Abstract
Background
Children born extremely preterm are at increased risk of learning limitations
Aim
To identify the antecedents of learning limitations of children born extremely preterm.
Study design
Prospective observational study from birth to age 10 years. Variables entered into the multinomial logistic regression analyses were ordered temporally, with the earliest occurring predictors/covariates of each learning limitation risk entered first and not displaced by later occurring covariates.
Subjects
874 children who were born before the 28th week of gestation
Outcome measures
A reading limitation was defined as a score one or more standard deviations below the expected mean on the WIAT-III Word Reading and a mathematics limitation was defined as a similarly low score on the Numerical Operations component.
Results
56 children had a ‘reading ONLY’ limitation, 132 children had a ‘math ONLY’ limitation and 89 children had ‘reading AND math’ limitations. All risk profiles included an indicator of socioeconomic disadvantage (e.g., mother’s “racial” identity and eligibility for government-provided health care insurance), an indicator of newborn’s immaturity/vulnerability (e.g., high illness severity score, receipt of hydrocortisone, and/or ventilator-dependence at 36 weeks post-menstruation), and all but the math only limitation included an indicator of fetal growth restriction and inflammation (i.e., pregnancy urinary tract infection or late ventilator-dependence).
Conclusions
The themes of socioeconomic disadvantage and immaturity/vulnerability characterize all three risk profiles, while the themes of fetal growth restriction and inflammation are characteristic of a reading limitation only, and the reading and math limitations entity.
Keywords: extremely preterm, school performance, learning disabilities, mathematics, reading, special educational needs
1. INTRODUCTION
Children born very preterm are at higher risk of reading and math limitations than children born at term [1]. Why should this be?
In the general pediatric population, the antecedents of mathematics limitations include low socioeconomic status (SES) [2], and its correlate African-American identification [2]. Among children born preterm, the antecedents of mathematics limitations include socioeconomic disadvantage [1], and its correlates cigarette smoking during pregnancy [3], as well as indicators of immaturity (low gestational age, and not receiving breast milk while in the neonatal unit) [1], and inflammation, including bronchopulmonary dysplasia/chronic lung disease of prematurity (BPD/CLD) [3], (need for) postnatal dexamethasone therapy to reduce the risk of BPD/CLD [4], and necrotizing enterocolitis (NEC) requiring surgery or drainage [1].
The antecedents of reading limitations in the general population include socioeconomic disadvantage [2], and a correlate, African-American identification [2]. Low socioeconomic position is also an antecedent of reading limitations among children who had very low birth weight [5], and among children born before the 26th week of gestation [1]. Other antecedents of low reading scores in this very high risk group of former extremely low gestational age newborns (X-ELGANs), included indicators of immaturity (low gestational age, no receipt of breast milk, neonatal illnesses, duration of stay in neonatal unit), and indicators of inflammation (NEC requiring surgery or drainage) [1].
Among children born preterm or at a very low birth weight, a larger literature is devoted to the correlates of broad, often variously-defined learning limitations categorized as grade failure [6], special educational needs [7], use of special services [6], learning problems [8], placement in a special classroom [9], educational impairment [9], low teacher rating of academic achievement [9], and school difficulties [10], Almost all of these identified low gestational age and/or low birthweight as the prime risk factor. “Small-for-gestational-age” (lowest decile birth weight for gestational age) also placed the very preterm newborn at increased risk of school difficulties [10], and need for special education [11]. In the studies that evaluated socioeconomic status, socioeconomic disadvantage [6, 9] were also associated with sub-optimal academic achievement.
We sought to identify the antecedents of reading and math limitations in X-ELGANs and to see in what ways the risk profiles of learning limitations among 10-year children born extremely preterm were similar to, or different from, the risk profiles of structural and functional indicators/correlates of brain damage. We were able to do this because the ELGAN Study evaluated 732 X-ELGANs at age 10 years who had an IQ ≥ 70 and assessments of the Word Reading and Numerical Operations components of the WIAT-III. We limited the analyses reported here to those with an IQ ≥ 70 because we wanted to identify the antecedents of reading and math limitations and not the antecedents of low IQ.
2. METHODS
2.1 Participants
The ELGAN study is a multi-center observational study designed to identify characteristics and exposures associated with increased risk of structural and functional neurologic disorders in extremely preterm infants [12]. During the years 2002–2004, women delivering before 28 weeks gestation at one of 14 participating institutions were asked to enroll in the study. A total of 1249 mothers of 1506 ELGANs consented to participate (Table 1). Enrollment and consent processes were approved by the individual institutional review boards.
Table 1.
Risk Ratios and 95% Confidence Intervals for the Association of Each Learning Limitation with the Antecedents Listed on the Left. These are based on a time-oriented logistic regression model that added variables sequentially as they were identified. Earlier occurring variables could not be displaced. The selection of variables to offer the model is based on what was seen in earlier tables. Children with each of the learning limitations are compared to the same referent group of children who did not have any learning limitation.
| READING only | Antenatal | Pregnancy | Early Postnatal | Late Postnatal |
|---|---|---|---|---|
| ANTENATAL | ||||
| Black | 2.1 (1.1, 3.9) | 1.8 (0.9, 3.5) | 1.6 (0.8, 3.1) | 1.6 (0.8, 3.2) |
| Public insurance | 1.6 (0.8, 2.9) | 1.6 (0.98, 2.9) | 1.6 (0.8, 3.0) | 1.6 (0.8, 3.0) |
| PREGNANCY | ||||
| Pregnancy UTI | 2.3 (1.1, 4.5) | 2.6 (1.2, 5.2) | 2.6 (1.3, 5.4) | |
| EARLY POSTNATAL | ||||
| SNAP-PE 45+ | 0.9 (0.4, 1.9) | 0.9 (0.4, 1.9) | ||
| Hydrocortisone | 2.9 (1.4, 6.0) | 2.6 (1.2, 5.5) | ||
| LATE POSTNATAL | ||||
| Severe BPD (vent & O2) | 2.0 (0.7, 5.7) | |||
|
| ||||
| MATH only | Antenatal | Pregnancy | Early Postnatal | Late Postnatal |
|
| ||||
| ANTENATAL | ||||
| Black | 2.1 (1.3, 3.2) | 2.1 (1.3, 3.4) | 2.1 (1.3, 3.4) | 2.1 (1.3, 3.4) |
| Public insurance | 1.8 (1.1, 2.7) | 1.7 (1.1, 2.7) | 1.7 (1.1, 2.6) | 1.8 (1.1, 2.8) |
| PREGNANCY | ||||
| Pregnancy UTI | 1.1 (0.6, 2.0) | 1.1 (0.6, 1.9) | 1.1 (0.6, 1.9) | |
| EARLY POSTNATAL | ||||
| SNAP-PE 45+ | 1.7 (2.2, 2.7) | 1.6 (1.00, 2.5) | ||
| Hydrocortisone | 1.2 (0.7, 2.3) | 1.2 (0.7 2.3) | ||
| LATE POSTNATAL | ||||
| Severe BPD (vent & O2) | 1.9 (0.95, 4.0) | |||
|
| ||||
| READING & MATH | Antenatal | Pregnancy | Early Postnatal | Late Postnatal |
|
| ||||
| ANTENATAL | ||||
| Black | 1.7 (0.99, 2.9) | 1.7 (1.00, 3.0) | 1.7 (0.9, 2.9) | 1.7 (0.9, 2.9) |
| Public insurance | 3.2 (2.0, 5.3) | 3.3 (2.0, 5.4) | 3.2 (1.9, 5.3) | 3.3 (2.0, 5.7) |
| PREGNANCY | ||||
| Pregnancy UTI | 1.2 (0.6, 2.2) | 1.1 (0.6, 2.2) | 1.2 (0.6, 2.3) | |
| EARLY POSTNATAL | ||||
| SNAP-PE 45+ | 3.1 (1.9, 5.2) | 2.7 (1.6, 4.6) | ||
| Hydrocortisone | 1.4 (0.7, 2.8) | 1.4 (0.7, 2.9) | ||
| LATE POSTNATAL | ||||
| Severe BPD (vent & O2) | 2.4 (1.1, 5.4) | |||
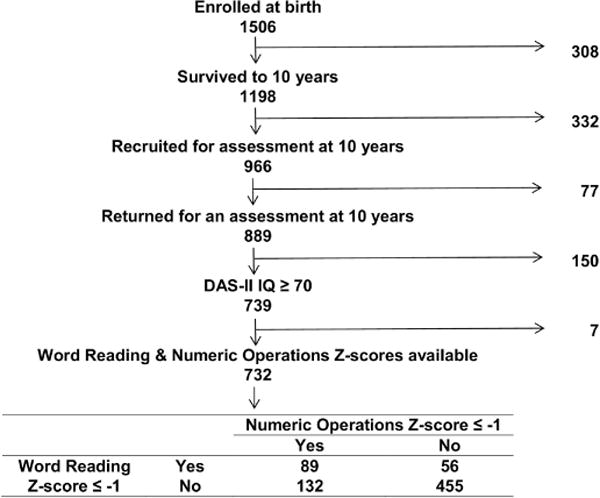
Ten years later, we invited 966 children to return for an age-appropriate assessment of cognition, executive function, behaviors, and achievement. They were selected because the concentrations of inflammation-related proteins in their blood collected during the first postnatal month had been measured. Of these 966 children, 889 (92%) returned for follow up and 874 were administered the neurocognitive tests. Enrollment and consent procedures for this follow up study were approved by the institutional review boards of all participating institutions.
2.2 Antedecents
Information about the characteristics and exposures considered potential antecedents is included in the appendix, as are tables that contain each of the candidates, and summary text describing the contents of the tables.
2.3 Procedures at age 10 years
The families of all children whose development was assessed at age 2 years were contacted by mail and then by phone to invite them to participate in the 10-year follow up. Lost to follow-up families were searched for on state vaccination registries, and other openly-available websites. Facebook was also used where approved by the local institution’s institutional review board.
Families willing to participate were scheduled for one visit during which all of the measures reported here were administered in 3 to 4 hours, including breaks. The assessments were selected to provide the most comprehensive information about neurocognitive and academic function in one testing session.
2.4 General cognitive ability
General cognitive ability (or IQ) was assessed with the School-Age Differential Ability Scales–II Verbal and Nonverbal Reasoning scales [13]. We required that children have scores of 70 or higher on both scales to be included in our sample for these analyses.
2.5 Academic Function
The Wechsler Individual Achievement Test-III (WIAT-III) provides grade- and age-adjusted standard scores for the Word Reading and Numeric Operations subtests [14]. As have others,[15–17] we defined each learning limitation as a Z-score ≤ −1 (i.e., below the 16th centile, which is equivalent to a score ≤ 85) on a grade-based WIAT-III achievement test. Thus, we identified four mutually-exclusive groups, reading limitation only (Word Reading Z-scores ≤ −1, Numerical Operations Z-scores > −1), math limitation only (Numerical Operations Z-scores ≤ −1, Word Reading Z-scores > −1), both reading and math limitations (Word Reading Z-scores ≤ −1, Numerical Operations Z-scores ≤ −1), and neither limitation (Word Reading Z-scores > −1, Numerical Operations Z-scores > −1) (Table 1).
2.6 Data analyses
We evaluated the generalized form of the null hypotheses that each of the three learning limitations is not associated with any maternal, pregnancy, delivery, or postnatal characteristic or exposure in children who did not have a major impairment in cognitive function (DAS ≥ 70). We began with univariate analyses (Appendix Tables 1–9), which identified candidate variables for the multivariate logistic regression analyses (Table 2). Because postnatal phenomena can be influenced by antepartum phenomena, the variables entered into the multinomial logistic regression analyses were ordered temporally, with the earliest occurring predictors/covariates of each learning limitation risk entered first and not displaced by later occurring covariates [18].
Table 2.
Single table summary of the three parts of Table 2.
| READING only | Antenatal | Pregnancy | Early Postnatal | Late Postnatal |
|---|---|---|---|---|
| ANTENATAL | ||||
| Black | Read, Math | Math | Math | Math |
| Public insurance | Math, Both | Math, Both | Math, Both | Math, Both |
| PREGNANCY | ||||
| Pregnancy UTI | Read | Read | Read | |
| EARLY POSTNATAL | ||||
| SNAP-PE 45+ | Math, Both | Both | ||
| Hydrocortisone | Read | Read | ||
| LATE POSTNATAL | ||||
| Severe BPD (vent & O2) | Both |
We used a step-down procedure seeking a parsimonious solution without interaction terms. The contributions of relevant variables are presented as risk ratios with 95% confidence intervals. The risk ratio for each variable expresses the increased or decreased risk of each learning limitation in one category of a characteristic or exposure relative to the other.
3. RESULTS
3.1 Sample description (Table 1)
Of the 889 children who returned at age 10 years, 732 had an IQ of at least 70 and completed the WIAT-III assessment. Of these children, who comprise the sample for this report, 56 are identified as having a reading limitation only (defined as a Word Reading Z-score ≤ −1 and a Numerical Operations Z-score > −1), 132 as having a math limitation only (i.e., Numerical Operations Z-score ≤ −1 + Word Reading Z-score > −1), and 89 as having both reading and math limitations (i.e., Word Reading Z-score ≤ −1 + Numerical Operations ≤ −1).
3.2 Univariable analyses (Appendix Tables 1–9)
The Appendix tables display the prevalences of the three learning limitation entities among children classified by maternal, pregnancy, and newborn characteristics.
In this sample, 8% of children had a reading limitation, 18% had a math limitation, and 12% had both reading and math limitations. We identify higher prevalences as a prevalence that is 5 percentage points higher than these (e.g., 13% for reading, 23% for math, and 17% for both).
Rather than go through each table individually, we grouped the findings in two ways. First, we identified all the variables that are associated with each learning limitation. These findings are presented in the Appendix.
3.3 Themes (Appendix Tables 1–9)
Then, we grouped the variables that fit each of several themes. This approach is especially important because the univariate analyses in Appendix Tables 1–9 are preparation for the multinomial, time-oriented regression (Table 2), which models the risk of the 3 mutually-exclusive but highly related learning limitations. In multinomial regression models, one set of antecedent variables is considered for all 3 learning limitation entities.
In presenting these themes, we retain the identification of each variable with each learning limitation with initials: R for reading, M for math, and B for both reading and math.
3.3.1 Theme #1: socioeconomic disadvantage
The indicators/correlates of mother’s low SES include Black self-identification (M, B), Hispanic self-identification (M, B), no more than a high school education (M, B), single marital status (B), public insurance (M,B), smoked during pregnancy (B) or exposure to the smoke of others during pregnancy (M).
3.3.2 Theme #2: inflammation
We divide inflammation into 2 categories, antenatal and postnatal. Among the indicators of antenatal inflammation are mother-reported vaginal/cervical (M), urinary tract (R), and/or periodontal infection (R,B), as well as consumption of a non-steroidal anti-inflammatory drug (which can be an indicator of the mother’s fever or malaise) (B), recovery from the placenta of two or more organisms (B), Mycoplasma species (B), and/or vaginal organisms (B). Cervical insufficiency (M), which is often accompanied by inflammation, especially when extremely preterm birth occurs [19], is also included here.
Among the indicators of postnatal inflammation are tracheal colonization (M), early (first postnatal week) bacteremia (M), necrotizing enterocolitis (B), and isolated intestinal perforation (which can have inflammation-related antecedents) [20] (M).
3.3.3 Theme # 3: immaturity
Low gestational age (B) has many correlates that we view as conveying risk information that is similar to that provided by low gestational age. Here we find learning limitations associated with low birth weight (M), high illness severity scores (SNAPPEs) (B), which we view as indicators of physiologic instability associated with immaturity [21], low blood pressure (lowest Q lowest MAP) (M), blood gas exchange instability [22] (highest quartile highest PaO2 on 2 of the first 3 postnatal days (M), lowest quartile PaO2 on day 7) (M), (need for) postnatal hydrocortisone (R), pneumothorax (M), pulmonary hemorrhage (M), necrotizing enterocolitis (B) and isolated intestinal perforation (M), prethreshold retinopathy of prematurity (R), and severe lung disease at 36 weeks post-menstrual age requiring ventilation assistance as well as supplemental oxygen (M, B). Of course, each of these has the potential to reflect risk information beyond that provided by immaturity.
3.3.4 Theme #3: fetal growth restriction
Although low birth-weight Z-score defines fetal growth restriction (M), several correlates of fetal growth restriction were also associated with learning limitations. These include maternal aspirin consumption during pregnancy [23] (M), fetal stem vessel thrombosis (fetal thrombotic vasculopathy) [24] (B), placenta infarct and decidual hemorrhage/fibrin deposition [25] (M), and fetal indication for extremely preterm delivery [26] (M,B).
3.4 Multinomial time-oriented risk models (Table 2)
3.4.1 Theme #1: Sociodemographic
On multivariable analyses, the four themes are represented by just a few variables. The low socioeconomic state is represented by just two. Black self-identification is identified as an antecedent of the reading only, and the math only limitations, while government-provided medical care insurance is an antecedent of the math only limitations and the both limitations entity.
3.4.2 Theme #2: inflammation
Only one indicator of antenatal inflammation, pregnancy urinary tract infection, was associated with the reading only limitation, and only one indicator of postnatal inflammation, severe bronchopulmonary dysplasia/chronic lung disease (BPD/CLD), was associated with the reading and math limitations entity.
3.4.3 Theme # 3: Immaturity/vulnerability
A high Score for Neonatal Acute Physiology with Perinatal Extension-II (SNAPPE-II), an indicator of physiologic instability/immaturity, was associated with increased risk of the math only limitation and the combined (reading and math) limitation, while severe BPD/CLD was associated with increased risk of the combined limitations entity, and the apparent need for postnatal hydrocortisone, a predictor of BPD/CLD [27], was associated with the reading only limitation.
3.4.4 Theme # 4: Fetal growth restriction
In the ELGAN Study larger sample, fetal growth restriction is a risk factor for BPD/CLD [28]. Thus, the association of severe BPD/CLD with the combined limitations entity probably conveys information about fetal growth restriction, just as the need for hydrocortisone, which is an antecedent of the reading only limitation, is likely an indicator that the newborn is at heightened risk of BPD/CLD [27], and therefore an indicator of fetal growth restriction.
4. DISCUSSION
Our main finding is that the risk profiles of the reading only limitation, math only limitation, and both reading and math limitations are similar in some ways, and different in other ways. All risk profiles include an indicator/correlate of low SES(i.e., Black identification or eligibility for government-provided medical care insurance) and an indicator of immaturity/vulnerability (i.e., high illness severity score, or receipt of hydrocortisone). Only the reading only limitation included an antenatal inflammation variable (i.e., maternal urinary tract infection during the pregnancy), while only the both reading and math limitations entity included a variable that reflected immaturity/vulnerability, fetal growth restriction, postnatal inflammation and other exposures (i.e., severe bronchopulmonary dysplasia, defined as the need for ventilation assistance and supplemental oxygen at 36 weeks post-menstrual age).
4.1 Methodologic issues
4.1.1 possible selection bias
Figure 1 indicates where sample attrition occurred. The 332 children not available at age 10 years overwhelmingly represent loss to follow-up. This loss of 28% of the sample over a 10 year interval is an attrition rate of <3% per year. This loss and the 8% loss of children of contacted parents who declined to bring their children for the 10-year assessment is higher among families with characteristics of those who are at socioeconomic disadvantage than among others. In light of this selection bias, the most likely implication of our findings is that they might not be generalizable to children of families with socioeconomic burdens.
Figure 1.
4.1.2 categorization of learning limitations
No discontinuity or sharp break in WIAT-III Word Reading scores separates those with a reading limitation from their peers who are better readers.[29] The same holds for scores on the WIAT-III Numerical Operations assessment. As epidemiologists most interested in disorders that limit function, we acknowledge that measures of a function represent a continuum, and yet feel the need to establish a cut-off that will allow us to identify the antecedents of dysfunction. In doing so, we are continuing the tradition established in the study of such other continuous measures as blood pressure (e.g., hypertension), blood sugar (e.g., diabetes mellitus), and eye pressure (e.g., glaucoma).
4.1.3. multinomial time-oriented risk models
Multinomial models are most appropriate when multiple, related, but mutually-exclusive entities are being evaluated. This allows those who have none of the disorders to serve as the common referent group.
Time-oriented models introduce variables in the sequence of their occurrence or identification. These models evaluate the contribution of later-occurring exposures and characteristics in light of earlier-occurring exposures and characteristics. For example, variables that are correlates/consequences of immaturity, such as the need for prolonged ventilation, convey information about low gestational age or other indicators of immaturity. If low gestational age or another very early indicator of immaturity is already in the model, prolonged ventilation will be identified as a risk factor only if is provides supplemental risk information.
4.1.4. heterogeneity of categorization of learning limitations
We acknowledge that each of the three learning limitation entities is an amalgam of dysfunctions. Because each form of dysfunction might have its own risk profile, our lumping them together impedes our ability to identify specific risk profiles, and increases the likelihood of our not identifying anything associated with the broader group of dysfunctions.
4.1.5. multifactorial view of risks of a disease, disorder, or dysfunction
The multifactorial view best explains the multiple contributions to the risk of any disorder. In the ELGAN Study, multifactorial etiology is probably best exemplified by low mental development index of the Bayley Scales-II, whose risk is increased among children who had indicators/correlates of low socioeconomic status [30, 31], immaturity [30], were growth restricted at birth [31, 32], were exposed to antenatal inflammation [33], and had systemic inflammation [33, 34]. These observations facilitate our acceptance of a multifactorial view of the etiology of learning disorders, even among children with an IQ in the normal range.
4.1.6. limitations and strengths
Among our limitations is our classifying all reading and all math limitations as if they were homogeneous entities. They are not. In addition, our definition of a limitation as one or more standard deviations below the mean on a single achievement assessment probably adds to the heterogeneity by including as limited, children who though not gifted, might not really be functionally limited. Because of the socioeconomic contributions to recruitment bias, our findings might not be generalizable to populations of children whose families are challenged by socioeconomic burdens. Our strengths include the prospective design, the quality of the antecedent information, and a large number of high-risk children.
4.2. Themes
We emphasize themes rather than individual variables because of the interrelatedness of many of the variables in this dataset. Each variable is seen as a surrogate for other variables subsumed within each theme. Our interpretations of our findings are in keeping with other studies. Here we review the studies that support each of the four themes.
4.2.1 Theme #1: Sociodemographic
Poverty and its correlates have been associated in many observational and correlational studies with sub-optimal academic success (presumably among children born at term) [35]. Academic limitations appear to be especially pronounced in very preterm children born into low socioeconomic families [1, 36, 37]. The available evidence suggests that the interaction between low SES and very preterm birth is at least additive (i.e., greater than the sum of the effects of each of these two variables/characteristics) [36], and possibly multiplicative [37].
4.2.2 Theme #2: Inflammation
Both antenatal [38] and postnatal [39] inflammation are associated with increased risk of brain damage among very preterm newborns, and preterm children who have brain abnormalities are more likely than others to have learning limitations. [1, 40] Thus, one would expect that children born extremely preterm and exposed to inflammatory stimuli are at increased risk of learning limitations. Nevertheless, we are not aware of studies of perinatal inflammation and learning disorders.
Pregnancy genitourinary tract infection was associated in one study with low scores on the Cognitive Composite, Language Composite, and Receptive Language components of the Bayley Scales-III at age 2 years among children born very preterm [41]. We know of no other report of antenatal inflammation antecedents of learning disorders.
Bronchopulmonary dysplasia, an indicator of postnatal inflammation [42], has been associated with multiple manifestations of impaired brain development [43], but to our knowledge, not with learning limitations among children whose IQ is in the normal range. On the other hand, postnatal receipt of a steroid, which is often a correlate of the risk of bronchopulmonary dysplasia [44], has been identified as a risk factor for low reading scores among children born before the 26th week of gestation [1]. Necrotizing enterocolitis requiring surgery or drainage has also been identified as a risk factor for low scores on reading and mathematics assessments in this very high risk group [1].
4.2.3 Theme # 3: Immaturity/vulnerability
We have argued elsewhere that low gestational age is a surrogate for many developmental processes associated with vulnerability to organ damage and limited repair. Others have reported that selected stages of cerebral white matter oligodendrocyte maturation are especially vulnerable [45].
In a large study of 11-year-old children born before the 26th week of gestation, admission temperature less than 35°C, and long duration of stay in the neonatal unit, two indicators of relative immaturity, were associated with increased risk of low scores on mathematics [1]. In addition, because the risks of cranial ultrasound abnormalities, bronchopulmonary dysplasia, and necrotizing enterocolitis are highest in the least mature, the association of neonatal disorders with low scores on both reading and mathematics assessments can also be viewed as additional support for contributions of immaturity/vulnerability to the risk of learning limitations among X-ELGANs who have IQs in the normal range.
4.2.4 Theme # 4: Fetal growth restriction
Not only is fetal growth restriction an antecedent of brain damage [46], and low scores on language components of the Bayley Scales-III at age 2 years among children born very preterm [41], it also appears to place the newborn at increased risk of systemic inflammation [47].
4.3 Limitations and strengths
4.4. Conclusion
A multinomial model that includes information about socioeconomic disadvantage, immaturity, fetal growth restriction, and inflammation best accounts for the heightened risk of learning limitations among X-ELGANs. The risk profiles of reading only, math only, and the combination of reading and math limitations appear more alike than different.
Supplementary Material
Highlights.
Among 10-year old children born extremely preterm, the risk profiles of a ‘reading ONLY’ limitation, a ‘math ONLY’ limitation, and the combination of ‘reading AND math’ have similarities and differences
All risk profiles included an indicator of socioeconomic disadvantage and an indicator of newborn’s immaturity/vulnerability
An indicator of fetal growth restriction and inflammation were characteristic of the risk profiles of a reading ONLY limitation, and the reading AND math limitations entity.
These findings provide support for the hypothesis that what applies to children born at term also applies to children born extremely preterm
The higher risks of learning limitations in children born extremely preterm than among children born at term probably reflect the increased frequency of immaturity/vulnerability and its consequences including heightened risk neonatal inflammatory processes.
Acknowledgments
The authors express their gratitude to the children and their families who participated in this study. They also gratefully acknowledge the contributions of the ELGAN Study Investigators, listed below.
Boston Children’s Hospital, Boston MA
Janice Ware, Taryn Coster, Brandi Henson, Rachel Wilson, Kirsten McGhee, Patricia Lee, Aimee Asgarian, Anjali Sadhwani
Tufts Medical Center, Boston MA
Ellen Perrin, Emily Neger, Kathryn Mattern, Jenifer Walkowiak, Susan Barron
University of Massachusetts Medical School, Worcester MA
Jean Frazier, Lauren Venuti, Beth Powers, Ann Foley, Brian Dessureau, Molly Wood, Jill Damon-Minow
Yale University School of Medicine, New Haven, CT
Richard Ehrenkranz, Jennifer Benjamin, Elaine Romano, Kathy Tsatsanis, Katarzyna Chawarska, Sophy Kim, Susan Dieterich, Karen Bearrs
Wake Forest University Baptist Medical Center, Winston-Salem NC
T. Michael O’Shea, Nancy Peters, Patricia Brown, Emily Ansusinha, Ellen Waldrep, Jackie Friedman, Gail Hounshell, Debbie Allred
University Health Systems of Eastern Carolina, Greenville, NC
Stephen C. Engelke, Nancy Darden-Saad, Gary Stainback
North Carolina Children’s Hospital, Chapel Hill, NC
Diane Warner, Janice Wereszczak, Janice Bernhardt, Joni McKeeman, Echo Meyer
Helen DeVos Children’s Hospital, Grand Rapids, MI
Steve Pastyrnak, Wendy Burdo-Hartman, Julie Rathbun, Sarah Nota, Teri Crumb
Sparrow Hospital, Lansing, MI
Madeleine Lenski, Deborah Weiland, Megan Lloyd
University of Chicago Medical Center, Chicago, IL
Scott Hunter, Michael Msall, Rugile Ramoskaite, Suzanne Wiggins, Krissy Washington, Ryan Martin, Barbara Prendergast, Megan Scott
William Beaumont Hospital, Royal Oak, MI
Judith Klarr, Beth Kring, Jennifer DeRidder, Kelly Vogt
Funding
This study was supported by grants from the National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069-06A2), the National Institute of Child Health and Human Development (5P30HD018655-34), and the Office of the NIH Director (1UG3OD023348-01).
Abbreviations
- BPD/CLD
Bronchopulmonary dysplasia/chronic lung disease
- NEC
Necrotizing enterocolitis
- SES
Socioeconomic status
- WIAT-III
Wechsler Individual Achievement Test-III
- X-ELGANs
Former extremely low gestational age newborns
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None.
Citations
- 1.Sillanpaa M, Saarinen M, Schmidt D. Long-term risks following first remission in childhood-onset epilepsy. A population-based study. Epilepsy Behav. 2012;25:145–9. doi: 10.1016/j.yebeh.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Morgan PL, Farkas G, Wu Q. Kindergarten children’s growth trajectories in reading and mathematics: who falls increasingly behind? Journal of learning disabilities. 2011;44:472–88. doi: 10.1177/0022219411414010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Pehboeck-Walser N, Fussenegger B. Early risk predictors for impaired numerical skills in 5-year-old children born before 32 weeks of gestation. Acta Paediatr. 2013;102:66–71. doi: 10.1111/apa.12036. [DOI] [PubMed] [Google Scholar]
- 4.Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–13. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 5.Hagen EW, Palta M, Albanese A, Sadek-Badawi M. School achievement in a regional cohort of children born very low birthweight. J Dev Behav Pediatr. 2006;27:112–20. doi: 10.1097/00004703-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105:325–31. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S, Hennessy E, Smith R, Trikic R, Wolke D, Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F283–9. doi: 10.1136/adc.2008.152793. [DOI] [PubMed] [Google Scholar]
- 8.Marlow N. Stratifying risk factors for learning problems in very preterm children. Dev Med Child Neurol. 2013;55:105–6. doi: 10.1111/dmcn.12023. [DOI] [PubMed] [Google Scholar]
- 9.D’Angio CT, Sinkin RA, Stevens TP, Landfish NK, Merzbach JL, Ryan RM, et al. Longitudinal, 15-year follow-up of children born at less than 29 weeks’ gestation after introduction of surfactant therapy into a region: neurologic, cognitive, and educational outcomes. Pediatrics. 2002;110:1094–102. doi: 10.1542/peds.110.6.1094. [DOI] [PubMed] [Google Scholar]
- 10.Guellec I, Lapillonne A, Renolleau S, Charlaluk ML, Roze JC, Marret S, et al. Neurologic outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics. 2011;127:e883–91. doi: 10.1542/peds.2010-2442. [DOI] [PubMed] [Google Scholar]
- 11.Kok JH, den Ouden AL, Verloove-Vanhorick SP, Brand R. Outcome of very preterm small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol. 1998;105:162–8. doi: 10.1111/j.1471-0528.1998.tb10046.x. [DOI] [PubMed] [Google Scholar]
- 12.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen-Pupp I, Hovel H, Lofqvist C, Hellstrom-Westas L, Fellman V, Huppi PS, et al. Circulatory insulin-like growth factor-I and brain volumes in relation to neurodevelopmental outcome in very preterm infants. Pediatr Res. 2013;74:564–9. doi: 10.1038/pr.2013.135. [DOI] [PubMed] [Google Scholar]
- 14.Fiks AG, Mayne S, Karavite DJ, DeBartolo E, Grundmeier RW. A shared e-decision support portal for pediatric asthma. The Journal of ambulatory care management. 2014;37:120–6. doi: 10.1097/JAC.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa DS, Miranda DM, Burnett AC, Doyle LW, Cheong JLY, Anderson PJ. Executive Function and Academic Outcomes in Children Who Were Extremely Preterm. Pediatrics. 2017;140 doi: 10.1542/peds.2017-0257. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131:e1053–61. doi: 10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- 17.Taylor HG, Hack M, Klein N, Schatschneider C. Achievement in children with birth weights less than 750 grams with normal cognitive abilities: evidence for specific learning disabilities. J Pediatr Psychol. 1995;20:703–19. doi: 10.1093/jpepsy/20.6.703. [DOI] [PubMed] [Google Scholar]
- 18.Laughon M, O’Shea MT, Allred EN, Bose C, Kuban K, Van Marter LJ, et al. Chronic lung disease and developmental delay at 2 years of age in children born before 28 weeks’ gestation. Pediatrics. 2009;124:637–48. doi: 10.1542/peds.2008-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SM, Park KH, Jung EY, Cho SH, Ryu A. Prediction of spontaneous preterm birth in women with cervical insufficiency: Comprehensive analysis of multiple proteins in amniotic fluid. J Obstet Gynaecol Res. 2016;42:776–83. doi: 10.1111/jog.12976. [DOI] [PubMed] [Google Scholar]
- 20.Omarsdottir S, Agnarsdottir M, Casper C, Orrego A, Vanpee M, Rahbar A, et al. High prevalence of cytomegalovirus infection in surgical intestinal specimens from infants with necrotizing enterocolitis and spontaneous intestinal perforation: A retrospective observational study. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2017;93:57–64. doi: 10.1016/j.jcv.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Logan JW, Dammann O, Allred EN, Dammann C, Beam K, Joseph RM, et al. Early postnatal illness severity scores predict neurodevelopmental impairments at 10 years of age in children born extremely preterm. J Perinatol. 2017;37:606–14. doi: 10.1038/jp.2016.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leviton A, Allred EN, Paneth N, Kuban KCK, Dammann O, O’Shea TM, et al. Early blood gas abnormalities and the preterm brain. Am J Epidemiology. 2010;172:907–16. doi: 10.1093/aje/kwq222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 24.Redline RW, Pappin A. Fetal thrombotic vasculopathy: the clinical significance of extensive avascular villi. Human pathology. 1995;26:80–5. doi: 10.1016/0046-8177(95)90118-3. [DOI] [PubMed] [Google Scholar]
- 25.Mifsud W, Sebire NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal diagnosis and therapy. 2014;36:117–28. doi: 10.1159/000359969. [DOI] [PubMed] [Google Scholar]
- 26.Marsal K. Obstetric management of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol. 2009;23:857–70. doi: 10.1016/j.bpobgyn.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Doyle LW, Ehrenkranz RA, Halliday HL. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014:CD001145. doi: 10.1002/14651858.CD001145.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, et al. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124:e450–6. doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Branum-Martin L, Fletcher JM, Stuebing KK. Classification and identification of reading and math disabilities: the special case of comorbidity. Journal of learning disabilities. 2013;46:490–9. doi: 10.1177/0022219412468767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan JW, O’Shea TM, Allred EN, Laughon MM, Bose C, Dammann O, et al. Early Postnatal Hypotension and Developmental Delay at 24 Months of Age among Extremely Low Gestational Age Newborns. Archives of Disease in Childhood. 2011 doi: 10.1136/adc.2010.183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helderman JB, O’Shea TM, Goldstein DJ, Hounshell G, Allred EN, Kuban KCK, et al. Antenatal antecedents of low scores on the Bayley Scales of Infant Development at 24 months among children born before the 28th post-menstrual week. The ELGAN Study. Pediatrics. 2012;129:494–502. [Google Scholar]
- 32.Streimish IG, Ehrenkranz RA, Allred EN, O’Shea TM, Kuban KC, Paneth N, et al. Birth weight- and fetal weight-growth restriction: Impact on neurodevelopment. Early Hum Dev. 2012;88:765–71. doi: 10.1016/j.earlhumdev.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanni D, Korzeniewski S, Allred EN, Fichorova RN, O’Shea TM, Kuban K, et al. Both antenatal and postnatal inflammation contribute information about the risk of brain damage in extremely preterm newborns. Pediatr Res. 2017;82:691–6. doi: 10.1038/pr.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Shea TM, Allred EN, Kuban K, Dammann O, Paneth N, Fichorova R, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at two years in extremely premature infants. J Pediatr. 2012;160:395–401.e4. doi: 10.1016/j.jpeds.2011.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan GJ, Magnuson K, Votruba-Drzal E. Moving Beyond Correlations in Assessing the Consequences of Poverty. Annu Rev Psychol. 2017;68:413–34. doi: 10.1146/annurev-psych-010416-044224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards JL, Chapple-McGruder T, Williams BL, Kramer MR. Does neighborhood deprivation modify the effect of preterm birth on children’s first grade academic performance? Soc Sci Med. 2015;132:122–31. doi: 10.1016/j.socscimed.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gisselmann M, Koupil I, De Stavola BL. The combined influence of parental education and preterm birth on school performance. J Epidemiol Community Health. 2011;65:764–9. doi: 10.1136/jech.2009.105569. [DOI] [PubMed] [Google Scholar]
- 38.Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014;41:83–103. doi: 10.1016/j.clp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014 doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50:3348–62. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee I, Neil JJ, Huettner PC, Smyser CD, Rogers CE, Shimony JS, et al. The impact of prenatal and neonatal infection on neurodevelopmental outcomes in very preterm infants. J Perinatol. 2014;34:741–7. doi: 10.1038/jp.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bose C, Laughon M, Allred EN, Van Marter LJ, O’Shea TM, Ehrenkranz RA, et al. Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatr Res. 2011;69:347–53. doi: 10.1203/PDR.0b013e31820a58f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120:1337–51. doi: 10.1213/ANE.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halliday HL. Update on Postnatal Steroids. Neonatology. 2017;111:415–22. doi: 10.1159/000458460. [DOI] [PubMed] [Google Scholar]
- 45.Penn AA, Gressens P, Fleiss B, Back SA, Gallo V. Controversies in preterm brain injury. Neurobiol Dis. 2015;92:90–101. doi: 10.1016/j.nbd.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller SL, Huppi PS, Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol. 2016;594:807–23. doi: 10.1113/JP271402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McElrath TF, Fichorova RN, Allred EN, Hecht JL, Ismail MA, Yuan H, et al. Blood protein profiles of infants born before 28 weeks differ by pregnancy complication. Am J Obstet Gynecol. 2011;204418:e1–e12. doi: 10.1016/j.ajog.2010.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


