Summary
Recent studies have demonstrated pleiotropic effects of statins in various mouse models of kidney disease. In this study, Townes humanized sickle cell mice were treated for 8 weeks with atorvastatin at a dose of 10 mg/kg/day starting at 10 weeks of age. Treatment with atorvastatin significantly reduced albuminuria, and improved both urine concentrating ability and glomerular filtration rate. Atorvastatin also decreased markers of kidney injury and endothelial activation, and ameliorated oxidant stress in renal tissues and peripheral macrophages. Atorvastatin downregulated the expression of mRNA levels of the NADPH oxidases, Cybb (also termed Nox2) and Nox4, which are major sources of oxidant stress in the kidney. These findings highlight the pleiotropic effects of atorvastatin and suggest that it may provide beneficial effects in sickle cell nephropathy.
Keywords: Sickle Cell Disease, Sickle Cell Nephropathy, Oxidant Stress, Statins, Albuminuria
Introduction
Sickle cell disease (SCD) affects approximately 100,000 people in the United States. It is an autosomal recessive disorder due to a single point mutation in the HBB (β-globin) gene of, with haemoglobin polymerization leading to characteristic red blood cell (RBC) sickling, vaso-occlusion and haemolysis. Beginning in infancy, individuals with SCD experience a broad range of acute and chronic complications due to vascular occlusion and endothelial damage by sickled RBCs and other cellular elements, resulting in end organ damage, including renal dysfunction (Ataga, et al 2014).
Sickle cell nephropathy (SCN) is the term used to describe the constellation of renal complications that results from SCD. Renal dysfunction begins early in the first decade of life and is commonly manifested as microalbuminuria; occurring in approximately 20% of SCD patients aged 18 years and under (McPherson Yee, et al 2011). The problem worsens with age and approximately 68% patients over the age of 40 years have evidence of SCN, proteinuria and decreased renal function (Guasch, et al 2006). End stage renal disease (ESRD) develops in 4.2 to 11.6% of adults with SCD and is an independent predictor of premature mortality in young adults (Powars, et al 2005, Powars, et al 1991). Furthermore, ESRD is associated with an increased risk of mortality compared with those without this diagnosis (Abbott, et al 2002).
The pathophysiology of SCN is not well-understood and commonly attributed to repeated vaso-occlusive crises (VOCs), intermittent hypoxia and impaired protective cellular mechanisms. Repeated episodes of hypoxia/reperfusion lead to endothelial activation, reduced nitric oxide bioavailability and augmented oxidative stress. Endothelial activation, defined by endothelial expression of cell-surface adhesion molecules, such as soluble vascular cell adhesion molecule-1 (sVCAM-1), perpetuates inflammation, resulting in vascular disease. SCD creates a state of increased oxidative stress, with increased production of reactive oxygen species (ROS), the effects of which contribute significantly to the long-term pathology of SCD (Kupesiz, et al 2012). NADPH oxidase (NOX), a membrane-bound enzymatic complex, is the most important source of ROS in the vasculature, and produces superoxide when activated (Margaritis, et al 2014). NOX activity has been implicated in SCD-induced endothelial dysfunction in sickle cell mice (Wood et al 2005).
Current treatment options for SCN aim to decrease albuminuria by initiating an angiotensin converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB) (Falk, et al 1992, Foucan, et al 1998). Hydroxycarbamide (also termed Hydroxyurea), a drug that increases fetal haemoglobin and reduces erythrocyte sickling, decreases albuminuria in patients with SCD (Bartolucci, et al 2016, Fitzhugh, et al 2005, Tehseen, et al 2017). However, available drug treatment options for albuminuria in patients with SCD remain limited, with no large, adequately controlled studies demonstrating the efficacy of any interventions (Quinn, et al 2017). Statins (3-hydroxy-3-methylgutaryl (HMG)-CoA reductase inhibitors) exert lipid-independent effects, including improvement of endothelial function, upregulation of nitric oxide (NO) and reduction in markers of oxidative stress (Margaritis, et al 2014). Nitric oxide donors have been shown to suppress the expression of endothelial adhesion molecules. Statins may restore endothelial function that is altered during repeated episodes of hypoxia-reperfusion associated with VOCs and inhibit vascular inflammation and oxidation (Zhou, et al 2004, Zhou, et al 2008, Zhou and Liao 2010). Statins have been used in various animal models of proteinuric kidney disease, in diabetic nephropathy and anti-glomerular basement membrane disease, demonstrating a decrease in albuminuria and associated pathological changes (Eller, et al 2010, Fujii, et al 2007, Zoja, et al 2010).
We hypothesized that atorvastatin would downregulate mediators of inflammation and oxidant stress, which aid in restoring cytoprotective balance, and slow the progression of SCN. Here we studied the effects of atorvastatin therapy on renal function and markers of oxidative stress in a murine model of SCD.
Materials and Methods
Animals
All mice were maintained and studied in accordance with the US National Research Council’s Guide for the Care and use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee of Emory University. Male and female homozygous humanized sickle cell mice aged of 8-10 weeks, expressing human beta-sickle globin (SS) were used (Ryan, et al 1997, Wu, et al 2006). Similar age-matched male and female Townes mice expressing normal human haemoglobin (AA) were controls. There were three experimental groups with 8 mice per group: AA and SS mice, which were treated with vehicle (controls), and SS mice treated with atorvastatin. Mice were housed under conditions of constant temperature and humidity and exposed to a 12-h light/dark cycle. All mice were given free access to food and water. Twenty-four hour urine samples were collected at two time points, baseline (prior to initiation of treatment) and at 8 weeks.
Mice were placed in standard metabolic cages and allowed to adapt for 24 h prior to obtaining a 24 h urine sample to measure urine albumin, creatinine, nephrin and kidney injury molecule-1 (KIM-1). Mice were water deprived for 12 h and first morning urine was obtained to measure maximal urine concentrating ability using a vapour pressure osmometer (VAPRO, Elitech Company, Logan, UT, USA). Urine osmolality was measured prior to the initiation of treatment and after 4 and 8 weeks of treatment.
Drug Treatments
Atorvastatin was obtained from Pfizer Inc. (New York, NY, USA), through its drug assistance programme and reconstituted in 0.5% methylcellulose, as recommended. SS mice were orally gavaged daily with atorvastatin for 8 weeks. Atorvastatin was administered at 10 mg/kg/day, a dose that has previously been shown to decrease albuminuria, lower cholesterol and decrease inflammation (Eller, et al 2010, Gotoh, et al 2013, Pan, et al 2016). Mice treated with the vehicle (0.5% methylcellulose) served as controls. Control mice were orally gavaged daily for 8 weeks.
Urine analysis
Creatinine
Urine creatinine was measured using the Beckman Creatinine analyser (Beckman, Brea, CA, USA), which determined urine creatinine by the Jaffe rate method as previously published (Cottrell 1979).
Albumin
Urine albumin was measured using the Albumin Mouse enzyme-linked immunosorbent assay (ELISA) kit (ab108792; Abcam, Cambridge, MA, USA). Urine was diluted 1:400, as per manufacturer recommendations. All samples and standards were run in duplicate. Unknown sample concentration was determined from a standard curve. Urine albumin was expressed as albumin/creatinine ratio and normalized to 24-h urine volume [albumin (μg/ml) × 24-h collected volume (ml)].
Urine biomarkers
The concentration of urine KIM-1, nephrin and neutrophil gelatinase-associated lipocalin (NGAL) was measured using the KIM-1 mouse ELISA kit (ab119596; Abcam), Cloud-Clone Corp (Katy, TX, USA) nephrin mouse ELISA kit (SEA937Mu) and BIOPORTO Diagnostics (Hellerup, Denmark) mouse NGAL ELISA Kit (KIT 042), respectively. All ELISA kits were processed following manufacturers directions. Unknown sample concentrations were determined from standard curves. Urine biomarkers were expressed as a ratio of urine creatinine (see Appendix S1).
Plasma Biomarkers
The concentration of plasma sVCAM-1 was measured using a mouse ELISA Kit (MCV00; R&D Systems, Minneapolis, MN, USA) (see Appendix S1).
Procedures
Blood was collected via retro-orbital bleeding into sodium EDTA tubes under isoflurane anesthesia at two time points. The first-time point occurred at initiation of the experiment (prior to receipt of study treatment) and the second-time point occurred at 8 weeks. Complete blood counts (CBC) were measured using a HemaTrue machine (HESKA Corp., Loveland, CO, USA) at the beginning of the experiment and after 8 weeks of treatment.
GFR
Glomerular filtration rate (GFR) was estimated at three time points, 0, 4 and 8 weeks. GFR was calculated using plasma clearance of 5% fluorescein isothiocyanate (FITC)-Inulin following a single bolus of intravenous injection as previously described (Qi 2003). Approximately 2 μl of blood was collected at 3, 7, 10,15, 35, 55, 75 and 95 min post-injection of FITC-inulin for determination of FITC concentration by fluorescence.
Renal Histology
The excised kidneys were fixed in formalin and processed by the Pathology Core at the Children’s Healthcare of Atlanta. 3-μm sections of kidney samples were stained with Masson trichrome, haematoxylin and eosin, and periodic-acid-Schiff stain. The same pathologist analysed all renal biopsies and was blinded to the treatment groups. The presence of glomerular changes, including basement membrane thickening, focal segmental sclerosis, mesangial hypercellularity, mesangial expansion, interstitial inflammation, interstitial fibrosis and tubular atrophy, were evaluated according to the Nephrotic Syndrome Study Network (NEPTUNE) digital pathology scoring system (Barisoni, et al 2016). AA mice served as controls. Mesangial hypercellularity was defined as greater than 3 mesangial cells: 0 - none (≤3 mesangial cells), 1 - mild (3-4 mesangial cells), 2- moderate (5-6 mesangial cells), and 3- severe (>6 mesangial cells).
Estimation of Glomerular Area
Glomerular area was estimated by tracing glomerular tufts using the NanoZoomer Digital Pathology computer program, 2015) (Hamamatsu Photonics K.K., Hamamatsu, Japan). Approximately 50 glomeruli were measured per mouse.
Flow Analysis
We collected blood as described above for red blood cell (RBC), white blood cell (WBC) and reticulocyte analysis. For RBC and reticulocyte analysis, blood was incubated with TER-119, CD45 and Thiazole at room temperature for 15 min (Table SI). The remaining blood was processed utilizing dextran sedimentation and hypotonic lysis (Appendix S1). For WBC analysis, antibodies CD45, CD19, Ly6G, CD3, Live/Dead, and CD11b (Table SI). For measurement of ROS, a DCFDA (2’,7’ – dichlorofluorescin diacetate) Cellular ROS Detection Assay Kit (ab113851; Abcam) was used, following manufacturer instructions. Cell-surface molecule measurements were performed on a Becton Dickinson (BD) LSRII collecting 50,000 events per sample and data were analysed using the FACSDIVA Software (BD, Franklin Lakes, NJ, USA).
mRNA isolation and measurement
mRNA was isolated using the Pure Link Mini RNA extraction kit (Ambion, Austin, TX) (Boesen, et al 2008) Real time polymerase chain reaction was carried out using iTaq Universal Probes Mastermix (Biorad, Hercules, CA, USA) and Taqman primer gene expression assays (Applied Biosystems, Foster City, CA, USA; CYBB (NOX2): Mm01287743_m1, NOX4: Mm00479246_m1, EDN1 (ET-1): Mm00438656_m1; GAPDH: Mm999999_g1). Results were quantified using 2-ΔΔCt and GAPDH served as the internal control (Schmittgen and Livak 2008).
GSH, GSSG, CYS and CYS levels in mouse kidney
Kidney tissue was used to measure reduced glutathione (GSH), glutathione disulphide (GSSG), reduced cysteine (CYS) and cystine (CYSS) as previously reported (Yeligar, et al 2014). The redox potential (Eh) of the thiol pair was calculated using the Nernst equation as previously reported. (Yeligar, et al 2014) A more positive Eh indicates greater oxidation of the thiol pair.
Statistical Analysis
A sample size of eight mice per group would be needed to achieve a power of 0.8, at an alpha of 0.05 with a 95% confidence interval. Data are presented as mean ±standard error of the mean (SEM). Comparisons between groups were made by paired t-tests, with p<0.05 being considered statistically significant. Welch’s correction was applied if F-test demonstrated significance. Mann-Whitney test was employed in datasets that did not demonstrate normal distribution, with p<0.05 considered significant. Two-factor repeated measures analysis of variance models with one within-factor (time: 0, 4, 8 weeks) and one between-factor (group: SS vehicle vs. SS statin) were employed. Models included a treatment-by-time interaction and adjusted least square mean estimates with reporting of standard errors. To determine which time points were significantly different, post-hoc pairwise comparisons between groups and across groups were performed. Unadjusted p-values were reported. Data analysis was carried out using Prism v6 (Graphpad Software Inc, La Jolla, CA, USA).
Results
Atorvastatin ameliorates renal dysfunction in sickle cell mice
Baseline renal function and haematological indices in AA and SS mice are shown in Table I. Ten-week-old sickle cell mice demonstrate SCN with abnormal renal function; impaired urine concentrating ability, and albuminuria. They have significant anaemia and profound reticulocytosis when compared to control mice. Urine concentrating defects significantly improved in SS mice treated with atorvastatin after 4 weeks and maintained at 8 weeks (SS statin 4 weeks: 2364±42 mmol/kg and 8 weeks: 2410 ± 50 mmol/kg vs. SS vehicle 4 weeks: 2165 ± 54 mmol/kg, 8 weeks: 2157 ± 72 mmol/kg, p< 0.01) (Fig 1A). Daily treatment of SS mice with atorvastatin significantly increased GFR, which was first observed after 4 weeks of treatment and was maintained after 8 weeks of treatment, (SS statin 4 weeks: 115.4 ± 3.2 μl/min and 8 weeks: 118.7 ± 1.8 μl/min vs. SS vehicle 4 weeks: 101.0 ± 2.0 μl/min (p<0.01) and 8 weeks: 103.6 ± 1.9 μl/min, p <0.001) (Figure 1B).
Table I. Characteristics of treatment groups at baseline and after 8 weeks of treatment.
AA mice vs SS mice, p<0.001 (vehicle and treated). No differences in Glomerular filtration rate (GFR), Urine Osmolality, Albumin excretion rate (AER), Haematocrit (Hct) and Reticulocyte (Retic) count between SS groups.
| Baseline | 8 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GFR (ul/min) | Urine Osmolality | AER (ug/day) | Hct (%) | Retic (%) | GFR (ul/min) | Urine Osmolality | AER (ug/day) | Hct (%) | Retic (%) | |
| AA vehicle | 132 ± 6 | 2854 ± 125 | 96 ± 8 | 31 ± 0.4 | 5 ± 0.3 | 162 ±8 | 3930 ±296 | 210 ±16 | 32 ±0.5 | 4.6 ±0.3 |
| SS vehicle | 98.0± 3 | 2077± 50 | 148 ± 14 | 28 ± 1.0 | 38 ± 1 | 103 ±2 | 2102 ± 72 | 321 ± 30 | 25 ±1.3 | 39± 2.3 |
| SS statin | 102± 2 | 2078 ± 51 | 151± 15 | 26 ± 0.5 | 43± 2 | 118 ±2 | 2427 ±119 | 219 ±21 | 34 ±1.3 | 24 ±0.6 |
Figure 1. Atorvastatin treatment improves renal function in Sickle Cell mice.
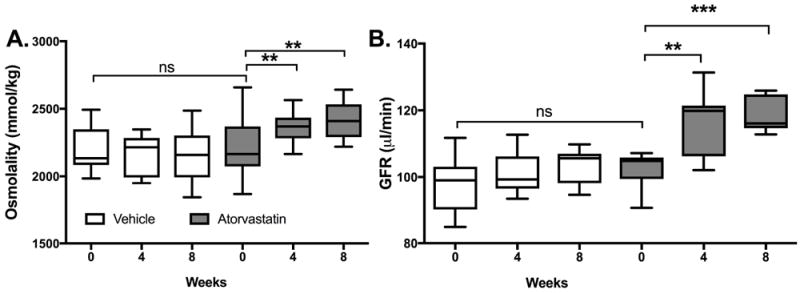
Sickle cell mice were treated with 10 mg/kg/day Atorvastatin for 8 weeks. Significant improvement in maximal urinary concentrating ability (water-deprived) and glomerular filtration rate (GFR) was demonstrated at 4 and 8 weeks (a) and (b), n =8. **p<0.01 and ***p<0.001
Treatment with atorvastatin over 8 weeks resulted in significant improvement in the albumin excretion rate, (AA: 228.9 ± 65.6 μg/day, SS vehicle: 321.5 ± 81.1 μg/day vs. SS statin 219.1 ± 60.9 μg/day) (Fig 2A). The albumin to creatinine ratio demonstrated a significant decrease in atorvastatin treated mice, p<0.05 (Fig 2B). SS mice have higher urine volume compared to AA mice (p<0.01), probably due to their poor urine concentrating abilities despite water deprivation, which will achieve maximum concentrating ability (Fig 2C). After 8 weeks of treatment, atorvastatin significantly improved urine volume in SS treated mice (p<0.05). No significant changes were observed in haematological indices, haematocrit, RBC count or reticulocyte count.
Figure 2. Biomarkers of Renal Function and Haematological indices.
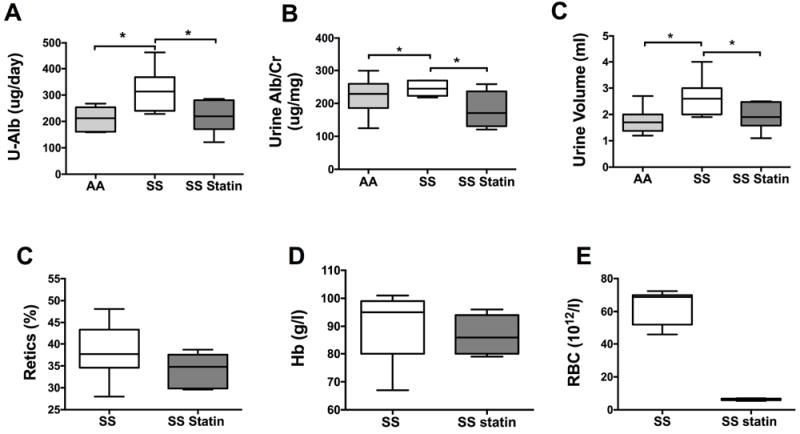
Sickle cell mice treated with Atorvastatin for 8 weeks demonstrated significant decrease in urine albumin excretion rate (a), albumin to creatinine ratio (b) and urine volume (24hr collection) (c) p<0.05, n=8. Haematological markers of sickle cell disease did not change with Atorvastatin treatment (c, d and e), n=8. Cr: creatinine; Hb: haemoglobin concentration; RBC: red blood cell count; Retics: reticulocytes; U-Alb: urine albumin.
Renal Histology
To determine changes in renal histology due to atorvastatin treatment, glomerular tuft size was assessed in the treatment groups. Non-sickle phenotype, AA mice demonstrate significantly smaller mean glomerular area (2595 ± 82.6 μm2) compared to SS mice (3712 ± 76.0 μm2), p<0.001 (Fig 3A). Treatment with atorvastatin significantly improved mean glomerular tuft area (3387 ± 76.36 μm2), p<0.01. Figure 3B, demonstrates left curve shift to smaller glomerular area in atorvastatin-treated mice. Mesangial hypercellularity and mesangial matrix expansion was identified in SS mice. There were no glomerular basement membrane changes or focal segmental sclerosis identified. Glomeruli that were most affected in the sections were used to assess for mesangial matrix expansion and mesangial hypercellularity. However, there were no qualitative differences found between the two treated SS mice groups.
Figure 3. Glomerular tuft area improved with Atorvastatin treatment.
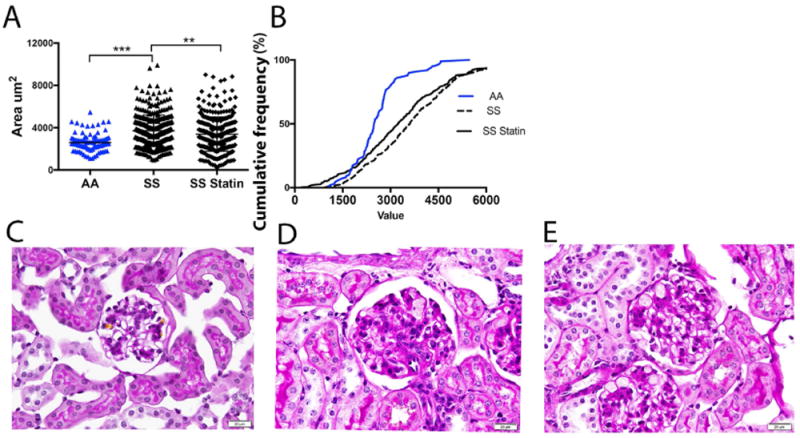
A. SS mice demonstrated significant glomerular hypertrophy; AA vs SS, ***p<0.001. Glomerular tuft area significantly decreased with Atorvastatin treatment in SS mice; **p<0.01 B. Histogram demonstrating shifts in cumulative size of glomerular tufts of SS treated mice. For assessment of sickle cell nephropathy Periodic-acid-Schiff stained sections (400x) were examined using light microscopy. C. No glomerular hypertrophy was seen in AA mice. SS vehicle (D) and SS statin-treated mice (E) exhibited sickle cell phenotype.
Biomarkers of renal injury in sickle cell nephropathy
We evaluated biomarkers of renal glomerular and tubular injury to assess the ability to predict early renal disease. We studied urinary KIM-1, nephrin and NGAL. Both urinary nephrin and KIM-1 were significantly lower in AA mice. After 8 weeks of treatment with atorvastatin, urine KIM-1 significantly improved in SS mice (p<0.05) (Fig 4). No significant decreases were observed in the levels of urine NGAL and nephrin following 8 weeks of treatment. However, there was a trend towards a significant decrease in urine nephrin in atorvastatin-treated SS mice (SS vehicle 6.75 ± 1.2 ng/day vs. SS statin 4.0 ± 0.5 ng/day, p= 0.056).
Figure 4. Urinary and Plasma Biomarkers in sickle cell neuropathy.
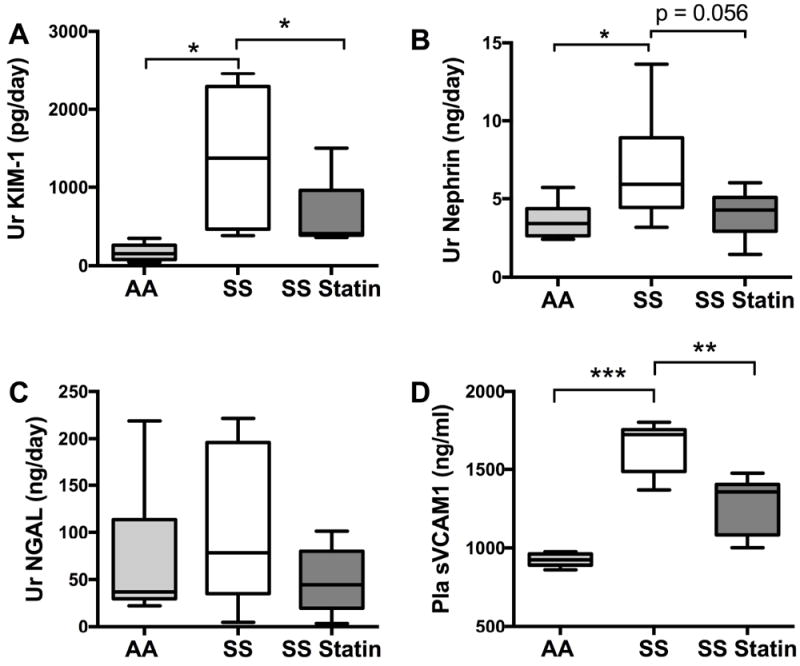
After 8 weeks of Atorvastatin treatment, sickle cell mice demonstrated significant decrease in KIM-1, *p<0.05 (A) without seeing significant changes in urine (Ur) Nephrin and NGAL (B and C). SS mice have elevated levels of Ur KIM-1 and Ur Nephrin (A and B), *p<0.05. C. Plasma (Pla) sVCAM1 is elevated in SS mice, ***p<0.001. After 8 weeks of statin treatment there is a significant decrease in plasma sVCAM1, **p<0.01.
In order to assess the effect of atorvastatin on endothelial activation, we analysed plasma levels of sVCAM-1, an adhesion molecule that is upregulated during endothelial activation. AA mice had significantly lower levels of sVCAM-1 compared with SS mice (Fig 4D). After treatment with atorvastatin, there was a significant decrease in the plasma level of sVCAM-1 compared with vehicle-treated SS mice (1256 ± 55.4 ng/ml vs. 1403 ± 21.4 ng/ml, p<0.05).
Renal Oxidative Stress
To further understand the role of oxidant stress in SCD, we employed flow cytometry studies for measurement of cellular ROS. Our analyses confirmed a significant burden of oxidant stress in RBC, WBC and neutrophils of SS mice compared to AA mice (Fig 5A). Specifically, after 8 weeks of treatment with atorvastatin, oxidant stress was significantly decreased in macrophages, p<0.05 (Fig 5B). We did not find significant changes in oxidant stress in either RBC, CD19 cells, CD3 cells or neutrophils. However, neutrophils and B cells showed a trend towards a decrease in oxidant stress, p= 0.052 and p= 0.069 respectively.
Figure 5. Oxidant stress is elevated in SS mice.
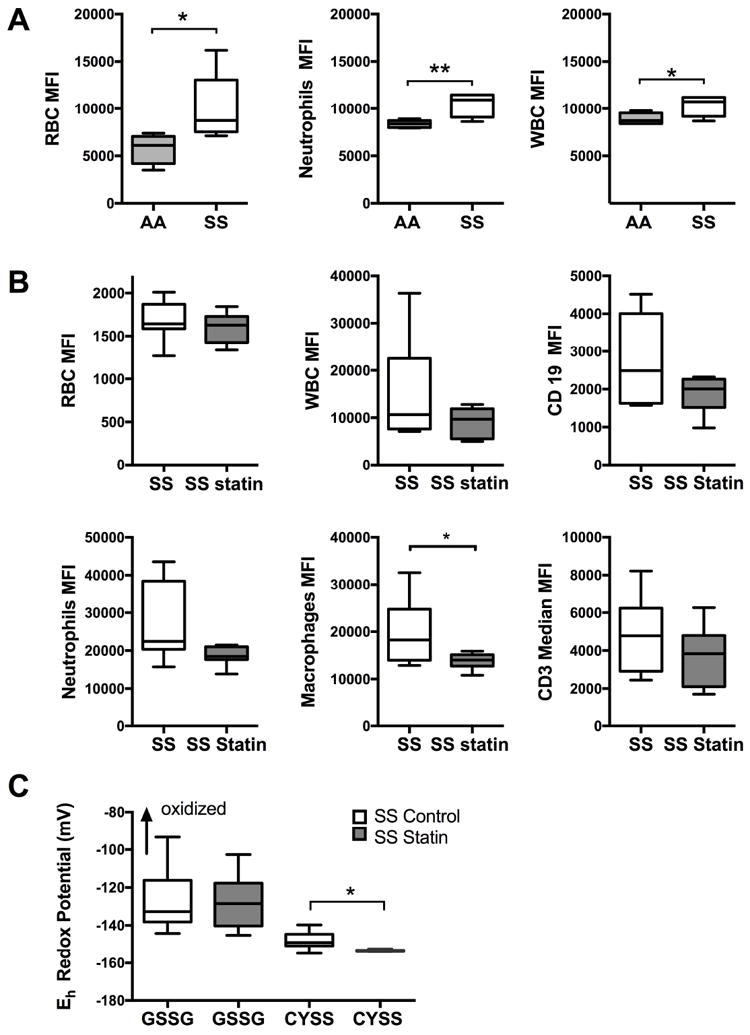
A. Oxidant stress measured by DCFDA stain in peripheral blood and white blood cells. Baseline increase in oxidant stress is significant in SS mice in RBC, WBC and neutrophils, *p<0.05 and **p<0.01. B. Significant decrease in oxidant stress in macrophages of Atorvastatin treated SS mice, *p<0.05. C. Redox potential of thiol pairs were calculated in renal tissue of SS vehicle and SS statin treated mice by high performance liquid chromatography. Results were normalized to kidney weight for each sample, *p<0.05.
CYS: cysteine; CYSS: cysteine; GSH: glutathione; GSSG: glutathione disulphide; MFI: mean fluorescence intensity; RBC: red blood cell; WBC: white blood cell.
We used high performance liquid chromatography to measure thiol/disulfide redox couples GSH/GSSG, CYS/CYSS levels in SS mice kidney tissue. There was no significant difference in the redox potential of GSH/GSSG with atorvastatin treatment. A reductive shift in CYS/CYSS pair was demonstrated following treatment with atorvastatin, indicating significant decrease in oxidant stress (Fig 5C).
mRNA expression of Cybb, Nox4 and Edn1 in renal tissue
To further determine the level of oxidative stress in the kidney of SS mice we measured mRNA levels of Cybb, Nox4 and Edn1. SS mice had significantly higher levels of Cybb, Nox4 and Edn1 mRNA in whole kidney than treated SS mice (Fig 6).
Figure 6. Expression of NADPH oxidase subunits, CYBB, NOX4 and endothelial expression of EDN1 (ET1) in whole kidney tissue in SS mice.
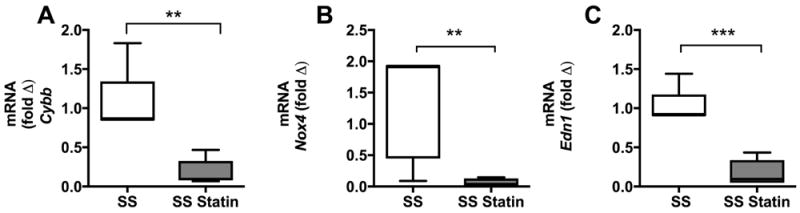
Expression of Cybb, Nox4 and Edn1 significantly decreased after 8 weeks of Atorvastatin treatment (A, B and C), **p<0.01 and ***p<0.001, n=6. mRNA expression of Cybb, Nox4 and Edn1 was normalized to SS mice and Gapdh served as internal control.
Discussion
We demonstrate that atorvastatin improves both GFR and urine concentrating ability and reduces albuminuria in mice with SCN. Although the effects of statins on the progression of kidney disease in human clinical trials remain controversial, studies have demonstrated significant improvement in proteinuria or albuminuria following treatment with statins as summarized in a recent meta-analysis (Su, et al 2016). A small pilot study examining the effect of simvastatin in SCD patients found significant decreases in markers of inflammation and reduction in the frequency of pain episodes (Hoppe, et al 2017). In this study, treatment with simvastatin for 3 months resulted in decreased plasma levels of the endothelial marker soluble E-selectin but not sVCAM-1. However, the effects of statins in patients with SCN have not been studied.
The humanized knock-in sickle cell mice utilized in this study provides an excellent model of SCD that recapitulates all the major features of sickle cell pathology in humans (Hyacinth, et al 2017, Sun, et al 2017, Wu, et al 2006). The experimental and control groups of mice studied in this experiment were similar at baseline. They demonstrated SCN with urine concentrating defect, proteinuria or albuminuria and decreased renal function at 10 weeks of age whereas the non-sickle mice had normal renal function. As such, our model adequately represents renal disease found in SCN. We demonstrate improvement in albuminuria after 4 weeks of daily treatment, which was maintained at 8 weeks. While our findings are limited to 8 weeks, we may have observed further improvement with more prolonged treatment. As albuminuria is a sensitive marker of glomerular damage, which precedes development of overt renal insufficiency, in patients with SCD (Guasch, et al 2006), treatment beginning prior to 10 weeks in our mice may also prevent the development of albuminuria.
In this study, GFR was assessed using FITC-inulin clearance in mice at 8-10 weeks of age. Glomerular hyperfiltration is seen early in children with SCD, with return to normal GFR values in the second decade of life (Wigfall 2000). While children usually present with hyperfiltration, we studied adult mice and believe that, at this age, renal dysfunction is already advanced and hyperfiltration would be seen at a younger age. It is tempting to posit that the improvement in renal function observed in these mice may also halt progression of disease.
Glomerular hypertrophy is an early sign of SCN and has been seen in children as young as 2 years of age (Sharpe and Thein 2011). Our histological examination demonstrated glomerular hypertrophy in SS mice in comparison to AA mice. We did not find other significant pathological differences, interstitial fibrosis or focal segmental sclerosis, between SS and AA mice. However, we found that treatment with atorvastatin significantly improved glomerular tuft size.
We also found that urine concentrating ability greatly improved following treatment with atorvastatin. Hyposthenuria occurs early in life in SCD patients and is one of the first manifestations of SCN in children. Chronic RBC transfusions were reported to improve urine osmolality (Statius van Eps, et al 1970) and albuminuria (Alvarez, et al 2017), however this modality carries the risk of multiple complications, including allo-immunization and iron overload.
Albuminuria is a reliable marker of glomerular disease, but novel markers are needed to predict early disease prior to development of albuminuria. Urine and serum biomarkers of renal injury have been studied to assess their ability to predict early disease. Recent studies demonstrated correlation between presence of albuminuria and elevated urine KIM-1 in SCD patients (Hamideh, et al 2014). In our study, we evaluated biomarkers of tubular and glomerular injury, KIM-1, nephrin and NGAL, in SS mice. KIM-1 is a trans-membrane protein that is not expressed in normal kidney but becomes expressed upon significant proximal tubular injury (Koyner, et al 2010). Nephrin is expressed in the glomerular podocytes of the kidneys and plays an important role in maintaining the barrier in the glomerular capillary. Chang et al (2012) found nephrin to be a sensitive marker of glomerular injury and that it was detected before albuminuria developed in animal models. NGAL is expressed and secreted by renal tubular cells, collecting duct and has been shown to correlate with measured GFR and may be a potential marker for chronic kidney disease (Vanmassenhove, et al 2013). We found that KIM-1 level correlated with albuminuria and showed significant improvement following treatment with atorvastatin. In contrast, no significant improvements in urine NGAL or nephrin were observed, although there was a trend to improvement with urine nephrin following treatment with atorvastatin. However, a longer dosing period or increased number of mice may need to be treated with atorvastatin to demonstrate significance. In recent study, Sundaram et al (2011) observed subnormal urine levels of NGAL were in most SCD patients, which did not correlate with increasing albuminuria. Our findings suggest that KIM-1 and nephrin maybe reliable biomarkers for identifying early SCN.
As SCD is characterized by a chronic state of oxidative stress, we evaluated ROS in peripheral cells and renal tissue. We found that non-sickle mice demonstrated low oxidant stress in peripheral RBCs and white blood cells. Although treatment with atorvastatin did not significantly decrease oxidant stress in RBCs, there was a significant decrease in oxidant stress in macrophages, with a trend towards decreased ROS in neutrophils of sickle mice. Increased white blood cell count is an independent predictor of adverse outcomes in SCD (Miller, et al 2000). In addition, macrophages may have a role in the activation of the endothelium as well as the perpetuation of pro-inflammatory markers (Vinchi, et al 2016). Both endothelial and monocytic cells in culture respond to oxidized cysteine-cystine with proinflammatory signalling and increased cell adhesion. The major thiol/disulfide redox couple in human plasma is cysteine and its disulfide form, cystine. In our study, we found significant reduction of this thiol pair in renal tissues between treated and non-treated sickle mice. Key redox events of inflammatory processes are controlled by the redox state of CYS/CYSS at the extracellular surface of endothelial cell (Go and Jones 2011). In addition to our observations of decreased oxidant stress in the kidney, both Cybb and Nox4 responded to atorvastatin treatment with decreased expression. Nox4 and Cybb have been implicated as contributing to oxidant stress in the kidney and in the vasculature, respectively. Indeed, a recent study of diabetic nephropathy in mice found decreased expression of Nox4 with statin treatment (Fujii, et al 2007). In addition, Cybb mRNA expression in sickle cell mice has been found to be significantly decreased in the kidney following treatment with an endothelin type A (ETA) receptor blocker (Heimlich, et al 2016).
Previous studies have demonstrated elevated levels of serum, urine and renal EDN1 (also termed ET1) levels in sickle mice when compared to control non-sickle phenotype (Sabaa, et al 2008). EDN1 is a potent vasoconstrictor and has been shown to exacerbate hypoxia/reoxygenation injury in sickle cell mice (Sabaa, et al 2008). EDN1 is synthesized in the endothelium of the renal vasculature, mesangial cells, peritubular capillaries, in the epithelium of the proximal tubule, thick ascending limb and the collecting duct, and in the inner medullary-collecting duct (Prabhakar 2013). These areas of EDN1 synthesis are quite susceptible to hypoxic injury, which in SCD frequently occurs due to recurrent episodes of vaso-occlusion. Although EDN1 (ETI) may bind to both ETA and ETB receptors, it has increased affinity to the ETA receptor binding in the renal vasculature (Sabaa, et al 2008). Recent work by Kasztan et al (2017) demonstrated reduced albuminuria, decreased oxidative stress and inflammatory markers in mice treated with an ETA antagonist. We found that mRNA expression of Edn1 significantly decreased in kidney tissue following treatment with atorvastatin. In addition to changes seen with EDN1 expression, plasma sVCAM-1 decreased with atorvastatin treatment, further supporting the role of atorvastatin in stabilizing the renal endothelium in SCD.
Our results demonstrate that atorvastatin may offer a potential therapeutic option for the treatment of SCN. The mechanism of improvement of renal pathologies in sickle mice may be related to the ability of atorvastatin to improve endothelial function and decrease ROS. Further studies exploring whether the addition of ACEI, ARB or hydroxycarbamide would provide additive or synergistic benefit in the treatment of SCN are a future target.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01HL111659 (KIA, DRA).
We thank Pfizer Inc. (New York, NY, USA) for providing atorvastatin via a compound transfer program. We would also like to acknowledge the Children’s Healthcare of Atlanta and Emory University Pediatric Flow Cytometry and Biomarkers Cores.
Footnotes
Author Contributions
RSZ, KIA and DRA conceived and designed the present work. RSZ performed the experiments, analysed data and wrote the first draft. PC performed the research and analysed the data. HY and LB analysed the data. All authors approved the final version.
Conflict of interest
KIA serves on clinical advisory boards of Global Blood Therapeutics and Novartis, and receives research support from Pfizer. DRA receives research support from Agios Pharmaceuticals.
References
- Abbott KC, Hypolite IO, Agodoa LY. Sickle cell nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol. 2002;58:9–15. doi: 10.5414/cnp58009. [DOI] [PubMed] [Google Scholar]
- Alvarez O, Nottage K, Simpson LM, Wood J, Davis BR, Fuh B, Sarnaik S, Aygun B, Helton K, Ware RE. Kidney function of transfused children with sickle cell anemia: Baseline data from the TWiTCH study with comparison to non-transfused cohorts. Am J Hematol. 2017;92:E637–E639. doi: 10.1002/ajh.24871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga KI, Derebail VK, Archer DR. The glomerulopathy of sickle cell disease. Am J Hematol. 2014;89:907–914. doi: 10.1002/ajh.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisoni L, Troost JP, Nast C, Bagnasco S, Avila-Casado C, Hodgin J, Palmer M, Rosenberg A, Gasim A, Liensziewski C, Merlino L, Chien HP, Chang A, Meehan SM, Gaut J, Song P, Holzman L, Gibson D, Kretzler M, Gillespie BW, Hewitt SM. Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol. 2016;29:671–684. doi: 10.1038/modpathol.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolucci P, Habibi A, Stehle T, Di Liberto G, Rakotoson MG, Gellen-Dautremer J, Loric S, Moutereau S, Sahali D, Wagner-Ballon O, Remy P, Lang P, Grimbert P, Audureau E, Godeau B, Galacteros F, Audard V. Six Months of Hydroxyurea Reduces Albuminuria in Patients with Sickle Cell Disease. J Am Soc Nephrol. 2016;27:1847–1853. doi: 10.1681/ASN.2014111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM. Interleukin-1beta, but not interleukin-6, enhances renal and systemic endothelin production in vivo. Am J Physiol Renal Physiol. 2008;295:F446–453. doi: 10.1152/ajprenal.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Paik SY, Mao L, Eisner W, Flannery PJ, Wang L, Tang Y, Mattocks N, Hadjadj S, Goujon JM, Ruiz P, Gurley SB, Spurney RF. Diabetic kidney disease in FVB/NJ Akita mice: temporal pattern of kidney injury and urinary nephrin excretion. PLoS One. 2012;7:e33942. doi: 10.1371/journal.pone.0033942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell Evaluation of the Beckman Creatinine Analyzer 2. Clin Biochem. 1979;12:159–161. doi: 10.1016/s0009-9120(79)80081-8. [DOI] [PubMed] [Google Scholar]
- Eller P, Eller K, Wolf AM, Reinstadler SJ, Tagwerker A, Patsch JR, Mayer G, Rosenkranz AR. Atorvastatin attenuates murine anti-glomerular basement membrane glomerulonephritis. Kidney Int. 2010;77:428–435. doi: 10.1038/ki.2009.478. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med. 1992;326:910–915. doi: 10.1056/NEJM199204023261402. [DOI] [PubMed] [Google Scholar]
- Fitzhugh CD, Wigfall DR, Ware RE. Enalapril and hydroxyurea therapy for children with sickle nephropathy. Pediatr Blood Cancer. 2005;45:982–985. doi: 10.1002/pbc.20296. [DOI] [PubMed] [Google Scholar]
- Foucan L, Bourhis V, Bangou J, Merault L, Etienne-Julan M, Salmi RL. A randomized trial of captopril for microalbuminuria in normotensive adults with sickle cell anemia. Am J Med. 1998;104:339–342. doi: 10.1016/s0002-9343(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Fujii M, Inoguchi T, Maeda Y, Sasaki S, Sawada F, Saito R, Kobayashi K, Sumimoto H, Takayanagi R. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int. 2007;72:473–480. doi: 10.1038/sj.ki.5002366. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh K, Masaki T, Chiba S, Ando H, Fujiwara K, Shimasaki T, Tawara Y, Toyooka I, Shiraishi K, Mitsutomi K, Anai M, Itateyama E, Hiraoka J, Aoki K, Fukunaga N, Nawata T, Kakuma T. Effects of hydrophilic statins on renal tubular lipid accumulation in diet-induced obese mice. Obesity Research & Clinical Practice. 2013;7:e342–e352. doi: 10.1016/j.orcp.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17:2228–2235. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- Hamideh D, Raj V, Harrington T, Li H, Margolles E, Amole F, Garcia-Buitrago M, Ruiz P, Zilleruelo G, Alvarez O. Albuminuria correlates with hemolysis and NAG and KIM-1 in patients with sickle cell anemia. Pediatr Nephrol. 2014;29:1997–2003. doi: 10.1007/s00467-014-2821-8. [DOI] [PubMed] [Google Scholar]
- Heimlich JB, Speed JS, O’Connor PM, Pollock JS, Townes TM, Meiler SE, Kutlar A, Pollock DM. Endothelin-1 contributes to the progression of renal injury in sickle cell disease via reactive oxygen species. Br J Pharmacol. 2016;173:386–395. doi: 10.1111/bph.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe C, Jacob E, Styles L, Kuypers F, Larkin S, Vichinsky E. Simvastatin reduces vaso-occlusive pain in sickle cell anaemia: a pilot efficacy trial. Br J Haematol. 2017;177:620–629. doi: 10.1111/bjh.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyacinth HI, Sugihara CL, Spencer TL, Archer DR, Shih AY. Higher prevalence of spontaneous cerebral vasculopathy and cerebral infarcts in a mouse model of sickle cell disease. J Cereb Blood Flow Metab. 2017 Sep 19;:2017. doi: 10.1177/0271678X17732275. https://doi.org/10.1177/0271678X17732275. [DOI] [PMC free article] [PubMed]
- Kasztan M, Fox BM, Speed JS, De Miguel C, Gohar EY, Townes TM, Kutlar A, Pollock JS, Pollock DM. Long-Term Endothelin-A Receptor Antagonism Provides Robust Renal Protection in Humanized Sickle Cell Disease Mice. J Am Soc Nephrol. 2017;28:2443–2458. doi: 10.1681/ASN.2016070711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, Bonventre JV, Murray PT. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154–2165. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupesiz A, Celmeli G, Dogan S, Antmen B, Aslan M. The effect of hemolysis on plasma oxidation and nitration in patients with sickle cell disease. Free Radic Res. 2012;46:883–890. doi: 10.3109/10715762.2012.686037. [DOI] [PubMed] [Google Scholar]
- Margaritis M, Channon KM, Antoniades C. Statins as regulators of redox state in the vascular endothelium: beyond lipid lowering. Antioxid Redox Signal. 2014;20:1198–1215. doi: 10.1089/ars.2013.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson Yee M, Jabbar SF, Osunkwo I, Clement L, Lane PA, Eckman JR, Guasch A. Chronic kidney disease and albuminuria in children with sickle cell disease. Clin J Am Soc Nephrol. 2011;6:2628–2633. doi: 10.2215/CJN.01600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ST, Sleeper LA, Pegelow CH, Enos LE, Wang WC, Weiner SJ, Wethers DL, Smith J, Kinney TR. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342:83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- Pan X, Hou R, Ma A, Wang T, Wu M, Zhu X, Yang S, Xiao X. Atorvastatin Upregulates the Expression of miR-126 in Apolipoprotein E-knockout Mice with Carotid Atherosclerotic Plaque. Cell Mol Neurobiol. 2016;37:29–36. doi: 10.1007/s10571-016-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991;115:614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- Prabhakar SS. Inhibition of renin-angiotensin system: implications for diabetes control and prevention. J Investig Med. 2013;61:551–557. doi: 10.2310/JIM.0b013e31828298ce. [DOI] [PubMed] [Google Scholar]
- Qi Z. Serial determination of glomerular fitration rate in conscious mice using FITC-inuin clearance. Am J Physiol Renal Physiol. 2003;286:F591–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- Quinn CT, Saraf SL, Gordeuk VR, Fitzhugh CD, Creary SE, Bodas P, George A, Raj AB, Nero AC, Terrell CE, McCord L, Lane A, Ackerman HC, Yang Y, Niss O, Taylor MD, Devarajan P, Malik P. Losartan for the nephropathy of sickle cell anemia: A phase-2, multicenter trial. Am J Hematol. 2017;92:E520–E528. doi: 10.1002/ajh.24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbabi H, Galaup A, Maier-Redelsperger M, Vandermeersch S, Scarpa A, Janin A, Levy B, Girot R, Beuzard Y, Leboeuf C, Henri A, Germain S, Dussaule JC, Tharaux PL. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest. 2008;118:1924–1933. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sharpe CC, Thein SL. Sickle cell nephropathy - a practical approach. Br J Haematol. 2011;155:287–297. doi: 10.1111/j.1365-2141.2011.08853.x. [DOI] [PubMed] [Google Scholar]
- Statius van Eps LW, Schouten H, Haar Romeny-Wachter CC, La Porte-Wijsman LW. The relation between age and renal concentrating capacity in sickle cell disease and hemoglobin C disease. Clin Chim Acta. 1970;27:501–511. doi: 10.1016/0009-8981(70)90305-0. [DOI] [PubMed] [Google Scholar]
- Su X, Zhang L, Lv J, Wang J, Hou W, Xie X, Zhang H. Effect of Statins on Kidney Disease Outcomes: A Systematic Review and Meta-analysis. Am J Kidney Dis. 2016;67:881–92. doi: 10.1053/j.ajkd.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Sun YY, Lee J, Huang H, Wagner MB, Joiner CH, Archer DR, Kuan CY. Sickle Mice Are Sensitive to Hypoxia/Ischemia-Induced Stroke but Respond to Tissue-Type Plasminogen Activator Treatment. Stroke. 2017;48:3347–3355. doi: 10.1161/STROKEAHA.117.018334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram N, Bennett M, Wilhelm J, Kim MO, Atweh G, Devarajan P, Malik P. Biomarkers for early detection of sickle nephropathy. Am J Hematol. 2011;86:559–566. doi: 10.1002/ajh.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehseen S, Joiner CH, Lane PA, Yee ME. Changes in urine albumin to creatinine ratio with the initiation of hydroxyurea therapy among children and adolescents with sickle cell disease. Pediatr Blood Cancer. 2017;64:e26665. doi: 10.1002/pbc.26665. [DOI] [PubMed] [Google Scholar]
- Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28:254–273. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- Vinchi F, Costa da Silva M, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, Cerwenka A, Tolosano E, Muckenthaler MU. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016;127:473–486. doi: 10.1182/blood-2015-08-663245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigfall D. Prevalence and clinical correlates of glomerulopathy in children with sickle cell disease. J Pediatr. 2000;136:749–753. [PubMed] [Google Scholar]
- Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB Journal. 2005;9:989–991. doi: 10.1096/fj.04-3218fje. [DOI] [PubMed] [Google Scholar]
- Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108:1183–1188. doi: 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM, Brown LA. Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by downregulating NADPH oxidases. Am J Physiol Lung Cell Mol Physiol. 2014;306:L429–441. doi: 10.1152/ajplung.00159.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MS, Jaimes EA, Raij L. Atorvastatin prevents end-organ injury in salt-sensitive hypertension: role of eNOS and oxidant stress. Hypertension. 2004;44:186–190. doi: 10.1161/01.HYP.0000136395.06810.cf. [DOI] [PubMed] [Google Scholar]
- Zhou MS, Schuman IH, Jaimes EA, Raij L. Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-beta, and fibronectin with concomitant increase in nitric oxide bioavailability. Am J Physiol Renal Physiol. 2008;295:F53–59. doi: 10.1152/ajprenal.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Liao JK. Pleiotropic Effects of Statins. Circulation Journal. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoja C, Corna D, Gagliardini E, Conti S, Arnaboldi L, Benigni A, Remuzzi G. Adding a statin to a combination of ACE inhibitor and ARB normalizes proteinuria in experimental diabetes, which translates into full renoprotection. Am J Physiol Renal Physiol. 2010;299:F1203–1211. doi: 10.1152/ajprenal.00045.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


