Abstract
Aging impairs development of new B cells and diminishes the expression of protective antibodies. Reduced numbers of B cell precursors generally occur in old (~2 yrs.) mice. At the pro-B to pre-B cell transition, the pre-B cell receptor (preBCR) checkpoint directs pre-B cell expansion and selection of the pre-B cell immunoglobulin (Ig) µ heavy chain variable region repertoire. The preBCR is comprised of Ig µ heavy chain + surrogate light chains (SLC; λ5/VpreB). In old B cell precursors, SLC is decreased and fewer pre-B cells form the preBCR. In pro-B cells, SLC is complexed with cadherin 17 to form a “pro-B cell receptor” whose signaling is postulated to increase apoptotic sensitivity. We propose that inflammation in old mice, in part mediated by the age-associated B cells (ABC), promotes apoptosis among pro-B cells, particularly those relatively high in SLC. The remaining pro-B cells, with lower SLC, now generate pre-B cells with limited capacity to form the preBCR. Ig µ heavy chains vary in their capacity to associate with SLC and form the preBCR. We speculate that limited SLC restricts formation of the preBCR to a subset of Ig µ heavy chains. This likely impacts the composition of the antibody repertoire among B cells.
B lymphocyte functions and B lymphopoiesis are compromised in old age
Old age is accompanied by decline in function in most organ systems and the immune system is no exception (reviewed in Cancro, et al., 2009; Scholz, et al., 2013). Dysfunction of the immune system is seen in elderly humans as well as in mouse models of aging. Phenotypic and functional changes are observed in most of the cell types that constitute the immune system, including T and B cells, NK cells, dendritic cells, as well as macrophages and neutrophils. This results in impaired cellular and humoral immunity to a variety of pathogens, complicates vaccine effectiveness, and may contribute to increased autoreactivity (reviewed in Cancro, et al., 2009; Scholz, et al., 2013).
This review will summarize our studies as to the mechanisms that alter the production, activity, and antigenic specificity (antibody repertoire) of B lymphocytes in old age. In old mice, the production of new B lymphocytes within the bone marrow is reduced (Riley, et al., 1991; Stephan, et al., 1996; Labrie, et al., 2004). However, surprisingly, the numbers of mature B cells in the spleens of old mice are roughly maintained, albeit the composition of these B cells is markedly different from that seen in young adults (Hao, et al., 2011; Rubtsov, et al., 2011; Ratliff, et al., 2013). In particular, the splenic B cells of old mice are enriched for phenotypes that suggest prior antigen exposure (Johnson, et al., 2002a), including “age-associated B cells” (ABC) (Hao, et al., 2011; Rubtsov, et al., 2011; Ratliff, et al., 2013; reviewed in Naradikian, et al., 2016). The ABC have a characteristic CD21/35low/neg CD23neg phenotype, expand numerically and proportionately (becoming up to one-half of mature B cells) in the spleens of old mice, are biased to react with certain apoptotic/oxidized lipid/bacterial antigens (phosphorylcholine; malondialdehyde) (Riley, et al., 2017), and express somatic hypermutation consistent with chronic antigen exposure (Russell Knode, et al., 2017). Typical follicular B2 B cells, which ordinarily make up the major (~90%) pool of naïve B cells in the spleen, decline by up to one-half in old mice (Ratliff, et al., 2013). Marginal zone B cells, likely another antigen-experienced subset, are increased in some strains (C57BL/6) and decreased in others (BALB/c) in old age (Johnson, et al., 2002a; Frasca, et al., 2012; Birjandi, et al., 2011).
It is likely that the differences in types of mature B cells populating the periphery in old mice affects both B cell activity and the specificity of antibodies elicited in immune responses. Analysis of anti-phosphorylcholine (PC) antibodies and their idiotypes have proven to be a useful tool in assessing changes in antibody/B cell repertoires in old age (Zharhary and Klinman, 1986; Riley, et al., 1989; Khomtchouck, et al., 2017). In particular, the T15 (TEPC 15) idiotype dominates the B cell responses to PC in young adult BALB/c mice (Zharhary and Klinman, 1986; Riley, et al., 1989), but is reduced as a percentage of the total anti-PC response in old age. This is seen both among total splenic B cells (Zharhary and Klinman, 1986; Riley, et al., 1989) and the B2 follicular splenic B cell subset (Khomtchouck, et al., 2017). In old mice, this results mainly from a marked increase in anti-PC B cells which are T15neg in their idiotype (Zharhary and Klinman, 1986; Riley, et al., 1989; Khomtchouk, et al., 2017). The T15 idiotype of anti-PC antibodies is required for effective immunity to pneumococcal bacteria (Briles, et al., 1982; Mi, et al., 2000), but is effectively “diluted” in old mice (Nicoletti, et al., 1993).
While B2 B cells may produce anti-PC antibodies bearing T15 as described above, the B1 B cell subset, which is generated primarily during fetal/neonatal life and is is maintained in adults via self-renewal (reviewed in Hardy, 2006), generally produces most of the T15 anti-PC antibodies during bacterial exposures (Masmoudi, et al., 1990; Martin, et al., 2001). However, in old mice, the B1 B cell repertoire may also undergo change. Typically, B1 B cells of fetal/neonatal origin have minimal N region nucleotide addition in their Ig heavy chain H-CDR3 sequences (reviewed in Hardy, 2006). This is a consequence of the poor expression of the enzyme terminal deoxynucleotidyl transferase (Tdt) at this point in ontogeny. In contast, peritoneal B1 B cells in old mice display increased N region addition in the H-CDR3 regions (Holodick, et al., 2016). This would mitigate against expression of the T15 idiotype which requires VHS107.1, with a germ-line encoded H-CDR3 Ig variable region sequence, paired with the Vκ22 light chain (Perlmutter, et al., 1984).
It may be that an increasing number of B1 B cells in old mice are derived from the bone marrow where, due to Tdt expression, N region additions are prevalent within H-CDR3 (Holodick, et al., 2015). This would be expected to impair the normal expression of the germ-line T15 idiotype within the B1 B cell pool. Notably, a limited pool of progenitors that generate B1 B cells is found in adult bone marrow (reviewed in Montecino-Rodriguez and Dorschkind, 2012). In old mice, these B1 progenitors may be maintained (Alter-Wolf, et al., 2009). Maintenance of a bone marrow pathway of B1 B cell generation in old mice, particularly with diminished B2 B lymphopoiesis, may contribute to alterations in B cell composition and antibody repertoires. Further studies to address these issues appear warranted.
While much remains to be determined regarding the alterations in development of various subsets of B cells during old age, it has been demonstrated that B cells in old mice reactive with PC express greater diversity of VH and VL gene usage, lower affinity, extensive self-reactivity/polyreactivity, reduced T15 idiotype, and poor protection against microbial pathogens (Nicoletti, et al., 1993; Zharhary and Klinman, 1986; Riley, et al., 1989; Khomtchouk, et al., 2017).
The activation of B cells in germinal centers, leading to both Ig isotype switching and somatic hypermutation, is impaired in old age and consequently this diminishes memory B cell responses (Yang, et al., 1996; Zheng, et al., 1997). This both reflects poor availability of T cell help, but also intrinsic B cell defects affecting expression of activation induced cytidine deaminase (AID) (Frasca, et al., 2008), important to both the processes of Ig isotype switching and somatic mutation of Ig variable region genes.
It is reasonable to hypothesize that changes in the peripheral B cell compartments in old age may, in part, reflect altered patterns of B cell development within the bone marrow. Indeed, the reduced expression of the T15 idiotype among splenic B cells reactive to PC is also seen among newly derived B cells in the bone marrow of old BALB/c mice (Zharhary and Klinman, 1986; Riley, et al., 1989). This supports the view that altered patterns of B lymphopoiesis in old age may contribute to peripheral B cell dysfunctions. In this review, we will focus primarily on the pro-B and pre-B cell stages of development in the aged mouse (Fig. 1). However, earlier progenitor cells, e.g., common lymphoid progenitors (Miller and Allman, 2003; Min, et al., 2006; Lescale, et al., 2010) and hematopoietic stem cells (reviewed in Van Zant and Liang, 2012; Pang, et al., 2017) also show limitations in development of lymphocytes in old age.
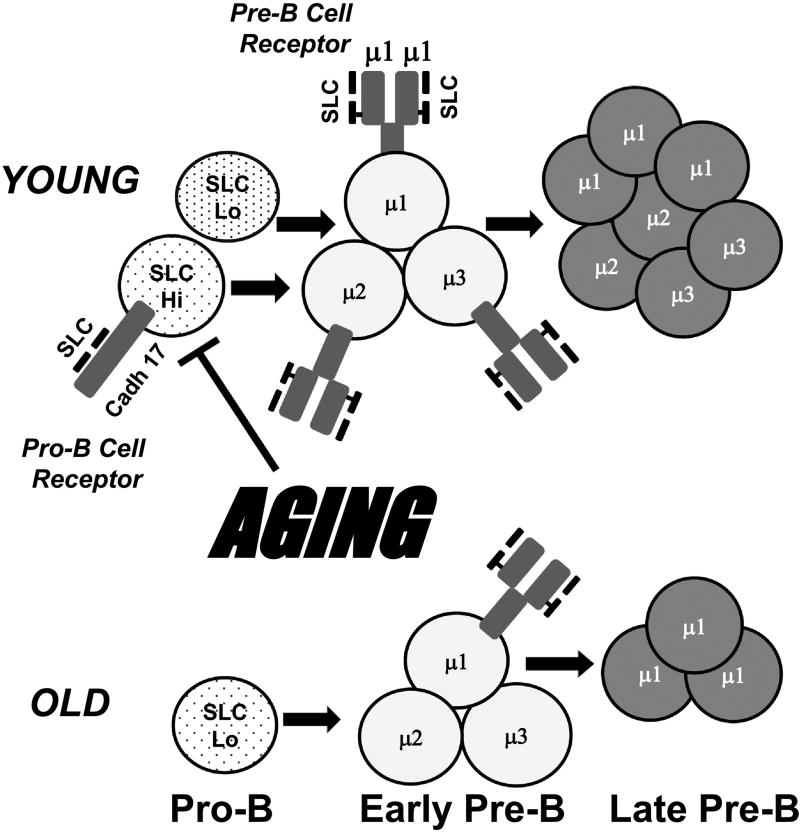
Figure 1. Aging reduces pro-B and pre-B cells, decreases surrogate light chains, and impairs pro-B cell receptor and pre-B cell receptor expression.
Bone marrow pro-B cells and pre-B cells are reduced in old (~2 yrs) vs. young adult (~3 mo.) mice. Lower surrogate light chain proteins, λ5 and VpreB, occur in old pro-B cells. SLC protein losses likely affect both the “pro-B cell receptor” (cadherin 17 [Cadh 17] + SLC) and the pre-B cell receptor (preBCR) (Ig µ heavy chain + SLC). Three early pre-B cells are shown which have different Ig µ heavy chain V region sequences. These µ heavy chains differ in their capacity to associate with SLC to form the preBCR (µ1>µ2 and µ3). In young adult mice, overall SLC is relatively high and all of these early pre-B cells express the preBCR, proliferate, and produce late stage pre-B cells. We propose that in old age pro-B cells with higher SLC levels preferentially are depleted, leaving pro-B cells lower in SLC. Under these conditions, only early pre-B cells with µ1 (higher binding to SLC) are capable of forming the preBCR, proliferating, and undergoing further B cell maturation.
The extent to which B lymphopoiesis is compromised varies with the individual mouse, but this can be quite profound with up to 2-fold decrease in pro-B cells and up to 90% decline in pre-B cells observed (Van der Put, et al., 2003). Overall there is a continuing decline in new B lymphocyte generation roughly beginning in “middle-age” (e.g., ~10–13 months old) and becoming more pronounced in advanced old age (~24–27 months old) (Miller and Allman, 2003; Riley, et al., 1991; Stephan, et al., 1996; Van der Put, et al., 2003). In particular, the reductions seen in newly generated (immature) bone marrow B cells, both in proportion and number, generally correlate with the losses seen in earlier precursors, e.g., the pre-B and pro-B cell stages (Van der Put, et al., 2003). The diminution seen in pro-B, pre-B, and immature B cell stages in old mice coincide with decreased rates of production as revealed by BrdU labeling studies (Kline, et al., 1999; Johnson, et al., 2002b; Labrie, et al., 2004).
The reductions seen in B lineage cells in old mice may result from a variety of reported changes in growth and differentiation acting in synergy. These include reduced responses to the growth factor IL-7 (Stephan, et al., 1997; Lescale, et al., 2010), as evidenced by diminished activation of Stat5 (Lescale, et al., 2010), and reduced bioavailability of IL-7 produced by bone marrow stromal cells (Stephan, et al., 1998); increased susceptibility to apoptosis due to diminished expression of the Bcl-xL survival factor (Kirman, et al., 1998; Sherwood, et al., 2003); and reduced crucial B lineage transcription factors (e.g., E47/E2A; EBF1) (Sherwood, et al., 2000; Frasca, et al., 2003; Lescale, et al., 2010). Poor E47/E2A expression is particularly important as this factor regulates transcription of the surrogate light chains (SLC) λ5 and VpreB, components of the pre-B cell receptor, and RAG1/2 enzymes that direct Ig variable region gene recombination. Notably, SLC proteins are reduced in old pro-B cells (Sherwood, et al., 1998; Sherwood, et al., 2000; Alter-Wolf, et al., 2009; Ratliff, et al. 2015; Khomtchouk, et al., 2017) (Fig. 1) and RAG1/2 proteins are also lower (Labrie, et al., 2004). E47/E2A operates together with the EBF1 transcription factor to regulate SLC and RAG1/2 expression (Sigvardsson, et al., 1997). E47/E2A promotes the expression of Early B cell Factor (EBF); in addition to E47/E2A, levels of EBF1 also are lower in pro-B cells in aged mice (Lescale, et al., 2010; Riley, 2013).
The SLC proteins form signaling complexes at the pro-B and pre-B cell stages (reviewed in Melchers, 2005; Martensson, et al., 2007) (Fig. 1). In pre-B cells, the SLC forms complexes with the Ig µ heavy chain to form the pre-B cell receptor (preBCR). In pro-B cells the SLC forms a different complex, associating with the non-classical cadherin, cadherin 17, to form a “pro-B cell receptor” (Ohnishi, et al., 2000). In pro-B cells from old mice, we hypothesize that reduced SLC proteins result in reduced “pro-B cell receptors” (cadherin 17 + SLC) and fewer preBCR in pre-B cells. This contributes to limit new B cell formation and alters the normal expression of the Ig antibody repertoire.
In old mice, there is a preferential loss of those pro-B cells that have higher levels of SLC proteins and this promotes an “SLClo” B cell developmental pathway
The signaling that occurs via the preBCR has been the subject of extensive experimentation. It is generally thought that aggregation of the preBCR on the early pre-B cell surface results in signaling and this requires a basic aminio acid motif in the non-immunoglobulin tail of SLC λ5 (reviewed in Clark, et al., 2014). While not yet clear, signaling via the preBCR may occur by either self-association mediated by the SLC proteins (reviewed in Vettermann, et al., 2010) or through binding to stromal cell/extracellular matrix ligands (e.g., galectin-1; heparin sulfate [Gautheir, et al., 2002; Bradl, et al., 2003]).
PreBCR signaling is complex. While preBCR signaling synergizes with IL-7 to promote proliferation at the pro-B to pre-B cell transition (Marshall, et al., 1998; Fleming and Paige, 2001), this is limited to 4-5 divisions (reviewed in Clark, et al., 2014). Continued preBCR signaling leads to down-regulation of pre-B cell proliferation (reviewed in Clark, et al., 2014). This is coincident with increased expression of the transcription factor Aiolos which is dependent upon RAS-MEK-ERK activation (Iritani, et al., 1997; Heng, et al., 2008; Yasuda, et al., 2008; reviewed in Clark, et al., 2014). Aiolos acts as a repressor of Myc30 and Ccnd3 gene transcription and, consequently, this decreases proliferation, increases survival, promotes Ig light chain rearrangement and furthers differentiation to the immature B cell stages (reviewed in Clark, et al., 2014).
Unlike the preBCR, the function of this “pro-B cell receptor” (cadherin 17/SLC) is not known. Mice with cadherin 17 knocked out have partially reduced early B cell precursors (Ohnishi, et al., 2005), suggesting an as yet ill-defined role of the pro-B cell receptor in normal B lymphopoiesis. More recently we have shown that deficits in SLC expression in pro-B cells renders them more susceptible to apoptosis induced by either TNFα or TGFβ cytokines (Ratliff, et al., 2015). Since most pro-B cells undergo aberrant Ig µ heavy chain rearrangements and cannot continue along the B cell developmental pathway, susceptibility to apoptosis is likely a normal requirement to insure that pro-B cells which do not undergo productive Ig VH gene rearrangements are rapidly removed. A possible function for the pro-B cell receptor may be to insure appropriate sensitivity to apoptosis, facilitate the removal of non-functional pro-B cells, and maintain normal pro-B cell homeostasis.
In old mice, pro-B cells in the bone marrow are often reduced numerically and generally express lower levels of SLC proteins and hence, presumably, reduced pro-B cell receptors (Sherwood, et al., 1998; Sherwood, et al., 2000; Alter-Wolf, et al., 2009; Ratliff, et al., 2015) (Fig. 1). The reductions seen in pro-B cells in old mice may, in part, result from increased “apoptotic stress” which increases apoptosis among the most susceptible (e.g., cadherin 17/SLChigh) pro-B cells. A source of increased “apoptotic stress” could be the chronic inflammation seen in old mice (reviewed in Frasca and Blomberg, 2016). We will discuss this and propose a basis for increased inflammation in old bone marrow below. Reduced expression of bioactive IL-7 growth factor as well as diminished responsiveness to IL-7 may also contribute to pro-B cell apoptosis and reduced growth in old age (Stephan, et al., 1997; Stephan, et al., 1998). Pro-B cells in old mice are deficient in IL-7-mediated recruitment into mitosis in vitro (Sherwood, et al., 2003) and show somewhat lower mitotic activity in vivo (Sherwood, et al., 1998; Sherwood, et al., 2000; Khomtchouck, et al. 2017). Signer, et al. (2008) have shown that B lineage precursors in aged mice have elevated levels of p16Ink4a and Arf and that these proteins are critical in inhibiting cell cycle, and induce apoptosis in a variety of aging and senescent cell types. Whether inflammation-associated signaling pathways lead to the increased expression of p16Ink4a and Arf remains to be determined.
Increased ERK MAPK activity: a common mechanism linking low SLC expression and apoptotic resistance in old pro-B cells
SLC expression is governed by the transcription factors E47/E2A and EBF1 (Sigvardson, et al., 1997) and SLC mRNA and protein levels are reduced in old pro-B cells (Sherwood, et al., 1998; Sherwood, et al., 2000; Ratliff, et al., 2015; Khomtchouk, et al., 2017). Prior studies by us (Sherwood, et al., 2000; Frasca, et al., 2003; Van der Put, et al., 2004; Riley, 2013) and others (Lescale, et al., 2010), have demonstrated that both E47/E2A and EBF1 expression are reduced in B cell precursors in old mice. The low expression of E47/E2A in old B cell precursors reflects increased rates of proteasomal protein degradation (Van der Put, et al., 2004). E47/E2A sits at the pinnacle of a hierarchy of key transcription factors that govern the B lymphopoietic program (reviewed in Kee, et al., 2000). Not surprisingly, the reduced levels of E47/E2A would be expected to impact further B lymphopoiesis in old mice.
Our studies focus on the basis for the increased turnover of E47/E2A proteins in old mice. Notably, phosphorylation of E47/E2A proteins is increased in old B cell precursors and this targets E47/E2A for ubiquitination and subsequent proteasomal degradation (King, et al., 2007). Old pro-B cells have increased ERK MAPK activation that leads to E47/E2A protein phosphorylation (King, et al., 2007). This also drives increased phosphorylation and subsequent degradation of the pro-apoptotic protein Bim (Ratliff, M., Blomberg, B.B., Riley, R.L., manuscript in preparation) and Bim is required for cytokine (TNFα)-induced apoptosis in pro-B cells (Ratliff, et al., 2015). Of considerable interest, deficiency in SLC, and hence the pro-B cell receptor, in either young adult λ5 hetereozygous or knock out mice or in old mice, results in increased ERK MAPK activation, decreased E47/E2A levels and decreased Bim levels (Ratliff, M., Blomberg, B.B., Riley, R.L., manuscript in preparation).
We propose, based on our preliminary studies, that the pro-B cell receptors (cadherin 17/SLC), provide signals which inhibit the activation of ERK MAPK (Fig. 2). Lower expression of SLC coincides with increased ERK MAPK activation in old pro-B cells (King, et al., 2007). Moreover, in young pro-B cells from λ5 knock out mice, ERK MAPK activation is increased, indicating that this is a property associated with low SLC rather than simply old age (Ratliff, M., Blomberg, B.B., Riley, R.L., manuscript in preparation). The pro-B cell receptor complexes possibly cause signaling, as yet uncharacterized, which dampen the activation of ERK MAPK in pro-B cells.
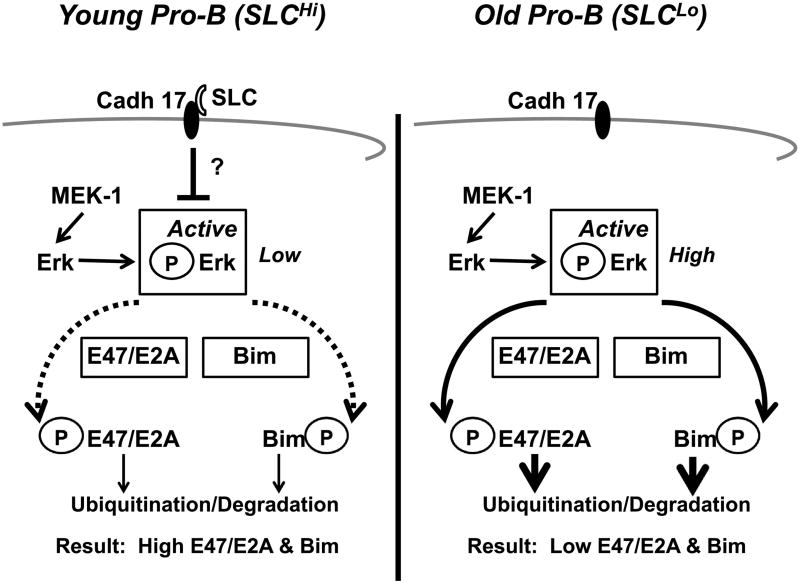
Figure 2. In old pro-B cells with low surrogate light chain, increased ERK MAPK activity reduces E47/E2A and Bim proteins.
Old pro-B cells have lower SLC and active E47/E2A and Bim proteins. We hypothesize that the “pro-B cell receptor” (cadherin 17 [Cadh 17] + SLC) in young adult pro-B cells inhibits ERK MAPK activation, resulting in lower levels of phosphorylated (active) ERK MAPK. This minimizes both phosphorylation and subsequent ubiquitination and degradation of E47/E2A and Bim proteins. Therefore, in young pro-B cells, E47/E2A and Bim levels are relatively high. In old SLCLo pro-B cells, cadherin 17/SLC complexes are reduced, signaling via the “pro-B cell receptor” is diminished, and phosphorylated (active) ERK MAPK is increased. Active ERK MAPK phosphorylates both E47/E2A and Bim proteins, targeting them for further ubiquitination and proteosomal degradation. This results in reduced levels of E47/E2A and Bim proteins in old SLCLo pro-B cells.
Reduced SLC in old mice may affect the B cell antibody repertoire
The reduced expression of SLC in old B cell precursors likely not only affects the development of pre-B cells within the bone marrow of old mice, but also the repertoire of Ig µ heavy chains that are observed. The capacity of individual Ig µ heavy chains to complex with SLC to form the preBCR varies substantially and this coincides with preBCR function (Kawano, et al., 2006). Some estimates indicate that only about one half of µ heavy chains may associate with SLC to form the preBCR (ten Boekel, et al., 1997). Possibly, low SLC expression in pro-B cells in old age only allows for preBCR assembly and function in those few pre-B cells whose Ig µ heavy chains have relatively high affinity for SLC. Our previous results showed that the reduced pool of early pre-B cells at the preBCR checkpoint undergo extensive proliferation consistent with preBCR signaling (Sherwood, et al., 1998; Khomtchouk, et al., 2017).
Are the Ig µ heavy chains significantly different in their variable regions in old pre-B cells compared to those in young adults? The answer to this question requires a comprehensive analysis of variable region sequences from individual old mice. However, we and others have shown that the recently generated immature B cells in old mice are enriched for reactivity to the self-antigen/bacterial antigen phosphorylcholine (PC) (Zharhary and Klinman, 1986; Riley, et al., 1989; Khomtchouk, et al., 2017). Moreover, immature B cells from old bone marrow are enriched for conventional λ light chain expression, are relatively low in surface IgM expression, and are generally anergic to BCR signaling (Alter-Wolf, et.al., 2009 and data not shown). We would like to speculate that these characteristics, taken together, are consistent with a skewing of the immature B cell antibody repertoire possibly to self-reactivity in old mice. Whether this is due to alteration in the preBCR checkpoint remains to be determined. However, studies from Keenan, et al. (2008) suggested that normal expression of the preBCR participates in establishment of self-tolerance.
As discussed above, a hallmark of old age in BALB/c mice is a decline in the incidence of the T15 idiotype among anti-PC reactive B cells and their antibodies. In old BALB/c mice, the extent of loss of pre-B cells and of reduced SLC also coincides with an increased frequency of T15neg PC reactive B cells (Khomtchouk, et al., 2017). Furthermore, in young adult mice, deficiency in SLC λ5 protein not only partially blocks new B cell development, but also can bias the PC reactive B cell repertoire in some mice in favor of T15neg clonotypes (Khomtchouk, et al., 2017). Therefore, we suggest that low SLC expression not only contributes to poor B lymphopoiesis in old age, but also alters the primary antibody specificity repertoire in ways that may affect immunity to pathogens.
Age-associated B cells (ABC) increase in old mice, are pro-inflammatory, and promote pro-B cell loss
The down-regulation of SLC in old mice likely affects both the production of B cell precursors and new B cells within the bone marrow and also may modify the repertoire of antibody specificities. But what causes the reductions in SLC seen in old B cell precursors? As discussed above, increased apoptosis, preferentially targeting those pro-B cells with relatively higher levels of SLC, contributes to the low SLC expression characteristic of the diminished pro-B cell pool in old mice. We have reported that B cells in old mice often are pro-inflammatory and secrete TNFα (Frasca, et al., 2012; Ratliff, et al., 2013). This includes the CD21/35low/neg CD23neg age-associated B cells (ABC) (Frasca, et al., 2012; Ratliff, et al., 2013).
We have shown that the numbers of ABC within the bone marrow of individual old mice correlates with reductions in B cell precursors (Ratliff, et al., 2013; Ratliff, et al., 2015). Moreover, adoptive transfer of ABC into young adult RAG-2 knockout mice results in diminished numbers of pro-B cells within the bone marrow and a skew to lower SLC expression among the remaining pro-B cells similar to what is seen in old mice (Ratliff, et al., 2013). It is notable that depletion of all mature B cells in old mice leads to restoration of B lymphopoiesis, indicating the importance of B cells to the mechanisms of impaired B cell development observed in old age (Keren, et al., 2011a; Keren, et al., 2011b). We would suggest that pro-inflammatory B cells, in particular ABC, may compromise B lymphopoiesis in old mice.
In addition to ABC, other cells within the bone marrow may also contribute to increased inflammation and down-regulate B lymphopoiesis in old age. Recently, Kennedy and Knight (2015; 2017) have determined that inflammation, mediated by adipocyte activation of myeloid suppressor cells, can inhibit early B cell precursors. Natural Killer (NK) cells from old mice can secrete TNFα (King, et al., 2009). NK cells are normally maintained or modestly increased in old bone marrow (King, et al., 2009; Fang, et al., 2010; Beli, et al., 2014), albeit in old bone marrow there is a bias to immature NK cells and the production of more mature NK cells is impaired (Fang, et al., 2010; Beli, et al., 2014). These studies suggest that possibly pro-inflammatory ABC and NK cells (and likely other cell types) may contribute to the loss of relatively SLChi pro-B cells in old bone marrow and skew the remaining pro-B cells to lower SLC expression.
A model for reduced B lymphopoiesis in old age and how this affects the primary B cell antibody repertoire
It is clear that, in mouse models of old age, B lymphopoiesis is impaired and the antibody repertoire of B cells is altered (Riley, et al., 1991; Stephan, et al., 1996; Zharhary and Klinman, 1986; Riley, et al., 1989; Khomtchouk, et al., 2017). However, the cellular and molecular mechanisms responsible for these alterations in B cell development and function remain to be fully elucidated. We suggest a hypothesis (see Fig. 3) in which the inflammatory bone marrow microenvironment in old mice promotes the apoptosis of early B cell precursors. Our results suggest that apoptotic susceptibility in pro-B cells may be determined, in part, by levels of SLC (as the cadherin 17/SLC complex) (Ratliff, et al., 2015). Consequently, heightened “apoptotic stress” in old bone marrow likely preferentially eliminates those pro-B cells with relatively higher levels of SLC and leaves a residual pro-B cell pool with lower SLC levels. If so, this would also likely affect the preBCR checkpoint and has implications for the selection of µ heavy chains that are utilized to form antibodies in new B cells. We have identified ABC as capable of TNFα secretion and inhibition of B lymphopoiesis in old mice (Ratliff, et al., 2013; Ratliff, et al., 2015). Additional cells, including NK cells, likely also are involved in this process (King, et al., 2009). We note that the generation of particular B cells (anti-PC/T15neg) is increased in old mice even as overall B cell production is reduced (Khomtchouk, et al., 2017). Whether this paradigm extends to other antibody specificities remains to be tested.
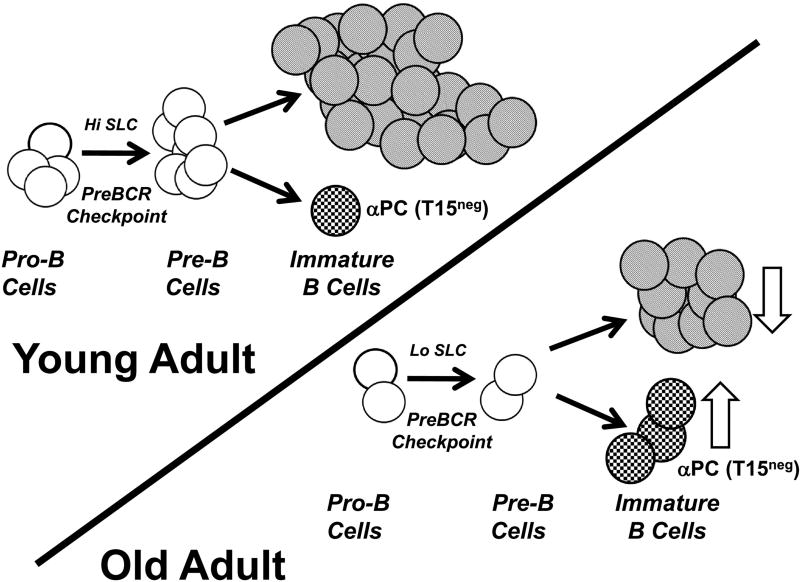
Figure 3. A hypothesis: Inflammatory ABC promote loss of B cell precursors in old mice, diminish new B cell production, and alter antibody repertoires.
In this model, ABC and likely other cell types (NK cells?) increase inflammation in old bone marrow. This promotes apoptosis in B cell precursors and reduces B lymphopoiesis. B cell precursors relatively high in surrogate light chain (SLC) are preferentially targeted for apoptosis. Remaining B cell development continues on an “SLCLo” pathway and the lower levels of SLC available may constrain preBCR assembly. As proposed in Figure 1, those µ heavy chains with greater capacity to form the preBCR under low SLC conditions will be favored and this may alter the frequency of particular B cell specificities in old age. While overall B cell production is reduced in old age (unfilled down arrow), the generation of the antibody repertoire is altered with increased representation of particular clonotypes, e.g., T15neg anti-PC (unfilled up arrow).
Among the unanswered questions are: 1) Does inflammation provide the major impetus for diminished B lymphopoiesis in old age and can this be reversed therapeutically? 2) How extensive are the changes in antibody repertoire that occur subsequent to down-regulation of B lymphopoiesis in aged mice and does this reflect changes in preBCR mediated VH selection? 3) What is the global effect on immunity that occurs due to poor B lymphopoiesis in old mice? And 4) To what extent are the results obtained in old mice regarding reduced B lymphopoiesis applicable to elderly humans? Only future experiments will illuminate these questions and undoubtedly reveal new and exciting aspects of the regulation of B cell immunity in the context of old age.
Highlights.
B lymphopoiesis is reduced in old mice, with losses seen in pro-B cells, pre-B cells, and new B cells.
Pro-inflammatory age-associated B cells (ABC) can cause apoptosis in pro-B cells in old mice and inhibit B lymphopoiesis.
In old mice, pro-B cells with relatively high surrogate light chain (SLC) levels are more susceptible to apoptosis and are preferentially deleted within the bone marrow. This leaves a reduced “SLClo” pro-B cell pool for further B cell development in old mice.
Low SLC expression in aged B cell precursors likely compromises the pre-B cell receptor checkpoint and alters the expression of B cell specificities. This is represented by the increased incidences of T15neg/anti-phosphorylcholine B cells in old mice and in young mice when SLC expression is low.
Acknowledgments
We wish to thank all members of the Riley and Blomberg laboratories, past and present, who have contributed to this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by NIH grants R01 AG025256 to RLR and R01 AG023717 to BBB
References
- Alter-Wolf S, Blomberg BB, Riley RL. Deviation of the B cell pathway in senescent mice is associated with reduced surrogate light chain expression and altered immature B cell generation, phenotype, and light chain expression. J Immunol. 2009;182:138–147. doi: 10.4049/jimmunol.182.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beli E, Duriancik DM, Clinthorne JF, Lee T, Kim S, Gardner EM. Natural killer cell development and maturation in aged mice. Mech. Ageing Dev. 2014;135:33–40. doi: 10.1016/j.mad.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol. 2011;186:3441–3451. doi: 10.4049/jimmunol.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl H, Wittmann J, Milius D, Vettermann C, Jack HM. Interaction of murine precursor B cell receptor with stroma cells is controlled by the unique tail of lambda 5 and stroma cell-associated heparan sulfate. J Immunol. 2003;171:2338–2348. doi: 10.4049/jimmunol.171.5.2338. [DOI] [PubMed] [Google Scholar]
- Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancro M, Hao Y, Scholz J, Riley R, Frasca D, Dunn-Walters D, Blomberg BB. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30:313–318. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol. 2014;14:69–80. doi: 10.1038/nri3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J. Exp. Med. 2010;207:2369–2381. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521–531. doi: 10.1016/s1074-7613(01)00216-3. [DOI] [PubMed] [Google Scholar]
- Frasca D, Nguyen D, Riley R, Blomberg BB. Decreased E12 and/or E47 transcription factor activity in the bone marrow as well as in the spleen of aged mice. J Immunol. 2003;170:719–726. doi: 10.4049/jimmunol.170.2.719. [DOI] [PubMed] [Google Scholar]
- Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- Frasca D, Romero M, Diaz A, Alter-Wolf S, Ratliff M, Landin A, Riley R, Blomberg B. A molecular mechanism for TNF-alpha-mediated downregulation of B cell responses. J Immunol. 2012;188:279–286. doi: 10.4049/jimmunol.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17:7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A. 2002;99:13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, O'Neill P, Naradikian M, Scholz J, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1300. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- Heng TS, Painter MW. Consortium, I. G. P. The Immunological Genome Project: networks of gene expression in immune cells. Nature Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- Holodick NE, Vizconde T, Hopkins TJ, Rothstein TL. Age-related decline in natural IgM function: diversification and selection of the B-1a cell pool with age. J. Immunol. 2016;196:4348–4357. doi: 10.4049/jimmunol.1600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodick NE, Rothstein TL. B cells in the aging immune system: time to consider B-1 cells. Ann NY Acad Sci. 2015;1362:176–187. doi: 10.1111/nyas.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Rozzo SJ, Cambier JC. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B cell repertoire. J Immunol. 2002a;168:5014–5023. doi: 10.4049/jimmunol.168.10.5014. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Owen K, Witte PL. Aging and developmental transitions in the B cell lineage. Int Immunol. 2002b;14:1313–1323. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Yoshikawa S, Minegishi Y, Karasuyama H. Pre-B cell receptor assesses the quality of IgH chains and tunes the pre-B cell repertoire by delivering differential signals. J Immunol. 2006;177:2242–2249. doi: 10.4049/jimmunol.177.4.2242. [DOI] [PubMed] [Google Scholar]
- Kee BL, Quong MW, Murre C. E2A proteins: essential regulators at multiple stages of B-cell development. Immunol Rev. 2000;175:138–149. [PubMed] [Google Scholar]
- Keenan RA, De Riva A, Corleis B, Hepburn L, Licence S, Winkler TH, Mårtensson IL. Censoring of autoreactive B cell development by the pre-B cell receptor. Science. 2008;321:696–699. doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- Kennedy DE, Knight KL. Inhibition of B lymphopoiesis by adipocytes and IL-1 producing myeloid-derived suppressor cells. J. Immunol. 2015;195:2666–2674. doi: 10.4049/jimmunol.1500957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DE, Knight KL. Inflammatory changes in bone marrow microenvironment associated with declining B lymphopoiesis. J Immunol. 2017;198:3471–3479. doi: 10.4049/jimmunol.1601643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren Z, Naor S, Nussbaum S, Golan K, Itkin T, Sasaki Y, Schmidt-Supprian M, Lapidot T, Melamed D. B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood. 2011a;117:3104–3112. doi: 10.1182/blood-2010-09-307983. [DOI] [PubMed] [Google Scholar]
- Keren Z, Averbuch D, Shahaf G, Zisman-Rozen S, Golan K, Itkin T, Lapidot T, Mehr R, Melamed D. Chronic B cell deficiency from birth prevents age-related alterations in the B lineage. J Immunol. 2011b;187:2140–2147. doi: 10.4049/jimmunol.1100999. [DOI] [PubMed] [Google Scholar]
- Khomtchouck K, Alter S, Ratliff M, Blomberg BB, Riley RL. In old BALB/c mice, bone marrow pre-B cell and surrogate light chain reduction is associated with increased B cell reactivity to phosphorylcholine, but reduced T15 idiotype dominance. Mech Ageing Dev. 2017;162:53–62. doi: 10.1016/j.mad.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM, Van der Put E, Blomberg BB, Riley RL. Accelerated Notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased ERK MAPK activation. J Immunol. 2007;178:3521–3529. doi: 10.4049/jimmunol.178.6.3521. [DOI] [PubMed] [Google Scholar]
- King AM, Keating P, Prabhu A, Blomberg BB, Riley RL. NK cells in the CD19- B220+ bone marrow fraction are increased in senescence and reduce E2A and surrogate light chain proteins in B cell precursors. Mech Ageing Dev. 2009;130:384–392. doi: 10.1016/j.mad.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirman I, Zhao K, Wang Y, Szabo P, Telford W, Weksler ME. Increased apoptosis of bone marrow pre-B cells in old mice associated with their low number. Int. Immunol. 1998;10:1385–1392. doi: 10.1093/intimm/10.9.1385. [DOI] [PubMed] [Google Scholar]
- Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol. 1999;162:3342–3349. [PubMed] [Google Scholar]
- Labrie JE, Sah A, Allman D, Cancro M, Gerstein R. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescale C, Dias S, Maes J, Cumano A, Szabo P, Charron D, Weksler ME, Dosquet C, Vieira P, Goodhardt M. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging Cell. 2010;9:410–419. doi: 10.1111/j.1474-9726.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J. Immunol. 1998;161:6038–6045. [PubMed] [Google Scholar]
- Mårtensson IL, Keenan RA, Licence S. The pre-B-cell receptor. Curr. Opin. Immunol. 2007;19:137–142. doi: 10.1016/j.coi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Caenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- Melchers F. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nat. Rev. Immunol. 2005;5:578–584. doi: 10.1038/nri1649. [DOI] [PubMed] [Google Scholar]
- Mi QS, Zhou L, Schulze DH, Fischer RT, Lustig A, Rezanka LJ, Donovan DM, Longo DL, Kenny JJ. Highly reduced protection against Streptococcus pneumonia after deletion of a single heavy chain gene in mouse. Proc Natl Acad Sci U S A. 2000;97:6031–6036. doi: 10.1073/pnas.110039497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J Immunol. 2006;176:1007–1012. doi: 10.4049/jimmunol.176.2.1007. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naradikian MS, Hao Y, Cancro MP. Age-associated B cells: key mediators of both protective and autoreactive humoral responses. Immunol Rev. 2016;269:118–129. doi: 10.1111/imr.12380. [DOI] [PubMed] [Google Scholar]
- Nicoletti C, Yang X, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150:543–549. [PubMed] [Google Scholar]
- Ohnishi K, Shimizu T, Karasuyama H, Melchers F. The identification of a nonclassical cadherin expressed during B cell development and its interaction with surrogate light chain. J Biol Chem. 2000;275:31134–31144. doi: 10.1074/jbc.M005901200. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Melchers F, Shimizu T. Lymphocyte-expressed BILL cadherin/cadherin-17 contributes to the development of B cells at two stages. Eur J Immunol. 2005;35:957–963. doi: 10.1002/eji.200425853. [DOI] [PubMed] [Google Scholar]
- Pang WW, Schrier SL, Weissman IL. Age-associated changes in human hematopoietic stem cells. Semin. Hematol. 2017;54:39–42. doi: 10.1053/j.seminhematol.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Perlmutter RM, Crews ST, Douglas R, Sorensen G, Johnson N, Nivera N, Gearhart PJ, Hood L. The generation of diversity in phosphorylcholine binding antibodies. Adv Immunol. 1984;35:1–37. doi: 10.1016/s0065-2776(08)60572-6. [DOI] [PubMed] [Google Scholar]
- Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors. Aging Cell. 2013;12:303–311. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff M, Alter S, McAvoy K, Frasca D, Wright JA, Zinkel SS, Khan WN, Blomberg BB, Riley RL. In aged mice, low surrogate light chain promotes pro-B cell apoptotic resistance, compromises the preBCR checkpoint, and favors generation of autoreactive, phosphorylcholine-specific B cells. Aging Cell. 2015;14:382–390. doi: 10.1111/acel.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley SC, Froscher BG, Linton PJ, Zharhary D, Marcu K, Klinman NR. Altered VH gene segment utilization in the response to phosphorylcholine by aged mice. J Immunol. 1989;143:3798–3805. [PubMed] [Google Scholar]
- Riley RL, Kruger MG, Elia J. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin Immunol Immunopathol. 1991;59:301–313. doi: 10.1016/0090-1229(91)90026-7. [DOI] [PubMed] [Google Scholar]
- Riley RL. Impaired B lymphopoiesis in old age: a role for inflammatory B cells? Immunol Res. 2013;57:361–369. doi: 10.1007/s12026-013-8444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RL, Khomtchouk K, Blomberg BB. Age-associated B cells (ABC) inhibit B lymphopoiesis and alter antibody repertoires in old age. Cell Immunol. 2017;321:61–67. doi: 10.1016/j.cellimm.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, Ford ML, Tobias JW, Cancro MP, Gearhart PJ. Age-associated B cells express a diverse repertoire of VH and Vκ genes with somatic hypermutation. J. Immunol. 2017;198:1921–1927. doi: 10.4049/jimmunol.1601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JL, Diaz A, Riley RL, Cancro MP, Frasca D. A comparative review of aging and B cell function in mice and humans. Curr Opin Immunol. 2013;25:504–510. doi: 10.1016/j.coi.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood EM, Blomberg BB, Xu W, Warner CA, Riley RL. Senescent BALB/c mice exhibit decreased expression of lambda5 surrogate light chains and reduced development within the pre-B cell compartment. J. Immunol. 1998;161:4472–4475. [PubMed] [Google Scholar]
- Sherwood EM, Xu W, King AM, Blomberg BB, Riley RL. The reduced expression of surrogate light chains in B cell precursors from senescent BALB/c mice is associated with decreased E2A proteins. Mech Ageing Dev. 2000;118:45–59. doi: 10.1016/s0047-6374(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Sherwood EM, Xu W, Riley RL. B cell precursors in senescent mice exhibit decreased recruitment into proliferative compartments and altered expression of Bcl-2 family members. Mech Ageing Dev. 2003;124:147–153. doi: 10.1016/s0047-6374(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Signer RA, Montecino-Rodriguez E, Witte ON, Dorshkind K. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 2008;22:3115–3120. doi: 10.1101/gad.1715808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigvardsson M, O'Riordan M, Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25–36. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- Stephan RP, Sanders VM, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: declilne in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood. 1998;91:75–88. [PubMed] [Google Scholar]
- ten Boekel E, Melchers F, Rolink AG. Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- Van der Put E, Sherwood EM, Blomberg BB, Riley RL. Aged mice exhibit distinct B cell precursor phenotypes differing in activation, proliferation and apoptosis. Exp Gerontol. 2003;38:1137–1147. doi: 10.1016/j.exger.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Van der Put E, Frasca D, King AM, Blomberg BB, Riley RL. Decreased E47 in senescent B cell precursors is stage specific and regulated posttranslationally by protein turnover. J Immunol. 2004;173:818–827. doi: 10.4049/jimmunol.173.2.818. [DOI] [PubMed] [Google Scholar]
- Van Zant G, Liang Y. Concise review: hematopoietic stem cell aging. Stem Cells Transl Med. 2012;1:651–657. doi: 10.5966/sctm.2012-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettermann C, Jäck HM. The pre-B cell receptor: turning autoreactivity into self-defense. Trends Immunol. 2010;31:176–183. doi: 10.1016/j.it.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183:959–970. doi: 10.1084/jem.183.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Sanjo H, Pagès G, Kawano Y, Karasuyama H, Pouysségur J, Ogata M, Kurosaki T. ERK kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Zharhary D, Klinman NR. A selective increase in the generation of phosphorylcholine-specific B cells associated with aging. J Immunol. 1986;136:368–370. [PubMed] [Google Scholar]
- Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol. Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]