Abstract
BACKGROUND
Clostridium difficile infection (CDI) presents a substantial economic burden and is associated with significant morbidity. While multiple treatment strategies have been evaluated, a cost-effective management strategy remains unclear.
OBJECTIVE
We conducted a systematic review to assess cost-effectiveness analyses of CDI treatment and to summarize key issues for clinicians and policy makers to consider.
METHODS
We searched PubMed and 5 other databases from inception to August 2016. These searches were not limited by study design or language of publication. Two reviewers independently screened the literature, abstracted data, and assessed methodological quality using the Drummond and Jefferson checklist. We extracted data on study characteristics, type of CDI, treatment characteristics, and model structure and inputs.
RESULTS
We included 14 studies, and 13 of these were from high-income countries. More than 90% of these studies were deemed moderate-to-high or high quality. Overall, 6 studies used a decision-tree model and 7 studies used a Markov model. Cost of therapy, time horizon, treatment cure rates, and recurrence rates were common influential factors in the study results. For initial CDI, fidaxomicin was a more cost-effective therapy than metronidazole or vancomycin in 2 of 3 studies. For severe initial CDI, 2 of 3 studies found fidaxomicin to be the most cost-effective therapy. For recurrent CDI, fidaxomicin was cost-effective in 3 of 5 studies, while fecal microbiota transplantation (FMT) by colonoscopy was consistently cost-effective in 4 of 4 studies.
CONCLUSIONS
The cost-effectiveness of fidaxomicin compared with other pharmacologic therapies was not definitive for either initial or recurrent CDI. Despite its high cost, FMT by colonoscopy may be a cost-effective therapy for recurrent CDI. A consensus on model design and assumptions are necessary for future comparison of CDI treatment.
Clostridium difficile infection (CDI) is one of the most common healthcare-associated infections in North America and Europe.1 In 2011, the estimated incidence of CDI in the United States was approximately 453,000.2 The management of CDI remains complicated because of epidemic strains (BI/NAP1/027) introduced in 2005 and because disease severity varies.3,4 In addition, patients often have multiple and frequent recurrences,5 which exacerbate the disease burden and increase medical costs. The most common risk factors for CDI recurrence include age ≥ 65 years, severe underlying comorbidities, and concomitant use of antibiotics.6,7 Clostridium difficile infection continues to impose a significant economic burden on the US healthcare system, estimated to be more than $5.4 billion (2014 US dollars).8
The current guidelines for CDI management recommend either metronidazole, vancomycin, fidaxomicin, or fecal microbiota transplantation (FMT), depending on disease severity and the presence and number of recurrences.3,9–11 Current treatment choices and available algorithms make it difficult for physicians to tailor individualized therapies for patients. While newer drugs and therapies may be more effective, they are also more expensive. In the past few years, several cost-effectiveness analyses of different CDI treatment strategies have been conducted to support evidence-based decision making,12–17 but the results were mixed. A previous review summarized the economics of CDI treatments, but it did not include study quality assessments and based recommendations on partial costing or comparative effectiveness studies.18 Therefore, the aim of this systematic review was to critically assess the available literature on economic evaluations of various treatment modalities for initial and recurrent CDI. Based on model comparison, we summarized the findings about treatment modalities and key issues for clinicians to consider when treating patients with CDI, to inform health policy makers, and to identify important areas for future cost-effectiveness research.
METHODS
We conducted a systematic review following the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) reporting guideline19 and a measurement tool for the Assessment of Multiple Systematic Reviews (AMSTAR) standard for quality of execution.20
Search Strategy
Studies were included if they (1) were original analyses; (2) were full cost-effectiveness analysis (CEA), cost-utility analysis (CUA), cost-benefit analysis (CBA), or a combination of CEA-CUA or CEA-CBA; and (3) examined treatment modalities that were approved for patient use. Studies were excluded if they (1) did not estimate cost per unit of health outcomes; (2) only addressed CDI diagnostic tests, prevention strategies, and hypothetical or under-investigation treatments; or (3) were an editorial, comment, review, letter to the editor, or conference abstract. In case of multiple publications using the same cost-effectiveness model and data, the more recent and comprehensive study was included. All studies using similar models for different treatments, populations, or types of CDI were included.
Independently, 2 investigators (P.L. and V.T.N.) identified relevant articles by searching PubMed/MEDLINE, Cochrane Library, Web of Science, EMBASE, and Scopus databases from inception through August 2016. We also searched the British National Health Service (NHS) Economic Evaluation database and the reference lists of included studies. The search terms were “Clostridium difficile,” “C. difficile,” “economic,” “economic evaluation,” “cost,” “cost-effectiveness,” “cost-utility,” and “cost-benefit.” The full PubMed search strategy is available as supplementary material. After reviewing the study title and abstract, P.L. and V.T.N. selected articles and independently reviewed the full text to determine inclusion. All disagreements were resolved through discussion with the third investigator (A.D.).
Data Extraction
Independently, 2 investigators (P.L. and V.T.N.) extracted relevant data using a uniform data extraction tool (available as Supplementary Table 1). We extracted information on study characteristics (authors, publication year, country, funding sources), type of CDI (initial, recurrent), treatment characteristics (types, medication dose and administration route, and mode of delivery of FMT), model structure (design, population, perspective, time horizon, discount rate), epidemiological data related to CDI and treatment effectiveness, types of costs and values, cost year and currency, outcome measures, the incremental cost-effectiveness ratio, decision threshold, and sensitivity analyses. We summarized data by type of CDI. Cost-effectiveness findings were additionally stratified by funding source.
Quality Assessment
We assessed study quality using the British Medical Journal’s Drummond and Jefferson checklist.21 We adapted the checklist to include 3 additional items: generalizability, source of funding, and conflict of interest based on the Consolidated Health Economic Evaluation Reporting Standards checklist.22 Each item in the checklist has a ‘Yes’, ‘No’ or ‘Not applicable’ (NA) option and was scored 1, 0, or no score, respectively (available as Supplementary Table 2). The overall quality score was then calculated as the percentage of ‘Yes’ responses out of the total criteria applicable to each individual study. For example, if a paper had 27 Yes, 7 No, and 4 NA, the quality score was calculated as 71% (27 of 34). Based on its quality score, each study was ranked as either low quality (<50%), moderate quality (50%–64%), moderate-to-high quality (65%–80%), or high quality (>80%).
Conversion of Outcomes to a Standard Metric
For US-based studies, we converted reported costs and incremental cost-effectiveness ratios into 2016 US dollars, using the medical care component of the Consumer Price Index. For other countries, we inflated data to 2016 using the country-specific Consumer Price Index23 and converted the result to US dollars using relevant exchange rates.
RESULTS
Search Results
We retrieved 556 unique citations and screened all titles and abstracts, as well as full texts of 21 potentially relevant study reports. We excluded 7 studies after a full text review because they did not consider both cost and health outcomes, conducted burden-of-illness analyses, or did not report data with their analytical frameworks. A total of 14 eligible studies remained (Figure 1). No additional studies were found after we reviewed references of included studies and searched the NHS database.
FIGURE 1.
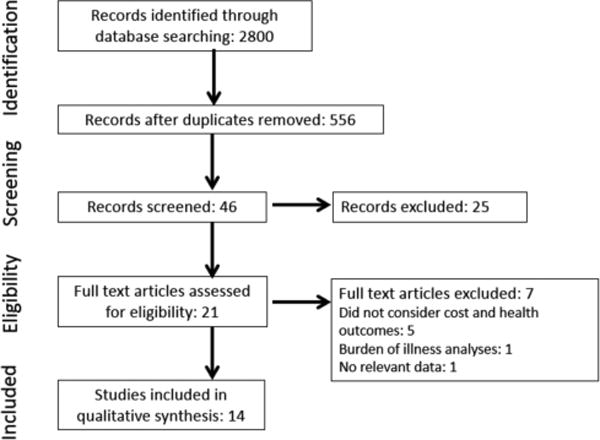
Flow diagram summarizing evidence search and selection
Study Characteristics
Of the 14 studies reviewed, 13 were conducted in high-income countries within the past 5 years (Table 1). Federal or local governments sponsored 6 studies, and the pharmaceutical industry funded 5 studies. Furthermore, 7 studies evaluated treatments for initial CDI, 2 of which focused solely on severe infection. In addition, 4 studies considered treatments for recurrent CDI, while 2 others investigated both initial and recurrent CDI. The final study evaluated C. difficile–induced colitis unresponsive to metronidazole. All available treatment modalities approved for patient use were evaluated for initial and recurrent CDI, irrespective of guideline recommendation. Notably, 1 study examined FMT use for initial CDI,15 and 2 others investigated metronidazole use for recurrent CDI.13,14 Fidaxomicin was evaluated in 10 studies, while vancomycin was examined in all studies.
TABLE 1.
Characteristics of Included Economic Evaluations
| Author (Year) Country Funding |
Model Design
|
||||||
|---|---|---|---|---|---|---|---|
| Comparisons | Population | Model Type No. of CDI recurrences | Perspective | Time Horizon Discount Rate | Decision Threshold, US$/QALY | Health Outcomes | |
| Initial CDI (no specific disease severity) | |||||||
| Gidengil31 (2014) United States Industry |
• Metronidazole, then metronidazole for 1st recurrence • Metronidazole, then vancomycin for 1st recurrence • Vancomycin, then vancomycin for 1st recurrence • Fidaxomicin, then fidaxomicin for 1st recurrence |
Adult inpatients | • Markov (no cycle length) • 0 |
Healthcare provider/health system | • 1 y • None |
NR | • No. of CDI recurrences • No. of persistent CDI cases requiring tx change • No. of readmissions • No. of CDI-related deaths • No. of VRE colonization cases • No. of VRE infections |
| Rubio-Terres24 (2015) Spain Industry |
• Fidaxomicin • Vancomycin |
Pts with cancer, concomitant antibiotic tx, renal impairment | • Markov (10-d cycle) • 1 |
Healthcare provider/health system | • 1 y • None |
$31,800 (€30,000) | QALY |
| Stranges27 (2013) United States None |
• Fidaxomicin • Vancomycin |
Mean age, 59.9y | • Decision tree • ≥ 2 |
Third-party payer | • 23 y • 3% |
$100,000 | QALY |
| Varier15 (2014) United States NGO |
• Metronidazole • Vancomycin • FMT colonoscopy |
Adult outpatients | • Decision tree • ≥2 |
Third-party payer | • 90 d • None |
$100 000 | QALY |
| Watt17 (2016) Germany Industry |
• Fidaxomicin • Vancomycin |
Pts with severe initial CDI, recurrent CDI, concomitant antibiotic tx, age ≥65 y, cancer, renal impairment | • Markov (10-d cycle) • ≥2 |
Healthcare provider/health system | • 1 year • None |
$63 000 (€50,000) | • QALY • No. of bed days saved • No. of CDI recurrences avoided |
| Initial CDI (severe) | |||||||
| Perras30 (2011) Canada Public |
• Metronidazole • Vancomycin • Fidaxomicin |
Pts with severe CDI | • Decision tree • 1 |
Healthcare provider/health system | • 50 d • None |
NR | Clinical cure |
| Wagner29 (2014) Canada Industry |
• Vancomycin | Pts with severe CDI | • Decision tree • 1 |
Healthcare provider/health system | • 2 months • None |
NR | No. of CDI recurrences avoided |
| Recurrent CDI | |||||||
| Konijeti13 (2014) United States Public |
• Metronidazole • Vancomycin • Fidaxomicin • FMT colonoscopy • FMT duodenal infusion • FMT enema |
Pts age ≥65 y | • Decision tree • ≥2 |
Societal | • 1 y • None |
$50,000 | QALY |
| Lapointe-Shaw14 (2011) Canada Public |
• Metronidazole and vancomycin for subsequent recurrences • Vancomycin and vancomycin for subsequent recurrences • Fidaxomicin and vancomycin for subsequent recurrences • FMT by enema and repeated therapy using a different donor for recurrence • FMT by nasogastric tube and repeated therapy using a different donor for recurrence • FMT by colonoscopy and repeated therapy using a different donor for recurrence |
Community-dwelling persons, age ≥ 70y | • Markov (6-week cycle) • ≥2 |
Healthcare provider/health system | • 18 weeks (CDI-related costs and complications); lifetime (QALYs) • 5% (health benefits); none (costs) |
$38,000 (CAD 50,000) |
QALY |
| Merlo26 (2016) Australia None |
• Vancomycin • Nasoduodenal FMT • Colorectal FMT |
Age ≥ 65y | • Markov (10-d cycle) • ≥2 |
NR | • Lifetime • 5% |
NR | • QALY • No. of life years saved |
| Varier16 (2015) United States Public |
• Vancomycin • FMT colonoscopy |
Outpatient adults | • Decision tree • 1 |
Third-party payer | • 90d • None |
NR | QALY |
| Initial and Recurrert CDI | |||||||
| Bartsch12 (2013) United States Public |
• Metronidazole (nonsevere) and vancomycin (severe) • Fidaxomicin • Fidaxomicin with strain typing |
Adults, age ≥18 y | • Microsimulation • 1 |
Third-party payer | • None • None (3% for cost conversion) |
$50,000 | QALY |
| Nathwani25 (2014) Scotland Industry |
• Fidaxomicin • Vancomycin |
Adults, age ≥18 y | • Markov (10-d cycle) • ≥2 |
Governmental | • 1 year • None |
$25,400 (£20,000) $38,100 (£30,000) |
QALY |
| Other | |||||||
| Markovic28 (2014) Serbia Public |
• Fidaxomicin • Vancomycin |
NR | • Markov (15-d cycle) • ≥2 |
Third-party payer | • 90 d • None |
$458,440/life saved (RSD 53,307,040/life saved) |
• No. of lives saved • No. of subtotal colectomies avoided |
NOTE. CDI, Clostridium difficile infection; d, day; FMT, fecal microbiota transplantation; NGO, nongovernmental organization; NR, not reported or not available; pt, patients; QALY, quality-adjusted life years; tx, treatment; VRE, vancomycin-resistant enterococci, venous thromboembolism; y, years.
Model Design
Overall, 13 studies employed either a Markov or decision-tree model. The common Markov cycle length was 10 days (Table 1). Also, 2 studies used the same model to evaluate CDI treatments in different patient populations.24,25 The analytical perspective was that of the healthcare provider/health system or third-party payer for most studies (k = 12). Discounting was not applied for most studies because of the short time horizon. Furthermore, 2 studies that followed patients throughout their lives used appropriate discounting rates,14,26 but 1 of these studies had discordant time frames for cost and quality-adjusted life years (QALY; 18 weeks for costs vs lifetime for QALY).14 Comorbidities (eg, cancer, concomitant antibiotics, renal impairment) were accounted for in 3 studies.17,24,27
Study Quality
Most of the studies were deemed moderate-to-high or high quality (k = 13). The mean and median quality scores were ~ 80% (data not shown but available upon request). Most studies provided detailed information on study design and population. In 1 study, the analytical perspective was societal, but indirect costs were not included.13 Another study did not specify the perspective,26 and 2 studies lacked information on cost year.28,29
Health Outcomes
Quality-adjusted life years was the most common health outcome reported (Table 1). Other outcome measures were CDI cases/recurrences avoided, clinical cure, life years, or bed days saved. Of the 10 studies that estimated QALY, 8 specified a cost-effectiveness decision threshold, but none conducted primary data collection for utility measurement. Because of the lack of CDI-specific utility weights,14–17,27 alternative weights for noninfectious diarrhea or for grade 3–4 diarrhea associated with chemotherapy were used. Utility weights generally varied substantially across studies; for example, utility for CDI was between 0.319 and 0.880.14,17,24–27
Treatment Effectiveness
Table 2 shows how reviewed studies differed on treatment effectiveness across CDI episode and severity. Studies used a range of probabilities (0.65–0.84) as the metronidazole cure rate. Perras et al30 used the lowest value (0.65) based on the success rate of metronidazole for initial severe CDI reported in a conference proceeding.30 In contrast, Varier et al15 used a higher cure rate of 0.80 based on 1997 American College of Gastroenterology guidelines. Bartsch et al12 derived the highest rate from a randomized clinical trial (RCT) and assumed it to be the same for both initial and recurrent CDI.
TABLE 2.
Effectiveness, Costs, Incremental Cost-Effectiveness Ratio, and Sensitivity Analyses of Included Economic Evaluations
| Author (Year) Country Funding |
Effectiveness
|
Cost Estimate
|
|||||
|---|---|---|---|---|---|---|---|
| Probability of Cure | Probability of Recurrence | Types Source Original Cost Year |
Treatment, 2016 US$ | Incremental Cost-Effectiveness Ratio, 2016 US$/QALY (Original Values) | Scenario Analysis, 2016 US$/QALY (Original Values) | Influential Variables | |
| Initial CDI (no specific severity) | |||||||
| Gidengil31 (2014) United States Industrya |
MET: 0.735 VAN: 0.817 FID: 0.841 |
MET Initial episode: 0.267 1st recurrence: 0.330 ≥ 2 recurrences: 0.308 VAN Initial episode: 0.253 1st recurrence: 0.355 ≥ 2 recurrences: 0.308 FID Initial episode: 0.154 1st recurrence: 0.197 ≥ 2 recurrences: 0.308 |
• Hospitalization, tx, lab test, outpatient visit • Manufacturer cost, expert interviews, literature • 2010 |
MET: $27 VAN: $1,255 FID: $3,316 |
NR | NR | • Recurrence probability for initial episode with MET and VAN tx • All VRE clinically related probabilities • Recurrence probability (beyond the 1st 2) with VAN tx • Cost of an invasive VRE infection • Cost of FID • PSA: yes |
| Rubio-Terres24 (2015) Spain Industry |
• Hospitalization, tx, outpt visit • Spanish public healthcare prices, literature • 2013 |
VAN: $40 FID: $1,577 |
FID dominant | NR | • Duration of excess stay attributable to CDI, initial CDI or recurrent CDI • PSA: yes |
||
| Stranges27 (2013) USA None |
VAN Inpatient: 0.781 Outpatient: 0.975 Mild-to-moderate CDI: 0.839 Severe CDI: 0.886 Concomitant antimicrobials: 0.794 NAP1/BI/027: 0.807 FID Inpatient: 0.814 Outpatient: 0.975 Mild-to-moderate CDI: 0.920 Severe CDI: 0.821 Concomitant antimicrobials: 0.900 NAP1/BI/027: 0.787 |
VAN Inpatient: 0.274 Outpatient: 0.227 With previous CDI episode: 0.312 Mild-to-moderate CDI: 0.244 Severe CDI: 0.266 Concomitant antimicrobials: 0.292 NAP1/Bl/027: 0.209 FID Inpatient: 0.179 Outpatient: 0.128 With previous CDI episode: 0.214 Mild-to-moderate CDI: 0.168 Severe CDI: 0.130 Concomitant antimicrobials: 0.169 NAP1/Bl/027: 0.271 |
• Hospitalization, tx, lab test • HCUP • 2011 |
VAN: $1,335 FID: $3,218 |
FID: $77,661 ($67,576) |
ICER of FID • Severe CDI: $405,676 ($352,994) • Mild-to-moderate CDI: $36,799 ($32,020) • Initial tx as outpatient: $44,328 ($38,571) • Initial tx as inpatient: $86,321 ($75,111) • NAP1/B1/027 strain typing: FID dominated • Concomitant antimicrobials: $1,709 ($1,487) |
• Recurrence rate of FID • Recurrence rate of VAN • First episode inpatient FID cure rate • Rate of hospitalization • Probability of CDI mortality • PSA: yes |
| Varier15 (2014) United States NGOc |
MET: 0.800 VAN: 0.900 FMT colonoscopy: 0.910 |
MET: 0.168 VAN: 0.084 FMT colonoscopy: 0.076 |
• Hospitalization, cost of tx • Medicare, literature • 2011 |
FMT: $1,248 MET: $66 VAN: $1,548 |
FMT colonoscopy: $143,614 ($124,964) |
NR | • Cure rate of MET • Cost of FMT • Cost of MET • PSA: yes |
| Watt17 (2016) Germany Industry |
VAN ≥ 1 recurrences: 0.889 Severe CDI: 0.826 Concomitant antibiotics: 0.755 Age ≥65 y: 0.845 Cancer: 0.740 Renal impairment: 0.760 FID ≥ 1 recurrence: 0.898 Severe CDI: 0.800 Concomitant antibiotics: 0.843 Age ≥65 y: 0.848 Cancer: 0.851 Renal impairment: 0.731 |
VAN 9 ≥ 1 recurrence: 0.325 Severe CDI: 0.283 Concomitant antibiotics: 0.255 Age ≥65 y: 0.293 Cancer: 0.296 Renal impairment: 0.316 FID ≥ 1 recurrence: 0.203 Severe CDI: 0.114 Concomitant antibiotics: 0.174 Age ≥65 y: 0.161 Cancer: 0.135 Renal impairment: 0.147 |
• Hospitalization, tx • German drug tariff • 2014 |
VAN: $69 FID: $1,564 |
ICER of FID • Pts with ≥1 recurrence: $49,482/QALY (€43,900) • Pts with severe CDI: $39,225/QALY (€34,800) • Pts with concomitant antibiotics: $34,603/QALY (€30,700) • Age ≥65 y $50,159/QALY (€44,500) • Pts with cancer: dominant • Pts with renal impairment: $30,320/QALY (€26,900) |
NR | • Recurrence rate • Clinical cure rate • CDI-attributable mortality rate • PSA: no |
| Initial CDI (severe) | |||||||
| Perras30 (2011) Canada Publicd |
MET: 0.649 VAN: 0.849 |
MET: 0.150 VAN: 0.150 |
• Hospitalization, tx, lab test, outpatient visit • Provincial drug formularies • 2010 |
MET: $3.10 VAN: $279 |
NR | NR | • Infection populations (with or without NAP1 strain), efficacy rates in initial therapy with MET • Cost of VAN • Complication rate among tx failures • PSA: yes |
| Wagner29 (2014) Canada Industrye |
VAN Severe CDI: 0.813 With recurrence: 0.926 NAP1/BI/027: 0.813 Non-NAPI/BI/027: 0.912 FID Severe CDI: 0.813 With recurrence: 0.926 NAP1/BI/027: 0.813 Non-NAP1/BI/027: 0.912 |
VAN Severe CDI: 0.283 With recurrence: 0.323 NAP1/BI/027: 0.282 Non-NAP1/BI/027: 0.278 FID Severe CDI: 0.114 With recurrence: 0.203 NAP1/BI/027: 0.248 Non-NAP1/BI/027: 0.097 |
• Hospitalization, tx, outpt visit • Canadian Agency for Drugs and Technologies in Health, Ontario Case Costing Initiative, Ontario Schedule for Physician Services • NR |
NR | NR | NR | • Recurrence rate of FID • Duration of tx, 10-14 d • PSA: no |
| Recurrent CDI | |||||||
| Konijeti13 (2014) United States Public |
MET: 0.710 VAN: 0.916 FID: 0.937 FMT colonoscopy: 0.945 FMT duodenal infusion: 0.813 FMT enema: 0.815 |
MET: 0.421 VAN: 0.355 FID: 0.197 FMT colonoscopy: 0.091 FMT duodenal infusion: 0.063 FMT enema: 0.091 |
• Hospitalization, tx, outpt visit • Clinical diagnostic laboratory fee schedule from Centers for Medicare and Medicaid Services, literature • 2012 |
MET: $25 VAN: $754 FID: $3,104 FMT colonoscopy: $2,495 FMT duodenal infusion: $2,386 FMT enema: $2,048 |
FMT colonoscopy: $18,865/QALY ($17,016) |
FMT duodenal available • FMT duodenal: $107,927/QALY ($97 352); • FID: $109,136/QALY ($98,443) FMT enema available • FID: $110.709/QALY ($99,862) All FMT routes available • FMT colonoscopy: $18,865/QALY ($17,016) No FMT available • FID: $204 012/QALY ($184,023) |
• Cure and recurrence rate of outpt oral VAN, FMT colonoscopy • Costs of colonoscopy, FID, outpt oral VAN • Probability of severe CDI if tx failure • PSA: no |
| Lapointe-Shaw14 (2011) Canada Publicf |
MET: 0.776 | MET: 0.400 VAN oral: 0.517 VAN pulse/taper: 0.178 FID: 0.321 FMT colonoscopy: 0.078 FMT duodenal infusion: 0.233 FMT enema: 0.185 |
• Hospitalization, tx, lab test, outpt visit, capital cost (equipment) • University Health Network outpt database, Ontario Schedule of Benefits • 2014 |
MET: $31.20 VAN: $278 FID: $1,923 FMT enema: $6,504 FMT nasogastric: $1,040 FMT colonoscopy: $4,083 |
FMT colonoscopy dominant | • Age ≥10 y: FMT colonoscopy dominant • FID off-patent: FMT colonoscopy dominant No FMT available • FID $20 757/QALY (CAD 25,968) • No FMT colonoscopy: FMT by enema $1,365/QALY (CAD 1,708) 2 recurrences considered • FMT colonoscopy: $411/QALY (CAD514) |
• Probability of recurrence following fecal transplantation by enema • PSA: yes |
| Merlo26 (2016) Australia Noneh,g |
VAN: 0.308 Colorectal FMT: 0.939 Nasoduodenal FMT: 0.939 |
• Hospitalization, tx, lab test • National databases, market prices, pharmaceutical benefits schedule, Queensland health wage rate • 2015 |
VAN: $494 Colorectal FMT: $1,688 Nasoduodenal FMT: $1,637 |
Nasoduodenal FMT and colorectal FMT dominant | NR | • None • PSA: yes |
|
| Varier16 (2015) United States Publicc |
VAN taper: 0.690 FMT colonoscopy: 0.910 |
VAN taper: 0.260 FMT colonoscopy: 0.076 |
• Tx, lab test (for recurrent CDI), hospitalization (only for tx of fulminant colitis) • Medicare • 2011 |
VAN taper: $2,378 FMT colonoscopy: $1,248 |
FMT colonoscopy dominant | NR | • Cure probability of FMT colonoscopy, cost of FMT colonoscopy, cure probability of VAN • PSA: yes |
| Initial and recurrent CDI | |||||||
| Bartsch12 (2013) United States Public |
MET: 0.835 VAN NAP1/BI/027: 0.820 Non-NAPI/BI/027: 0.897 FID NAP1/BI/027: 0.859 Non-NAP1/BI/027: 0.926 |
MET: 0.136 VAN NAP1/BI/027: 0.295 Non-NAP1/BI/027: 0.278 FID NAP1/BI/027: 0.247 Non-NAP1/BI/027: 0.098 |
• Hospitalization, tx, lab test • HCUP, Redbook • 2012 |
MET: $65 VAN: $1,144 FID: $3,725 |
NR, “No FID" was best | NR | • Proportion of C. diff infections caused by NAP1 strain versus other strains • Cost of FID • Tx guidelines recommends VAN as the first-line tx • PSA: yes |
| Nathwani25 (2014) Scotland Industryc,e |
VAN Severe CDI: 0.853 Recurrence: 0.889 FID Severe CDI: 0.853 Recurrence: 0.889 |
VAN Severe CDI: 0.267 Recurrence: 0.325 FID Severe CDI: 0.122 Recurrence: 0.172 |
• Hospitalization, tx, outpatient visit • British National Formulary • 2011 |
VAN: $312 FID: $1,568 |
For severe CDI: FID $27 225/QALY (£16 529) For recurrence CDI: FID dominant |
NR | • OR of experiencing a recurrence with FID in pts who had already experienced >1 recurrence • OR of experiencing a recurrence with FID in pts with severe CDI • OR of having recurrent CDI if treated with FID • Probability of a recurrence being treated in hospital • PSA: yes |
| Other | |||||||
| Markovic28 (2014) Serbia Public |
VAN: 0.652 FID: 0.741 |
VAN: 0.221 FID: 0.130 |
• Hospitalization, tx, lab test, surgery • Literature • NR |
NR | NR | NR | • Cost of FID • PSA: no |
NOTE. C. diff, Clostridium difficile; CDI, Clostridium difficile infection; d, day; FID, fidaxomicin; FMT, fecal microbiota transplantation; HCUP, Healthcare Cost and Utilization Project; ICER, incremental cost-effectiveness ratio; MET, metronidazole; NGO, nongovernmental organization; NR, not reported or not available; outpt, outpatients; OR, odds ratio; PSA, probabilistic sensitivity analysis; pt, patients; QALY, quality-adjusted life-years; tx, treatment; VAN, vancomycin; VRE, vancomycin-resistant enterococci, venous thromboembolism; y, years.
Probabilities of cure estimated from published reports.
Insufficient information to derive the probability of either cure or recurrence from the published report.
Probabilities of recurrence estimated from published reports.
Probabilities of recurrence assumed to be similar for both treatments.
Probabilities of cure assumed to be similar for both treatments.
Probability of cure not listed for other treatments in the report.
Probability of recurrence not available from the published report.
The vancomycin probability of cure for 10-d cycles. The FMT probability of cure assumed to be the same regardless of delivery modes.
The studies that compared vancomycin and metronidazole generally used higher cure rate estimates for vancomycin, from 0.817 to 0.916.13,15,30,31 These rates were, however, lower than that of fidaxomicin, except for severe CDI (NAP1/BI/027 strain) or patients with renal impairment.17,27 For recurrent CDI, Varier et al used a vancomycin cure rate of 0.69,16 which was lower than the 0.889–0.926 range used in other studies.13,17,29 Furthermore, 2 studies assumed that vancomycin and fidaxomicin were similarly effective,25,29 and in 1 study, both drugs had much lower cure rates for C. difficile–induced colitis.28 The fidaxomicin cure rates for the NAP1/BI/027 strain were considerably different in 2 studies,12,27 whereas the cure rate of FMT was high (0.910–0.945) when delivered via colonoscopy but not other modes.13
Similarly, the probability of CDI recurrence after treatment varied significantly across studies. Recurrence rates after treatment with metronidazole ranged from 0.150 to 0.421 and were higher for recurrent CDI than for initial CDI. The CDI recurrence rate after vancomycin was lower than after metronidazole but higher than after fidaxomicin. While 2 studies modeled vancomycin with a higher recurrence rate for the NAP1/BI/027 strain than fidaxomicin,12,27,29 another study did the opposite.27 The probability of recurrence after FMT via colonoscopy was comparable among studies but differed noticeably for other modes of delivery. Specifically, the recurrence rate of FMT by duodenal infusion or enema was 2–4 times higher in a study than in another, although the same reference source was cited in both.13,14 In some studies, recurrence rates were not stated explicitly.26
Economic Parameters
Costs of CDI therapies and hospitalizations were included in all studies. Costs of laboratory tests were included in most studies, and costs of outpatient visits were included much less often (Table 2). Although excluding costs of treatment-related adverse events would bias results, only 3 studies included such costs.15,16,31 Most studies used official sources for cost estimates, and US studies had higher per-unit costs than studies in other countries. The cost of FMT therapy varied depending on the route of administration and often included associated pretreatment cost of oral vancomycin.
Cost-Effectiveness of CDI treatments
Table 2 summarizes the incremental cost-effectiveness ratios in 2016 US dollars per QALY gained stratified by type of CDI, wherever available. For initial CDI with no specific disease severity, fidaxomicin was cost-effective compared to vancomycin in 2 studies17,27 but not in the study accounting for severity.12 For initial CDI in patients with concomitant antibiotics use, cancer, or renal impairment, 2 studies found fidaxomicin to be cost-effective.17,24 Although FMT has not been recommended for initial CDI, the study that examined the use of colonoscopy-delivered FMT found it not cost-effective.15 Also, 2 studies found fidaxomicin cost-effective for severe initial CDI,17,25 but another concluded differently.27 While many factors might have influenced results, a much higher cure rate of vancomycin (0.886) and the double cost for fidaxomicin,27 compared with the other 2 studies, were notable. For recurrent CDI, studies consistently reported that FMT via colonoscopy was a cost-effective treatment, whereas findings on other FMT delivery routes were inconsistent.13,14,16,26 When FMT was not available, fidaxomicin was a cost-effective option compared to other drugs in 3 studies14,17,25 but not in 2 other studies.12,13
Stratified by funding source, all 5 industry-funded studies examined fidaxomicin, 3 of which concluded that fidaxomicin was either cost-effective or cost saving compared to metronidazole or vancomycin.17,24,25 The remaining 2 studies did not measure QALYs and made no conclusion about its cost-effectiveness.29,31 For studies with other types of or no funding, fidaxomicin was found cost-effective in one study27 but not the other,12 whereas FMT was favored in most of them.13,14,16,26
Sensitivity Analysis
Most studies reported that treatment effectiveness was an important factor in 1-way sensitivity analysis (Table 2). For example, if the cure rate after vancomycin was >95.5%, it would be the preferred treatment for recurrent CDI.13 Cost of therapy was another influential parameter; FMT would no longer be dominant if its cost was > $3,20516 or if the fidaxomicin cost was < $1,359.13 Some other important variables were treatment duration, complication rates, and CDI mortality rate.
Probabilistic sensitivity analysis was conducted in 79% of the studies, but final results were not reported in 2 of them.12,13 Some studies presented a cost-effectiveness acceptability curve, while others reported 95% CI around the mean cost and effectiveness. The probability of being cost-effective at a pre-specified willingness to pay, defined as the maximum amount of dollars spent for an additional QALY gained, was between 60% and 96% for fidaxomicin, depending on CDI severity and population.24,25,27 FMT was either dominant or had a probability of cost-effectiveness between 38% and 87%.14,15
DISCUSSION
Our study is one of the first systematic reviews to critically assess the quality of studies and cost-effectiveness of CDI treatment modalities, and we found substantial differences among the included studies. Because fidaxomicin is a newer drug, it was examined extensively for use in treating initial CDI. Results for fidaxomicin were inconclusive, however, except being cost-effective in some special and/or selective populations. The 3 studies of fidaxomicin for severe, initial CDI treatment had divergent conclusions, as did the 5 studies of fidaxomicin for recurrent CDI. FMT by colonoscopy was cost-effective for recurrent infection, but not for initial CDI. These cost-effectiveness findings did not hold true when FMT was delivered by other routes.
We identified important differences in study design among the included studies. Although QALY has become the most common outcome measure, one-third of the studies reviewed did not estimate QALY. Furthermore, studies accounted for CDI complications differently, and while some included costs of treating adverse events, none accounted for complications such as renal failure, which might bias the results in either direction. Another source of divergence was differences in healthcare resource utilization and costs among different settings. In particular, assumptions about treatment effectiveness contributed significantly to the diverging results. Two randomized controlled trials examined fidaxomicin.32,33 Both trials were conducted by OPT-80-003 Clinical Study Group investigators, and although the times and settings differed, they reported comparable cure and recurrence rates. These studies excluded patients with > 1 CDI occurrence within 3 months before studies started, and only 16% of enrolled patients had 1 previous CDI. Therefore, it is possible that the results applied to patients with initial CDI and not to those with recurrence. To date, there has been no published RCT on fidaxomicin effectiveness in recurrent CDI. Similarly, 2 other RCTs investigated the efficacy and safety of FMT in patients with recurrent but not initial CDI,34,35 and there were no RCTs comparing delivery routes when conducting this systematic review. Therefore, any study that examined fidaxomicin for recurrent CDI or FMT for initial CDI or compared delivery routes for FMT would have assumed their effectiveness or used data sources other than the available RCTs.36–38 Previous studies showed that comorbidities (eg, cancer, inflammatory bowel disease, and surgical burden) were strongly associated with increased risks for development and recurrence of CDI.39–43 However, most included studies did not account for such comorbidities in their models, which potentially biased the results. Lastly, studies modeled various numbers of recurrences following the initial episode, which might be another reason the results differed.
Our study has several limitations. Although we searched a wide range of databases, we may have missed some unpublished studies. In addition, because these studies differed in terms of study design, target population, model structure and input, our conclusions on the cost-effectiveness of CDI treatments were speculative. Finally, because we included industry-sponsored studies, which tend to be published only when results are favorable,44 our synthesis and interpretation of results might be biased toward positive findings.
Our review has highlighted certain areas that could be improved in future CDI cost-effectiveness analyses. While some of the models followed patients in the short term, those examining the long-term impact would present a more comprehensive assessment of interventions. Because there has been no widely accepted decision threshold for cost-effectiveness using effectiveness measures other than QALY, future studies should preferably estimate QALY change to facilitate comparison. The cost-effectiveness of fidaxomicin compared with other pharmacologic therapies was not definitive for either initial or recurrent CDI, and different studies have used different values for its effectiveness. Therefore, future research might include a comprehensive literature review and provide rationale for choosing specific effectiveness values. A wide range for effectiveness and threshold analyses could also help understand the impact of fidaxomicin in various treatment scenarios. More prospective studies are needed to establish the efficacy and safety of fidaxomicin for recurrent CDI. There is also an urgent need for specific CDI utility weights that consider different complications, other comorbidities, or infection/severity stages. Given that a validated instrument for CDI-specific, health-related, quality-of-life assessment is now available,45 future research on utility weights will facilitate a more precise estimate of QALY change across CDI treatments.
In conclusion, CDI is a complex condition with a high recurrence rate, resulting in a significant burden of morbidity and mortality, as well as economic costs. Metronidazole and vancomycin have long been standard CDI treatment, but they are often associated with high rates of recurrence. New medications, such as fidaxomicin, and novel treatment modalities, such as FMT, have opened a new arena in CDI management. Because new treatments often come with a high cost, cost-effectiveness analyses are important to aid clinicians in rational decision making and health policy makers. Our review has identified an important divergence in research findings, especially in cost-effectiveness of fidaxomicin for either initial or recurrent CDI, which arose from discrepancies in model design and methods. Finally, our review informs future research of areas that need improvement and may help policymakers and physicians to critically assess the cost-effectiveness of different CDI treatments.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Financial support: The study received no external funding. Van T. Nghiem was supported by a predoctoral fellowship from the Cancer Education and Career Development Program (NCI/NIH grant no. R25 CA057712) and research funding from the Center for Health Promotion and Prevention Research at the University of Texas School of Public Health. Patricia Dolan Mullen reported research funding from the National Cancer Institute and the Cancer Prevention Research Institute of Texas for research not related to this study. Abhishek Deshpande is supported by a grant from the Agency for Healthcare Research and Quality (AHRQ grant no. K08 HS025026). He has also received research support from 3M, Clorox, Steris for research not related to this study.
Footnotes
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.303.
References
- 1.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Winston LG, McDonald LC, Emerging Infections Program C. difficile Surveillance Team Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369–2370. doi: 10.1056/NEJMc1505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913–1924. doi: 10.1053/j.gastro.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 5.McFarland LV. Alternative treatments for Clostridium difficile disease: what really works? J Med Microbiol. 2005;54:101–111. doi: 10.1099/jmm.0.45753-0. [DOI] [PubMed] [Google Scholar]
- 6.Eyre DW, Walker AS, Wyllie D, et al. Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis. 2012;55(Suppl 2):S77–S87. doi: 10.1093/cid/cis356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(Suppl 6):21–27. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 8.Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303. doi: 10.1186/s12879-016-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 10.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch SM, Umscheid CA, Fishman N, Lee BY. Is fidaxomicin worth the cost? An economic analysis. Clin Infect Dis. 2013;57:555–561. doi: 10.1093/cid/cit346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konijeti GG, Sauk J, Shrime MG, Gupta M, Ananthakrishnan AN. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis. 2014;58:1507–1514. doi: 10.1093/cid/ciu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapointe-Shaw L, Tran KL, Coyte PC, et al. Cost-effectiveness analysis of six strategies to treat recurrent Clostridium difficile infection. PLoS One. 2016;11:e0149521. doi: 10.1371/journal.pone.0149521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varier RU, Biltaji E, Smith KJ, et al. Cost-effectiveness analysis of treatment strategies for initial Clostridium difficile infection. Clin Microbiol Infect. 2014;20:1343–1351. doi: 10.1111/1469-0691.12805. [DOI] [PubMed] [Google Scholar]
- 16.Varier RU, Biltaji E, Smith KJ, et al. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2015;36:438–444. doi: 10.1017/ice.2014.80. [DOI] [PubMed] [Google Scholar]
- 17.Watt M, McCrea C, Johal S, Posnett J, Nazir J. A cost-effectiveness and budget impact analysis of first-line fidaxomicin for patients with Clostridium difficile infection (CDI) in Germany. Infection. 2016;44:599–606. doi: 10.1007/s15010-016-0894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639–650. doi: 10.1007/s40273-014-0161-y. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 20.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313:275–283. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16:e1–e5. doi: 10.1016/j.jval.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Consumer price indices. Organisation for Economic Cooperation and Development (OECD) website. http://stats.oecd.org/index.aspx?datasetcode=mei_prices. Accessed August 25, 2016.
- 24.Rubio-Terres C, Cobo Reinoso J, Grau Cerrato S, et al. Economic assessment of fidaxomicin for the treatment of Clostridium difficile infection (CDI) in special populations (patients with cancer, concomitant antibiotic treatment or renal impairment) in Spain. Eur J Clin Microbiol Infect Dis. 2015;34:2213–2223. doi: 10.1007/s10096-015-2472-0. [DOI] [PubMed] [Google Scholar]
- 25.Nathwani D, Cornely OA, Van Engen AK, Odufowora-Sita O, Retsa P, Odeyemi IA. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother. 2014;69:2901–2912. doi: 10.1093/jac/dku257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merlo G, Graves N, Brain D, Connelly L. Economic evaluation of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection in Australia. J Gastroenterol Hepatol. 2016;31:1927–1932. doi: 10.1111/jgh.13402. [DOI] [PubMed] [Google Scholar]
- 27.Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16:297–304. doi: 10.1016/j.jval.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Markovic V, Kostic M, Ilickovic I, Jankovic SM. Cost-effectiveness comparison of fidaxomicin and vancomycin for treatment of Clostridium difficile infection: a Markov model based on data from a South West Balkan country in socioeconomic transition. Value in Health Regional Issues. 2014;4C:87–94. doi: 10.1016/j.vhri.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Wagner M, Lavoie L, Goetghebeur M. Clinical and economic consequences of vancomycin and fidaxomicin for the treatment of Clostridium difficile infection in Canada. Can J Infect Dis Med Microbiol. 2014;25:87–94. doi: 10.1155/2014/793532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perras C, Tsakonas E, Ndegwa S, Conly J, Valiquette L, Farrah K. Vancomycin or metronidazole for treatment of Clostridium difficile infection: clinical and economic analyses. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2011. ((Technology report; no. 136)). [PubMed] [Google Scholar]
- 31.Gidengil CA, Caloyeras JP, Hanson M, Hillestad R, Mattke S. Comparative effectiveness of fidaxomicin for treatment of Clostridium difficile infection. Am J Pharmacy Benefit. 2014;6:161–170. [Google Scholar]
- 32.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–289. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 33.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 34.Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2016;315:142–149. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 35.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 36.Li YT, Cai HF, Wang ZH, Xu J, Fang JY. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther. 2016;43:445–457. doi: 10.1111/apt.13492. [DOI] [PubMed] [Google Scholar]
- 37.Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172:191–193. doi: 10.1001/archinte.172.2.191. [DOI] [PubMed] [Google Scholar]
- 38.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 39.Hebbard AIT, Slavin MA, Reed C, et al. Risks factors and outcomes of Clostridium difficile infection in patients with cancer: a matched case-control study. Support Care Cancer. 2017;25:1923–1930. doi: 10.1007/s00520-017-3606-y. [DOI] [PubMed] [Google Scholar]
- 40.Abrahamian FM, Talan DA, Krishnadasan A, et al. Clostridium difficile infection among US emergency department patients with diarrhea and no vomiting. Ann Emerg Med. 2017;70:19–27. doi: 10.1016/j.annemergmed.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–210. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 43.McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175:784–791. doi: 10.1001/jamainternmed.2015.42. [DOI] [PubMed] [Google Scholar]
- 44.Bell CM, Urbach DR, Ray JG, et al. Bias in published cost-effectiveness studies: systematic review. BMJ. 2006;332:699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality-of-life questionnaire. J Clin Gastroenterol. 2016;50:631–637. doi: 10.1097/MCG.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


