Abstract
A primary challenge facing the development of interventions for dyslexia is identifying effective predictors of intervention response. While behavioral literature has identified core cognitive characteristics of response, the distinction of reading versus executive cognitive contributions to response profiles remains unclear, due in part to the difficulty of segregating these constructs using behavioral outputs. In the current study we used functional neuroimaging to piece apart the mechanisms of how/whether executive and reading network relationships are predictive of intervention response. We found that readers who are responsive to intervention have more typical pre-intervention functional interactions between executive and reading systems compared to nonresponsive readers. These findings suggest that intervention response in dyslexia is influenced not only by domain-specific reading regions, but also by contributions from intervening domain-general networks. Our results make a significant gain in identifying predictive bio-markers of outcomes in dyslexia, and have important implications for the development of personalized clinical interventions.
Keywords: Dyslexia, Intervention, fMRI, Prefrontal Cortex, Intervention Response Prediction
1. Introduction
Dyslexia is the most prevalent learning disorder, estimated to affect 6–17% of the population (Fletcher, 2009); it is characterized by impaired word reading deficits despite intact cognition and adequate instruction (Lyon et al., 2003). Though studies have identified key interventional targets for dyslexia, current interventions are ineffectual for approximately 2–3% of readers with dyslexia (Mathes et al., 2005). These intervention limitations are due in part to inconsistent behavioral profiles of response prediction (Al Otaiba & Fuchs, 2002; Stuebing et al., 2015; Cho et al., 2015; Fletcher et al., 2011; Miciak et al., 2014, 2015). While studies have identified core reading characteristics that predict response—including phonological awareness, knowledge of the alphabetic principle, rapid naming of words, and demographics (Fletcher et al., 2011; Nelson, Benner, & Gonzalez, 2003)—the extent to which response is dependent on baseline executive functions is unclear. The distinction between reading versus executive contributions to prediction is critical, as the answer addresses a fundamental question on the nature of intervention response in learning disabilities: do responsive learners simply have greater baseline cognitive efficacy in domain-specific skills (e.g. reading, math, etc.), or do they have a more intact executive “scaffold” (e.g. working memory, meta-cognition, and planning ability) that provides support for domain-specific skills?
The distinction between executive versus reading contributions consequently has large implications for the development of effective interventions, and potential for identifying additional population sub-groups. Notably, developmental research appears to report paradoxical findings in regard to executive function and its role in educational gains. Broader behavioral studies on school readiness have found that executive functions are indeed critical predictors of school readiness and achievement (Blair & Razza, 2007; Diamond, 2013; St Clair-Thompson & Gathercole, 2006). However, executive function ability is generally not considered to be a good predictor of dyslexia intervention response, with domain-specific skills instead being the best predictors (Cho et al., 2015; Miciak et al., 2015; Stuebing et al., 2015). This discrepancy has partially been attributed to the fact that the extent of executive function contributions may be concealed by overlapping variance with reading-related behavioral metrics (Schatschneider, Fletcher, Francis, Carlson, & Foorman, 2004; Stuebing et al., 2015; Wagner, 1996). This explanation ties nicely into recent work in the psychiatric literature that provides a more nuanced explanation of how executive function may relate to other cognitive functions. This literature has revealed that the interaction between executive and other cognitive systems, rather than executive ability alone, is what engenders positive behavioral outcomes (Cole et al., 2011, 2014). Thus, as applied to learning outcomes, neuroimaging allows for a window into executive function and reading relationships that may otherwise be obscured, particularly how executive functions may facilitate reading systems1, and, in this case, how such coordination may predict intervention response in dyslexia. Such knowledge may be critical for understanding how executive systems play a role in intervention response and academic growth more generally.
Previous work in neuroimaging that has examined baseline activation/structure in responders and nonresponders overall characterizes responders as having more intact reading systems that are more like typically developing readers (Farris et al., 2011; Rezaie et al., 2011a, 2011b), including some possible evidence to suggest that responders recruit compensatory right inferior frontal gyrus (IFG; Farris et al. 2016; Hoeft et al., 2011). However, no one has tested the hypothesis that these more typical reading network connections may be traced to greater utilization of a top-down executive scaffold. In the current study, we apply the concept that the interaction of executive systems with reading systems may also be important for academic outcomes. Specifically, we used functional magnetic resonance imaging (fMRI) to examine neurobiological network interactions that predict intervention response. This approach allowed us to move beyond general patterns of response prediction (as have been characterized by Farris et al., 2011; Hoeft et al., 2011; Rezaie et al., 2011a, 2011b; for review see Barquero et al., 2014), and specifically test whether responders have greater baseline utility of executive systems to facilitate activation of typical reading networks. Of particular relevance to the current study was the potential contributions of the frontoparietal control network (FPN)—a neural system known to subserve executive functions including working memory, cognitive control, and attention (Cole et al., 2014; Ptak, 2012). Higher integrity of the FPN has been found to be predictive of better clinical outcomes in neural vulnerabilities in the neural disorder and psychiatric literature (Borstad et al. 2016; Cole et al., 2011). The convergent implication of the FPN across highly disparate disorders has led some to suggest that a healthy FPN regulates other neural systems in a goal-directed manner in both typical and pathological states; worse clinical outcomes may consequently reflect both a primary, disease-specific neural deficit and a secondary failure of the FPN to direct the vulnerable systems (Cole et al., 2011, 2014).
The involvement of the FPN in reading and dyslexia is not unfounded. Behavioral models of word reading offer a few possibilities for when executive areas would be necessary in directing reading processes. For instance, Balota’s two-part verification model of lexical decision-making suggests that a reader must engage in executive processes if the familiarity/meaningfulness of a word-form is insufficient to resolve a word-form (Balota & Chumbley, 1984), and more generally that attention processes regulate the necessarily flexible pathways that support lexical access across varying task demands (Balota & Chumbley, 1999). Recent neuroimaging work (not in the context of intervention) has connected subcomponents of these word-reading attentional control processes to areas in the FPN (Ihnen, Petersen, & Schlaggar, 2015), and additional studies have pointed to FPN differences as a marker of dyslexia (Finn et al., 2013; Koyama et al., 2013; Norton et al., 2014). These latter studies include findings of internal connectivity reductions within the FPN in dyslexia (Finn et al., 2013; Koyama et al., 2013), as well as aberrance of specific structures within the FPN. In particular, the left dorsolateral prefrontal cortex (dlPFC)—a structure associated with working memory and the top-down planning/organization of information (Reynolds et al., 2012)—appears to be linked to reading ability. Although not highlighted in their findings, in a seminal study Shaywitz et al. (1998) found overactivation of dlPFC in readers with dyslexia. Others, however, have found that children with dyslexia have different patterns of anomalies in the dlPFC, including hypoactivation compared to reading-matched controls during a phonological decision task (Kovelman et al., 2012); hypo-connectivity between dlPFC and occipitotemporal cortex (OT; Vogel et al., 2012); and decreased gray matter related to the interaction of reading delay and family risk of dyslexia (Raschle et al., 2015). Consequently, convergent findings provide evidence for a key role of the FPN, and more specifically the dlPFC, in the neural profile of readers with dyslexia. However, no studies to date have examined the role of the FPN in response prediction, or how the FPN may interact with reading areas impacted by dyslexia, namely, OT, left IFG, and other left temporoparietal areas (Maisog, Einbinder, Flowers, Turkeltaub, & Eden, 2008; Richlan, Kronbichler, & Wimmer, 2009).
Given previous findings that (1.) in the early-childhood cognitive literature, behavioral executive function measures (including cognitive control measures linked to the FPN) predict a variety of academic outcomes (Blair & Razza, 2007; Diamond, 2013); (2.) the FPN seems to have a role in dyslexia; and (3.) recent clinical studies suggest that executive systems mitigate clinical symptoms and outcomes in neural and psychiatric disorders (Borstad et al. 2016; Cole et al., 2011), the current study aimed to test the hypothesis that the FPN may serve to mitigate poor reading outcomes in dyslexia. More specifically, we hypothesized that the findings within the psychiatric literature (Cole et al., 2011, 2014) would extend to the realm of learning, such that compared to nonresponders, responders would be characterized by greater pre-intervention interactions between nodes of executive and typical reading networks—not simply by reading network connections alone. We additionally aimed to examine whether these differences (if any) serve as compensatory mechanisms (in which case the same connectivity differences in responders versus nonresponders would also be evident in responders versus typically developing readers), or simply reflected a more normalized baseline network (in which case the same connectivity differences in responders versus nonresponders would also be evident in typically developing readers versus nonresponders). To examine our hypothesis, we implemented a short-term, intensive intervention in children with developmental dyslexia, and examined how pre-intervention functional brain interactions between the FPN and the typical reading network predicted intervention response. Better understanding how executive systems may interplay with reading systems in the context of response to intervention in dyslexia could have significant implications for the development of effective interventions and further characterization of dyslexia. More broadly, it has implications for understanding the role that executive systems may play in academic outcomes.
2. Materials and Methods
2.1 Participants
The current study consisted of a subsample from a larger clinical trial (see ClinicalTrials.gov: NCT00624234), and included native English-speaking children (n = 45), ages 8–14 years, who had either typical reading development (TD; n = 19) or dyslexia (DYS; n = 26). All participants had no history of major psychiatric illness or developmental and/or genetic disorders, and were required to have an IQ of ≥ 70 standard score. Of 19 TD participants with scan data, 4 were excluded due to excessive motion (see below). Of 26 DYS participants with scan data, 6 were excluded in total; exclusions were due to excessive motion (n = 2), missing pretest scores (n = 2), screening scores that were incongruent with reported DYS history (n = 1), and post-testing deemed invalid by the test administrator due to extreme off task behavior (n = 1). After exclusion criteria were applied, final groups included n = 37 subjects, with 22 DYS and 15 TD participants. Groups were matched in nonverbal IQ, age, gender, handedness, executive function, and ADHD diagnosis (1 participant in DYS and 1 in TD had a diagnosis of ADHD; for full description of behavioral comparisons, see Supplemental Table 1 and Supplemental Material). Participants received compensation, and all study procedures were carried out in accordance with Johns Hopkins University and Vanderbilt University’s Institutional Review Board.
2.2 Criteria for DYS
To qualify for entry into the larger clinical trial (see Barquero, Sefcik, Cutting, & Rimrodt, 2015; see ClinicalTrials.gov: NCT00624234), DYS had to score < 25%ile on 1 of three tests, and TD had to score > 35th %ile on two out of three tests and ≥ 27%ile on all three tests: Wechsler Individual Achievement Test-II Word Reading Subtest (WIAT-II; Wechsler, 2005), Word Attack (WA), or Letter Word Identification (LWID) of Woodcock Johnson-III Normative Update (WJ-III; McGrew, Schrank, & Woodcock, 2007; Woodcock, McGrew, & Mather, 2001). To be included for the current study, we applied further inclusionary criteria: DYS had to meet the clinical trial entry criteria, as well as score ≤ 25th percentile on a composite measure of word reading (WR-COMP) which consisted of the WIAT-II Word Reading Subtest, Test of Word Reading Efficiency Phonemic Decoding Efficiency (TOWRE-PDE; Torgesen, Wagner, & Rashotte, 1997), Word Identification and Spelling Test (WIST; Wilson & Felton, 2004) Sound Symbol Subtest, and WJ-III Basic Reading (BR); TD also had to meet the clinical trial entry criteria as well as have a WR-COMP score > 27%ile. Average WR-COMP scores for the DYS group were 8th %ile, and the average WR-COMP scores for the TD group were 53%ile (p <.05; see Supplementary Table 1) and there was no overlap between group scores on either WR-COMP or the WJ-III BR.
2.3 Intervention and post-intervention testing
DYS participants were randomly assigned to one of two tutorial reading interventions and received 15 hours of one-to-one instruction, administered over 3 to 5 consecutive days. Both interventions incorporated systematically structured, research-based principles of reading instruction, largely derived from Orton-Gillingham (Orton, 1937) methods. The sequence of activities in each intervention was standardized across participants, but the pace of instruction was modified based on participant needs. Treatment A emphasized a multisensory approach to strengthen sound-symbol correspondence, using a combination of visual, auditory, and kinesthetic/tactile strategies to teach phonological awareness and sound-symbol correspondences. Treatment B focused upon building word-reading skills through repetition, using visual strategies to train sound-symbol correspondences to the point of automaticity.2
As found in the larger intervention study, there were significant behavioral gains related to intervention (see Supplemental Table 1; Supplemental Figure 1; Supplemental Material) on our primary outcome measure (WJ-III BR). Consistent with the larger clinical trial in which WJ-III BR was the primary outcome measure (Barquero et al., 2015), responders (DYS-R) and nonresponders (DYS-NR) were defined based up on a median split of change scores on WJ-III BR (BR ss change median score = 2). A median split definition of groups has previously been employed in neuroimaging of reading intervention studies (e.g., Davis et al., 2011; Simos et al., 2007). For details on behavioral group profiles for DYS-R and DYS-NR, see Supplemental Table 1.
2.4 fMRI Task
The current study was interested in examining functional activation and connectivity patterns related to the naturalistic gains of the intervention—i.e. non-manipulated word and pseudoword reading. As such, during the fMRI scan, participants viewed individual words that appeared in the center of the screen. As in Cutting et al. (2013), the stimuli consisted of real words (80%) and decodable pseudowords (20%). Pseudowords were included as low-probability, random stimuli for the in-scanner lexical decision task. Because of the unequal ratio of words to pseudowords, accuracy was calculated as A′, a non-parametric measure of sensitivity that has been shown to be more robust than d′ when performance may be biased (Donaldson, 1993). Both real words and pseudowords ranged from three to six letters in length. Each pseudoword was created by replacing one letter of a decodable real word with another letter to produce a decodable nonsense word. For each stimulus, the participant decided whether it was a real word (indicated by right-thumb button press) or a pseudoword (indicated by left-thumb button press). This was an event related design, with two separate runs. Each run consisted of 50 “words.” Stimuli were presented in random order, with each stimulus appearing on the screen for 2000 ms, with a jittered blank inter-stimulus interval ranging in duration from 1000ms to 3000 ms (mean 2000 ms). For each trial, reaction time and accuracy (calculated using A’) were measured. MANOVA analysis revealed that in-scanner accuracy (TD: mean = .94, SE = .04; DYS: mean = .82, SE =.03) and reaction time (TD: mean = 895.25, SE = 32.39; DYS: mean = 967.68, SE = 26.11) were non-significant for TD vs. DYS (Wilks’ Λ = .83, F(2, 30) = 3.12; p = .06). Two subjects had missing in-scanner behavioral data due to operator error. During each run, three 10 second periods of crosshair fixation were included. This period of time in addition to jitter totaled to 130 s of implicit baseline per run.
2.5 fMRI Acquisition
All fMRI scans were acquired at either the Kennedy Krieger Institute (KKI) in Baltimore, Maryland, United States, or at Vanderbilt University Institute of Imaging Science (VUIIS) in Nashville, Tennessee, United States, on a 3.0 T Philips Achieva MR scanner with an 8-channel head coil (see Supplemental Table 2 for participant demographics per site). Functional imaging used a single-shot echo planar sequence to acquire 40 slices (transversely oriented, ascending order, 3mm thick with a 1-mm interslice gap). Task sessions consisted of 2 runs, each 3 minutes and 40 seconds (94 dynamics per run). Other relevant imaging parameters for the functional images are TE=30 msec (for optimal BOLD contrast at 3T), 75 degree flip angle, TR=2200 msec, FOV 240 × 216 × 159 mm, and a reconstruction matrix size of 128×128 yielding 1.88×1.69×3.00 mm voxels. All analyses included site as a covariate.
2.6 fMRI Data Analysis
All functional data were analyzed using MATLAB 2013a (The MathWorks, Natick, Massachusetts, United States) and SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). At the individual level, functional data were corrected for slice timing, aligned to the mean functional image, normalized to MNI space using the EPI template, and spatially smoothed with an 8-mm FWHM Gaussian filter. Motion related outlying volumes for each participant were identified using Artifact Detection Tools (ART; Whitfield-Gabrieli, 2009, http://www.nitrc.org/projects/artifact_detect/). Using a motion threshold of 3 mm translation and 3° rotation, participants with ≥ 20% of the total volumes exceeding this threshold in a run were excluded from analyses. A total of 4 TD and 2 DYS subjects were excluded due to excessive motion. There was no significant difference in global mean signal change (t(35) = 0.56, p = 0.58) or total outlier percentage (t(35) = 0.22, p = 0.83) between TD and DYS. The first-level event-related model included estimated hemodynamic response (HRF) for each condition, six motion parameters (translational and rotational x, y, z), and outlying volumes as determined by ART; motion-related parameters were added to the design matrix as regressors of no interest. Individual contrast maps were created to establish relative activation for the task condition, single word reading (words and pseudowords) versus baseline. To account for multiple comparisons, Monte Carlo simulations were performed for all analyses, using AFNI 3dClustSim to find appropriate cluster-correction values for p-values equivalent to < .05 (using p = 0.005 height threshold, compilation date in 2016).
Group-level Region of Interest (ROI) analysis
Region of interest (ROI) analyses were performed to compare DYS-R and DYS-NR groups. Additionally, to address concerns of dichotomization of near-median data points, ROI analyses also explored activations that corresponded with the continuous measure of response (covariate analyses with the WJ-III BR change in standard score). ROIs were selected in the traditional left-lateralized reading network that have previously been found to be associated with word-level reading in TD (Price, 2010, 2012), and included: left IFG, left middle temporal gyrus (MTG), left superior temporal gyrus (STG), left angular gyrus (AG; including BA 39), left supramarginal gyrus (SMG; including BA 40), left fusiform, and the putative visual word form area (pVWFA; sphere with 6 mm radius centered at MNI coordinates of [−42, −54, −17]; Bach, Richardson, Brandeis, Martin, & Brem, 2013) located within the fusiform gyrus. For the FPN node, we additionally tested a seed in the left dorsolateral prefrontal cortex (dlPFC) that has previously been associated with both working memory and reading ability (identified by masking reading activations with the Neurosynth working memory meta-analysis image; Yarkoni et al., 2011; Aboud, Bailey, Petrill, & Cutting, 2016). Supplemental analysis revealed that the left dlPFC seed significantly correlated with the FPN and not with the TD reading network, indicating that the left dlPFC seed was appropriately characterized as an executive function area (see Supplemental Material). With the exception of the pVWFA and dlPFC, all ROIs were defined anatomically using the Automated MNI Atlas Label (AAL) and TD Brodmann in the WFU PickAtlas toolbox (http://fmri.wfubmc.edu/research/PickAtlas). All ANCOVA analyses included scan site as a covariate of no interest.
Connectivity analysis
Connectivity analysis was performed using SPM8 conn toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012). Seed regions were derived from ROI activations that were significant in the TD activation map (see GLM results and Table 1). For each seed, voxel time-series were extracted and averaged. Word stimuli were modeled as events. Confounding signals were estimated from white matter and CSF (derived from T1 images) through the CompCor method (Behzadi, Restom, Liau, & Liu, 2007); the CompCor output, motion outliers and six movement parameters (as determined by ART) were regressed out from all ROI time series, and a high-pass filter of .008 Hz was applied. To remove correlations driven by general, task-related co-activations (e.g. onset/offset effects), task effects and their first temporal derivative were also removed from the signal (Whitfield-Gabrieli & Nieto-Castanon, 2012), an approach that has been referred to as “background connectivity” analysis (Al-Aidroos, Said, & Turk-Browne, 2012; Westphal, Wang, & Rissman, 2017). Linear detrending and despiking algorithms were additionally applied. For every subject, bivariate correlation maps were generated for each ROI, and converted to Fisher’s z-scores. All results were run using a mask that included traditional left-lateralized reading areas (listed above) as well as their right hemisphere homologues that have been previously implicated as potential compensatory circuits in DYS (right IFG, right MTG, right STG, and right fusiform; Hoeft et al., 2011; Pugh et al., 2000; Shaywitz et al., 1998; Waldie, Haigh, Badzakova-Trajkov, Buckley, & Kirk, 2013), and the FPN (defined from Yeo et al., 2011). To identify group differences, ANCOVA models were run comparing group correlation maps.
Table 1.
Seed regions used in connectivity analysis.
| Seed | MNI Coordinates | Observed Function | ||
|---|---|---|---|---|
| x | y | z | ||
| pVWFA | −44 | −56 | −20 | Orthographic processing |
| Left dorsal IFG (45, 44) | −54 | 8 | 24 | Phonological processing |
| Left dorsal MTG | −50 | −44 | 8 | Phonological processing |
| Left ventral IFG/insula (45/47) | −36 | 26 | −4 | Semantic processing |
| Left ventral MTG | −64 | −42 | −14 | Semantic processing |
| Left DLPFC | −52 | 10 | 30 | Cognitive control |
Physio-physiological interaction analysis
To test the potential interaction effects of dlPFC activation and reading network connectivity (see Introduction for hypotheses), a physio-physiological interaction analysis was run for DYS-R versus DYS-NR. As described in Friston et al. (1997), physio-physiological interaction analysis examines the interaction effect between the time series of two seeds. In the current analysis, we tested how the interaction effect of left dlPFC and seed-of-interest time series predicted the voxel time series for each voxel in the brain (e.g. how a one-unit change in left dlPFC activation corresponded with left vMTG whole-brain connectivity), as represented in the following model:
Where ϕβ1 and ϴβ2 are the main effects of the left dlPFC and the seed region of interest activations, respectively, and the metric of interest is their interaction (ϕ × ϴ)β3 (Friston et al., 1997). As in the direct connectivity metrics mentioned above, group differences in interaction value maps were identified using ANCOVA models.
Additional analyses
ROI-to-ROI analyses were run to (a) confirm that the DYS subgroup connectivity differences that we found were also present when considering individual differences; e.g. in the continuous measure of intervention response (WJ-III BR standard score change) and (b) compare DYS subgroups to TD, in order to address in particular whether DYS-R findings were more indicative of compensatory or “normalized” baseline networks.
3. Results
To confirm expected pre-intervention deficits in DYS subgroups, pre-intervention activations in the reading task versus implicit baseline were examined across groups using an ANCOVA region of interest (ROI) analysis that modelled response group and scanner site. As a first pass, initial characterization of each group via ANCOVAs confirmed expected left-lateralized reading/executive activations in TD (e.g. pVWFA, left fusiform gyrus, left IFG, left MTG, and left dlPFC), and a reduced number of reading activations in the DYS groups (e.g., activation in left fusiform only; see Supplemental Figure 2). Significant regions in TD (which were used as seed regions for connectivity analyses) were consistent with previous literature on word reading (Price, 2012) and included: left ventral IFG (vIFG), left dorsal IFG (dIFG), left ventral MTG (vMTG), left dorsal MTG (dMTG), left pVWFA, and left dlPFC. Direct statistical comparisons of the groups revealed findings consistent with the literature, with significant activation differences observed only between TD and DYS-NR, with DYS-NR showing reduced activation in left OT and left SMG (see Supplemental Table 3). Additionally, covariate analysis using our continuous measure of intervention response (WJ-III BR) showed significant positive correlations between basic reading change score and activation in left dorsal SMG/BA 40, replicating findings from Rezaie et al. (2011; see Supplemental Table 3). Consequently, both our group comparisons and our covariate GLM findings were very much consistent with previous findings.
3.1 Functional connectivity during single word reading
DYS-R versus DYS-NR
Comparisons of DYS-R and DYS-NR revealed that DYS-R had significantly greater connectivity than DYS-NR between the left ventral MTG semantic seed and right dorsal IFG, a region previously implicated as a predictor of word reading improvement in readers with dyslexia, potentially through compensatory support mechanisms (Hoeft et al., 2011; see Figure 1 and Table 2; see Supplemental Figure 3 for boxplot comparisons; see Supplemental Material and Supplemental Table 4 for significant differences in DYS-NR > DYS-R). Analyses with the continuous measure further affirmed findings, revealing that a greater reading change score (i.e., the continuous measure of response) corresponded with greater correlation between the left ventral MTG and right dorsal IFG (see Table 2). With regard to TD comparisons, DYS-R showed significantly greater connectivity than TD between left ventral MTG and right dorsal IFG (DYS-R vs. TD: F(24) = 4.46; p = 0.0001), but TD showed similar connectivity to DYS-NR (p > 0.05). Findings therefore point to the left ventral MTG to right dorsal IFG correlations in DYS-R as a compensatory mechanism.
Figure 1. Left ventral MTG has greater connectivity to right IFG in DYS-R > DYS-NR.
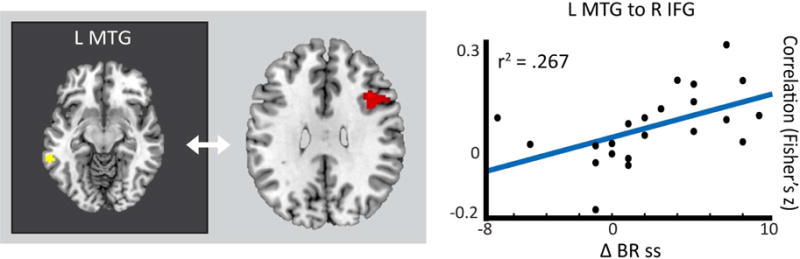
(a.) In DYS-R > DYS-NR, left ventral MTG activation correlates with right IFG activation, an area which has previously been implicated in long-term reading gains (Hoeft et al., 2011). (b.) Scatterplot of intervention response (BR ss change) and direct connectivity between the left ventral MTG and right IFG regions. Results displayed at p-corrected < 0.05 (p-uncorrected < 0.005; k = 107).
Table 2.
Functional connectivity clusters within ROI and FPN mask for DYS-R > DYS-NR (p-corrected < 0.05). r values are reported for the correlation between ROI-to-ROI z-transformed connectivity and BR ss change scores.
| Seed region | Whole-Brain Correlation Regions | MNI Coordinates | k | Max T | BA | r | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| DYS-R > DYS-NR | ||||||||
|
| ||||||||
| pVWFA | n.s. | – | – | – | – | – | ||
| L dIFG | n.s. | – | – | – | – | – | – | – |
| L dMTG | n.s. | – | – | – | – | – | – | – |
| L vIFG | n.s. | – | – | – | – | – | – | – |
| L vMTG | R IFG | 40 | 8 | 30 | 320 | 4.37 | 44 | 0.52 |
| L dlPFC | n.s. | – | – | – | – | – | – | – |
3.2 Physio-physiological interaction analysis
To examine whether left dlPFC plays a mediating role in less efficient reading network patterns specifically in DYS-R, we ran a physio-physiological interaction analysis for DYS-R > DYS-NR. This analysis examined how increased activity in a left dlPFC seed previously implicated in reading ability (Aboud et al., 2016) predicted connectivity of functional connectivity analysis. Significant interaction differences in DYS-NR > DYS-R are reported in Supplemental Material and Supplemental Table 5.
DYS-R versus DYS-NR
In DYS-R > DYS-NR, a one-unit increase of left dlPFC activation predicted increased connectivity from two of the reading seeds (left vMTG and vIFG) to other reading areas.
Left dlPFC activation corresponds with increased correlations between left vMTG and left inferior parietal lobule (IPL) in DYS-R > DYS-NR (see Table 3 and Figure 2; see Supplemental Figure 4 for boxplot comparisons). Analyses with the continuous measure of response further affirmed findings, revealing that greater reading change score also corresponded with greater correlation between the left vMTG and left IPL (see Table 3 for descriptive values). With regard to TD comparisons, DYS-R was not significantly different from TD (p > 0.05), but TD was also significantly different than DYS-NR (TD vs. DYS-NR: F(21) = 3.67; p = 0.0007). Thus, results point to more typical (rather than compensatory) interactions between reading and executive networks in DYS-R.
Left dlPFC activation corresponds with increased correlations between left vIFG and (1.) right IFG and (2.) left IPL in DYS-R > DYS-NR (see Table 3 and Figure 2; see Supplemental Figures 5 and 6 for boxplot comparisons). Of note, the left IPL area found in the interaction findings from the left vIFG overlapped with the interaction findings from the left vMTG seed. Analyses with the continuous measure of response further affirmed results, revealing that greater reading change score corresponded with greater correlation between the left vIFG and right IFG (see Table 3). Comparisons to TD revealed that DYS-R was not significantly different from TD (p > 0.05), but TD was significantly different than DYS-NR (TD vs. DYS-NR: F(21) = 16.06; p = 0.0001). Thus, results point to more typical (rather than compensatory) interactions between reading and executive networks in DYS-R.
Table 3.
Significant clusters in the physio-physiological interaction analysis for DYS-R > DYS-NR, with the left dlPFC seed as the mediating variable (p-corrected < 0.05). r values are reported for the dlPFC-related change in correlation between ROIs and BR ss change scores.
| Seed region | Whole-Brain Correlation Regions | MNI Coordinates | k | Max T | BA | r | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| DYS-R > DYS-NR | ||||||||
|
| ||||||||
| pVWFA | n.s. | – | – | – | – | – | – | – |
| L dIFG | n.s. | – | – | – | – | – | – | – |
| L dMTG | n.s. | – | – | – | – | – | – | – |
| L vIFG | L IPL | −46 | −48 | 54 | 190 | 5.43 | 40 | 0.75 |
| R IFG | 52 | 28 | 28 | 122 | 4.13 | 44, 46 | 0.50 | |
| L vMTG | L IPL | −46 | −40 | 34 | 117 | 4.57 | 40 | 0.66 |
| L dlPFC | n.s. | – | – | – | – | – | – | – |
Figure 2. In DYS-R > DYS-NR, left dlPFC activation has greater interactions with reading network connectivity.
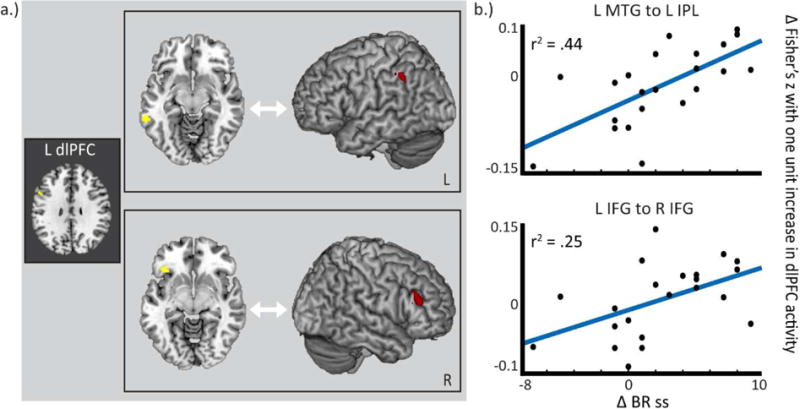
(a.) In DYS-R > DYS-NR, a one-unit increase in dlPFC activation corresponds with increased correlations in the reading network, including between left ventral MTG and left IPL (top), left IFG and right IFG (bottom), and left IFG and L IPL (not shown). (b.) The interaction effect seen in (a.) is also reflected in a continuous measure of response, as shown by a scatterplot of intervention response (BR ss change) and the interaction between dlPFC activation and connectivity between the seed areas represented in (a.). Results displayed at p-corrected < 0.05 (p-uncorrected < 0.005; k = 107).
4. Discussion
In this study, we used functional connectivity interaction analysis to identify baseline network predictors of intervention response in dyslexia. We were specifically interested in disambiguating whether responsiveness to intervention is marked by greater baseline involvement of the frontoparietal control network (FPN), an executive brain system that has been implicated as a facilitator of neural health in domain-specific systems, or is restricted to reading regions alone. We found that intervention response is marked by greater pre-intervention utility of a key area in the FPN—left dlPFC—in supporting the reading network. Of note, these findings represent patterns that remain even after tightly controlling for attention deficit hyperactivity disorder (ADHD), and thus reflect more subtle executive characterizations of responsiveness beyond comorbid attention problems. The current study consequently re-frames neural resilience to learning disorders, such as dyslexia, in the context of the presence or absence of an appropriate executive “scaffold”. By identifying neural circuits of intervention response, the current findings take steps to help identify neurobiological patterns of distinct reader subpopulations—groups which likely have different interventional needs.
Direct comparisons of DYS-R and DYS-NR, as well as continuous measures of response, revealed key differences in executive and reading network interactions between the DYS subgroups. Specifically, in DYS-R alone, the left dlPFC (an executive seed specifically implicated in reading ability; see Aboud, Bailey, Petrill, & Cutting, 2016) appeared to support greater communication within the reading network. Interestingly, examinations of DYS-R vs. TD revealed that the use of this executive scaffold may not be compensatory, but rather an important interaction that marks DYS-R as more typical than DYS-NR. Specifically, in DYS-R only, left dlPFC activation corresponded with increased correlations between left vMTG/left vIFG semantic seeds and the left IPL. The left IPL clusters mapped closely to coordinates that are functionally and structurally reduced in readers with dyslexia (Hoeft et al., 2007). In the context of word reading, this area is thought to support phonological processing, as well as mapping orthographic and phonological information (Celsis et al., 1999; Price, 2012; Vigneau et al., 2006). The current results point to a unique relationship in DYS-R, as compared to DYS-NR, between left dlPFC activity and attainment of stronger phonological-semantic communication— processes that are degraded in the face of poor orthographic representations of words (Perfetti, 2007).
Functional interaction analysis additionally showed that compared to DYS-NR, DYS-R had a unique correspondence between the left dlPFC and bilateral IFG connectivity. Previous studies that have examined reading network predictors of intervention response indicate that direct correlations between bilateral IFG is critical for reading outcomes. Farris et al (2011; 2016) found that pre- and post-intervention connectivity between bilateral IFG distinguished both TD and DYS-R from DYS-NR. The current findings point to a critical role of the left dlPFC in facilitating these necessary, typical homotopic connections in DYS-R (and TD) groups, which are absent in DYS-NR. Interestingly, comparisons of DYS-NR revealed that nonresponsive readers are also primarily characterized by interactions of the left dlPFC and reading areas (see Supplemental Material). However, DYS-NR appears to recruit the left dlPFC to support classic compensatory reading circuits in the right hemisphere (Pugh et al., 2000; Shaywitz et al., 1998; Waldie, Haigh, Badzakova-Trajkov, Buckley, & Kirk, 2013). Consequently, unlike the utilization of the left dlPFC in DYS-R, these pathways do not result in increased communication between canonical reading areas, possibility reflecting less efficiency in DYS-NR.
The role of the right IFG in DYS-R appears to extend beyond bilateral communication. Our direct connectivity measures showed that DYS-R had compensatory correlations between left MTG and right IFG as compared to DYS-NR and, in our confirmatory analysis, TD. The structure and function of the right IFG has previously been implicated in natural, long-term reading gains in readers with dyslexia: work by Hoeft et al. (2011) found that an overlapping right IFG area was highly predictive of long-term outcomes of DYS readers (without intervention). This included activation of right IFG and integrity of surrounding white matter tracts, specifically in the superior longitudinal fasciculus. The authors suggested that resilient readers rely on an alternate, compensatory right-hemisphere pathway—an interpretation that is supported by findings that interventions improve activation in both left hemisphere and homotopic right hemisphere reading structures, including the IFG (Eden et al., 2004; Shaywitz et al., 2004).
Together, these results suggest that even before intervention, DYS-R exhibit both more typical and compensatory network correlations compared to DYS-NR, including (1.) greater typical interactions between the left dlPFC and the reading network, and (2.) compensatory, direct correlations between a semantic processing area and the right IFG—a region consistently implicated in positive learning outcomes in dyslexia.
5. Conclusions and Future Directions
These results suggest a broader relationship between educational resilience and FPN involvement in domain-specific processes. Recent work has highlighted this relationship in the context of mental health outcomes and suggests that the widespread cortical connections of the FPN allow individuals to regulate the health of other neural systems (Cole et al., 2014). In this context, readers with high intervention response may have more in-tact executive support of the reading network than nonresponsive readers. Additionally, our findings offer an explanation as to the seemingly paradoxical findings that executive function is critical for learning on the one hand (Blair & Razza, 2007; Diamond, 2013), but on the other hand has inconsistent predictive validity for intervention response (Stuebing et al., 2015): results suggest that these cognitive control networks may not themselves in isolation be predictive of intervention response. Rather, it is their ability to capitalize on interactions with critical reading systems that is key. It is perhaps for this reason that some of the most effective reading interventions include executive components such as explicit strategy instruction and self-regulated learning (Wanzek, Wexler, Vaughn, & Ciullo, 2010). We therefore propose that cognitive control systems play an essential, albeit a “behind the scenes” role in resiliency in learning. In this context, behavioral measures of cognitive control would not powerfully directly predict intervention responsiveness, nor would training cognitive control systems in isolation result in far transfer to academic domains – both of which have been reported (Al Otaiba & Fuchs, 2002; Cho et al., 2015; Melby-Lervåg, Redick, & Hulme, 2016; Stuebing et al., 2015). Rather, the focus would need to shift in terms of how to strengthen and facilitate connections and interactions between critical cognitive control networks and reading systems that may allow for alternate learning pathways. In particular, readers who are non-responsive to traditional interventions may benefit from existing interventions that emphasize both reading and executive functions.
The current study has several limitations. First, the intervention used in this study is short-term, and consequently, the gains seen likely do not reflect the ultimate desired outcome of automation of intervention-supported skills. Future studies will need to build upon the current study by using varying intervention intensities and durations, as well as a larger sample size, in order to examine how to best facilitate the interface between cognitive control and reading networks. Additionally, the current study examined functional patterns related to non-manipulated word and pseudoword stimuli. An important next step in the examination of intervention response prediction will be to manipulate dimensions of word reading (including attention and working memory demands), to examine and specify the nature of the executive and reading interactions reported in the present paper. In particular, we would anticipate that task difficulty (as captured, for instance, by word familiarity/meaningfulness manipulation in a lexical decision task; Balota & Chumbley, 1984) may be associated with an increase in typical executive mediation of the left language network in responders, but that nonresponders would show increased reliance on right hemisphere, compensatory homologues. A better understanding of reader response to these manipulations will allow for more specific intervention development. More broadly, empirical examination of the mediating role of cognitive control networks in a variety of disorders is needed in order to fully understand the potentially powerful role that cognitive control networks may play in treatment response across different learning and psychiatric disorders.
Supplementary Material
Highlights.
The role of executive networks in reading intervention response is unclear.
Intervention response is predicted by prefrontal mediation of the reading network.
At baseline, responders have more typical prefrontal mediation than nonresponders.
Executive areas provide a scaffold for better intervention response in dyslexia.
Acknowledgments
This research was supported by the following funding sources: 7R01NS049096, U54 HD083211, UL1 TR000445, P41 RR15241, NIH M01-RR00052
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Heretofore we use “reading systems” or “reading networks” to refer to brain areas that are known to contribute to, but may not be specific to, reading, including the putative visual word form area, and areas in the canonical left-lateralized language network such as inferior frontal and middle temporal gyri (see Methods for specific information).
As found previously with a larger sample size in Barquero et al. (2015), intervention efficacy for the two Orton-Gillingham based intervention programs was equivalent (repeated measures ANOVA analysis showed no time × intervention type effect: F(1, 20) = .06, p = .82, ηp2 = .003) and that intervention response classification (responder/nonresponder) was not dependent on intervention type: F(1, 18) = .04, p = .83. Therefore, for all analyses intervention type was not further considered.
Author Contributions. Conceptualization, K.S.A., L.A.B. & L.E.C., Methodology, K.S.A., L.A.B. & L.E.C., Writing—Original Draft, K.S.A., L.A.B., L.E.C.; Funding Acquisition, L.E.C., Supervision, L.E.C.
References
- Aboud KS, Bailey SK, Petrill SA, Cutting LE. Comprehending text versus reading words in young readers with varying reading ability: Distinct patterns of functional connectivity from common processing hubs. Developmental Science. 2016:1–25. doi: 10.1111/desc.12422. http://doi.org/10.1111/desc.12422. [DOI] [PMC free article] [PubMed]
- Al-Aidroos N, Said CP, Turk-Browne NB. Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(36):14675–80. doi: 10.1073/pnas.1202095109. http://doi.org/10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Otaiba S, Fuchs D. Characteristics of children who are unresponsive to early literacy interventi … Education. 2002;23(5):300–316. http://doi.org/10.1177/07419325020230050501. [Google Scholar]
- Bach S, Richardson U, Brandeis D, Martin E, Brem S. Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. Neuroimage. 2013;82:605–615. doi: 10.1016/j.neuroimage.2013.05.062. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23727320. [DOI] [PubMed] [Google Scholar]
- Balota DA, Chumbley JI. Are lexical decisions a good measure of lexical access? The role of word frequency in the neglected decision stage. Journal of Experimental Psychology: Human Perception and Performance. 1984;10(3):340–357. doi: 10.1037//0096-1523.10.3.340. http://doi.org/10.1037/0096-1523.10.3.340. [DOI] [PubMed] [Google Scholar]
- Balota DA, Paul S, Spieler D. Attentional control of lexical processing pathways during word recognition and reading. Language Processing. 1999 Retrieved from http://psychnet.wustl.edu/coglab/wp-content/uploads/2015/01/Attentional-control-of-lex-process-1999.pdf.
- Barquero LA, Sefcik AM, Cutting LE, Rimrodt SL. Teaching reading to children with Neurofibromatosis Type 1: A clinical trial with random assignment to different approaches. Developmental Medicine and Child Neurology. 2015 doi: 10.1111/dmcn.12769. http://doi.org/10.1111/dmcn.12769. [DOI] [PMC free article] [PubMed]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. http://doi.org/10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating Effortful Control, Executive Function, and False Belief Understanding to Emerging Math and Literacy Ability in Kindergarten. Child Development. 2007;78(2):647–663. doi: 10.1111/j.1467-8624.2007.01019.x. http://doi.org/10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Borstad AL, Choi S, Schmalbrock P, Nichols-Larsen DS. Frontoparietal white matter integrity predicts haptic performance in chronic stroke. NeuroImage Clinical. 2016;10:129–39. doi: 10.1016/j.nicl.2015.11.007. http://doi.org/10.1016/j.nicl.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celsis P, Boulanouar K, Doyon B, Ranjeva JP, Berry I, Nespoulous JL, Chollet F. Differential fMRI Responses in the Left Posterior Superior Temporal Gyrus and Left Supramarginal Gyrus to Habituation and Change Detection in Syllables and Tones. NeuroImage. 1999;9(1):135–144. doi: 10.1006/nimg.1998.0389. http://doi.org/10.1006/nimg.1998.0389. [DOI] [PubMed] [Google Scholar]
- Cho E, Roberts GJ, Capin P, Roberts G, Miciak J, Vaughn S. Cognitive Attributes, Attention, and Self-Efficacy of Adequate and Inadequate Responders in a Fourth Grade Reading Intervention. Learning Disabilities Research & Practice: A Publication of the Division for Learning Disabilities, Council for Exceptional Children. 2015;30(4):159–170. doi: 10.1111/ldrp.12088. http://doi.org/10.1111/ldrp.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biological Psychiatry. 2011;70(1):43–50. doi: 10.1016/j.biopsych.2011.02.010. http://doi.org/10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Repov G, Anticevic A. The Frontoparietal Control System: A Central Role in Mental Health. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2014 doi: 10.1177/1073858414525995. http://doi.org/10.1177/1073858414525995. [DOI] [PMC free article] [PubMed]
- Conners CK. Conners’ Rating Scale-Revised. North Tonawanda, NY, United States: Multi-Health Systems, Inc; 2002. [Google Scholar]
- Cutting LE, Clements-Stephens A, Pugh KR, Burns S, Cao A, Pekar JJ, Rimrodt SL. Not all reading disabilities are dyslexia: distinct neurobiology of specific comprehension deficits. Brain Connectivity. 2013;3(2):199–211. doi: 10.1089/brain.2012.0116. http://doi.org/10.1089/brain.2012.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis Kaplan executive function system. San Antonio, Texas, USA: The Psychological Corporation; 2001. [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–68. doi: 10.1146/annurev-psych-113011-143750. http://doi.org/10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson W. Accuracy of d′ and A′ as estimates of sensitivity. Bulletin of the Psychonomic Society. 1993;31(4):271–274. [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44(3):411–22. doi: 10.1016/j.neuron.2004.10.019. http://doi.org/10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Farris EA, Odegard TN, Miller HL, Ring J, Allen G, Black J. Functional connectivity between the left and right inferior frontal lobes in a small sample of children with and without reading difficulties. Neurocase. 2011;17(5):425–439. doi: 10.1080/13554794.2010.532141. [DOI] [PubMed] [Google Scholar]
- Farris EA, Ring J, Black J, Lyon GR, Odegard TN. Predicting Growth in Word Level Reading Skills in Children With Developmental Dyslexia Using an Object Rhyming Functional Neuroimaging Task. Developmental Neuropsychology. 2016;41(3):145–161. doi: 10.1080/87565641.2016.1158264. http://doi.org/10.1080/87565641.2016.1158264. [DOI] [PubMed] [Google Scholar]
- Finn ES, Shen X, Holahan JM, Scheinost D, Lacadie C, Papademetris X, Constable RT. Disruption of Functional Networks in Dyslexia: A Whole-Brain, Data-Driven Analysis of Connectivity. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.031. http://doi.org/10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed]
- Fletcher JM. Dyslexia: The evolution of a scientific concept. Journal of the International Neuropsychological Society: JINS. 2009;15(4):501–508. doi: 10.1017/S1355617709090900. http://doi.org/10.1017/S1355617709090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Stuebing KK, Barth AE, Denton CA, Cirino PT, Francis DJ, Vaughn S. Cognitive Correlates of Inadequate Response to Reading Intervention. School Psychology Review. 2011;40(1):3–22. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23125475. [PMC free article] [PubMed] [Google Scholar]
- Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R. Psychophysiological and Modulatory Interactions in Neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. http://doi.org/10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive function. Odessa, FL, United States: Psychological Assessment Resources; 2000. [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Gabrieli JDE. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. http://doi.org/DOI10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Gabrieli JDE. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):361–6. doi: 10.1073/pnas.1008950108. http://doi.org/10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, Gabrieli JDE. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(10):4234–9. doi: 10.1073/pnas.0609399104. http://doi.org/10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihnen SKZ, Petersen SE, Schlaggar BL. Separable roles for attentional control sub-systems in reading tasks: a combined behavioral and fMRI study. Cerebral Cortex (New York, NY: 1991) 2015;25(5):1198–218. doi: 10.1093/cercor/bht313. http://doi.org/10.1093/cercor/bht313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, Gabrieli JDE. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cerebral Cortex (New York, NY: 1991) 2012;22(4):754–64. doi: 10.1093/cercor/bhr094. http://doi.org/10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, Schwartz SJ, Milham MP. Cortical Signatures of Dyslexia and Remediation: An Intrinsic Functional Connectivity Approach. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0055454. http://doi.org/10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, Shaywitz BA, Sl Ba, Catts H, Dickman E, Viall T. A Definition of Dyslexia. Annals of Dyslexia. 2003;53(1):1–14. http://doi.org/10.1007/s11881-003-0001-9. [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences. 2008;1145:237–59. doi: 10.1196/annals.1416.024. http://doi.org/10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Mathes PG, Denton CA, Fletcher JM, Anthony JL, Francis DJ, Schatschneider C. The effects of theoretically different instruction and student characteristics on the skills of struggling readers. Reading Research Quarterly. 2005;40(2):148–182. http://doi.org/10.1598/RRQ.40.2.2. [Google Scholar]
- McGrew KS, Schrank FA, Woodcock RW. Woodcock-Johnson III Normative Update. Rolling Meadows, Illinois, United States: Riverside Publishing; 2007. [Google Scholar]
- Melby-Lervåg M, Redick TS, Hulme C. Working Memory Training Does Not Improve Performance on Measures of Intelligence or Other Measures of "Far Transfer": Evidence From a Meta-Analytic Review. Perspectives on Psychological Science: A Journal of the Association for Psychological Science. 2016;11(4):512–34. doi: 10.1177/1745691616635612. http://doi.org/10.1177/1745691616635612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miciak J, Stuebing KK, Vaughn S, Roberts G, Barth AE, Fletcher JM, VanDerHeyden A. Cognitive Attributes of Adequate and Inadequate Responders to Reading Intervention in Middle School. School Psychology Review. 2014;43(4):407–427. doi: 10.17105/SPR-13-0052.1. http://doi.org/10.17105/SPR-13-0052.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miciak J, Williams JL, Taylor WP, Cirino PT, Fletcher JM, Vaughn S. Do Processing Patterns of Strengths and Weaknesses Predict Differential Treatment Response? Journal of Educational Psychology. 2015;108(6):898–909. doi: 10.1037/edu0000096. http://doi.org/10.1037/edu0000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JR, Benner GJ, Gonzalez J. Learner characteristics that influence the treatment effectiveness of early literacy interventions: A meta-analytic review. Learning Disabilities Research & Practice. 2003;18(4):255–267. [Google Scholar]
- Norton ES, Black JM, Stanley LM, Tanaka H, Gabrieli JDE, Sawyer C, Hoeft F. Functional neuroanatomical evidence for the double-deficit hypothesis of developmental dyslexia. Neuropsychologia. 2014;61:235–46. doi: 10.1016/j.neuropsychologia.2014.06.015. http://doi.org/10.1016/j.neuropsychologia.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton S. Reading, writing, and speech problems in children. New York, New York, United States of America: Norton; 1937. [Google Scholar]
- Perfetti C. Reading Ability: Lexical Quality to Comprehension. Scientific Studies of Reading. 2007;11(4):357–383. http://doi.org/10.1080/10888430701530730. [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. http://doi.org/10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–47. doi: 10.1016/j.neuroimage.2012.04.062. http://doi.org/10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2012;18(5):502–15. doi: 10.1177/1073858411409051. http://doi.org/10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Gore JC. The Angular Gyrus in Developmental Dyslexia: Task-Specific Differences in Functional Connectivity Within Posterior Cortex. Psychological Science. 2000;11(1):51–56. doi: 10.1111/1467-9280.00214. http://doi.org/10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Larkin B, Becker C, Smith S, Fehlbaum LV, Wang Y, Gaab N. Investigating the Influences of Language Delay and/or Familial Risk for Dyslexia on Brain Structure in 5-Year-Olds. Cerebral Cortex. 2015:1–13. doi: 10.1093/cercor/bhv267. http://doi.org/10.1093/cercor/bhv267. [DOI] [PMC free article] [PubMed]
- Reynolds JR, O’Reilly RC, Cohen JD, Braver TS. The function and organization of lateral prefrontal cortex: a test of competing hypotheses. PloS One. 2012;7(2):e30284. doi: 10.1371/journal.pone.0030284. http://doi.org/10.1371/journal.pone.0030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie R, Simos PG, Fletcher JM, Cirino PT, Vaughn S, Papanicolaou AC. Engagement of temporal lobe regions predicts response to educational interventions in adolescent struggling readers. Developmental Neuropsychology. 2011a;36(7):869–88. doi: 10.1080/87565641.2011.606404. http://doi.org/10.1080/87565641.2011.606404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie R, Simos PG, Fletcher JM, Cirino PT, Vaughn S, Papanicolaou AC. Temporo-parietal brain activity as a longitudinal predictor of response to educational interventions among middle school struggling readers. Journal of the International Neuropsychological Society: JINS. 2011b;17(5):875–85. doi: 10.1017/S1355617711000890. http://doi.org/10.1017/S1355617711000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–308. doi: 10.1002/hbm.20752. http://doi.org/10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatschneider C, Fletcher JM, Francis DJ, Carlson CD, Foorman BR. Kindergarten Prediction of Reading Skills: A Longitudinal Comparative Analysis. Journal of Educational Psychology. 2004;96(2):265–282. http://doi.org/10.1037/0022-0663.96.2.265. [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically- based intervention. Biological Psychiatry. 2004;55(9):926–33. doi: 10.1016/j.biopsych.2003.12.019. http://doi.org/10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz Ba, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Gore JC. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(5):2636–2641. doi: 10.1073/pnas.95.5.2636. http://doi.org/10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. The human connectome: A complex network. Annals of the New York Academy of Sciences. 2011;1224(1):109–125. doi: 10.1111/j.1749-6632.2010.05888.x. http://doi.org/10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- St Clair-Thompson HL, Gathercole SE. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Quarterly Journal of Experimental Psychology (2006) 2006;59(4):745–759. doi: 10.1080/17470210500162854. http://doi.org/10.1080/17470210500162854. [DOI] [PubMed] [Google Scholar]
- Stuebing KK, Barth AE, Trahan LH, Reddy RR, Miciak J, Fletcher JM. Are Child Cognitive Characteristics Strong Predictors of Responses to Intervention? A Meta-Analysis Review of Educational Research. 2015;85(3):395–429. doi: 10.3102/0034654314555996. http://doi.org/10.3102/0034654314555996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PA, Hulme C, Nash HM, Gooch D, Hayiou-Thomas E, Snowling MJ. Developmental dyslexia: predicting individual risk. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2015;56(9):976–87. doi: 10.1111/jcpp.12412. http://doi.org/10.1111/jcpp.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency. Austin, TX, United States: Pro-ED; 1997. [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houd?? O, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. http://doi.org/10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, Schlaggar BL. The putative visual word form area is functionally connected to the dorsal attention network. Cerebral Cortex (New York, NY: 1991) 2012;22(3):537–49. doi: 10.1093/cercor/bhr100. http://doi.org/10.1093/cercor/bhr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. The Comprehensive Test of Phonological Processing. Austin, TX: Pro-ED; 1999. [Google Scholar]
- Wagner RK. Expertise in Reading. In: Lyon N, Krasnegor R, editors. Attention, memory, and executive functions. Baltimore, MD: Paul H. Brooks Publishing; 1996. pp. 139–156. [Google Scholar]
- Waldie KE, Haigh CE, Badzakova-Trajkov G, Buckley J, Kirk IJ. Reading the wrong way with the right hemisphere. Brain Sciences. 2013;3(3):1060–75. doi: 10.3390/brainsci3031060. http://doi.org/10.3390/brainsci3031060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanzek J, Wexler J, Vaughn S, Ciullo S. Reading interventions for struggling readers in the upper elementary grades: a synthesis of 20 years of research. Reading and Writing. 2010;23(8):889–912. doi: 10.1007/s11145-009-9179-5. http://doi.org/10.1007/s11145-009-9179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children, Fourth. San Antonio, Texas, USA: The Psychological Corporation; 2003. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children (Fourth) London, UK: Pearson Assessment; 2004. WISC IV Integrated. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test (Second) San Antonio, TX, USA: The Psychological Corporation; 2005. [Google Scholar]
- Westphal AJ, Wang S, Rissman J. Episodic memory retrieval benefits from a less modular brain network organization. The Journal of Neuroscience. 2017;(310):2509–16. doi: 10.1523/JNEUROSCI.2509-16.2017. http://doi.org/10.1523/JNEUROSCI.2509-16.2017. [DOI] [PMC free article] [PubMed]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. http://doi.org/10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Felton RH. Word Identification and Spelling Test (WIST) Austin, TX, United States: PRO-ED; 2004. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock Johnson - III Complete Battery. Itasca, IL, USA: Riverside Publishing Company; 2001. [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. NeuroSynth: a new platform for large-scale automated synthesis of human functional neuroimaging data. Frontiers in Neuroinformatics. 2011;5 doi: 10.1038/nmeth.1635. http://doi.org/10.3389/conf.fninf.2011.08.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(3):1125–65. doi: 10.1152/jn.00338.2011. http://doi.org/10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


