Abstract
Post-viral pneumococcal pneumonia is a leading morbidity and mortality in older patients (≥ 65 years of age). The goal of our current study is to understand the impact of chronological aging on innate immune responses to a secondary, post viral infection with Streptococcus pneumoniae, a causative agent of bacterial pneumonia. Using aged murine models of infection, our findings demonstrate increased morbidity and mortality in aged mice within 48 hours post secondary S. pneumoniae infection. Increased susceptibility of aged mice was associated with decreased TLR1, TLR6, and TLR9 mRNA expression and diminished IL1β mRNA expression. Examination of NLRP3 inflammasome expression illustrated decreased NLRP3 mRNA expression and decreased IL1β production in aged lung in response to secondary S. pneumoniae infection.
1. Introduction
Secondary pneumococcal infections are a leading cause of community-acquired pneumonia, sepsis, and death in older patients (≥ 65 years of age), with Streptococcus pneumoniae being the most causative organism (Heron 2011; McBean and Hebert 2004; Sousa and others 2013). Previous studies have illustrated an increase in hospitalization rates for pneumonia, with significant increases occurring among older adults diagnosed with chronic cardiac disease, chronic pulmonary disease, or diabetes mellitus (Fry and others 2005). It is estimated that older patients, account for the most serious cases of pneumococcal infections with the majority of direct medical costs as well as the highest rate of hospitalizations, number of days hospitalized, emergency department visits, outpatient visits, and deaths (Heron 2011; Huang and others 2011).
It has been well established that the process of chronological aging affects various components of the immune response, leading to impaired host defense, defective vaccine responses, and a significantly higher risk of elderly persons developing life-threatening bacterial infections (Miyashita and others 2012; Pawelec and others 2005; Weng 2006). Due to increased prevalence of comorbidities in older persons, impaired adaptive immune responses to vaccination, and the pervasiveness of antibiotic resistant bacterial strains, there is a pressing need to understand the molecular mechanisms that underlie these impairments and develop cutting-edge therapies that specifically target and amplify innate immune responses in older persons (Simonsen and others 2009).
The NLRP3 inflammasome is a multiprotein complex consisting of the nucleotide-binding domain leucine-rich repeat containing (NLR) family member NLRP3, the adaptor protein ASC, and the cysteine protease caspase 1 (Agostini and others 2004). The NLRP3 inflammasome can activate caspase 1 in response to cellular danger, resulting in the processing and secretion of proinflammatory cytokines IL1β and IL18 (Kanneganti and others 2006; Mariathasan and others 2006; Martinon and Tschopp 2007). A diverse array of stimuli can activate the NLRP3 inflammasome including both pathogen-associated molecular patterns (PAMPs) and endogenous host-derived molecules indicative of cellular damage (Nakahira and others 2011; Petrilli and others 2007). Recent work has illustrated the importance of caspase 1 dependent responses, mediated by the NLRP3 inflammasome, in modulating innate immunity to S. pneumoniae (Fang and others 2014; Karmakar and others 2015; Koedel and others 2002; Mariathasan and others 2006; McNeela and others 2010; Mitchell and Mitchell 2010; Shoma and others 2008; Witzenrath and others 2011).
Our previous findings demonstrate that the NLRP3 inflammasome is needed for protection and activation of the inflammasome is decreased and/or delayed in aged lung during influenza infection (Stout-Delgado and others 2012). To expand upon these findings, as detailed in the findings of our current study, we examined the impact of chronological aging on inflammasome expression and production of IL1β in response to a secondary post influenza S. pneumoniae infection. Using murine models of infection, our findings demonstrate increased morbidity and mortality in aged mice within 48 hours post secondary S. pneumoniae infection relative to young mice. Increased susceptibility of aged mice was associated with decreased TLR1, TLR6, and TLR9 mRNA expression and diminished IL1β mRNA expression compare to young controls. Examination of NLRP3 inflammasome expression illustrated decreased NLRP3 mRNA expression and decreased IL1β production in aged lung in response to secondary S. pneumoniae infection when compare to young.
2. Materials and Methods
2.1. Mice
Young (2 months) and aged (19 months) male and female BALB/c mice were purchased from the NIA rodent facility (Charles River Laboratories). Upon receipt, mice were handled under identical husbandry conditions and fed certified commercial feed. Body weights were measured daily and mice were humanely euthanized if they lost more than 15% of their starting body weight. The IACUC at Weill Cornell Medical College approved the use of animals in this study. No animals were used in the study if they had evidence of skin lesions, weight loss, or lymphadenopathy.
2.2. Pathogen propagation and culture
Influenza A virus (A/PR/8/1934(H1N1)) (PR8) was purchased from ATCC and grown in MDCK cells (ATCC, Manassas, VA) as previously described (Szretter and others 2006). Streptococcus pneumoniae (ATCC 6303, ATCC, Manassas, VA) was grown on 10% sheep blood agar plates (BD Biosciences, San Jose, CA) overnight or for 4–24 hours in brain heart infusion (BHI) broth (BD Biosciences). Colony forming units (CFU) were assessed by dilution of samples in BHI and titers were determined by colony counts X dilution.
2.3. In vivo procedures
As detailed in Figure 1A: Primary influenza viral infection (INF + PBS): All mice were anesthetized with isoflurane (5% for induction and 2% for maintenance) prior to intranasal instillation with 1×103 PFU of PR8 (50 μL volume in PBS). On day 14-post influenza instillation, mice received an intranasal instillation of 50 μL of PBS. Our previous work has illustrated that there are undetectable levels of influenza present in aged lung at day 14 post infection (Stout-Delgado and others 2012). Primary S. pneumoniae infection (PBS + S. pne): All mice were anesthetized with isoflurane (5% for induction and 2% for maintenance) prior to intranasal instillation with 1×103 CFU of S. pneumoniae (ATCC 6303) (50 μL volume in PBS). Secondary S. pneumoniae infection (INF + S. pne): All mice were anesthetized with isoflurane (5% for induction and 2% for maintenance) prior to intranasal instillation with 1×103 PFU of PR8 (50 μL volume in PBS). On day 14-post influenza instillation, mice received an intranasal instillation of 50 μL of 1×103 CFU of S. pneumoniae (ATCC 6303) (50 μL volume in PBS). As previously described, the following clinical scores were assigned: 0=normal, 1=slightly ruffled, 2=ruffled fur, 3= ruffled fur and inactive, 4=hunched/moribund, and 5= dead (Dimmock and Marriott 2006; Stout-Delgado and others 2012).
Figure 1. Increased Morbidity and Mortality of Aged Mice in Response to Secondary S. pneumoniae Infection.
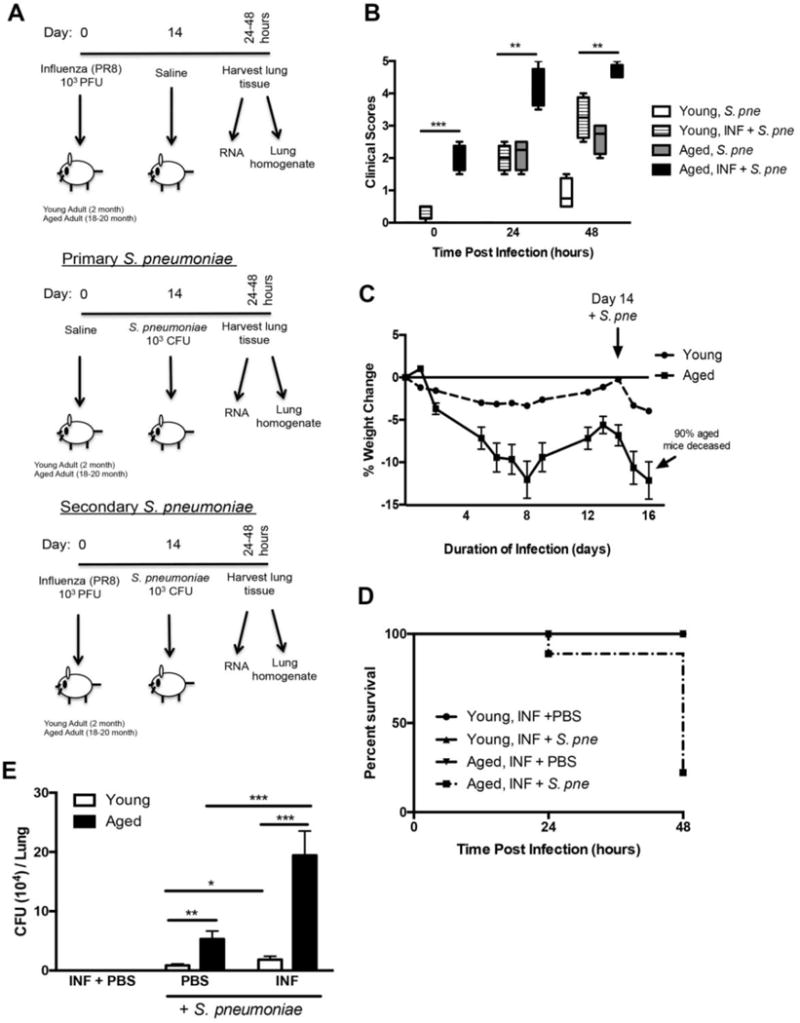
(A) Experimental layout of primary influenza (day 0 instillation with 103 PFU influenza A/PR/8/34 H1N1 (PR8) followed by day 14 instillation with PBS), primary S. pneumoniae (day 0 instillation with PBS followed by day 14 instillation with 103 CFU of ATCC 6303), and secondary S. pneumoniae (day 0 instillation with PR8 103 PFU followed by day 14 instillation with 103 CFU of ATCC 6303) infection in young (2 months) and aged (19 months) adult male and female BALB/c mice. (B) Clinical scores (0hr: P=0.0005, 24hr: P=0.0013, and 48hr: P=0.0073), (C) weight changed (P<0.0001), and (D) survival (P=0.0004) in young and aged mice in response to secondary S. pneumoniae infection. (E) Bacterial titer in lung was assessed in lung homogenates collected at 24 hours post infection (primary S. pneumoniae (PBS + S. pne): P=0.0028, secondary S. pneumoniae (INF + S. pne): P=0.0001, young primary vs. secondary S. pneumoniae: P=0.0461, and aged primary vs. secondary S. pneumoniae: P=0.0006). Similar results were obtained from at least two or more independent experiments with an N=5 per experiment. Data are expressed as the mean ± SEM.
2.4. RNA purification and real time PCR
RNA was extracted from lung tissue at 24 hours post-secondary control (influenza + PBS) or S. pneumoniae (influenza + S. pneumoniae) infection using previously published methods (Stout-Delgado and others 2012). QuantiTect Primer Assays were used to assess gene expression (Qiagen). All reactions were performed in triplicate and an endogenous control was used to ensure experimental reproducibility. Relative levels of messenger RNA (mRNA) were calculated by the comparative cycle threshold method and either β–actin mRNA levels were used as the invariant control for each sample. Briefly, for these calculations, we used young or aged lung from influenza infected mice (day 14 post infection) at 24 hours post PBS instillation (as detailed in Figure 1A) as the calibrator. mRNA expression values in young and aged lung in response to secondary S. pneumoniae infection are relative to age-matched calibrator samples.
2.5. ELISA
Culture supernatants and lung homogenates were analyzed for IL1β and TNFα production using ELISA kits purchased from eBioscience (San Diego, CA) per manufacturer’s instructions. Changes in 450nm absorbance and correction at 570nm were assessed using GloMax Multi-Detection System (Promega, Fitchburg, WI). Results were analyzed using Graph Pad Prism software (San Diego, CA).
2.6. Statistical Analysis
Survival analysis between groups was calculated using the Mantel Cox test. Comparison of groups was performed using a two-tailed t-test, one-way or two-way ANOVA, when appropriate. Samples obtained were normally or approximately normally distributed. All samples were independent and contained the same sample size for analysis. Variances of the populations were equal. All data were analyzed using GraphPad Prism software. Statistical significance was considered by a p value < 0.05.
3. Results
3.1. Increased Morbidity and Mortality in Aged Mice in Response to Secondary S. pneumoniae Infection
It is well established that the elderly have increased morbidity and mortality to primary Streptococcus pneumoniae infections (de Cunto Brandileone and others 1998; Kurtti and others 1997). Given the prevalence for post-viral pneumonia in the elderly, our experiments were designed to examine the impact of aging on NLRP3 inflammasome expression in response to a secondary infection with S. pneumoniae. Based on our previous work, we chose to use S. pneumoniae ATCC 6303, a highly virulent type 3 strain of S. pneumoniae commonly associated with an increased relative risk of death in older persons (Martens and others 2004; Mitzel and others 2014). As illustrated in Figure 1A, young (2 months of age) and aged (19 months of age) adult mice were instilled with saline or influenza (PR8). Mice were monitored for the duration of influenza infection and on day 14, a time point in which influenza is no longer detectable in both young and aged lung, mice received an instillation of PBS or S. pneumoniae (Figure 1A) (Stout-Delgado and others 2012). When compared to young, in response to secondary S. pneumoniae, there was a significant increase in baseline and pathogen-associated morbidity in aged mice (Figure 1B: 0hr P=0.0005, 24hr P=0.0013, 48hr P=0.0073). In addition, when compared to young, there is a significant increase in weight loss (Figure 1C: P<0.0001) and mortality (Figure 1D: P<0.0001) in aged mice in response to secondary S. pneumoniae infection. In response to secondary S. pneumoniae infection, there were also significantly higher bacterial titers present in aged lung (Figure 1E, Young vs. aged: PBS + S. pneumoniae, P=0.0028 and INF + S. pneumoniae, P=0.0001).
3.2. TLR Signaling in Aged Lung in Response to Secondary S. pneumoniae Infection
To gain a better understanding of how the process of chronological aging might contribute to increased susceptibility of older persons to secondary S. pneumoniae, we first examined the impact of aging on the activation of pathogenic recognition receptors. Previous work has illustrated an important role for toll like receptors (TLR) in the innate immune response to S. pneumoniae and dysregulated TLR signaling in aged lung contributes to increased susceptible to infection (Albiger and others 2007; Boyd and others 2012; Branger and others 2004; Hinojosa and others 2009; Knapp and others 2004; Nguyen and others 2015). We isolated lung from young and aged mice at 24 hours post secondary S. pneumoniae infection and examined TLR mRNA expression by real time PCR. Changes in mRNA expression were relative to PBS instilled age-matched lung at day 14 post influenza infection (Figure 2). When compared to young, we detected a significant decrease in TLR1, TLR6, and TLR9 mRNA expression in aged lung in response to secondary S. pneumoniae infection (Figure 2A: TLR1 P=0.0011, TLR6 P=0.0223, and TLR9 P=0.0041). Similar expression of TLR2, TLR4, and MYD88 mRNA expression was detected in both young and aged lung tissue (Figure 2A).
Figure 2. Dysregulated TLR Signaling in Aged Lung in Response to Secondary S. pneumoniae Infection.
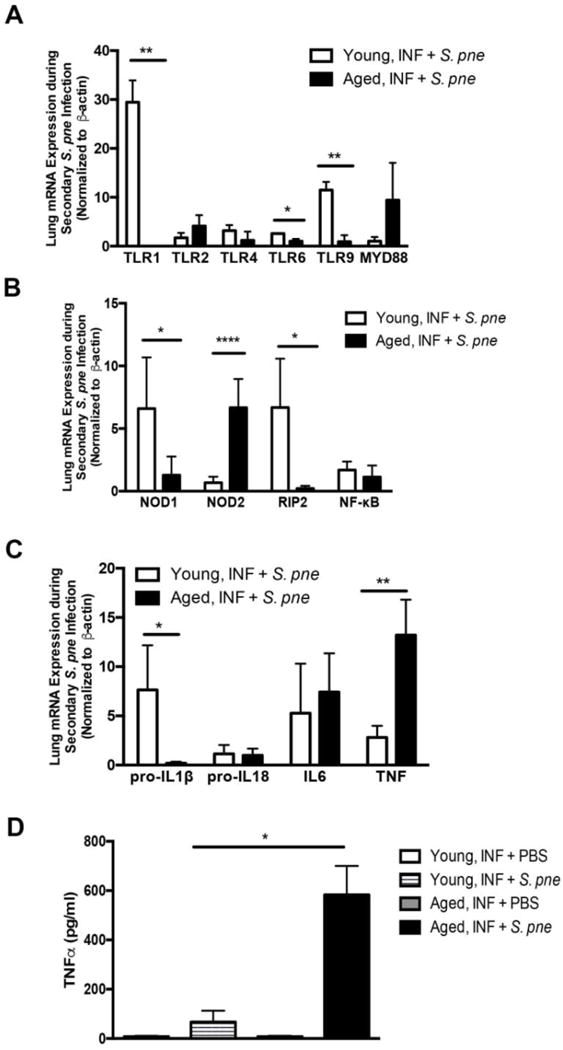
(A–C) Young (2 months) and aged (19 months) male and female BALB/c mice received influenza (day 0: PR8, 103 PFU-INF) prior to secondary instillation with S. pneumoniae (day 14, 103 CFU, ATCC 6303- S. pne). Lung tissue was collected at 24 hours post-secondary S. pneumoniae infection. Young and aged lung from influenza-infected mice (day 14 post infection) at 24 hours post PBS instillation were used as calibrator samples. mRNA expression values in young and aged lung in response to secondary S. pneumoniae infection are relative to age-matched calibrator samples. (A) Changes in lung mRNA expression of TLR1 (P=0.0011), TLR2, TLR4, TLR6 (P=0.0223), and TLR9 (P=0.0041) in young and aged lung was assessed by real time PCR. (B) Changes in mRNA expression of NOD1 (P=0.0201), NOD2 (P<0.0001), RIP2 (P=0.0455), and NF-κB at 24 hours post infection were assessed by real time PCR. (C) Inflammatory cytokine expression was investigated in young and aged lung and changes in pro-IL1β (P=0.0169), pro-IL18, IL6, and TNF (P=0.0089) were assessed by real time PCR. (D) TNFα production in lung homogenates was assessed at 24 hours secondary instillation with PBS (day 0 instillation with influenza followed by day 14 instillation with PBS: INF + PBS) or S. pneumoniae (day 0 instillation with influenza followed by day 14 instillation with S. pneumoniae: INF + S. pne) (P=0.0284). Similar results were obtained from at least three or more independent experiments with an N=5 per experiment. Data are expressed as the mean ± SEM.
Previous work has illustrated that in response to streptococcal peptidoglycan NOD-like receptor (NLR), NOD2, plays an important role in the production of inflammatory mediators, such as TNFα, IL1β, and IL6 (Chamaillard and others 2003; Davis and others 2011). Given the importance of the NOD1/NOD2 pathway on innate immune responsiveness to multiple pulmonary pathogens, we next examined if changes in NOD1, NOD2, or RIP2 mRNA expression might be associated with increased morbidity and mortality of aged mice to a secondary infection with S. pneumoniae. There was decreased upregulation of NOD1 and RIP2 mRNA expression in aged lung relative to young control (Figure 2B: NOD1 P=0.0201, RIP2 P=0.0455). When compared to young, there was a significant upregulation in NOD2 mRNA expression in aged lung in response to secondary infection with S. pneumoniae (Figure 2B: NOD2 P<0.0001). We next evaluated the impact of chronological aging on inflammatory cytokine expression in response to secondary infection with S. pneumoniae. Expression of IL6 and TNFα mRNA was augmented in both young and aged lung, with significantly higher levels TNFα expression being detected in aged lung relative to young control (Figure 2C: P=0.0089). While the increase in pro-IL18 mRNA expression in lung was similar, pro-IL1β mRNA expression was significantly decreased in aged lung in response to secondary S. pneumoniae infection when compare to young (Figure 2C: P=0.0169). To expand these findings, we examined TNFα expression in lung homogenates collected from young and aged mice in response to secondary S. pneumoniae infection. When compared to young, there is a significant increase in TNFα production in aged lung in response to a secondary S. pneumoniae infection (Figure 2D: P=0.0284).
3.3. NLRP3 mRNA Expression in Response to Secondary S. pneumoniae Infection is diminished in Aged Lung
Based upon these findings, we next evaluated the impact of chronological aging on NLRP3 inflammasome expression during secondary S. pneumoniae infection. Despite similar pro-caspase 1 and ASC mRNA upregulation, there is a significant decrease in NLRP3 mRNA expression in aged lung in response to a secondary infection with S. pneumoniae when compared to young (Figure 3A: P=0.001). Given these findings, we next examined the impact of decreased NLRP3 mRNA expression on IL1β production. At 24 hours post secondary S. pneumoniae infection, there was a similar detectable increase in IL1β production in serum collected from both young and aged mice (Figure 3B). While IL1β production was also detectable in lung homogenates, when compared to young, expression in aged lung at 24 hours post secondary infection was markedly reduced (Figure 3C: P<0.05).
Figure 3. NLRP3 mRNA Expression in Response to Secondary S. pneumoniae Infection is diminished in Aged Lung.
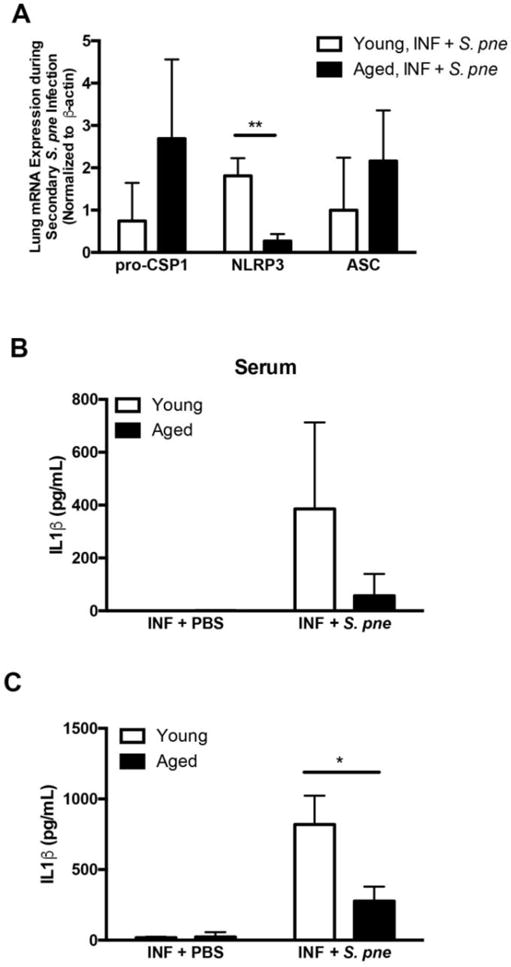
(A–C) A–C) Young (2 months) and aged (19 months) male and female BALB/c mice received influenza (day 0: PR8, 103 PFU-INF) prior to secondary instillation with S. pneumoniae (day 14, 103 CFU, ATCC 6303- S. pne). Serum and lung tissue was collected at 24 hours post-secondary S. pneumoniae infection. Young and aged lung from influenza-infected mice (day 14 post infection) at 24 hours post PBS instillation were used as calibrator samples. mRNA expression values in young and aged lung in response to secondary S. pneumoniae infection are relative to age-matched calibrator samples. (A) Changes in lung mRNA expression of pro-caspase 1, NLRP3 (P=0.001), and ASC in young and aged lung was assessed by real time PCR. IL1β production in (B) serum and (C) lung homogenates (P=0.0147) was assessed by ELISA. Similar results were obtained from at least three or more independent experiments with an N=5 per experiment. Data are expressed as the mean ± SEM.
4. Discussion
At present, very little is known regarding the role of the NLRP3 inflammasome in mediating innate immune responses to a secondary S. pneumoniae infection. The results of our current study demonstrate that there is increased morbidity and mortality of aged mice in response to secondary post-viral S. pneumoniae infection. In addition, there is an age-associated decrease in TLR1, TLR6, and TLR9 mRNA expression that may contribute to decreased pro-IL1β expression, and diminished IL1β production in aged lung in response to secondary S. pneumoniae infection.
While previous work has illustrated impaired innate immune responses in aged lung to primary influenza or S. pneumoniae infection, very little is known how the process of chronological aging contributes to increased susceptibility to secondary bacterial infections. Results of our current study illustrate that there is decreased TLR1, TLR6, and TLR9 mRNA expression in aged lung in response to secondary S. pneumoniae infection. It is possible that in agreement with previously published work, a chronic inflammatory environment contributes to changes in TLR expression and dysfunction (Hinojosa and others 2009). Despite similar levels of influenza clearance by day 14 post influenza, upregulation of TLR expression may remain significantly altered in aged lung and thereby contribute to dysregulated innate immune responses to secondary pathogenic stimuli (Stout-Delgado and others 2012). An alteration in the level of TLR expression and function can result in abnormal innate signaling and associated inflammatory responses. It is evident by the results of our current study that a previous viral infection can significantly impact the responsiveness of innate immune responses to the next infectious stimuli. Based upon our previous work, it is plausible that the increased length of influenza infection in aged lung might influence NLRP3 inflammasome responsiveness to secondary pathogenic stimuli (Stout-Delgado and others 2012).
Dysregulated regulation of proinflammatory cytokine production can result in detrimental innate immune signaling in aged lung, thereby contributing to increased morbidity and mortality in response to infectious stimuli. Results of our study illustrate that TNFα expression is significantly upregulated in aged lung in response to a secondary infection with S. pneumoniae. While essential for bacterial clearance, overly heightened expression of TNFα can contribute to increased neutrophil recruitment and activation. Excessive and sustained TNFα expression may also contribute to increased lung damage and downstream imbalances in cytokine responses. Future work to understand the impact of increased TNFα expression in aged lung on neutrophil activation and function in response to secondary pneumococcal infection will need to be performed.
Recent work, using a model of pneumococcal meningitis, has illustrated that the NLRP3 inflammasome can contribute to increased host pathology instead of pathogen protection and clearance (Hoegen and others 2011). Our recent work has illustrated that in response to heightened ROS and DNA damage in response to bleomycin sulfate instillation, aged macrophages can contribute to harmful NLRP3-mediated inflammation and development of pulmonary fibrosis (Stout-Delgado and others 2016). Similarly, in the presence of high levels of free fatty acids, excessive NLRP3 activation can contribute to increased morbidity in response to S. pneumoniae (Moon and others 2016). NLRP3 inflammasome responses are tightly regulated and the balance between harmful and beneficial responses to secondary pathogenic stimuli has not been fully elucidated. Similar to other innate immune pathways, excessive inflammasome activation in response to a secondary infection with S. pneumoniae can be detrimental to the host, resulting in extensive tissue damage. It is plausible, that the threshold for early IL1β production in response to a secondary pathogen may be significantly altered in aged lung. Future work will need to examine the impact of location, magnitude, the context of inflammasome activation, and the impact of chronological aging and primary influenza infection on these responses.
It is important to note that our study investigated a highly virulent serotype 3 strain of S. pneumoniae that has been shown to have a higher incidence of occurrence in older adults. Given the divergent nature and secretion of pneumolysin by different S. pneumoniae strains, it will be of great interest to investigate if specific changes in TLR mRNA expression post influenza infection are conserved in response to multiple S. pneumoniae serotypes. These avenues of discovery may aid in the development of targeted therapeutics that can improve innate antibacterial responses against multiple pathogens.
The usage of antibiotics is essential for the management of pneumococcal infections. A systematic review of twenty antibiotic therapy studies illustrated antibiotic treatment initiated within four to eight hours of hospital arrival resulted in improved survival (Lee and others 2016). Results from our current study illustrate pneumococcal disease severity in aged is rapid and is associated with enhanced bacterial growth in lung. Given these findings, future studies to understand the impact of antibacterial therapies on post viral pneumococcal disease progression in aged lung will need to be performed.
It is important to note that gene expression patterns detailed in the results of our current study are representative of whole lung tissue and thereby, reflect a heterogeneous cell population present in the lung in response to a secondary S. pneumoniae infection. Neutrophil recruitment and activation in response to macrophage and epithelial cell cytokine and chemokine production is essential to the control of bacterial replication. The results of our current study illustrate decreased inflammasome gene expression and diminished IL1β production in aged lung in response to a secondary bacterial infection. As resident and infiltrating macrophages play an important role in the lung’s innate host defense against S. pneumoniae infection, future work will be pursued to examine the impact of primary influenza infection on cellular migration to lung and the role of chronological age of these frequencies.
In sum, findings of our current study demonstrate a potential mechanism by which an age associated decrease in NLRP3 inflammasome expression and IL1β production contributes to increased morbidity and mortality in response to secondary pneumococcal infection.
Highlights.
Our current study was designed to understand the impact of chronological aging on innate immune responses to a secondary, post influenza infection with Streptococcus pneumoniae, a causative agent of bacterial pneumonia.
Increased susceptibility of aged mice to a secondary S. pneumoniae infection was associated with decreased TLR1, TLR6, and TLR9 mRNA expression and diminished IL1β mRNA expression.
Examination of NLRP3 inflammasome expression illustrated decreased NLRP3 mRNA expression and decreased IL1β production in aged lung in response to secondary S. pneumoniae infection.
Acknowledgments
Funding sources: K01AG034999 (H.W.S), R21AG044755 (H.W.S), R01AG052530 (H.W.S), WCMC Department of Medicine Seed Grant for Innovative Research (H.W.S), American Lung Association Biomedical Grant (S.J.C), K08HL138285 (S.J.C), and WCMC Pre-K Award Department of Medicine (M.P).
Abbreviations
- S. pne
Streptococcus pneumoniae
- NLRP3
NACHT LRR, and PYD domains-containing protein 3
- ASC
apoptosis-associated speck-like protein containing a CARD
- NOD
nucleotide-binding oligomerization domain-containing protein
- RIP2
receptor-interacting serine/threonine-protein kinase 2
- TLR
toll like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Albiger B, Dahlberg S, Sandgren A, Wartha F, Beiter K, Katsuragi H, Akira S, Normark S, Henriques-Normark B. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol. 2012;47:507–518. doi: 10.1016/j.exger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, Florquin S, van der Poll T. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect Immun. 2004;72:788–794. doi: 10.1128/IAI.72.2.788-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamaillard M, Girardin SE, Viala J, Philpott DJ. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 2003;5:581–592. doi: 10.1046/j.1462-5822.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- Davis KM, Nakamura S, Weiser JN. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest. 2011;121:3666–3676. doi: 10.1172/JCI57761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cunto Brandileone MC, Simonsen DVV, Tadeu Casagrande S, Cobo Zanella R, Leopoldo Silva Guerra ML, Pires Brandao A, de Andrade Melles CE, AC CP, Di Fabio JL, Austrian R. Characteristics of Isolates Streptococcus pneumoniae from Middle-Aged and Elderly Adults in Brazil: Capsular Serotypes and Antimicrobial Sensitivity to Invasive Infections. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 1998;2:90–96. [PubMed] [Google Scholar]
- Dimmock NJ, Marriott AC. In vivo antiviral activity: defective interfering virus protects better against virulent Influenza A virus than avirulent virus. J Gen Virol. 2006;87:1259–1265. doi: 10.1099/vir.0.81678-0. [DOI] [PubMed] [Google Scholar]
- Fang R, Hara H, Sakai S, Hernandez-Cuellar E, Mitsuyama M, Kawamura I, Tsuchiya K. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect Immun. 2014;82:2310–2317. doi: 10.1128/IAI.01572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294:2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2007. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2011;59:1–95. [PubMed] [Google Scholar]
- Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegen T, Tremel N, Klein M, Angele B, Wagner H, Kirschning C, Pfister HW, Fontana A, Hammerschmidt S, Koedel U. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J Immunol. 2011;187:5440–5451. doi: 10.4049/jimmunol.1100790. [DOI] [PubMed] [Google Scholar]
- Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, Finkelstein JA. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–3412. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, Pearlman E. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol. 2015;194:1763–1775. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S, Wieland CW, van ‘t Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;2:721–736. doi: 10.1016/s1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- Kurtti P, Isoaho R, von Hertzen L, Keistinen T, Kivela SL, Leinonen M. Influence of age, gender and smoking on Streptococcus pneumoniae, Haemophilus influenzae and Moraxella (Branhamella) catarrhalis antibody titres in an elderly population. Scandinavian journal of infectious diseases. 1997;29:485–489. doi: 10.3109/00365549709011859. [DOI] [PubMed] [Google Scholar]
- Lee JS, Giesler DL, Gellad WF, Fine MJ. Antibiotic Therapy for Adults Hospitalized With Community-Acquired Pneumonia: A Systematic Review. JAMA. 2016;315:593–602. doi: 10.1001/jama.2016.0115. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martens P, Worm SW, Lundgren B, Konradsen HB, Benfield T. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC infectious diseases. 2004;4:21. doi: 10.1186/1471-2334-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell death and differentiation. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- McBean AM, Hebert PL. New estimates of influenza-related pneumonia and influenza hospitalizations among the elderly. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2004;8:227–235. doi: 10.1016/j.ijid.2004.04.013. [DOI] [PubMed] [Google Scholar]
- McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Petrilli V, Andrew PW, Kadioglu A, Lavelle EC. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010;6:e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Mitchell TJ. Streptococcus pneumoniae: virulence factors and variation. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2010;16:411–418. doi: 10.1111/j.1469-0691.2010.03183.x. [DOI] [PubMed] [Google Scholar]
- Mitzel DN, Lowry V, Shirali AC, Liu Y, Stout-Delgado HW. Age-enhanced endoplasmic reticulum stress contributes to increased Atg9A inhibition of STING-mediated IFN-beta production during Streptococcus pneumoniae infection. J Immunol. 2014;192:4273–4283. doi: 10.4049/jimmunol.1303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita N, Kawai Y, Akaike H, Ouchi K, Hayashi T, Kurihara T, Kawanaka N, Okimoto N. Influence of age on the clinical differentiation of atypical pneumonia in adults. Respirology. 2012 doi: 10.1111/j.1440-1843.2012.02188.x. [DOI] [PubMed] [Google Scholar]
- Moon JS, Nakahira K, Chung KP, DeNicola GM, Koo MJ, Pabon MA, Rooney KT, Yoon JH, Ryter SW, Stout-Delgado H, Choi AM. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med. 2016;22:1002–1012. doi: 10.1038/nm.4153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CT, Kim EH, Luong TT, Pyo S, Rhee DK. TLR4 mediates pneumolysin-induced ATF3 expression through the JNK/p38 pathway in Streptococcus pneumoniae-infected RAW 264.7 cells. Mol Cells. 2015;38:58–64. doi: 10.14348/molcells.2015.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell death and differentiation. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Shoma S, Tsuchiya K, Kawamura I, Nomura T, Hara H, Uchiyama R, Daim S, Mitsuyama M. Critical involvement of pneumolysin in production of interleukin-1alpha and caspase-1-dependent cytokines in infection with Streptococcus pneumoniae in vitro: a novel function of pneumolysin in caspase-1 activation. Infect Immun. 2008;76:1547–1557. doi: 10.1128/IAI.01269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L. Influenza vaccination and mortality benefits: new insights, new opportunities. Vaccine. 2009;27:6300–6304. doi: 10.1016/j.vaccine.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Sousa D, Justo I, Dominguez A, Manzur A, Izquierdo C, Ruiz L, Nebot M, Bayas JM, Celorrio JM, Varona W, Llinares P, Miguez E, Sanchez E, Carratala J. Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19:187–192. doi: 10.1111/j.1469-0691.2012.03765.x. [DOI] [PubMed] [Google Scholar]
- Stout-Delgado HW, Cho SJ, Chu SG, Mitzel DN, Villalba J, El-Chemaly S, Ryter SW, Choi AM, Rosas IO. Age-Dependent Susceptibility to Pulmonary Fibrosis Is Associated with NLRP3 Inflammasome Activation. American journal of respiratory cell and molecular biology. 2016;55:252–263. doi: 10.1165/rcmb.2015-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol. 2012;188:2815–2824. doi: 10.4049/jimmunol.1103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szretter KJ, Balish AL, Katz JM. Influenza: propagation, quantification, and storage. Current protocols in microbiology. 2006:11. doi: 10.1002/0471729256.mc15g01s3. Chapter 15:Unit 15G. [DOI] [PubMed] [Google Scholar]
- Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, Holmes A, Trendelenburg G, Heimesaat MM, Bereswill S, van der Linden M, Tschopp J, Mitchell TJ, Suttorp N, Opitz B. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J Immunol. 2011;187:434–440. doi: 10.4049/jimmunol.1003143. [DOI] [PubMed] [Google Scholar]


