–22). We also did not use allergic sensitisation as a criterion as we had incomplete information for allergic sensitisation at 1 and 2 years and no information on food allergen specific IgE. Hence, we cannot reliably comment on the prevalence of whether these were IgE or non-IgE mediated food allergy. However, we have reported IgE mediated allergy as a subset of those with food allergy if they had a positive skin prick test to food allergens. The prevalence of FA in our cohort varied between 2.3%–5.3%, in children between 1–18 years, while IgE mediated food allergy varied between 0.8% and 2.2%. Similar prevalence has been reported in other birth cohort studies which have used open and DBPCFC for diagnosis of FA (6, 20, 23–27).
Self-reported FA rates were lower compared to other studies; both cross-sectional and birth cohort (7, 28). This could be explained by the fact that targeted FA symptom specific questionnaires were used to collect data in person (where possible) at every assessment, rather than from an anonymous FA questionnaire survey. Similar prevalence rates for FA in children and adolescents have been reported in other studies that have used symptom based surveys like our study (22, 29–31). In the food allergic sub-group, SPT availability rates varied between 37.2% and 85.5 %, which may explain the low SPT positivity in our food allergic population. However, similar low SPT positivity has been found in other studies.(7)
FA prevalence according to our study criteria were in line with other studies and suggest that our symptom based criteria was reasonably robust. The FA prevalence at 10 years (2.3%) was the same as previously reported in a cross sectional study in the IOW for 11 years old children which used DBPCFC (32), The 18 years prevalence of 4% is similar to that shown in previous cross-sectional studies in adult populations i.e. 3.2% and 3.6% (33). In a large Swedish birth cohort, the prevalence of FA and food related symptoms in early childhood was slightly higher (6.8% and 12.2% respectively) compared to this study where FA in early childhood ranged from 4.4% to 5.3% and reported FA was ~9% (34). However, when compared to the Food Allergy and Intolerance Research (FAIR) cohort, which was also recruited on the IOW, but a decade later (born between 2001–2002), the prevalence in our study is slightly higher (7, 27). The variance may be attributable to the use of DBPCFC in the FAIR cohort. Similarly, Osterballe et al reported a lower prevalence of 2.3% in children under the age of 3 years old when using food challenges (25). A recent population based study from Australia, where participants were recruited at a year of age, found an oral challenges defined FA prevalence of 10% in 2848 one year olds (egg allergy 8.9%, peanut allergy 3% and sesame allergy 0.8%) (35).
There were no significant overall changes during the prevalence of food allergy during the 1–2 year and 2–4 year transition windows; however, there was a significant reduction in FA between 4–10 years (5.3% to 2.3%) followed by significant increase between 10 and 18 years (2.3 to 4.1%). This negative transition at 10 years may represent the gradual acquisition of natural tolerance of childhood FA’s by 10 years of age. The subsequent positive transition at 18 years suggests the resurgence of new and possibly persistent forms of FA. These transitions were similar in the stable sub-group (n=843) of the cohort, where FA information was available throughout the 5 points of assessment.
Cow’s milk and eggs were the most common allergens at 1, 2, 4 and 10 years, and peanut, tree nuts, and shellfish became more prevalent at 18 years. Fruit, kiwi, mushrooms and mustard were generally new allergies at 10 and 18 years.
The rate of cow’s milk allergy varied between 1.6% and 3.5% between 1–4 years, 0.5% at 10 and 0.3% at 18 years. Other studies have shown a similar milk allergy prevalence of 2–3% based on OFC/DBPCFC in the first 3 years of life (4, 23, 36, 37), although a study by Gerrard et al (38) reported a much higher milk allergy prevalence of 7.4% on the basis of OFC. A large cross sectional survey of 38,380 children (39) showed a self-reported milk allergy prevalence of 1.5% between 6–10 years, 1.4% between 11–13 years, and 1.6% in children over 14 years, which is significantly higher than our study (0.5% at 10 years and 0.3% at 18 years).
Egg allergy rates varied from 1.1% to 1.4% between the ages of 1–4 years, similar to that reported by other population studies at 1–3 years (1–1.6%) using OFC/ DBPCFC (4, 27). Prevalence at 10 years (0.6%) and 18 years (0.3%) was also similar to that reported in the cross-sectional survey by Gupta et al for children over 6 years (39).
Peanut allergy data from this cohort has been published previously (9, 11, 16, 26, 40). However, using a different case ascertainment criteria in this study (i.e. not using allergen sensitisation as a requirement), peanut allergy was 0.08%–1% between the ages of 1–18 years. This is similar to previous reports up to the age of 10 years but higher at 18 years (1% compared to 0.5% reported previously)(16). Other studies have reported a similar prevalence (0.2% to 1.6%) (25, 41, 42).
Fish allergy was 0.2% at all ages, while shellfish allergy started to emerge at 10 and 18 years (0.2%). A recent meta-analysis (3) reported an overall prevalence of fish allergy of <0.5%, and shellfish allergy between 0.0% to 1.4% (based on symptoms and specific IgE). Our study reports lower rates for shellfish allergy at 10 and 18 years, which probably captures a period of gradual emergence of shellfish allergy in its’ natural history where it is more often associated with adult FA.
Wheat allergy prevalence in our study varied between 0.1 to 0.5% between 1 and 18 years. Gupta et al, in their US cross-sectional survey, reported higher prevalence (0.3 to 0.7%), with a peak at 11–13 years (39) whereas our study shows lowest prevalence at 10 years (0.1%). Allergy to fruits varied between 0.3% and 0.9%. Kiwi allergy was mainly reported at 10 and 18 years (0.1%–0.2%). Other studies have reported fruit allergy between 0.1 and 4.3% (43). Among miscellaneous food allergy, chocolate was commonly suspected. This may be due to undiagnosed nut allergy or cross-contamination with nuts.
Focusing on sensitisation, we showed a rise in overall prevalence, more prominent for food allergen sensitisation than for FA. Kulig et al (44) report diminishing sensitisation rates to food allergens (from 10% at 1 year to 3% at 6 years) but this study did not look at sensitisation rates beyond 6 years. Pereira et al (32) reported food allergen sensitisation rates of 5.1% and 4.9% in 11 and 15 year old children in cross sectional studies conducted on the IOW (independent of the IOW cohort). This is similar to our findings at 10 years (4.5%) but we found a higher prevalence at 18 years (21.4%), which may indicate that the rise in food allergic sensitisation occurs in later adolescence.
Wheat, peanut and milk were the most common sensitising foods. The high rates of wheat sensitisation could be due to cross-reactivity with grass pollen, as increasing wheat sensitisation (1%, 2.3%, and 19.3% at 4, 10 and 18 years) did not mirror our wheat allergy rates (which instead remained static) but did mirror grass pollen sensitisation. Using the FAIR cohort, we have recently shown the significance of cross reactivity between grass and wheat pollen (45). Similarly, the increase in peanut sensitisation between 10 and 18 years was likely to be due to cross reactivity between peanut and grass or birch pollen (16).
Our study has a number of strengths. It is the first to investigate the longitudinal trends in prevalence and the dynamics of change in FA and sensitisation patterns in childhood and adolescence. The unselected nature of the cohort, the high retention rate (84% to 94.3%) and prospective and repeated collection of food allergy information (5 specific time points) are strengths of this study.
The study has a few limitations. There was a significant gap in terms of number of years between 4th (10 year) and 5th (18 years) study assessments. However, different individuals progress through puberty at different times so by assessing at 10 and 18 years we have been able to compare the population when most were pre- and post-pubertal. Clinical studies show that milk and egg allergy continue to decline during teenage years (Savage 2007, Skripak 2007) with the trajectories following a straight line and thus we have likely captured transition changes before and after puberty (46, 47). A birth cohort study, where participants were assessed during adolescence, shows similar FA prevalence (34). The birth cohort was dynamic, and some children did not participate at one time point, but re-joined at another, which made it somewhat difficult to interpret transitions (emergence of new allergies and the resolution of existing allergies) in the whole population, but this is unavoidable in a study spanning over 18 years. Although lack of oral challenge remains a limitation, we have used previously published robust clinical criteria (15), which have provided results comparable to studies using the ‘gold standard’. Although our definition included reactions up to 4 hours after exposure, this is unlikely to have resulted in much misclassification as other adverse reactions to food would have not presented with the IgE-mediated symptoms in our definition. With lack of SPT data on all children at 1 and 2 years and of specific IgE, we were unable to assess the proportion of IgE mediated allergy in this report or patterns of sensitisation in the whole cohort in the first 2 years of life.
In conclusion, our study provides valuable information on the prevalence and longitudinal patterns of FA and food sensitisation not only in childhood but also during adolescence. There is little available data on this age group even though the adolescent population are at the highest risk of severe allergic reactions and death as a consequence of FA (48). The prevalence of FA’s was high in early childhood with a significant decrease in prevalence at 10 years, suggesting resolution of early childhood allergies followed by onset of new food allergies, resulting in higher prevalence at 18 years.
Supplementary Material
Acknowledgments
We would like to acknowledge the help of the staff at The David Hide Asthma and Allergy Research Centre in undertaking the assessments of the Isle of Wight Birth Cohort. We would also like to acknowledge the participants and their families who helped us with this project over the last two decades. The Isle of Wight Birth Cohort assessments have been supported by the National Institutes of Health USA (Grant no. R01 HL082925) and Asthma UK (Grant no. 364).
Footnotes
Conflict of interest statement
None of the authors have any conflicts of interests to declare.
References
- 1.Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology. 2004;113(5):832–6. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 2.Roberts G, Lack G. J Allergy Clin Immunol. Vol. 115. United States: 2005. Diagnosing peanut allergy with skin prick and specific IgE testing; pp. 1291–6. [DOI] [PubMed] [Google Scholar]
- 3.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: A meta-analysis. Journal of Allergy and Clinical Immunology. 2007;120(3):638–46. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69(1):62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 5.Venter C, Patil V, Grundy J, Glasbey G, Twiselton R, Arshad SH, et al. Prevalence and cumulative incidence of food hyper-sensitivity in the first 10 years of life. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2016;27(5):452–8. doi: 10.1111/pai.12564. [DOI] [PubMed] [Google Scholar]
- 6.Dean T, Venter C, Pereira B, Arshad SH, Grundy J, Clayton CB, et al. Patterns of sensitization to food and aeroallergens in the first 3 years of life. The Journal of allergy and clinical immunology. 2007;120(5):1166–71. doi: 10.1016/j.jaci.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63(3):354–9. doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62(1):91–6. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tariq SM, Stevens M, Matthews S, Ridout S, Twiselton R, Hide DW. Cohort study of peanut and tree nut sensitisation by age of 4 years. Bmj. 1996;313(7056):514–7. doi: 10.1136/bmj.313.7056.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheikh A, Alves B. Hospital admissions for acute anaphylaxis: time trend study. BMJ. 2000;320(7247):1441. doi: 10.1136/bmj.320.7247.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: Data from 2 sequential cohorts. The Journal of allergy and clinical immunology. 2002;110(5):784–9. doi: 10.1067/mai.2002.128802. [DOI] [PubMed] [Google Scholar]
- 12.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. J Allergy Clin Immunol. Vol. 122. United States: 2008. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy; pp. 984–91. [DOI] [PubMed] [Google Scholar]
- 13.Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010;65(3):258–62. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts G, Zhang H, Karmaus W, Raza A, Scott M, Matthews S, et al. Trends in cutaneous sensitization in the first 18 years of life: results from the 1989 Isle of Wight birth cohort study. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2012;42(10):1501–9. doi: 10.1111/j.1365-2222.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 15.Venkataraman D, Soto-Ramirez N, Kurukulaaratchy RJ, Holloway JW, Karmaus W, Ewart SL, et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. The Journal of allergy and clinical immunology. 2014;134(4):876–82. e4. doi: 10.1016/j.jaci.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arshad SH, Venter C, Roberts G, Dean T, Kurukulaaratchy R. The natural history of peanut sensitization and allergy in a birth cohort. The Journal of allergy and clinical immunology. 2014;134(6):1462–3. e6. doi: 10.1016/j.jaci.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HFW, PJA . Committee on toxicity of chemicals in foods, consumer products and the environment: Allergic reactions to food and food ingredients. Crwon Publications; 2000. [Google Scholar]
- 18.Niggemann B. Allergy. Vol. 65. Denmark: 2010. When is an oral food challenge positive? pp. 2–6. [DOI] [PubMed] [Google Scholar]
- 19.Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, et al. Allergy. Vol. 59. Denmark: 2004. Standardization of food challenges in patients with immediate reactions to foods--position paper from the European Academy of Allergology and Clinical Immunology; pp. 690–7. [DOI] [PubMed] [Google Scholar]
- 20.Roehr CC, Edenharter G, Reimann S, Ehlers I, Worm M, Zuberbier T, et al. Clin Exp Allergy. Vol. 34. England: 2004. Food allergy and non-allergic food hypersensitivity in children and adolescents; pp. 1534–41. [DOI] [PubMed] [Google Scholar]
- 21.Rancé F, Grandmottet X, Grandjean H. Prevalence and main characteristics of schoolchildren diagnosed with food allergies in France. Clin Exp Allergy. 2005;35(2):167–72. doi: 10.1111/j.1365-2222.2005.02162.x. [DOI] [PubMed] [Google Scholar]
- 22.Ostblom E, Wickman M, van Hage M, Lilja G. Reported symptoms of food hypersensitivity and sensitization to common foods in 4-year-old children. Acta paediatrica (Oslo, Norway : 1992) 2008;97(1):85–90. doi: 10.1111/j.1651-2227.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 23.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987;79(5):683–8. [PubMed] [Google Scholar]
- 24.Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. Lancet. Vol. 343. England: 1994. A population study of food intolerance; pp. 1127–30. [DOI] [PubMed] [Google Scholar]
- 25.Osterballe M, Hansen TK, Mortz CG, Host A, Bindslev-Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2005;16(7):567–73. doi: 10.1111/j.1399-3038.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 26.Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65(1):103–8. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 27.Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. The Journal of allergy and clinical immunology. 2006;117(5):1118–24. doi: 10.1016/j.jaci.2005.12.1352. [DOI] [PubMed] [Google Scholar]
- 28.Roehr CC, Edenharter G, Reimann S, Ehlers I, Worm M, Zuberbier T, et al. Food allergy and non-allergic food hypersensitivity in children and adolescents. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2004;34(10):1534–41. doi: 10.1111/j.1365-2222.2004.02080.x. [DOI] [PubMed] [Google Scholar]
- 29.Steinke M, Fiocchi A, Kirchlechner V, Ballmer-Weber B, Brockow K, Hischenhuber C, et al. Perceived food allergy in children in 10 European nations. A randomised telephone survey. Int Arch Allergy Immunol. 2007;143(4):290–5. doi: 10.1159/000100575. [DOI] [PubMed] [Google Scholar]
- 30.Brugman E, Meulmeester JF, Spee-van der Wekke A, Beuker RJ, Radder JJ, Verloove-Vanhorick SP. Prevalence of self-reported food hypersensitivity among school children in The Netherlands. Eur J Clin Nutr. 1998;52(8):577–81. doi: 10.1038/sj.ejcn.1600609. [DOI] [PubMed] [Google Scholar]
- 31.Venter C, Pereira B, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: a population-based study. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2006;17(5):356–63. doi: 10.1111/j.1399-3038.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 32.Pereira B, Venter C, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. The Journal of allergy and clinical immunology. 2005;116(4):884–92. doi: 10.1016/j.jaci.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 33.Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, et al. Allergy. 2010. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma; p. 65. [DOI] [PubMed] [Google Scholar]
- 34.Protudjer JL, Vetander M, Kull I, Hedlin G, van Hage M, Wickman M, et al. Food-Related Symptoms and Food Allergy in Swedish Children from Early Life to Adolescence. PloS one. 2016;11(11):e0166347. doi: 10.1371/journal.pone.0166347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. J Allergy Clin Immunol. Vol. 127. United States: Mosby, Inc; 2011. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants; pp. 668–76 e1.pp. 2 Asthma & Immunology. [DOI] [PubMed] [Google Scholar]
- 36.Host A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990;45(8):587–96. doi: 10.1111/j.1398-9995.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 37.Schrander JJ, van den Bogart JP, Forget PP, Schrander-Stumpel CT, Kuijten RH, Kester AD. Cow’s milk protein intolerance in infants under 1 year of age: a prospective epidemiological study. Eur J Pediatr. 1993;152(8):640–4. doi: 10.1007/BF01955238. [DOI] [PubMed] [Google Scholar]
- 38.Gerrard JW, MacKenzie JW, Goluboff N, Garson JZ, Maningas CS. Cow’s milk allergy: prevalence and manifestations in an unselected series of newborns. Acta Paediatr Scand Suppl. 1973;234:1–21. [PubMed] [Google Scholar]
- 39.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 40.Venter C, Maslin K, Patil V, Kurukulaaratchy R, Grundy J, Glasbey G, et al. The prevalence, natural history and time trends of peanut allergy over the first 10 years of life in two cohorts born in the same geographical location 12 years apart. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2016 doi: 10.1111/pai.12616. [DOI] [PubMed] [Google Scholar]
- 41.Kagan RS, Joseph L, Dufresne C, Gray-Donald K, Turnbull E, Pierre YS, et al. J Allergy Clin Immunol. Vol. 112. United States Canada: 2003. Prevalence of peanut allergy in primary-school children in Montreal, Canada Peanut allergy: an overview; pp. 1223–8. [DOI] [PubMed] [Google Scholar]
- 42.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. The Journal of allergy and clinical immunology. 2003;112(6):1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 43.Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers C, et al. The prevalence of plant food allergies: a systematic review. The Journal of allergy and clinical immunology. 2008;121(5):1210–8. e4. doi: 10.1016/j.jaci.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. The Journal of allergy and clinical immunology. 1999;103(6):1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 45.Venter C, Maslin K, Arshad SH, Patil V, Grundy J, Glasbey G, et al. Very low prevalence of IgE mediated wheat allergy and high levels of cross-sensitisation between grass and wheat in a UK birth cohort. Clin Transl Allergy. 2016;6:22. doi: 10.1186/s13601-016-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. The Journal of allergy and clinical immunology. 2007;120(5):1172–7. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. The Journal of allergy and clinical immunology. 2007;120(6):1413–7. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 48.Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30(8):1144–50. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.