Abstract
The opioid crisis has stimulated renewed interest in analgesic drug development. This effort will involve preclinical-to-clinical translational research and will benefit from a focus on endpoints that are both clinically relevant and shared across laboratory animals and humans. Measures of pain-related functional impairment and behavioral depression could serve this purpose.
Keywords: Opioid, pain, analgesic, drug development, translation
Introduction
The United States is suffering from a crisis in opioid abuse and overdose deaths fueled in part by prescription opioid analgesics. One response to this crisis has been a call by the National Institutes of Health for research to develop new pain treatments that would match or exceed opioid effectiveness while producing fewer and/or less severe side effects such as abuse liability and dangerous levels of respiratory depression [1]. If discovered, such new medications might permit a reduction in opioid use for pain treatment and protect patients and their communities from the addictive and lethal effects of prescribed or diverted prescription opioids.
As with drug development for many indications, efforts to develop new analgesics will rely on translational research in both laboratory animals and humans; however, the diversity of endpoints for pain assessment within and across species has historically been a complicating factor in analgesic development (Figure 1) [2]. In human studies, pain is most commonly measured using verbal reports guided by instruments such as the 0-to-10 Numeric Rating Scale (NRS). Under this scale, 0 reflects “no pain,” 10 reflects the “worst pain imaginable,” and analgesics are evaluated for their effectiveness to reduce self-reported pain-intensity scores [3]. In laboratory animals, by contrast, pain is most commonly inferred from measures of unconditioned reflexive behaviors, such as withdrawal responses from stimuli applied to the hind paw or tail, and analgesics are evaluated for their effectiveness to reduce these behaviors [4]. These endpoints have their own inherent weaknesses, and the discordance in endpoints across species is an obstacle to translation. Together, these limitations have likely contributed to past high-profile failures in development of new analgesics with novel mechanisms of action [5–8], and new initiatives to discover improved analgesics in response to the opioid crisis will face similar obstacles. The identification and use of endpoints that are both clinically relevant and translatable across species could facilitate preclinical-to-clinical translation and increase the likelihood of success in analgesic drug discovery. Measures of pain-related functional impairment and behavioral depression could serve this purpose.
Figure 1. Diversity of pain endpoints in laboratory animals and humans.
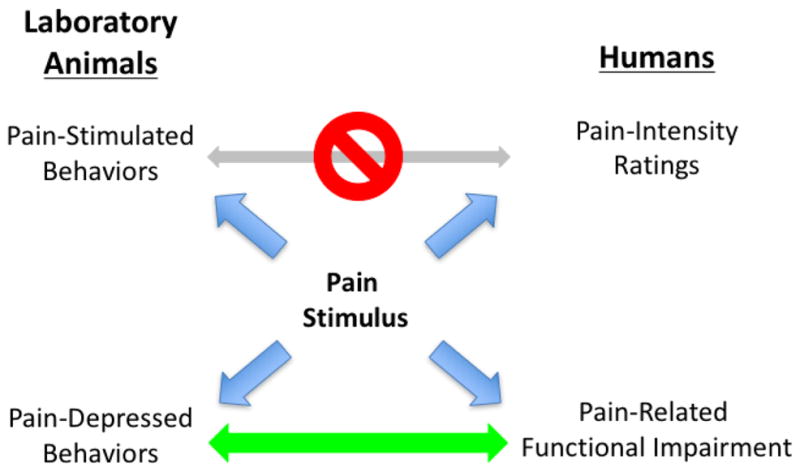
Pain stimuli produce a range of behavioral effects in laboratory animals and humans, and any of these behaviors can serve as endpoints in research. The principle endpoints are pain-stimulated behaviors in animals (e.g. withdrawal responses) and verbal reports of pain-intensity ratings in humans (e.g. numeric rating scale scores). These endpoints are different across species, potentially mediated by different neural mechanisms, and potentially modulated by different environmental variables and pharmacological treatments. Pain states also produce pain-depressed behaviors in animals (e.g. depression of feeding, locomotion, or positively reinforced operant behavior) and pain-related functional impairment in humans (e.g. impairment of activities of daily living). These endpoints are clinically relevant in pain diagnosis and treatment, more homologous across species, and potentially useful for translational research in analgesic development programs.
Pain-Related Functional Impairment in Humans
Although NRS metrics are often the principle endpoint in human pain assessment, the primacy of this type of measure has been challenged, and alternatives are available [3, 9, 10]. One obvious limitation to the use of NRS scores is that they cannot be objectively validated and may be influenced by factors other than pain, such as psychiatric co-morbidities or drug seeking. Additionally, excessive reliance on NRS scores to guide treatment may lead to excessive opioid dosing in patients at greatest risk of opioid-induced harm, a phenomenon that has been called “adverse selection” [10]. Lastly, verbal NRS reports are not useful for translational research because animals lack the prerequisite repertoire of verbal behavior.
An alternative source of information in pain assessment can be derived from measures of physical function [3, 9]. Clinically relevant pain states are commonly associated with functional impairment (e.g. impaired ability to walk, work, or accomplish other activities of daily living). Moreover, a common and important goal of pain treatment is the prevention of, or recovery from, impaired function. The degree of pain-related functional impairment can be measured with existing instruments such as the Brief Pain Inventory or the McGill Pain Questionnaire, and these and related measures have been recommended as complementary endpoints to NRS ratings in clinical trials of treatments for chronic pain [3]. Indeed, some pain specialists are suggesting that pain assessment and treatment should be guided more by measures of physical function and its impact on quality of life than by NRS scores of perceived pain intensity [10].
Pain-Depressed Behavior in Laboratory Animals
The reflexive behaviors at the heart of most preclinical pain research can be categorized as “pain-stimulated behaviors,” or behaviors that increase in rate, frequency, or intensity after delivery of a putative pain stimulus [4, 11]. Common examples include overt withdrawal responses from escapable pain stimuli (e.g. tail or paw withdrawal from thermal or mechanical stimuli) as well as pseudo-withdrawal behaviors from inescapable stimuli (e.g. stretching behaviors elicited by injection of dilute acid or other chemical irritants into the abdominal cavity). The appeal of these endpoints lies in their relative ease of measure, their low incidence in the absence of a pain stimulus, their sensitization under conditions of inflammation or neuropathy, their utility for basic in vivo pharmacological assessments, and their reliable expression without a requirement for training [12]. However, these measures are problematic for at least three reasons. First, although these behaviors do not require explicit training, their expression can be influenced by a host of variables independent of the pain stimulus, and this can complicate experimental design and interpretation of drug effects [13]. Second, pain-stimulated behaviors can be reduced not only by treatments that reduce sensory sensitivity to the pain stimulus (i.e. true analgesia), but also by treatments that produce sedation, paralysis, or other types of motor impairment that can result in “false positive” effects. Lastly, although humans also display pain-stimulated behaviors (e.g. withdrawal from a hot stove or scalpel blade), these behaviors are rarely used as endpoints in clinical trials of analgesics, perhaps because clinical reductions in pain-stimulated behaviors are usually accomplished with local or general anesthetics rather than with analgesics. As a result, pain-stimulated behaviors are not ideal endpoints for translational research to develop novel, non-opioid analgesics.
In addition to stimulating some behaviors, pain stimuli can also decrease the rate, frequency, or intensity of other behaviors in laboratory animals [4, 11]. These behaviors have been described as “pain-depressed behaviors,” and examples include pain-related decreases in feeding, locomotion, wheel running, nesting, burrowing, or positively reinforced operant behavior. Preclinical assays of pain-depressed behavior can serve as useful complements to conventional assays of pain-stimulated behavior for two major reasons. First, effective analgesic drugs restore function and increase expression of the pain-depressed behavior. As a result, treatments that produce motor impairment do not produce false-positive analgesic-like effects. Instead, such drugs merely exacerbate pain-related behavioral depression. Second, pain-depressed behaviors serve as diagnostic indicators of pain and targets of treatment in veterinary medicine [14], just as they do in human medicine. The shared usefulness of these signs for pain diagnosis and treatment in veterinary and human medicine suggests that pain-depressed behaviors may also serve as a category of clinically relevant endpoints that can be harmonized across studies in laboratory animals and humans to promote translational research.
As one example of a preclinical assay of pain-depressed behavior, we recently evaluated the effects of pain stimuli and drug treatments on nesting behavior in mice (Figure 2) [15]. Nesting is a naturally occurring and adaptive behavior in mice, and laboratory mice are routinely provided with nesting material as a part of standard animal husbandry. We found that either an acute visceral pain stimulus (intraperitoneal injection of dilute acid) or a more sustained inflammatory pain stimulus (injection of the hindpaw with complete Freund’s adjuvant) was sufficient to depress nesting behavior. Moreover, pain-depressed nesting could be blocked or reversed by clinically effective analgesics (the mu opioid agonist morphine and the nonsteroidal anti-inflammatory drug ketoprofen), but not by a non-analgesic (the kappa opioid receptor agonist U69,593) from a drug class that produces sedation and false-positive effects in many conventional preclinical assays of pain-stimulated behavior. Moreover, both morphine and ketoprofen failed to block depression of nesting produced by a non-pain stimulus, demonstrating that these drugs selectively relieved pain-depressed behavior without producing a more general and non-selective stimulant effect. These results illustrate the utility of this type of assay both to model pain-related functional impairment in laboratory animals and to dissociate analgesics from non-analgesics during drug evaluation. One goal of our research program is to determine whether these types of assays could be useful in the development of novel, non-opioid analgesics.
Figure 2. Pain-related depression of nesting in mice.
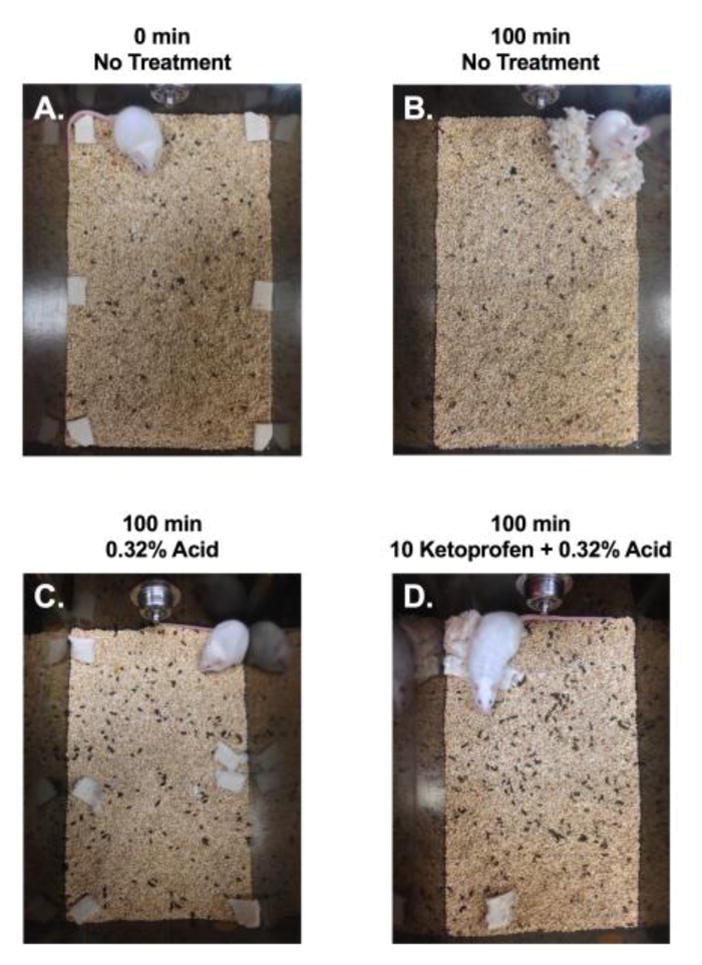
As one example of a preclinical assay of pain-depressed behavior, we have examined pain-related depression of nesting in mice. At the beginning of each 100-min test session, squares of pressed-cotton nesting material are distributed around the perimeter of the subject’s home cage (Panel A). By the end of the session, untreated mice typically consolidate and shred the nesting material to build a nest in one corner of the cage (Panel B). A visceral pain stimulus (intraperitoneal injection of dilute lactic acid) depresses this nesting behavior, and mice fail to consolidate or shred the nesting material (Panel C). Pretreatment with the non-steroidal anti-inflammatory drug ketoprofen blocks acid-induced depression of nesting, and ketoprofen-treated mice exhibit relatively normal nesting behavior (Panel D). Pretreatment with appropriate doses of opioid analgesics like morphine produces similar effects, whereas non-analgesics like the centrally acting kappa opioid agonist U69,593 do not. [15]
Toward Translational Research Platforms
The opioid crisis has given a new sense of urgency to the task of analgesic drug development. Unfortunately, recent efforts to develop new analgesics with novel mechanisms of action have produced several high-profile failures as drugs with promising constellations of preclinical effects failed to produce adequately safe and effective analgesia in humans. Future advances in analgesic development are nonetheless likely to involve translational research across laboratory animals and humans, and the fidelity of this translation will benefit from the use endpoints that are both clinically relevant and shared across species. Preclinical and clinical studies that share a focus on expression, mechanisms, and treatment of pain-related functional impairment and behavioral depression could serve this purpose. Incorporation of endpoints that measure functional impairment and behavioral depression need not replace existing endpoints, such as NRS scores in humans or assays of pain-stimulated behaviors in animals. Rather, these endpoints can complement existing endpoints to provide a more comprehensive assessment of drug effects on pain-related behaviors while strengthening the bridge between preclinical and clinical studies in the search for safe alternatives to opioid analgesics for the treatment of pain.
Acknowledgments
This work was supported by R01NS070715 and R01DA030404. The author has no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 2017;377(4):391–394. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- 2.Vierck CJ, et al. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135(1–2):7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin RH, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Negus SS, et al. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319(2):507–14. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 5.Fallon MT, et al. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. Br J Pain. 2017;11(3):119–133. doi: 10.1177/2049463717710042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21(7):244–6. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 7.Huggins JP, et al. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153(9):1837–46. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Pande AC, et al. Analgesic efficacy of the kappa-receptor agonist, enadoline, in dental surgery pain. Clin Neuropharmacol. 1996;19(1):92–7. doi: 10.1097/00002826-199619010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, et al. Report of the NIH Task Force on research standards for chronic low back pain. J Pain. 2014;15(6):569–85. doi: 10.1016/j.jpain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan MD, Ballantyne JC. Must we reduce pain intensity to treat chronic pain? Pain. 2016;157(1):65–9. doi: 10.1097/j.pain.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 11.Negus SS. Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 2013;42(8):292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteside GT, et al. Preclinical Pharmacological Approaches in Drug Discovery for Chronic Pain. Adv Pharmacol. 2016;75:303–23. doi: 10.1016/bs.apha.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–94. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 14.Brown DC, et al. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc. 2008;233(8):1278–83. doi: 10.2460/javma.233.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negus SS, et al. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156(6):1153–60. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]


