Abstract
Inhibition of the mechanistic target of rapamycin (mTOR) pathway by rapamycin (RAPA), an FDA-approved immunosuppressive drug used as a clinical therapy to prevent solid organ allograft rejection, enhances longevity in mice. Importantly, RAPA was efficacious even when initiated in relatively old animals, suggesting that mTOR inhibition could potentially slow the progression of aging-associated pathologies in older humans (Harrison et al., 2009; Miller et al., 2011). However, the safety and tolerability of RAPA in older human subjects have not yet been demonstrated. Towards this end, we undertook a placebo-controlled pilot study in 25 generally healthy older adults (aged 70–95 years); subjects were randomized to receive either 1 mg RAPA or placebo daily. Although three subjects withdrew, 11 RAPA and 14 controls completed at least 8 weeks of treatment and were included in the analysis. We monitored for changes that would indicate detrimental effects of RAPA treatment on basic metabolism, including both standard clinical laboratory assays (CBC, CMP, HbA1c) and oral glucose tolerance tests. In addition, we asked whether there were RAPA-induced modifications in parameters typically associated with aging. These included cognitive function which was assessed by three different tools: Executive Interview-25 (EXIT25); Saint Louis University Mental Status Exam (SLUMS); and Texas Assessment of Processing Speed (TAPS). In addition, physical performance was measured by handgrip strength and 40-foot timed walks. Lastly, changes in general parameters of healthy immune aging, including serum pro-inflammatory cytokine levels and blood cell subsets, were assessed. Five subjects reported potential adverse side effects; in the RAPA group, these were limited to facial rash (1 subject), stomatitis (1 subject) and gastrointestinal issues (2 subjects) whereas placebo treated subjects only reported stomatitis (1 subject). Although no other adverse events were reported, statistically significant decrements in several erythrocyte parameters including hemoglobin (HgB) and hematocrit (Hct) as well as in red blood cell count (RBC), red blood cell distribution width (RDW), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) were observed in the RAPA-treatment group. None of these changes manifested clinically significant effects during the short duration of this study. Similarly, no changes were noted in any other clinical laboratory, cognitive, physical performance, or self-perceived health status measure over the study period. Immune parameters were largely unchanged as well, possibly due to the advanced ages of the cohort (70–93 yrs; mean age 80.5). RAPA-associated increases in a myeloid cell subset and in TREGS were detected, but changes in most other PBMC cell subsets were not statistically significant. Importantly, the OGTTs revealed no RAPA-induced increase in blood glucose concentration, insulin secretion, and insulin sensitivity. Thus, based on the results of our pilot study, it appears that short-term RAPA treatment can be used safely in older persons who are otherwise healthy; a larger trial with a larger samples size and longer treatment duration is warranted.
Keywords: Rapamycin, mTOR inhibition, Geriatrics, Cytokines, Blood cell subsets, Physiological outcomes
Introduction
Pharmacological inhibition of the mechanistic (mammalian) target of rapamycin (mTOR) pathway by rapamycin (RAPA) is associated with lifespan and healthspan extension in mice (Harrison et al., 2009; Miller et al., 2011). RAPA delayed, but did not alter, the range of illnesses at death; it also attenuated the age-related decline of spontaneous activity consistent with increased healthspan. Importantly, these effects were achieved even if RAPA treatment was initiated late in life (Harrison et al., 2009), suggesting that RAPA could be used therapeutically to slow the effects of aging in older humans. Beneficial findings in cognition, immunological function, and physical function provide further justification for studies of this FDA-approved agent on similar parameters in humans. For example, RAPA administration to transgenic hAPP(J20) mice that model Alzheimer’s Disease (AD) after the onset of moderate AD-like cognitive deficits, resulted in memory improvement (Lin et al., 2013). Similarly, 18-month old mice treated with RAPA starting at 2 months of age performed significantly better on a spatial learning and memory task compared to age-matched animals on the control diet; the performance enhancement of learning and memory was associated with decreased IL-1β levels (Majumder et al., 2012). In humans, immunosuppression with an mTOR antagonist improved several psychopathological features in transplant patients, including memory performance, mood, and global psychiatric symptoms (Lang et al., 2009). Specifically, immunosuppressive regimens of nine cardiac transplant patients were modified to exclude calcineurin inhibitors (CNI) and to include the rapalog, everolimus. All patients underwent psychological testing while taking CNIs and after four weeks of CNI-free treatment on everolimus, significant improvement was noted in several tests. These included the Wechsler Memory Scale-Revised, Beck Depression Inventory, Symptom Checklist-90-Revised, and Trail Making Tests A and B, but the Digit Span and Hamilton Depression Scale remained unchanged. These findings suggest that rapalogs may exert beneficial psychological and perhaps cognitive effects. However, the cessation of CNIs may have also contributed to the positive outcomes seen in this uncontrolled study.
Preservation of physical function or possible enhancement thereof by RAPA has been suggested by prior research in animal models. For example, the finding that RAPA slowed age-dependent declines in spontaneous in-cage activity in mice suggests that physical activity could be enhanced in humans with this agent (Miller et al., 2011; Wilkinson et al., 2012). Similarly, a recent study of 24 healthy, middle-aged companion dogs administered either one of 2 doses of RAPA or placebo for 10 weeks, noted that owners reported a trend toward increased activity for those animals receiving RAPA (Urfer et al., 2017).
Both beneficial and detrimental effects of mTOR inhibition on immunological function have been reported. RAPA was first shown to be immunosuppressive in rodent models of induced autoimmunity (Martell, 1976); it was speculated that the depressed immune reactivity was due to RAPA’s inhibition of T cell proliferative responses (Araki et al., 2011; Weichhart and Saemann, 2009). However, subsequent studies suggested that the drug’s effects are more complex, affecting both innate and adaptive immunity (reviewed in Janes and Fruman, 2009). The effects of RAPA on immune regulation and the control of T cell differentiation, migration, and expansion have been well studied. One of the most striking findings is an expansion of viral specific memory CD8+ T cells (Araki et al., 2009; He et al., 2011; Rao et al., 2010; Turner et al., 2011). In addition, RAPA exposure is reported to skew the CD4+ T cell compartment, with a decrease in pro-inflammatory TH1 and TH17 subsets (Basu et al., 2008; Battaglia et al., 2006; Delgoffe et al., 2011; Liu et al., 2010; Strauss et al., 2009). This is consistent with the reported efficacy of RAPA in suppressing autoimmunity in rodents (Chen et al., 2010; Mushaben et al., 2011; Ramos-Barron et al., 2007; Teachey et al., 2009a) and in humans (type I diabetes, multiple sclerosis, etc.) (Piemonti et al., 2011; Teachey et al., 2009b). However, it may also explain the increased susceptibility of treated mice infected with West Nile virus (Goldberg et al., 2015).
Since older individuals are less able to generate effective immune responses to vaccination, one might speculate that RAPA would further compromise the antibody response to standard vaccines (like those for seasonal flu). However, data from murine models suggest the opposite. Specifically, young and old C57BL/6 mice were fed a diet containing either encapsulated rapamycin or a control diet (capsular material lacking RAPA) for seven months. The mice were then challenged subcutaneously with an inoculum containing Aggregatibacter actinomycetemcomitans, a human periodontal pathogen that does not naturally infect mice. The antibody response to bacterial challenge was enhanced significantly by RAPA in older animals, 28 months of age at challenge. No RAPA enhancement was seen in adult mice. These data (Benavides and Kraig, in preparation), suggest that RAPA may have different immune outcomes when tested in old vs. young subjects.
Similarly, in humans, increased susceptibility to infection has not been found and moreover, immunity to certain pathogens, like cytomegalovirus (CMV), may even be augmented by RAPA (Demopoulos et al., 2008; Ozaki et al., 2005). Improved responses to other viral challenges have also been reported in transplant recipients and in rodent and nonhuman primate models (Barozzi et al., 2008; Nicoletti et al., 2009). Effects of RAPA on autophagy, dendritic cells, and antigen processing also appear to enhance responses to intracellular bacteria (Fabri et al., 2011; Jagannath et al., 2009). Thus, the effects on cellular immunity appear to be surprisingly beneficial in many cases. Where tested, humoral immunity was also unaffected by RAPA treatment. Specifically, the addition of RAPA to a therapeutic regimen did not appear to negatively impact vaccine efficacy as shown by comparing antibody production to influenza vaccines in transplant patients (reviewed in Kumar et al., 2011). In fact, when compared to other immunosuppressive drugs, RAPA treatment correlated with improved efficacy in one study of lung transplant patients (Hayney et al., 2004) and with broader serum reactivity in another study involving liver and kidney recipients (Willcocks et al., 2007). In one recent study, the response to influenza vaccine showed modest, but significant improvements. Specifically, Mannick and colleagues showed that treatment in human volunteers of age 65 and older with the rapalog RAD001 for six weeks at doses of 0.5 mg daily or 5 mg weekly enhanced the response to the influenza vaccine administered two weeks after mTOR inhibition was discontinued (Mannick et al., 2014).
Despite the promising results of mTOR antagonists outlined above, concern exists within the research community regarding the safe use of RAPA and rapalogs in older persons (Apelo and Lamming, 2016). These concerns are primarily based on the hypothetical extension of clinical experience gleaned from transplant or oncology patients to healthy older adults. For example, there are concerns about the safety of giving an immunosuppressant to individuals who may be experiencing age-related immunological decline. At present, there is a paucity of studies that have actually examined the effects of mTOR inhibition in healthy humans, whether young or old. Instead, human studies with RAPA and rapalogs are primarily related to the FDA approved uses of these agents in patients with solid organ transplants, autoimmunity, or cancer (Eiden et al., 2016; Sankhala et al., 2009). Work in these patient groups shows that RAPA and rapalogs have similar profiles with commonly reported toxicities of mucositis, stomatitis, diarrhea, and nausea. Acneiform rash involving mainly the face and neck is a relatively common toxicity as well. Changes in clinical laboratory values reported with RAPA include increased levels of serum high-density lipoproteins (HDL), low-density lipoproteins (LDL), and triglycerides (TRIG). Thrombocytopenia and anemia have also been reported (Sofroniadou et al., 2010). Increased incidence of bacterial infections has been observed in patients with cancer as have been cases of pneumonitis, which are relatively rare with an incidence of 4–5% (Sankhala et al., 2009). These were not observed in healthy subjects in the vaccine trial (Mannick et al., 2014). Most adverse side effects are dose dependent. However pneumonitis and mucositis have been reported even at low doses. RAPA was shown to induce hyperglycemia in some animal models, though not in nonhuman primates (Ross et al., 2015). In humans, hyperglycemia was reported in several Phase I and II studies with mTOR inhibitors in patients with recurrent tumors although in most cases the severity was not clinically significant (Sankhala et al., 2009). Reports from transplant patients suggest that immunosuppressive regimens that included RAPA in combination with CNI were diabetogenic but that use of RAPA alone may not be (Pavlakis and Goldfarb-Rumyantzev, 2008). Given the potential adverse reactions summarized above and that older persons are at increased risk of adverse drug reactions both due to age related physiological changes and increased use of other pharmacological agents, these safety concerns warrant attention in this age group. Being cognizant that RAPA inhibition of mTOR has pleiotropic effects on metabolism and numerous physiological systems and that the effects seen in the older subjects may differ from those previously found in younger individuals, patients with transplants or cancer, we undertook a pilot study of RAPA in healthy older persons. Our intent was to assess the feasibility of enrolling generally healthy older voluntters to study tolerability, safety and effects of RAPA on parameters of general, physical, cognitive, and immune health to determine whether mTOR inhibition could be of potential benefit to the aging population.
Methods
Study design
We conducted a randomized, placebo controlled, prospective trial (NCT02874924 at clinicaltrials.gov) of orally-administered RAPA (sirolimus) in healthy older persons. Rapamune was obtained through either Pfizer, Inc. or its subsidiary, Greenstone LLC. As the human subject sample size was relatively small, we chose to test a single dose, 1 mg daily, and to then monitor blood levels obtained in the first 4 subjects. The goal was to achieve a relatively low therapeutic dose for safety that would still be in the range expected to exact clinical effects. This initial phase of the trial, designated “phase 1”, consisted of 8 subjects (half in the treatment group and half receiving placebo) and was designed to be a 4 month treatment protocol. The maximum levels of blood RAPA attained in the treatment group subjects was 6.1–8.2 ng/ml (Table 2) which was within the desired range. Thus, a slightly larger Phase 2 cohort was developed (10 subjects in each group) using the same dosing, 1 mg RAPA delivered orally daily. The Phase 2 study was shorter in duration with only an 8 week treatment period. Both phase 1 and phase 2 were similarly designed with baseline measurements made after subjects consented to participate, but prior to the initiation of RAPA/placebo. Measurements were repeated at several time points over the course of the studies as outlined in Table 1 and in the CONSORT flow diagrams (Supplemental Figures 1A, B).
TABLE 2.
Ages and genders of subjects completing the study
| PLACEBO SUBJECTS | RAPA SUBJECTS | ||||||
|---|---|---|---|---|---|---|---|
| Subject | Age | Sex | Subject | Age | Sex | RAPA levelb | |
| STUDY 1 | 001 | ≥90a | M | 002 | ≥90 | M | 8.2 ng/ml |
| 003 | ≥90 | M | 004 | 88 | M | 6.1 ng/ml | |
| 006 | 80 | M | 005 | 89 | M | 6.7 ng/ml | |
| 007 | 89 | M | 009 | ≥90 | M | 7.4 ng/ml | |
| STUDY 2 | 010 | 79 | M | 011 | 70 | M | 4.7 ng/ml |
| 013 | 86 | M | 015 | 77 | M | 6.8 ng/ml | |
| 014 | 83 | M | 016 | 81 | M | 11.8 ng/ml | |
| 018 | ≥90 | M | 021 | 75 | M | 6.1 ng/ml | |
| 023 | 74 | F | 024 | 81 | F | 8.8 ng/ml | |
| 027 | 71 | F | 030 | 70 | F | 7.8 ng/ml | |
| 028 | 70 | F | 033 | 70 | M | 4.8 ng/ml | |
| 029 | 73 | F | |||||
| 032 | 75 | M | |||||
| 034 | 75 | F | |||||
| Mean age with standard deviation | 80.6 +/− 7.9 yrs | 80.4 +/− 8.6 yrs | |||||
. Age over 89 yrs is considered an “identifier” by our IRB, so subjects 90 or above are indicated “≥90”
. The highest RAPA level in the blood attained during the course of the study is indicated for each subject in that treatment group.
TABLE 1.
Study design and timing of procedures.
| TIME ON TREATMENT (weeks): | 0a | 1 | 6–8 | 16 |
|---|---|---|---|---|
| PROCEDURES: | ||||
| Group assignment RAPA/placebod | 1b,2c | |||
| Physical exam, history | 1,2 | 1,2 | 1,2 | 1 |
| RAPA level | 1,2 | 2 | 1 | |
| Complete blood count | 1,2 | 1,2 | 1 | |
| Metabolic profile | 1,2 | 1,2 | 1 | |
| Fasting blood sugar (glucose) | 1,2 | 1,2 | 1 | |
| Fasting lipid profile | 1,2 | 1,2 | 1 | |
| Oral glucose tolerance test | 2 | 2 | ||
| Hand grip test | 1,2 | 1,2 | 1 | |
| 40-ft walk test | 1,2 | 1,2 | 1 | |
| Cognitive tests – EXIT, SLUMS, TAPS | 1,2 | 2 | 1 | |
| Cytokine profiles | 1,2 | 2 | 1,2 | 1 |
| Blood cell subsets by flow | 2 | 2 | 2 |
. RAPA/placebo treatment was initiated at week 0.
. “1” indicates a procedure performed for phase 1 of the study.
. “2” indicates a procedure performed for phase 2 of the study.
. randomization/blinding was handled by the Research Pharmacy with Graphpad QuickCalcs (https://www.graphpad.com/quickcalcs/randomize1/).
All other study participants and staff were blinded as to treatments until after completion of the project.
Prior to study participation, all subjects attended a screening visit to ensure that all inclusion and exclusion criteria were met. Tests done at screening included a history, physical exam, ECG, a test of cognitive function sufficient to provide informed consent (CLOX1≥10), CBC, CMP, as well as fasting glucose and lipid profile. Volunteers enrolled met the following inclusion criteria: older males or females of any ethnicity who were in relatively good health with all chronic diseases (hypertension, coronary artery disease, etc.) clinically stable. The exclusion criteria were: evidence of diabetes (A1c ≥6.5), being treated with a medication that would affect glucose homeostasis, history of skin ulcers or poor wound healing, smoking, warfarin anticoagulation treatment, on a drug known to affect cytochrome P450 3A due to its role in RAPA metabolism, or treatment with an immunosuppressant agent (including glucocorticoids) within the last year, liver disease, recent history (within 6 months) of myocardial infarction, active coronary disease, or intestinal disorders. The ages, genders, and outcomes for each of the 28 enrolled subjects are summarized in Supplemental Table 1.
Once enrolled, RAPA (or placebo) was added without any modification to the subjects’ regular medications. Any changes in treatment during the study period were noted in the record, but the data were not selected or analyzed based on the subjects’ other co-morbidities or medications (Supplemental Table 2). Due to the small sample size in this pilot study, it was not possible to statistically control for the drugs being taken by an individual subject. Thus, comparison to the placebo group (which had a similar age distribution and medical profile) was the best approach and is reported for all data discussed. The first study phase included subjects of more advanced age, 80–95 years old, and continued treatment, as tolerated, for 4 months. The second study phase extended the age range down to 70, had a treatment phase of 2 months, and included the addition of the glucose tolerance test (OGTT) and flow cytometry of PBMCs since changes in glucose metabolism and blood cell subsets had been reported elsewhere (Apelo et al., 2016; Hurez et al., 2015; Lamming et al., 2013).
Participants were randomized to receive either placebo or RAPA. Those subjects receiving at least 8 weeks of treatment in either phase were considered “completers” and are listed in Table 2. CONSORT Flow Diagrams for the two phases of this study are included as Supplemental Figures 1A and 1B. All clinical aspects of the project were performed at the South Texas Veterans Health Care System Frederic C. Bartter General Clinical Research Center under the auspices of a protocol approved by the UTHSCSA IRB.
Routine tests and clinical labs
A medical history and physical examination was done at every visit to assess general health and detect adverse reactions and subclinical sequelae of participation. In addition, at the time points indicated in Table 1, blood was also collected for standard clinical measures; these included: 1) fasting glucose and HbA1c levels; 2) fasting lipid profiles for HDL, LDL, TRIG, and VLDL; 3) complete metabolic panel [CHEM-20=sodium (Na), potassium (K), chloride (Cl), CO2 (bicarbonate), creatinine, glucose, urea nitrogen (BUN), albumin, calcium, alkaline phosphatase, ALT/SGPT, AST/SGOT, total bilirubin, and total protein]; and 4) complete blood counts to assess any hematological effects.
Glucose tolerance tests
For subjects in phase 2 of the trial, OGTTs were also performed to detect any RAPA-related diabetogenic effects. The OGTT was performed in the fasted state at the pre-treatment screen and repeated after 6 weeks of treatment. A catheter was placed in an antecubital vein and plasma glucose, insulin, and free fatty acid (FFA) concentrations were measured at baseline and every 30 minutes for 2 h after the ingestion of 75 g glucose. The plasma glucose concentration was determined by the glucose oxidase method with a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA), FFA were measured by colorimetric assay (Wako, Richmond, VA) and insulin by RIA (Diagnostic Products Corp., Los Angeles, CA). The HOMA-insulin resistance (HOMA-IR) index and the Matsuda index for insulin sensitivity were calculated as described (Matthews et al., 1985; Matsuda and DeFronzo, 1999).
Cognitive and physical function tests
Three tools commonly used to assess cognitive function in older subjects were performed at indicated intervals in both phases of this trial (Table 1). These included the Executive Interview (EXIT25) which includes letter fluency (Royall et al., 1992), SLUMS (St. Louis University Mental Status exam) comprised of a memory test, digit span, and animal fluency (Tariq et al., 2006), and TAPS (Texas Assessment of Processing Speed) which is a digit/symbol coding test (Grosch et al., 2012). The cognitive tests were performed at only two time points, at the initiation and termination of the study period, to circumvent complications due to learning. Physical function was assessed longitudinally to detect changes in the subjects’ capabilities using two independent physical performance tests. First, to measure grip strength, subjects were seated in a chair with the forearm at a 90° elbow bend and grip strength was measured four times for each hand using a standard grip strength dynamometer; the highest value obtained was reported. Second, each participant did four walking trials, timed with a stopwatch, at his/her preferred walking speed over a measured 40-foot path; the fastest time recorded was used. All performance measures were done in identical fashion longitudinally to offer the greatest potential to detect changes.
RAPA assays
At the indicated time points (Table 1), one 2 ml EDTA tube (purple top; BD367841; Becton Dickinson & Company) was collected, kept on ice and then aliquoted into 2 cryovials which were stored at −80°C. The blood RAPA levels were ascertained by mass spectroscopy performed by the Biological Psychiatry Analytical Lab, as described previously (Tardif et al., 2014).
Cytokine assays
One 8.5 ml serum tube of blood (black/red tiger top; BD367988, Becton Dickinson) was collected from each subject, mixed by inversion, and then allowed to clot at room temperature for 30’. The tubes were spun for 10’ at 1100×g, and the serum was then frozen in 0.7 ml aliquots and stored at −20°C. Serum samples were thawed and spun at 10,000g for 10 minutes before use. Multiplex cytokine array assays were performed using Luminex-based technology on a BioPlex 200 system (Bio-Rad Industries, Hercules CA) as directed by the kit and instrument directions. Briefly, subject samples (25 µl) were analyzed in duplicate using a magnetic Milliplex kit (Millipore, HCYTMAG-60K-PX29) allowing simultaneous quantitation of 29 human cytokines/chemokines. The Bio-Plex software (Bio-Plex Manager 5.0 build 531, Bio-Rad Industries) was used to determine serum concentrations (pg/ml) based on standard curves for each cytokine. In addition, a high sensitivity IL-6 assay (Invitrogen BMS213HS, Thermo Fisher Scientific) was performed on a subset of serum samples (50 µl each) according to the manufacturer’s instructions.
Flow cytometry
PBMCs were purified from five 8 ml CPT/heparin tubes (green/red tiger tops, BD362753, Becton Dickinson) for use in flow cytometry and for the repository. The CPT tubes were spun for 30 min at 1700×g and the PBMC layer was collected into PBS. Cells not needed for flow cytometry were viably frozen at 6–8×106 cells/cryovial in 90% fetal bovine serum (Hyclone) and 10% DMSO (Sigma). One aliquot of PBMCs was used for flow cytometry; included was approximately 3 × 106 for the three panels and another 5–10 × 106 cells for generating the pooled control sample. Briefly, cells were resuspended in 50 µl blocking solution [1×PBS, 5% FBS, 5% mouse serum (24-5544-94, eBioscience); 2.5 µl human TruStainFcX (1:20 dilution; 422302, Biolegend)]. After a 20–30 min incubation at room temperature, 50 µl (106) of blocked cells were added to 50 µl of a cocktail containing the following antibodies: PANEL 1: CD3-PacBlue (Biolegend 300442), CD20-AF647 (Biolegend 302318), and CD11b-PE (Biolegend 301306); PANEL 2: CD3-PacBlue, CD4-AF488 (Biolegend 317420), CD8-BV510 (Biolegend 344732), FoxP3-APC (eBioscience 17-4777-42), CD127-PE (Biolegend 351304), and CD25-PerCp-Cy5.5 (Biolegend 302626); and PANEL 3: CD3-PacBlue, CD4-AF488, CD8-BV510, CD45RA-PE (Biolegend 304108), CD45RO-PE-Cy7 (Biolegend 304230), CD28-PerCp-Cy5.5 (eBioscience 45-0289-42), and PD-1-APC (Biolegend 329908). After staining 20 minutes in the dark at 4°C, the cells were washed twice with FACS buffer containing 5% FBS in PBS. Cells with only surface staining were resuspended in 150 µl of Flow Cytometry Staining Buffer (eBioscience 00-4222) and 150 µl IC Fixation Buffer (eBioscience 00-8222). Cells were stored at 4°C in the dark overnight. For PANEL 2, the cells were stained with all antibodies to surface markers and then treated with the permeabilization buffer (eBiosciences) and subsequently stained with the intracellular foxp3 antibody before the final washes and resuspension in fixation buffer. For all panels, single color and FMO controls were included. Stained cells were analyzed on an LSRII (BD Bioscience San Jose, CA) and the BD FACSDiva software (v.8.0.1.1, Becton Dickinson) was used for quantitating the cell subsets.
Data Analysis
For most measures, the primary hypothesis was that RAPA treatment would alter clinical, functional, laboratory, or immune outcomes, so changes between the pre-treatment values and the post-treatment values were assessed for statistical significance using a paired t-test. The placebo group data were similarly analyzed. To determine whether the effects with RAPA were group specific, differences (POST – PRE) for each individual were calculated and tested for association with treatment status; the statistical significance was assessed using Welch’s t-test. For all t-tests, p<0.05 was considered significant. However, given the relatively small sample size in this study, it is recognized that additional testing will be required to validate these findings.
The OGTT data on glucose, insulin, and FFA had repeated measures (−15 to 120 minutes) for both pre- and post-treatment periods. These were analyzed using linear mixed effects models with a random intercept to account for within-subject correlation. A treatment by time interaction was used to evaluate changes in analyte trajectories related to RAPA relative to placebo. The total area under the curve AUC (proportional to the average value over −15 to 120 minutes) for these OGTT biomarkers was also assessed for pre/post changes within a treatment group and between treatment groups using Mann-Whitney. All testing was 2-tailed and a p-value <0.05 was considered significant. All analysis was conducted either in R (Vienna, Austria) or in GraphPad Prism (version 7.03 for Windows, GraphPad Software, La Jolla California www.graphpad.com).
Results
Rapamycin dosing
Older, generall healthy volunteers were recruited to participate in a placebo controlled trial of RAPA. Assignment to a treatment group was randomized and the age ranges were similar (median age for the placebo group was 79.5 while the median for the RAPA subjects was 81). Detailed information on the subjects who completed at least 8 weeks of treatment is found in Table 2 (age, sex, and treatment group) and in Supplemental Table 2 (co-morbidities and medications). The treatment group received a daily dose of 1 mg RAPA and their blood levels were analyzed at intervals thereafter. The highest concentration attained for each subject is shown in Table 2; the levels varied from 2.5–11.8 ng/ml, within the range shown to be physiologically relevant (Kahana et al., 2000; Moes et al., 2015; Trepanier et al., 1998). The 25 subjects completing at least 8 weeks of treatment were followed for parameters of physiological function, general health, cognition, and immunity which are often seen to diminish with age.
In Phase 1 of the trial, which involved male volunteers 80 years old and above, seven of the eight subjects experienced no significant adverse events during the treatment period. One subject on RAPA (#2, age ≥90) developed nocturnal diarrhea after 11 weeks of treatment. He then withdrew and his symptoms resolved. In Phase 2, the age range was expanded to include individuals as young as 70 yrs old; 20 subjects were enrolled and randomized to RAPA or placebo groups; both males and females were included (Supplemental table 1). Of the 17 individuals who completed 8 weeks of treatment, most had no significant adverse events during their participation. Two subjects reported 2–3 days of self-limited stomatitis symptoms; one of these received RAPA and one was in the placebo group; both chose to remain in the cohort with symptoms resolving spontaneously in a few days. One subject who received RAPA for 3 days, developed diarrhea during travel to an underdeveloped country and elected to cease trial participation. One subject who received RAPA for 1 week was dropped from the trial due to development of an acneiform facial rash. In both cases, the symptoms resolved once treatment was terminated. Lastly, one subject withdrew due to an unrelated medical condition requiring steroid treatment. Thus, although this is a small trial, it is unique in its inclusion of an older cohort that is generally healthy and not being treated for either cancer or to prevent transplant rejection. These outcomes are elaborated in the CONSORT Flow Diagrams found in Supplementary Figures 1A (trial phase 1) and 1B (trial phase 2).
Clinical parameters
To assess the safety and tolerability of RAPA in older human subjects, several standard laboratory tests were performed at the time points shown in Table 1. We focused on parameters previously reported to be affected by mTOR inhibition (Sankhala et al., 2009). In addition, we included general tests of health since it was possible that RAPA treatment would have different outcomes, either positive or negative, in these older individuals. Our overarching hypothesis was that RAPA treatment would alter clinical, functional, laboratory, or immune measures relative to placebo. The results from the blood chemistry tests are shown in Table 3, which includes the PRE and POST treatment values for subjects in the two treatment groups. To determine whether RAPA effected a significant change in any of the analytes measured, paired t tests were performed comparing the POST values for each of the 11 subjects relative to his/her PRE values. A similar analysis was carried out for the 14 individuals in the placebo group. None of the components tested showed a statistically significant change during the 6–8 weeks of RAPA treatment, yet analysis of a larger cohort with higher power might reveal small, but statistically significant, effects on blood analytes.
TABLE 3.
Changes in blood chemistry during treatment
| Treatment groupa | Group differenceb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLACEBO (n=14) | RAPA (n=11) | |||||||||
| Pre | Post | P value | Difference (95%CI) | Pre | Post | P value | Difference (95%CI) | P value | Difference (95%CI) | |
| BUN | 18.71 ± 4.39 | 18.36 ± 6.1 | 0.74 | −0.36 [−2.63, 1.91] | 22.27 ± 7.72 | 21.45 ± 7.17 | 0.64 | −0.82 [−4.58, 2.94] | 0.82 | 0.46 [−3.73, 4.65] |
| CREAT | 0.99 ± 0.28 | 1.05 ± 0.34 | 0.05 | 0.06 [0, 0.12] | 1.2 ± 0.5 | 1.21 ± 0.59 | 0.8 | 0.01 [−0.08, 0.1] | 0.31 | 0.05 [−0.05, 0.15] |
| Sodium | 140 ± 2.39 | 139.14 ± 2.07 | 0.1 | −0.86 [−1.89, 0.18] | 139.91 ± 1.3 | 138.91 ± 1.92 | 0.08 | −1 [−2.16, 0.16] | 0.84 | 0.14 [−1.33, 1.61] |
| Potassium | 4.02 ± 0.45 | 4.05 ± 0.36 | 0.69 | 0.03 [−0.12, 0.18] | 4.05 ± 0.34 | 3.93 ± 0.42 | 0.34 | −0.13 [−0.41, 0.15] | 0.29 | 0.16 [−0.15, 0.46] |
| Chloride | 105.29 ± 4.16 | 104.07 ± 4.3 | 0.09 | −1.21 [−2.63, 0.2] | 103.91 ± 3.24 | 104.55 ± 3.53 | 0.47 | 0.64 [−1.25, 2.52] | 0.1 | −1.85 [−4.08, 0.38] |
| CO2 | 26.64 ± 2.5 | 27 ± 2.22 | 0.58 | 0.36 [−1.01, 1.73] | 26.73 ± 2.65 | 26.64 ± 2.69 | 0.91 | −0.09 [−1.9, 1.72] | 0.67 | 0.45 [−1.7, 2.6] |
| Calcium | 9.33 ± 0.38 | 9.39 ± 0.36 | 0.42 | 0.06 [−0.1, 0.23] | 9.35 ± 0.34 | 9.23 ± 0.2 | 0.22 | −0.12 [−0.32, 0.08] | 0.14 | 0.18 [−0.06, 0.43] |
| PROTEIN | 6.76 ± 0.43 | 7.07 ± 0.44 | 0.004 | 0.31 [0.12, 0.51] | 6.92 ± 0.32 | 6.93 ± 0.47 | 0.96 | 0.01 [−0.37, 0.39] | 0.13 | 0.31 [−0.1, 0.72] |
| ALBUMIN | 3.97 ± 0.34 | 4.16 ± 0.29 | 0.002 | 0.19 [0.08, 0.29] | 3.96 ± 0.17 | 3.82 ± 0.35 | 0.23 | −0.15 [−0.4, 0.11] | 0.018 | 0.33 [0.07, 0.6] |
| ALK PHOS | 64.64 ± 8.71 | 68.29 ± 9.6 | 0.07 | 3.64 [−0.3, 7.58] | 58.09 ± 16.05 | 61.36 ± 13.34 | 0.29 | 3.27 [−3.28, 9.83] | 0.92 | 0.37 [−6.93, 7.67] |
| TBILI | 0.66 ± 0.19 | 0.69 ± 0.21 | 0.51 | 0.03 [−0.06, 0.12] | 0.78 ± 0.35 | 0.69 ± 0.27 | 0.14 | −0.09 [−0.22, 0.03] | 0.11 | 0.12 [−0.03, 0.27] |
| AST | 20.57 ± 4.29 | 20.79 ± 3.89 | 0.81 | 0.21 [−1.72, 2.15] | 20.36 ± 4.06 | 23.18 ± 5.69 | 0.11 | 2.82 [−0.73, 6.37] | 0.17 | −2.6 [−6.48, 1.27] |
| ALT | 16.36 ± 3.54 | 16.5 ± 3.92 | 0.82 | 0.14 [−1.16, 1.44] | 17.64 ± 5.89 | 18.09 ± 5.43 | 0.7 | 0.45 [−2.08, 2.99] | 0.81 | −0.31 [−3.05, 2.43] |
. To compare changes in blood chemistry over the treatment period, the “Pre” values (taken before treatment was initiated) were subtracted from the “Post” values to obtain the “Difference” within a treatment group and were assessed by paired t-tests to identify any statistically significant changes in either the RAPA or Placebo groups. The p-values are shown. The direction of the change is indicated in the “Difference column” where a positive number indicates a higher mean value after 6–8 weeks of treatment and a negative value reflects a lower value after treatment. The 95% confidence interval is shown in brackets. The mean values measured are shown ± standard deviation. Individual values for several of these markers and some additional data are shown graphically in Figure 1A,B,C.
. To determine whether there was a difference in the RAPA vs. the placebo group, the mean “difference” values were compared using a Welch’s t-test. The resulting p-values are shown. The difference mean changes seen in the RAPA and placebo groups are shown with the 95% confidence interval in brackets.
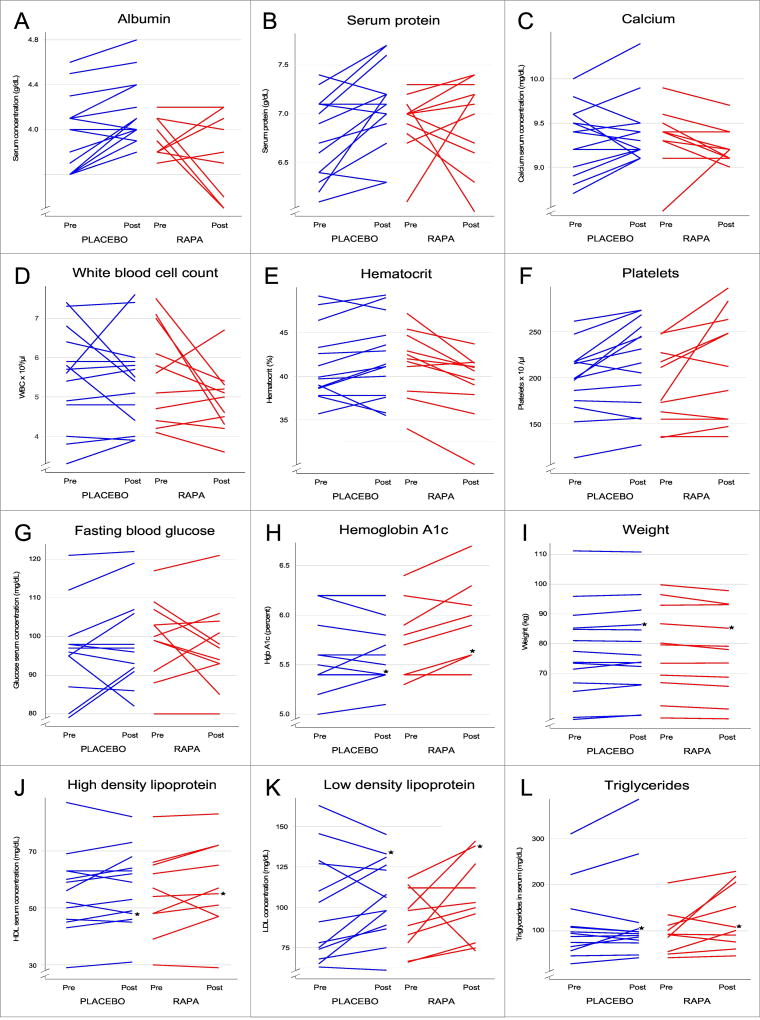
Hematological studies (Table 4) revealed RAPA-associated decrements in several clinical parameters of erythrocytes. These included reductions in hemoglobin (HgB) and hematocrit (Hct), which both decreased during RAPA treatment and included values slightly below the clinical reference range; the Hct data are shown in Figure 1E. The red blood cell count (RBC) decreased from values within the normal reference range to below the reference range in the RAPA-treated group and was below the reference range at the beginning and end of the study in the placebo-treated group. Red blood cell distribution width (RDW), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) were reduced over the course of the study in the RAPA-treated group but remained within the normal reference range. None of these changes manifested clinically significant effects although anemia has been seen in other populations treated with mTOR inhibitors. Besides an unexpected increase in platelets in the placebo group, no statistically significant changes were noted in other hematological parameters (Table 4).
TABLE 4.
Changes in CBC counts during treatment
| Treatment groupa | Group differenceb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLACEBO (n=14) | RAPA (n=11) | |||||||||
| Pre | Post | P value | Difference (95%CI) | Pre | Post | P value | Difference (95%CI) | P value | Difference (95%CI) | |
| RBC | 4.42 ± 0.47 | 4.45 ± 0.43 | 0.55 | 0.03 [−0.08, 0.15] | 4.43 ± 0.48 | 4.31 ± 0.53 | 0.043 | −0.12 [−0.24, 0] | 0.05 | 0.15 [0, 0.31] |
| MCV | 93.75 ± 4.2 | 93.74 ± 4.35 | 0.99 | −0.01 [−0.9, 0.89] | 94 ± 3.23 | 91.36 ± 4.19 | <0.001 | −2.64 [−3.52, −1.75] | <0.001 | 2.63 [1.44, 3.82] |
| MCH | 31.34 ± 1.89 | 31.2 ± 1.73 | 0.5 | −0.14 [−0.58, 0.3] | 31.47 ± 1.27 | 30.56 ± 1.29 | <0.001 | −0.91 [−1.3, −0.51] | 0.009 | 0.77 [0.21, 1.33] |
| MCHC | 33.41 ± 0.78 | 33.26 ± 0.6 | 0.31 | −0.15 [−0.46, 0.16] | 33.47 ± 0.56 | 33.46 ± 0.43 | 0.96 | −0.01 [−0.36, 0.35] | 0.52 | −0.14 [−0.58, 0.3] |
| RDW | 13.49 ± 0.82 | 13.53 ± 0.86 | 0.63 | 0.04 [−0.12, 0.19] | 13.73 ± 0.77 | 12.85 ± 0.44 | <0.001 | −0.88 [−1.14, −0.63] | <0.001 | 0.92 [0.63, 1.2] |
| WBC | 5.51 ± 1.26 | 5.39 ± 1.16 | 0.64 | −0.12 [−0.68, 0.43] | 5.6 ± 1.22 | 4.9 ± 0.81 | 0.09 | −0.7 [−1.54, 0.14] | 0.22 | 0.58 [−0.38, 1.54] |
| HgB | 13.81 ± 1.53 | 13.98 ± 1.63 | 0.33 | 0.16 [−0.19, 0.51] | 13.94 ± 1.35 | 13.12 ± 1.22 | 0.003 | −0.82 [−1.29, −0.35] | 0.001 | 0.98 [0.43, 1.54] |
| HCT | 41.29 ± 4.13 | 41.96 ± 4.54 | 0.21 | 0.67 [−0.42, 1.76] | 41.57 ± 3.79 | 39.24 ± 3.75 | 0.002 | −2.34 [−3.57, −1.1] | <0.001 | 3.01 [1.45, 4.56] |
| PLTS | 197.36 ± 37.88 | 215.21 ± 48.32 | 0.009 | 17.86 [5.17, 30.54] | 189.73 ± 41.9 | 211.82 ± 58.85 | 0.06 | 22.09 [−1.14, 45.32] | 0.73 | −4.23 [−29.58, 21.12] |
. To compare changes in CBC counts over the treatment period, the “Pre” values (taken before treatment was initiated) were subtracted from the “Post” values to obtain the “Difference” within a treatment group and were assessed by paired t-tests to identify any statistically significant changes in either the RAPA or Placebo groups. The p-values are shown. The direction of the change is indicated in the “Difference column” where a positive number indicates a higher mean value after 6–8 weeks of treatment and a negative value reflects a lower value after treatment. The 95% confidence interval is shown in brackets. The mean values measured are shown ± standard deviation. Individual values for several of these markers and some additional data are shown graphically in Figure 1D,E,F.
. To determine whether there was a difference in the RAPA vs. the placebo group, the mean “difference” values were compared using a Welch’s t-test. The resulting p-values are shown. The difference mean changes seen in the RAPA and placebo groups are shown with the 95% confidence interval in brackets.
Figure 1. Effects of RAPA on clinical parameters.
Shown are the changes in several measures of clinical health for individual subjects in the RAPA (red) and placebo (blue) treatment groups. The “pre” points were taken at time 0, prior to the initiation of treatment. Most of the “post” values were taken at the 6–8 week visit, but for a few subjects, data were only available at 16 weeks (indicated with a star, ★). All of the 6–8 week values for these and other parameters measured are summarized, with statistical analyses summarized in Tables 3–7.
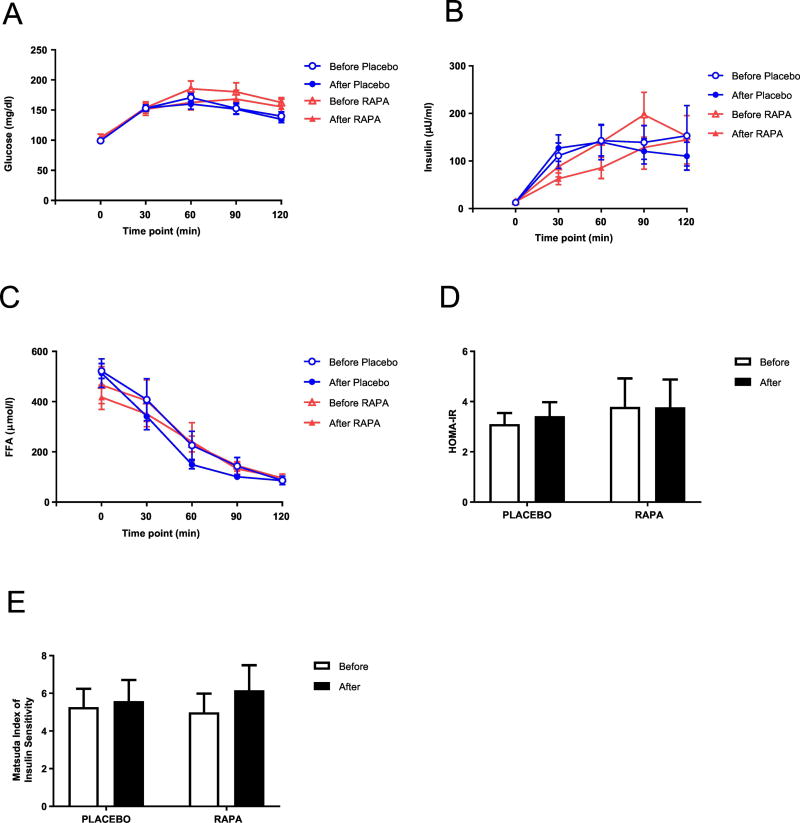
Examination of the metabolic profile measurements of fasting lipids, glucose, and HbA1c showed no RAPA-specific changes over the course of the study (Table 5, Figures 1G,H,J,K,L). A slight increase in A1c levels was seen in the RAPA treatment group (p=0.032 on paired t-test). To assess whether there were other indications of metabolic effects, a 2-hour oral glucose tolerance test was performed on subjects in phase 2 and the profiles for blood glucose, insulin, and free fatty acid levels were statistically unaltered by RAPA treatment (Figure 2). RAPA did not affect insulin sensitivity assessed with either the HOMA-IR or the Matsuda index which closely correlates (r=0.73, p<0.0001) with insulin sensitivity measured with the euglycemic clamp (Matsuda and DeFronzo, 1999). The analysis of the response curves did not indicate a significant difference between RAPA and placebo groups. For glucose, insulin, and FFA, respectively, the treatment by time interaction p-values were 0.27, 0.45, and 0.29. The differences between rapamycin and placebo in pre/post changes in area under the curve (AUC) for glucose, insulin, and FFA also yielded nonsignificant p-values [0.99, 0.48, and 0.52, respectively]. Thus, the concern that RAPA would pre-dispose older subjects to diabetes was not realized, at least in an eight-week treatment period.
TABLE 5.
Changes in metabolic markers during treatment
| Treatment groupa | Group differenceb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLACEBO | RAPA | |||||||||
| Pre | Post | P value | Difference (95%CI) | Pre | Post | P value | Difference (95%CI) | P value | Difference (95%CI) | |
| Glucose | 96.43 ± 10.86 | 97.71 ± 12.19 | 0.56 | 1.29 [−3.35, 5.92] | 99.64 ± 10.31 | 97.45 ± 10.93 | 0.42 | −2.18 [−7.93, 3.56] | 0.31 | 3.47 [−3.51, 10.44] |
| AIC | 5.67 ± 0.44 | 5.69 ± 0.36 | 0.68 | 0.02 [−0.09, 0.13] | 5.81 ± 0.4 | 6 ± 0.43 | 0.032 | 0.19 [0.02, 0.35] | 0.07 | −0.17 [−0.34, 0.01] |
| HDL | 55.83 ± 14.79 | 57.92 ± 13.91 | 0.13 | 2.08 [−0.71, 4.88] | 55.22 ± 15.74 | 58.11 ± 16.58 | 0.18 | 2.89 [−1.62, 7.39] | 0.73 | −0.81 [−5.79, 4.18] |
| LDL | 95.5 ± 31.46 | 103.9 ± 24.45 | 0.14 | 8.4 [−3.06, 19.86] | 89.47 ± 17.75 | 100.51 ± 23.52 | 0.23 | 11.04 [−8.7, 30.79] | 0.8 | −2.64 [−24.19, 18.9] |
| TRIG | 116.25 ± 78.74 | 123.42 ± 100.14 | 0.4 | 7.17 [−11.05, 25.38] | 92.78 ± 48.33 | 131.22 ± 71.53 | 0.05 | 38.44 [0.04, 76.85] | 0.12 | −31.28 [−71.83, 9.27] |
| VLDL | 19.82 ± 10.39 | 21.27 ± 13.02 | 0.26 | 1.45 [−1.24, 4.15] | 18.44 ± 9.76 | 26.09 ± 14.61 | 0.06 | 7.64 [−0.3, 15.59] | 0.12 | −6.19 [−14.33, 1.95] |
. To compare changes in metabolic markers over the treatment period, the “Pre” values (taken before treatment was initiated) were subtracted from the “Post” values to obtain the “Difference” within a treatment group and were assessed by paired t-tests to identify any statistically significant changes in either the RAPA or Placebo groups. The p-values are shown. The direction of the change is indicated in the “Difference column” where a positive number indicates a higher mean value after 6–8 weeks of treatment and a negative value reflects a lower value after treatment. The 95% confidence interval is shown in brackets. The mean values measured are shown ± standard deviation. Individual values for several of these markers and some additional data are shown graphically in Figure 1G,H,J,K,L.
. To determine whether there was a difference in the RAPA vs. the placebo group, the mean “difference” values were compared using a Welch’s t-test. The resulting p-values are shown. The difference mean changes seen in the RAPA and placebo groups are shown with the 95% confidence interval in brackets.
Figure 2. Effect of RAPA on metabolic profile.
Glucose (A), Insulin (B), and FFA (C) concentrations during OGTT, HOMA-IR (D), and Matsuda index (E) were determined as described under “Methods”. Values are mean ± SEM. No statistically significant differences between RAPA and placebo subjects were found.
General parameters of healthy aging were also examined and included body weight, pulse, and blood pressure (Table 6, Figure 1). The only statistically significant finding was a slight decrease in body weight in the RAPA group as compared to the placebos. Given that the differential is quite small, it is not likely to be clinically significant, but weight should be followed in future tests of mTOR inhibition in older subjects. Weight loss was also reported in mice (Miller et al., 2014).
TABLE 6.
Changes in weight and blood pressure during treatment
| Treatment groupa | Group differenceb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLACEBO (n=14) |
RAPA (n=11) | |||||||||
| Pre | Post | P value | Difference (95%CI) | Pre | Post | P value | Difference (95%CI) | P value | Difference (95%CI) | |
| Weight | 76.82 ± 16 | 77.22 ± 15.72 | 0.27 | 0.4 [−0.35, 1.15] | 77.28 ± 15.44 | 76.18 ± 15 | 0.011 | −1.1 [−1.88, −0.32] | 0.006 | 1.5 [0.48, 2.52] |
| Systolic bp | 132.92 ± 17.93 | 131.31 ± 14.17 | 0.78 | −1.62 [−14.22, 10.99] | 134.5 ± 18.22 | 135.1 ± 11.18 | 0.91 | 0.6 [−11.29, 12.49] | 0.78 | −2.22 [−18.47, 14.04] |
| Diastolic bp | 71.46 ± 10.14 | 72.92 ± 11.2 | 0.48 | 1.46 [−2.89, 5.81] | 76.6 ± 9.62 | 73.1 ± 7.74 | 0.09 | −3.5 [−7.7, 0.7] | 0.08 | 4.96 [−0.71, 10.63] |
| Pulse | 72.27 ± 10.88 | 68.82 ± 12.66 | 0.37 | −3.45 [−11.71, 4.8] | 64.62 ± 8.55 | 65.12 ± 9.51 | 0.87 | 0.5 [−6.52, 7.52] | 0.42 | −3.95 [−13.98, 6.07] |
. To compare changes in weight and blood pressure over the treatment period, the “Pre” values (taken before treatment was initiated) were subtracted from the “Post” values to obtain the “Difference” within a treatment group and were assessed by paired t-tests to identify any statistically significant changes in either the RAPA or Placebo groups. The p-values are shown. The direction of the change is indicated in the “Difference column” where a positive number indicates a higher mean value after 6–8 weeks of treatment and a negative value reflects a lower value after treatment. The 95% confidence interval is shown in brackets. The mean values measured are shown ± standard deviation. Individual values for weight are shown graphically in Figure 1I.
. To determine whether there was a difference in the RAPA vs. the placebo group, the mean “difference” values were compared using a Welch’s t-test. The resulting p-values are shown. The difference mean changes seen in the RAPA and placebo groups are shown with the 95% confidence interval in brackets.
Cognitive and physical function testing
Previous studies have suggested that mTOR inhibition could result in improved cognitive function which would be of particular value in this older cohort (Lang et al., 2009; Lin et al., 2013; Majumder et al., 2012). Three different cognitive tests were performed to assess various aspects of memory and executive function as noted in the Methods section (Lang et al., 2009; Lin et al., 2013; Majumder et al., 2012); these data are summarized in Table 7. No significant changes between initial test scores and scores at completion were observed, regardless of the treatment group. Likewise, no statistically significant changes were seen in handgrip strength or walking speed (Table 7). Although RAPA treatment did not improve cognition or physical function, it was also not detrimental. It is possible that longer treatment times will be necessary and/or perhaps treatment must be started at a somewhat younger age in order to be efficacious.
TABLE 7.
Changes in cognitive and physical function during treatment
| Treatment groupa | Group differenceb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLACEBO | RAPA | |||||||||
| Pre | Post | P value | Difference (95%CI) | Pre | Post | P value | Difference (95%CI) | P value | Difference (95%CI) | |
| Cognitive testsc | (n=13) | (n=10) | ||||||||
| EXIT | 6.54 ± 3.73 | 6.92 ± 4.23 | 0.71 | 0.38 [−1.84, 2.61] | 7.3 ± 4.92 | 7.2 ± 4.52 | 0.95 | −0.1 [−3.31, 3.11] | 0.78 | 0.48 [−3.2, 4.17] |
| SLUMS | 24.92 ± 3.23 | 24.54 ± 3.04 | 0.62 | −0.38 [−2.03, 1.26] | 24.3 ± 6.25 | 23.5 ± 4.7 | 0.58 | −0.8 [−3.92, 2.32] | 0.8 | 0.42 [−2.96, 3.79] |
| TAPS | 26.15 ± 6.99 | 25.15 ± 5.94 | 0.34 | −1 [−3.18, 1.18] | 25.67 ± 7.48 | 27.2 ± 8.69 | 0.32 | 1.44 [−1.68, 4.57] | 0.17 | −2.44 [−6.02, 1.13] |
| Physical function | (n=14) | (n=11) | ||||||||
| Grip strength | 22.27 ± 7.54 | 24.07 ± 5.57 | 0.15 | 1.79 [−0.75, 4.33] | 24.33 ± 8.2 | 25.63 ± 6.65 | 0.4 | 1.3 [−1.98, 4.59] | 0.8 | 0.49 [−3.44, 4.41] |
| Walking speed | 7.72 ± 1.6 | 7.17 ± 1.12 | 0.047 | −0.55 [−1.09, −0.01] | 8.8 ± 3.64 | 7.75 ± 1.12 | 0.34 | −1.04 [−3.38, 1.3] | 0.66 | 0.49 [−1.88, 2.86] |
. To compare changes over the treatment period, the “Pre” values (taken before treatment was initiated) were subtracted from the “Post” values to obtain the “Difference” within a treatment group and were assessed by paired t-tests to identify any statistically significant changes in either the RAPA or Placebo groups. The p-values are shown. The direction of the change is indicated in the “Difference column” where a positive number indicates a higher mean value after treatment and a negative value reflects a lower value after treatment. The 95% confidence interval is shown in brackets.
. To determine whether there was a difference in the RAPA vs. the placebo group, the mean “difference” values were compared using a Welch’s t-test. The resulting p-values are shown. The difference mean changes seen in the RAPA and placebo groups are shown with the 95% confidence interval in brackets.
. The “post” values are taken from the 16 week time point for phase 1 and from the 6–8 week timepoint for phase 2. Cognitive tests were done only twice on each subject to avoid complications due to learning.
Cytokines and systemic inflammation
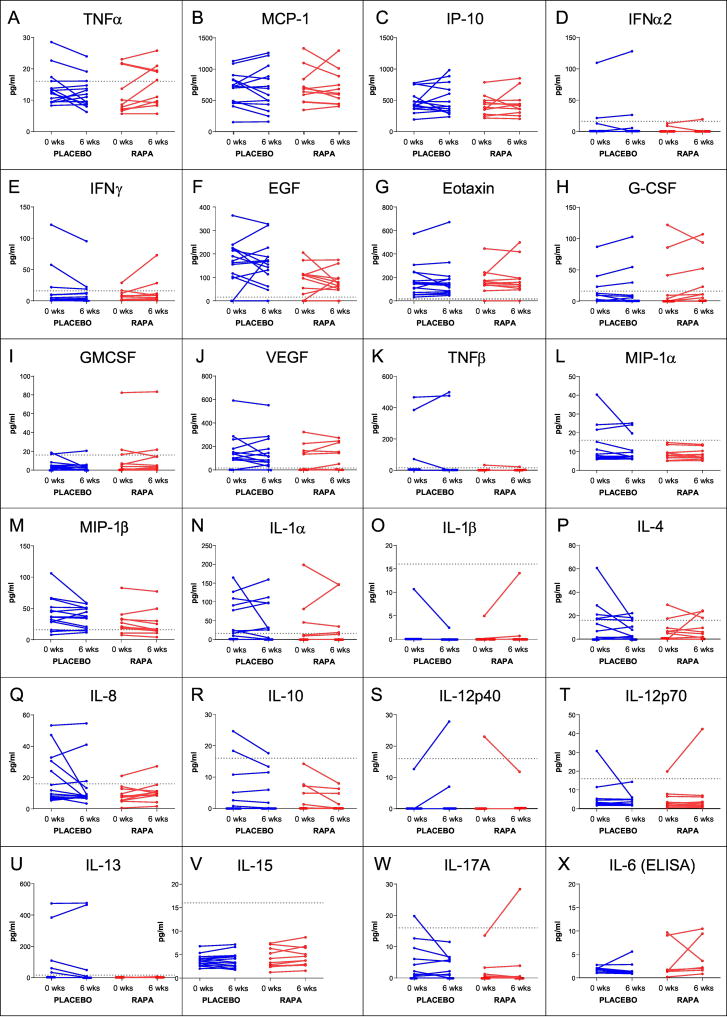
With aging, baseline inflammatory mediators are often increased and it has been suggested that RAPA might be eliciting positive outcomes on several physiological systems through a general anti-inflammatory effect (Buron et al., 2013; De Luna-Preitschopf et al., 2017; Jørgensen et al., 2001; Salehi et al., 2016; Thomson et al., 2009). To address this possibility, cytokine and chemokine levels were measured by a Luminex-based multiplex array prior to initiation of treatment and at 6 weeks. The data showed non-normal distributions where certain cytokines were present at very low levels in serum (as expected), but were much higher in a few of the subjects (Figure 3). Thus, we first analyzed changes in each treatment group (Post minus Pre) using paired t-tests and then used Welch’s t-tests to compare between the groups (Table 8). No statistically significant RAPA (or placebo) effects were seen in serum cytokine levels. However, it was noted that for one pro-inflammatory cytokine, TNF-α, more than half of the RAPA treated subjects showed increases and the remaining individuals either maintained or had very small declines. The placebo group had a more disparate profile showing the typical heterogeneity seen with serum cytokines (Figure 3). Given the small size of our test group and the lack of a uniform effect, the effect of RAPA on TNF-α concentration should be pursued further in a larger cohort.
Figure 3. Effects of RAPA on serum cytokines.
As described in the Methods, the levels of serum cytokines were measured using either a Luminex-based multiplex assay (panels A–W) or a high sensitivity ELISA (panel X) for samples collected either before initiation of treatment (0 weeks) or during the treatment phase (6 weeks). All individual subjects, from both phase 1 and phase 2, are shown. RAPA subjects are shown in red and placebo subject lines are blue. For the Luminex data, the horizontal dotted line on each graph indicates the concentration value for the lowest point on the standard curve; all values below that were extrapolated by the BioRad software. Any point assigned a value of “OOR<” (out of range less than) was considered to be 0 pg/ml for the analysis. RAPA subjects (red) and placebo subjects (blue) are shown and the p-values for RAPA vs. placebo differences are found in Table 8.
TABLE 8.
Changes in serum cytokines during treatment
| Treatment groupa | Group differenceb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLACEBO | RAPA | |||||||||
| Pre | Post | P value | Difference (95%CI) | Pre | Post | P value | Difference (95%CI) | P value | Difference (95%CI) | |
| Luminex | (n=14) | (n=11) | ||||||||
| EGF | 161.60 ± 96.32 | 160.30 ± 92.72 | 0.95 | −1.34 [−44.98, 42.30] | 91.53 ± 65.97 | 82.39 ± 49.52 | 0.62 | −9.14 [−49.31, 31.03] | 0.78 | −7.80 [−63.81, 48.21] |
| Eotaxin | 173.20 ± 140.60 | 175.00 ± 161.10 | 0.90 | 1.859 [−29.12, 32.84] | 172.90 ± 110.90 | 187.20 ± 144.90 | 0.61 | 14.35 [−45.79, 74.48] | 0.69 | 12.49 [−52.48, 77.45] |
| G−CSF | 13.41 ± 24.11 | 15.03 ± 29.74 | 0.40 | 1.62 [−2.43, 5.66] | 23.88 ± 42.16 | 27.65 ± 39.24 | 0.36 | 3.77 [−5.05, 12.59] | 0.63 | 2.15 [−7.22, 11.52] |
| GM-CSF | 4.95 ± 5.88 | 3.69 ± 5.27 | 0.35 | −1.26 [−4.07, 1.54] | 12.58 ± 24.26 | 13.47 ± 24.32 | 0.49 | 0.88 [−1.88, 3.65] | 0.24 | 2.15 [−1.57, 5.86] |
| INFα | 10.27 ± 29.30 | 11.42 ± 34.31 | 0.52 | 1.15 [−2.57, 4.87] | 1.97 ± 4.47 | 1.78 ± 5.82 | 0.85 | −0.20 [−2.51, 2.12] | 0.51 | −1.35 [−5.53, 2.836] |
| INFγ | 17.53 ± 33.50 | 13.49 ± 24.64 | 0.21 | −4.05 [−10.70, 2.60] | 8.55 ± 8.26 | 13.99 ± 20.93 | 0.23 | 5.44 [−4.00, 14.87] | 0.09 | 9.49 [−1.46, 20.43] |
| IL-1rα | 91.31 ± 200.10 | 96.22 ± 237.90 | 0.80 | 4.90 [−35.19, 44.99] | 6.35 ± 15.40 | 11.01 ± 26.49 | 0.21 | 4.66 [−3.10, 12.41] | 0.99 | −0.24 [−40.77, 40.28] |
| IL-1α | 45.88 ± 56.35 | 39.71 ± 53.53 | 0.57 | −6.17 [−29.18, 16.84] | 31.47 ± 61.12 | 32.57 ± 57.15 | 0.89 | 1.10 [−16.91, 19.11] | 0.59 | 7.27 [−20.42, 34.96] |
| IL-1β | 0.76 ± 2.85 | 0.18 ± 0.66 | 0.34 | −0.59 [−1.86, 0.68] | 0.47 ± 1.49 | 1.36 ± 4.23 | 0.31 | 0.89 [−0.95, 2.73] | 0.16 | 1.48 [−0.64, 3.60] |
| IL-2 | 0.00 ± 0.00 | 0.01 ± 0.04 | 0.34 | 0.01 [−0.01, 0.03] | Difference = 0; cannot calculate p value | 0.34 | −0.01 [−0.03, 0.01] | |||
| IL-3 | 1.11 ± 0.51 | 0.98 ± 0.30 | 0.31 | −0.13 [−0.40, 0.14] | 1.13 ± 0.37 | 1.22 ± 0.39 | 0.37 | 0.09 [−0.13, 0.31] | 0.17 | 0.23 [−0.10, 0.55] |
| IL-4 | 11.88 ± 17.04 | 7.05 ± 8.11 | 0.18 | −4.83 [−12.28, 2.62] | 6.84 ± 9.25 | 8.15 ± 9.54 | 0.63 | 1.31 [−4.60, 7.22] | 0.17 | 6.14 [−2.86, 15.15] |
| IL-5 | 7.38 ± 17.18 | 7.96 ± 19.93 | 0.66 | 0.58 [−2.21, 3.37] | 0.00 ± 0.00 | 0.08 ± 0.20 | 0.23 | 0.08 [−0.06, 0.21] | 0.70 | −0.51 [−3.30, 2.28] |
| IL-6 | 2.37 ± 8.87 | 0.41 ± 1.53 | 0.34 | −1.96 [−6.20, 2.28] | 0.56 ± 1.86 | 2.04 ± 4.02 | 0.32 | 1.47 [−1.69, 4.64] | 0.17 | 3.44 [−1.583, 8.454] |
| IL-7 | Difference = 0; cannot calculate p value | |||||||||
| IL-8 | 19.00 ± 16.09 | 14.34 ± 14.81 | 0.17 | −4.66 [−11.53, 2.21] | 8.86 ± 5.54 | 10.72 ± 6.57 | 0.10 | 1.86 [−0.40, 4.12] | 0.07 | 6.52 [−0.57, 13.61] |
| IL-10 | 4.45 ± 7.94 | 3.59 ± 6.07 | 0.18 | −0.86 [−2.17, 0.46] | 3.21 ± 4.74 | 1.86 ± 3.01 | 0.10 | −1.36 [−3.02, 0.31] | 0.61 | −0.50 [−2.50, 1.50] |
| IL-12p40 | 0.90 ± 3.38 | 2.49 ± 7.54 | 0.19 | 1.59 [−0.92, 4.10] | 2.09 ± 6.94 | 1.07 ± 3.56 | 0.34 | −1.02 [−3.29, 1.25] | 0.11 | −2.61 [−5.80, 0.59] |
| IL-12p70 | 5.41 ± 7.72 | 3.73 ± 3.36 | 0.36 | −1.68 [−5.55, 2.18] | 4.63 ± 5.50 | 6.77 ± 11.99 | 0.32 | 2.14 [−2.44, 6.72] | 0.17 | 3.82 [−1.84to 9.48] |
| IL-13 | 75.71 ± 154.00 | 71.32 ± 169.80 | 0.62 | −4.39 [−23.23, 14.45] | 0.44 ± 1.31 | 0.55 ± 1.82 | 0.88 | 0.11 [−1.47, 1.70] | 0.62 | 4.51 [−14.37, 23.39] |
| IL-15 | 3.81 ± 1.22 | 3.70 ± 1.70 | 0.61 | −0.11 [−0.53, 0.32] | 4.10 ± 2.12 | 4.46 ± 2.13 | 0.17 | 0.35 [−0.18, 0.89] | 0.16 | 0.46 [−0.19, 1.11] |
| IL-17A | 4.07 ± 6.02 | 2.83 ± 6.62 | 0.24 | −1.24 [−3.41, 0.93] | 1.75 ± 4.04 | 3.04 ± 8.47 | 0.36 | 1.29 [−1.74, 4.32] | 0.15 | 2.54 [−1.00, 6.07] |
| IP−10 | 480.20 ± 177.10 | 504.40 ± 241.10 | 0.64 | 24.20 [−84.89, 133.30] | 418.00 ± 164.00 | 445.30 ± 201.30 | 0.56 | 27.30 [−74.66, 129.30] | 0.96 | 3.09 [−137.9, 144.1] |
| MCP-1 | 689.40 ± 270.60 | 674.50 ± 344.90 | 0.75 | −14.92 [−113.10, 83.24] | 717.80 ± 285.40 | 696.10 ± 274.70 | 0.73 | −21.72 [−157.80, 114.30] | 0.93 | −6.81 [−165.8, 152.2] |
| MIP-1α | 12.44 ± 10.01 | 10.71 ± 6.90 | 0.28 | −1.73 [−5.08, 1.61] | 8.63 ± 3.12 | 8.44 ± 2.85 | 0.53 | −0.19 [−0.83, 0.46] | 0.34 | 1.54 [−1.84, 4.92] |
| MIP-1β | 41.47 ± 25.68 | 35.73 ± 16.88 | 0.16 | −5.74 [−14.09, 2.62] | 28.33 ± 20.57 | 24.97 ± 21.12 | 0.06 | −3.37 [−6.97, 0.24] | 0.58 | 2.37 [−6.46, 11.2] |
| TNFα | 14.07 ± 5.66 | 12.45 ± 4.84 | 0.11 | −1.63 [−3.68, 0.43] | 12.08 ± 6.80 | 14.00 ± 6.59 | 0.10 | 1.91 [−0.41, 4.23] | 0.02 | 3.54 [0.62, 6.47] |
| TNFβ | 65.94 ± 154.50 | 69.62 ± 177.00 | 0.72 | 3.68 [−17.71, 25.07] | 3.03 ± 10.06 | 2.03 ± 6.72 | 0.34 | −1.01 [−3.25, 1.24] | 0.65 | −4.69 [−26.14, 16.77] |
| VEGF | 151.80 ± 153.40 | 138.90 ± 147.60 | 0.47 | −12.90 [−50.32, 24.53] | 90.65 ± 113.70 | 100.10 ± 112.40 | 0.40 | 9.47 [−14.74, 33.69] | 0.29 | 22.37 [−20.16, 64.89] |
| ELISA | (n=8) | (n=7) | ||||||||
| IL6 | 1.79 ± 0.56 | 1.92 ± 1.61 | 0.80 | 0.13 [−1.08, 1.34] | 3.56 ± 4.00 | 4.33 ± 3.94 | 0.64 | 0.76 [−3.02, 4.55] | 0.71 | 0.63 [−3.12, 4.45] |
. Changes in serum cytokine levels were assessed either by Luminex-based array assays or by high sensitivity ELISA, as indicated. Values that were “OOR<” (out of range low) were converted to 0 (zero) for the statistical analysis. Values below 16 pg/ml (the concentration of the lowest standard) were extrapolated. The differences between pre-treatment values and those seen at 6 weeks of treatment are shown for both the RAPA and placebo groups. “Differences” due to treatment were assessed by paired t-tests to identify any statistically significant changes in either group and the p-values are shown. The direction of the change is indicated in the “Difference column” where a positive number indicates a higher mean value after treatment and a negative value reflects a decrease in that cell population. The 95% confidence interval is shown in brackets.
. To determine whether there was a difference in the RAPA vs. the placebo group, the mean “difference” values were compared using a Welch’s t-test. The resulting p-values are shown; the mean differences and 95% confidence interval are shown.
We did not detect a general RAPA-associated decline in pro-inflammatory serum cytokines. Yet, the Luminex-based assays are not designed to have sufficiently high sensitivity to detect small changes in cytokines, like IL-6, that are present in relatively low levels in serum. Since IL-6 is often seen to increase with aging and reversal of this effect by RAPA would be of interest, sera samples from 15 of the phase 2 subjects were analyzed using a high sensitivity ELISA for IL-6. As shown in Figure 3 (data analysis summarized in Table 8), RAPA did not affect IL-6 levels in the serum.
Blood cell subsets
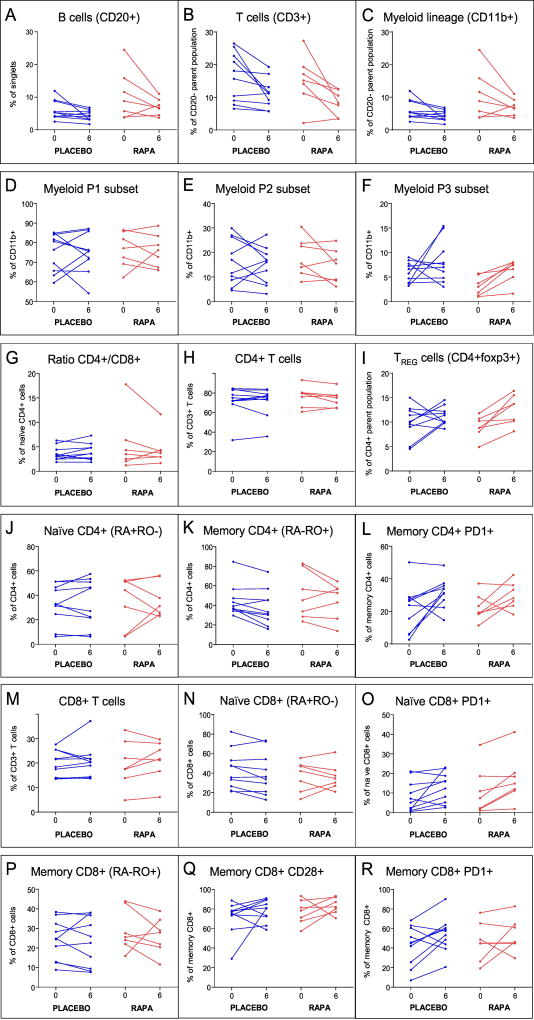
To assess whether short-term RAPA treatment could elicit significant changes in T cell subsets or other blood cell lineages, flow cytometry was performed on the second phase trial subjects using PBMCs harvested either before treatment or at the 6-week time point. The cell subsets examined and resulting p-values comparing the RAPA treatment group to placebo are shown in Table 9 and a subset of the data is shown in Figure 4. Although we had expected to see a RAPA effect on memory T cells, particularly those in the CD8+ subset, no statistically significant change (relative to placebo) was observed. Similarly, we did not detect a decrease in the proportion of cells expressing PD-1, a marker of T cell exhaustion, which had been previously reported both in mice and humans (Hurez et al, 2015; Mannick et al., 2014). Of note, the only cell population in this older cohort that was consistently increased by RAPA treatment and had a significant p value when compared to the placebo was a small myeloid subset defined by CD11b+, CD3low, and a unique side scatter profile (subset P3, Figure 4F); the gating strategy is shown in Supplemental Figure 2. This cell subset may be unique to this older cohort. Of note, Dr. Pan Zheng and colleagues (Childrens National Medical Center, Washington, DC) have shown RAPA effects on a specific myeloid subset in mice (personal communication, in press); efforts are underway to assess whether the cell type affected in our treated human subjects is similar.
TABLE 9.
Changes in blood cell subsets during treatment
| Treatment groupa | Group differenceb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLACEBO (n=10) | RAPA (n=7) | |||||||||
| Pre | Post | P value | Difference (95%CI) | Pre | Post | P value | Difference (95%CI) | P value | Difference (95%CI) | |
| PANEL 1 | ||||||||||
| B cells (CD20+) | 2.72 ± 1.58 | 2.24 ± 1.64 | 0.002 | −0.48 [−0.73, −0.23] | 3.2 ± 1.42 | 2.43 ± 1.54 | 0.13 | −0.77 [−1.83, 0.29] | 0.54 | −0.29 [−1.36, 0.77] |
| T cells (CD3+) | 16.3 ± 7.55 | 11.24 ± 4.45 | 0.02 | −5.06 [−9.14, −0.98] | 15.2 ± 7.7 | 8.33 ± 3.82 | 0.032 | −6.87 [−12.91, −0.83] | 0.56 | −1.81 [−8.48, 4.86] |
| monocyte (CD11b+) | 6.07 ± 2.92 | 4.35 ± 1.62 | 0.08 | −1.72 [−3.68, 0.24] | 10.59 ± 7.52 | 7.04 ± 2.54 | 0.14 | −3.54 [−8.7, 1.62] | 0.45 | −1.82 [−7.08, 3.43] |
| P1 subset (see Supplemental Figure 2) | 75.11 ± 9.77 | 75.06 ± 10.27 | 0.99 | −0.05 [−7.03, 6.93] | 76.47 ± 9 | 76.2 ± 8.25 | 0.93 | −0.27 [−7.14, 6.6] | 0.96 | −0.22 [−9.12, 8.68] |
| P2 subset (see Supplemental Figure 2) | 16.04 ± 9.35 | 14.79 ± 7.44 | 0.62 | −1.25 [−6.83, 4.33] | 17.97 ± 7.88 | 14.5 ± 6.91 | 0.21 | −3.47 [−9.49, 2.55] | 0.53 | −2.22 [−9.67, 5.23] |
| P3 subset (see Supplemental Figure 2) | 6 ± 2.09 | 7.1 ± 3.47 | 0.43 | 1.1 [−1.9, 4.1] | 3.13 ± 1.94 | 6.31 ± 2.33 | 0.005 | 3.19 [1.37, 5] | 0.19 | 2.09 [−1.18, 5.35] |
| PANEL 2 | ||||||||||
| TH cells (CD4+ CD3+) | 72.1 ± 15.26 | 71.5 ± 14.62 | 0.71 | −0.6 [−4.19, 2.99] | 76.49 ± 10.77 | 74.11 ± 8.61 | 0.21 | −2.37 [−6.5, 1.76] | 0.46 | −1.77 [−6.74, 3.2] |
| TC cells (CD8+ CD3+) | 19.91 ± 5.28 | 20.6 ± 6.82 | 0.59 | 0.69 [−2.09, 3.47] | 19.74 ± 9.53 | 21.27 ± 8.02 | 0.33 | 1.53 [−2, 5.06] | 0.67 | 0.84 [−3.25, 4.93] |
| dp: CD4+ CD8+ CD3+ | 5.65 ± 15.06 | 5.46 ± 13.36 | 0.77 | −0.19 [−1.61, 1.23] | 1.36 ± 1.42 | 1.84 ± 1.64 | 0.2 | 0.49 [−0.35, 1.32] | 0.36 | 0.68 [−0.86, 2.21] |
| dn: CD4−CD8− CD3+ | 2 ± 1.08 | 1.87 ± 0.89 | 0.79 | −0.13 [−1.21, 0.95] | 1.93 ± 0.87 | 2.46 ± 1.31 | 0.23 | 0.53 [−0.45, 1.5] | 0.31 | 0.66 [−0.67, 1.98] |
| TREGS Foxp3+ CD4+ | 9.93 ± 3.28 | 11.2 ± 1.85 | 0.28 | 1.27 [−1.23, 3.77] | 9.03 ± 2.24 | 12.59 ± 3.05 | 0.008 | 3.56 [1.35, 5.76] | 0.13 | 2.29 [−0.75, 5.33] |
| PANEL 3 | ||||||||||
| T cells (CD3+) | 11.45 ± 5.43 | 9.69 ± 5.32 | 0.22 | −1.76 [−4.77, 1.25] | 10.34 ± 5.72 | 5.93 ± 3.01 | 0.12 | −4.41 [−10.39, 1.56] | 0.36 | −2.65 [−8.89, 3.58] |
| CD4+ T cell subset | 70.44 ± 15.06 | 71.05 ± 15.57 | 0.73 | 0.61 [−3.3, 4.52] | 72.87 ± 13.67 | 74.3 ± 8.82 | 0.67 | 1.43 [−6.3, 9.16] | 0.82 | 0.82 [−7.26, 8.89] |
| Naïve CD4+ (CD45RA+RO−) | 32.85 ± 16.24 | 33.91 ± 19.19 | 0.65 | 1.06 [−4.01, 6.13] | 34.8 ± 20.61 | 36.29 ± 14.33 | 0.81 | 1.49 [−13.19, 16.17] | 0.95 | 0.43 [−14.45, 15.3] |
| Naïve CD4+ expressing PD1 | 0.85 ± 0.66 | 2.4 ± 4.65 | 0.33 | 1.55 [−1.87, 4.97] | 1.79 ± 2.08 | 1.39 ± 1.12 | 0.36 | −0.4 [−1.39, 0.59] | 0.24 | −1.95 [−5.42, 1.52] |
| Memory CD4+ (CD45RA−RO+) | 44.43 ± 16.01 | 37.67 ± 18.05 | 0.016 | −6.76 [−11.95, −1.57] | 50.2 ± 24.21 | 44.87 ± 18.48 | 0.32 | −5.33 [−17.38, 6.73] | 0.8 | 1.43 [−10.95, 13.81] |
| Memory CD4+ expressing PD1 | 21.49 ± 14.37 | 31.54 ± 9.04 | 0.047 | 10.05 [0.18, 19.92] | 22.36 ± 8.38 | 29.66 ± 8.19 | 0.11 | 7.3 [−2.38, 16.98] | 0.65 | −2.75 [−15.31, 9.81] |
| CD8+ T cell subset | 20.95 ± 5.24 | 19.72 ± 5.56 | 0.34 | −1.23 [−3.97, 1.51] | 22.57 ± 12.11 | 21.27 ± 8.31 | 0.62 | −1.3 [−7.39, 4.79] | 0.98 | −0.07 [−6.35, 6.21] |
| Naïve CD8+ T cells (CD45RA+RO−) | 43.72 ± 20.08 | 39.49 ± 21.43 | 0.06 | −4.23 [−8.63, 0.17] | 37 ± 15.43 | 36.4 ± 13.13 | 0.88 | −0.6 [−9.85, 8.65] | 0.41 | 3.63 [−5.96, 13.22] |
| Naïve CD8+ expressing CD28 | 12.95 ± 10.31 | 9.34 ± 11.58 | 0.41 | −3.61 [−13.15, 5.93] | 15.97 ± 13.62 | 18.21 ± 12.84 | 0.67 | 2.24 [−9.91, 14.4] | 0.39 | 5.85 [−8.2, 19.91] |
| Naïve CD8+ expressing PD1 | 8.34 ± 7.86 | 12.8 ± 7.69 | 0.07 | 4.46 [−0.51, 9.43] | 10.91 ± 12.18 | 17.1 ± 12.2 | 0.016 | 6.19 [1.64, 10.73] | 0.56 | 1.73 [−4.41, 7.86] |
| Memory CD8+ T cells (CD45RA−RO+) | 24.04 ± 10.34 | 23.39 ± 12.62 | 0.75 | −0.65 [−5.19, 3.89] | 28.86 ± 10.67 | 26.29 ± 9.2 | 0.55 | −2.57 [−12.44, 7.3] | 0.68 | −1.92 [−12.12, 8.27] |
| Memory CD8+ expressing CD28 | 71.71 ± 16.8 | 78.41 ± 11.32 | 0.29 | 6.7 [−6.92, 20.32] | 76.91 ± 12.45 | 83.17 ± 8.19 | 0.31 | 6.26 [−7.45, 19.96] | 0.96 | −0.44 [−18, 17.11] |
| Memory CD8+ expressing PD1 | 42.61 ± 20.21 | 53.67 ± 18.1 | 0.07 | 11.06 [−0.9, 23.02] | 46.54 ± 19.99 | 53.5 ± 17.24 | 0.38 | 6.96 [−10.99, 24.91] | 0.66 | −4.1 [−23.85, 15.65] |
| Transitional CD8+ (CD45RA+RO+) | 31.08 ± 14.36 | 36.6 ± 16.37 | 0.11 | 5.52 [−1.62, 12.66] | 32.99 ± 11.87 | 36.5 ± 12.57 | 0.56 | 3.51 [−10.28, 17.3] | 0.76 | −2.01 [−16.46, 12.44] |
| Transitional CD8+ expressing CD28 | 49.61 ± 25.99 | 59.74 ± 16.99 | 0.1 | 10.13 [−2.53, 22.79] | 64.83 ± 15.97 | 71.79 ± 15.99 | 0.36 | 6.96 [−10.13, 24.05] | 0.73 | −3.17 [−22.56, 16.21] |
| Transitional CD8+ expressing PD1 | 23.55 ± 24.93 | 40.51 ± 17.2 | 0.01 | 16.96 [5.07, 28.85] | 35.06 ± 13.47 | 45.93 ± 15.04 | 0.1 | 10.87 [−2.98, 24.73] | 0.44 | −6.09 [−22.67, 10.49] |
| Ratio of CD4+/CD8+ | 3.59 ± 1.45 | 3.93 ± 1.7 | 0.27 | 0.34 [−0.31, 0.99] | 5.38 ± 5.72 | 4.47 ± 3.29 | 0.4 | −0.92 [−3.4, 1.57] | 0.27 | −1.25 [−3.75, 1.24] |
. Changes in PBMC populations were assessed by flow cytometry for the subjects in the Phase 2 study. The differences between pre-treatment values and those seen at 6 weeks of treatment are shown for both the RAPA and placebo groups. “Differences” due to treatment were assessed by paired t-tests to identify any statistically significant changes in either group and the p-values are shown. The direction of the change is indicated in the “Difference column” where a positive number indicates a higher mean value after treatment and a negative value reflects a decrease in that cell population. The 95% confidence interval is shown in brackets.
. To determine whether there was a difference in the RAPA vs. the placebo group, the mean “difference” values were compared using a Welch’s t-test. The resulting p-values are shown; the mean differences and 95% confidence interval are shown.
Figure 4. Effects of RAPA on blood cell subsets.
PBMCs from the phase 2 subjects, purified from blood collected at the indicated time points, were stained using the three antibody panels described in the Methods section. The flow cytometry data were analyzed using Diva software. Shown are results from Panel 1 (A–F), Panel 2 (H,I), and Panel 3 (G,J–R). RAPA subjects (red) and placebo subjects (blue) are shown and the p-values for RAPA vs. placebo differences are found in Table 9 for these subsets and for additional subsets not shown.
Another shift in immune function-related parameters caused by RAPA was an increase in circulating TREGS (foxp3+CD4+). The calculated p value for the difference between “post” and “pre” for the RAPA group was highly significant at 0.008. However, when Welch’s t-test was performed to ask whether the RAPA effect was significant when compared to the placebo group; the p-value was 0.13. The sample size in this pilot study was not sufficiently powered to meet the higher stringency required of the latter test. Nonetheless, the trend towards an expanded TREG population was already evident at six weeks of RAPA treatment.
Discussion
We undertook a randomized, placebo controlled trial to define the feasibility of enrolling generally healthy older (aged 70–93 years) volunteers to study tolerability, safety and effects of RAPA on several functional parameters relevant to aging. Most prior studies that assessed in humans were performed in populations of somewhat younger individuals. Moreover, many of them were focused on subjects with serious health conditions such as cancer or organ transplant and the results could have therefore been confounded by the underlying condition or other medications being taken (Eiden et al., 2016; Sankhala et al., 2009). In the current study of older persons, whose medical conditions are stable, RAPA was well tolerated with no unanticipated side effects.
Subjects received either 1 mg of RAPA daily (or placebo) with resultant plasma levels of 7.2±2ng/ml. The toxicities seen were consistent with those previously reported in populations where the agent was used as an adjunct immunosuppressant. For example, the reductions in erythrocyte-related parameters we noted in our RAPA cohort were similar to those reported in renal transplant patients treated with RAPA, although subjects in this trial showed milder microcytosis/anemia as compared to the marked microcytosis with mild anemia seen in the transplant recipients (Sofroniadou et al., 2010). The changes we noted did not manifest themselves in any clinically remarkable ways. Whether these changes would become more marked with prolonged administration is unclear pending a larger trial of longer duration.
One objection to the use of RAPA in older adults derives from the diabetogenic effects that have been seen to occur in treated transplant patients and indeed, a very slight increase in A1c was observed in the RAPA treatment group. However, we found no statistically significant difference in plasma glucose, insulin, and FFA levels at baseline and during OGTT between those subjects receiving RAPA or the placebo. Likewise, indices of insulin sensitivity/resistance (HOMA-IR, Matsuda) were similar in RAPA and placebo groups, including decreases in FFA level during OGTT (caused by insulin), which denotes insulin sensitivity in the fat cell. Lastly, we found no significant increase in plasma lipids including serum triglycerides which had been reported in younger patient populations (Sankhala et al., 2009).
Tests of cognitive function were performed prior to treatment with RAPA or placebo and repeated only at the end of the trials to minimize learning effects that could confound results. We found no significant changes in either the EXIT-25, SLUMS, or TAPS. Interestingly, the person who scored most poorly on initial testing among all the subjects appeared to improve in these cognitive measures and also showed an increase in walking speed of nearly 10 seconds to walk 40 feet. Anecdotally, this subject’s family also reported that “we saw a slight increase in his cognitive and memory abilities on the med”. Furthermore, they noted that since termination of the drug “his short term memory…much worse”. Given these anecdotal observations and the results of uncontrolled pilot study by Lang et al. suggesting that the rapalog, everolimus improved cognition in human cardiac transplant patients (Lang et al., 2009), additional investigation into cognitive effects of RAPA and rapalogs with a larger samples size may be warranted.
The effects of RAPA are pleiotropic and the consequences of treatment may be different in older subjects than younger ones. Work in animal models suggests that RAPA is often devoid of effects in young but not older animals. For example, Lesniewski, et al. (Lesniewski et al., 2017) found that RAPA treatment led to significant improvement in several cardiovascular parameters in older mice, yet failed to elicit a similar response in younger animals. In line with these findings, our investigations into the effects of mTOR antagonism on endothelial function suggested increased endothelium-mediated vasodilation in middle-aged persons that was not observed in younger individuals (Kellogg, in preperation).
As already discussed, similar age-related differences in RAPA effects have been reported for immunological function. Indeed, many of the expected alterations in cytokine profiles and PBMC subsets were not seen in this older cohort. Specifically, PD-1, a cell surface marker of T cell exhaustion, previously shown to decrease expression in RAPA treated mice (Hurez et al., 2015), appeared to increase on naïve CD8+ cells and was not decreased on any of the populations analyzed. Similarly, there was no significant change detected in the naïve/memory T cell profile when post-treatment values were compared to baseline frequencies. Lastly, although it had been reported that mTOR inhibition led to decreased levels of TNF-α (Yard et al., 1993) and de-stabilization of its mRNA (Park et al., 2012), we did not observe diminished levels in our study. Therefore, it would appear that immune consequences of RAPA treatment in older individuals may differ from those seen in younger subjects. Importantly, RAPA had no unanticipated detrimental effects in this cohort, therefore trials of longer duration and larger size with emphasis on specific parameters that improve in animal models should be feasible in older persons and will be necessary to better understand the potential to modulate aging-related outcomes by mTOR inhibitors.
Supplementary Material
Acknowledgments
We thank Dr. Ben Daniel, Ms. Karla Gorena, and the UTHSCSA Core Flow Cytometry Facility for their helpful insights. We also appreciated extensive assistance from Ms. Joan Hecht, Ms. Lauri Che Kelly, Ms. Deborah Mote, and Mr. (Michael) Tony Biaglow who handled the billing, IRB, and pharmaceutical logistics, respectively. We also thank the staff of the South Texas Veterans Health Care System Frederic C. Bartter General Clinical Research Center. This study was supported by the UTHSA IIMS-CTSA (UL1 TR001120), NIDDK (R01-DK80157 and R01-DK089229 to NM), the American Diabetes Association (NM), the San Antonio Claude D. Pepper Older Americans Independence Center (P30 AG044271) and the San Antonio Nathan Shock Center of Excellence in the Biology of Aging (P30 AG021890).
Abbreviations
- ALK PHOS
Alkaline phosphatase
- ALT
alanine aminotransferase (serum glutamate-pyruvate transaminase (SGPT))
- AST
aspartate aminotransferase (serum glutamic oxaloacetic transaminase (SGOT))
- BUN
blood urea nitrogen
- CA
calcium
- CI
confidence interval
- CLOX
CLOX test (clock drawing test of executive function used as screening tool)
- CMP
complete metabolic panel
- CNI
calcineurin inhibitors
- COPD
chronic obstructive pulmonary disease
- CPT
cell preparation tube
- CREAT
creatinine
- dn
double negative (CD4−CD8−)
- dp
double positive (CD4+CD8+)
- ECG
electrocardiogram
- EXIT25
Executive Interview-25
- FBS
fetal bovine serum
- GERD
gastroesophageal reflux disease
- HCTZ
hydrochlorothiazide
- Hct
hematocrit
- HDL
high-density lipoproteins
- HgB
hemoglobin
- HbA1c
hemoglobin A1c
- LDL
low-density lipoprotein
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin content
- MCV
mean corpuscular volume
- mTOR
mechanistic target of rapamycin
- OGTT
Oral glucose tolerance test
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffered saline
- PLTS
platelets
- RAPA
rapamycin
- RBC
red blood cell count
- RDW
red blood cell distribution width
- RIA
radioimmunoassay
- SLUMS
Saint Louis University Mental Status Exam
- TAPS
Texas Assessment of Processing Speed
- TBILI
total bilirubin
- TRIG
triglycerides
- VLDL
very low density lipoprotein
- WBC
white blood cell count
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Harrison DE, Strong R, Sharp ZD, Nelson JD, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MJ, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MJ, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin but not resveratrol or simvastatin, extends life span of genetically heterogenous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A-L, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih Y-YI, Muir E, Fonseca RS, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J. Cerebral Blood Flow & Metab. 2013;33:1412–1421. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 2012;11:326–35. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang UE, Heger J, Willbring M, Domula M, Matschk K, Tugtekin SM. Immunosuppression using the Mammalian Target of Rapamycin (mTOR) Inhibitor Everolimus: Pilot study shows significant cognitive and affective improvement. Transplant. Proceed. 2009;41:4285–4288. doi: 10.1016/j.transproceed.2009.08.050. [DOI] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan C-C, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. GeroScience. 2017;39:117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell R, Klicus J, Galet S. Inhibition of the immune response by rapamycin a new antifungal antibiotic. Can J Physiol Pharmacol. 1976;55:48–51. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- Araki K, Ellebedy A, Ahmed R. TOR in the immune system. Curr Opinion in Cell Biology. 2011;23:1–9. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T, Saemann M. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Janes M, Fruman D. Immune regulation by rapamycin: moving beyond T cells. Sci Signal. 2009;2:pe25. doi: 10.1126/scisignal.267pe25. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner A, Shaffer V, Gangappa S, Keller S, Bachmann M, Larsen C, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–111. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Kato K, Jiang J, Wahl D, Mineish iS, Fisher E, Murasko D, Glick G, Zhang Y. Characterization of the metabolic phenotype of rapamycin-treated CD8+ T cells with augmented ability to generate long-lasting memory cells. PLoS ONE. 2011;6:e20107. doi: 10.1371/journal.pone.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Li Q, Shrikant P. Fine-tuning CD8(+) T cell functional responses: mTOR acts as a rheostat for regulating CD8(+) T cell proliferation, survival and differentiation? Cell Cycle. 2010;9:2996–3001. doi: 10.4161/cc.9.15.12359. [DOI] [PubMed] [Google Scholar]
- Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, Ford ML, Ahmed R, Kirk AD, Larsen CP. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Amer Journal of Transplantation. 2011;11:613–618. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Golovina T, Mikheeva T, June C, Riley J. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J. Immunol. 2008;180:5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo M. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- Delgoffe G, Pollizzi K, Waickman A, Heikamp E, Meyers D, Horton M, Xiao B, Worley P, Powell J. The kinase mTOR regulates the differentiation of helper T cells through the activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1057. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside T. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS ONE. 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu Y, Zheng P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. Journal of Clin Invest. 2010;120:4091–4101. doi: 10.1172/JCI43873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushaben E, Kramer E, Brandt E, Khurana Hershey G, Le Cras T. Rapamycin attenuates airway hyperreactivity, goblet cells, and IgE in Experimental Asthma. J. Immunol. 2011;187:5756–5763. doi: 10.4049/jimmunol.1102133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Barron A, Pinera-Haces C, Gomez-Alamillo C, Santiuste-Torcida I, Ruiz J, Buelta-Carrillo L, Merino R, deFrancisco A, Arias M. Prevention of lupus in NZBxW mice by sirolimus. Lupus. 2007;16:775–781. doi: 10.1177/0961203307081401. [DOI] [PubMed] [Google Scholar]
- Teachey DT, Obzut DA, Axsom K, Choi JK, Goldsmith KC, Hall J, Hulitt J, Manno CS, Maris JM, Rhodin N, Sullivan KE, Brown VI, Grupp SA. Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS) Blood. 2009a;108:1965–1971. doi: 10.1182/blood-2006-01-010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piemonti L, Maffi P, Monti L, Lampasona V, Perseghin G, Magistretti P, Secchi A, Bonifacio E. Beta cell function during rapamycin monotherapy in long term type 1 diabetes. Diabetologia. 2011;54:433–439. doi: 10.1007/s00125-010-1959-6. [DOI] [PubMed] [Google Scholar]
- Teachey D, Jubelirer T, Baluarte H, Wade A, Manno C. Treatment with sirolimus ameliorates tacrolimus-induced autoimmune cytopenias after solid organ transplant. Pediatr Blood Cancer. 2009b;53:1114–1116. doi: 10.1002/pbc.22183. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Romero-Aleshire M, Renkema K, Ventevogel M, Chew W, Uhrlaub J, Smithey M, Limesand K, Sempowsk G, Brooks H, Nickolich-Zugich J. Lifespan extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell. 2015;14:130–138. doi: 10.1111/acel.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos L, Polinsky M, Steele G, Mines D, Blum M, Caulfield M, Adamkovic A, Liu Q, Harler MB, Hahn C, Singh A. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplantation Proceedings. 2008;40:1407–1410. doi: 10.1016/j.transproceed.2008.03.084. [DOI] [PubMed] [Google Scholar]
- Ozaki KS, Camara NO, Galante NZ, Camargo LF, Pacheco-Silva A. Decreased Cytomegalovirus infection after antilymphocyte therapy in sirolimus-treated renal transplant patients. International Immunopharmacology. 2005;5:103–106. doi: 10.1016/j.intimp.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Barozzi P, Bonini C, Potenza L, Masetti M, Cappelli G, Gruarin P, Whitby D, Gerunda GE, Mondino A, Riva G, Vallerini D, Quadrelli C, Bosco R, Ciceri F, Bordignon C, Schulz TF, Torelli G, Luppi M. Changes in the immune responses against human herpesvirus-8 in the disease course of posttransplant Kaposi sarcoma. Transplantation. 2008;86:738–744. doi: 10.1097/TP.0b013e318184112c. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Lapenta C, Donati S, Spada M, Ranazzi A, Cacopardo B, Mangano K, Belardelli F, Perno C, Aquaro S. Inhibition of human immunodeficiency virus (HIV-1) infection in human peripheral blood leucocytes-SCID reconstituted mice by rapamycin. Clinical and Experimental Immunology. 2009;155:28–34. doi: 10.1111/j.1365-2249.2008.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri M, Realegeno S, Jo E, Modlin R. Role of autophagy in the host response to microbial infection and potential for therapy. Curr Opin Immunol. 2011;23:65–70. doi: 10.1016/j.coi.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath C, Lindsey D, Dhandayuthapani S, Xu Y, Hunter R, Eissa N. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267. doi: 10.1038/nm.1928. Nat Med 15, 267–276. [DOI] [PubMed] [Google Scholar]
- Kumar D, Blumberg E, Danziger-Isakov L, Kotton C, Halasa N, Ison M, Avery R, Green M, Allen U, Edwards K, Miller G, Michaels M. Influenza vaccination in the organ transplant recipient: Review and summary recommendations. Amer Journal of Transplantation. 2011;11:2020–2030. doi: 10.1111/j.1600-6143.2011.03753.x. [DOI] [PubMed] [Google Scholar]
- Hayney M, Welter D, Francois M, Reynolds M, Love R. Influenza vaccine antibody responses in lung transplant recipients Prog Transplant. 2004;14:346–351. doi: 10.1177/152692480401400410. [DOI] [PubMed] [Google Scholar]
- Willcocks L, Chaudhry A, Smith J, Ojha S, Doffinger R, Watson C, Smith K. The effect of sirolimus therapy on vaccine responses in transplant recipients. Amer Journal of Transplant. 2007;7:2006–2011. doi: 10.1111/j.1600-6143.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- Mannick JB, Giudice GD, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB. mTOR inhibition improves immune function in the elderly. Science Translational Medicine. 2014;6:1–5. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- Apelo SIA, Lamming DW. Rapamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island. J Gerontol A Biol Sci Med Sci. 2016;71:841–849. doi: 10.1093/gerona/glw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden AM, Zhang S, Gary JM, Simmons JK, Mock BA. Molecular Pathways: Increased Susceptibility to Infection Is a Complication of mTOR Inhibitor Use in Cancer Therapy. Clin. Cancer Res. 2016;22:277–283. doi: 10.1158/1078-0432.CCR-14-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankhala K, Mita A, Kelly K, Mahalingam D, Giles F, Mita M. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Targ. Oncol. 2009;4:135–142. doi: 10.1007/s11523-009-0107-z. [DOI] [PubMed] [Google Scholar]
- Sofroniadou S, Kassimatis T, Goldsmith D. Anaemia, microcytosis and sirolimus—is iron the missing link? Nephrol. Dial. Transplant. 2010;25:1667–1675. doi: 10.1093/ndt/gfp674. [DOI] [PubMed] [Google Scholar]
- Ross C, Salmon A, Strong R, Fernandez E, Javors M, Richardson A, Tardif S. Metabolic consequences of long- term rapamycin exposure on common marmoset monkeys (Callithrix jacchus) Aging. 2015;7:964–973. doi: 10.18632/aging.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis M, Goldfarb-Rumyantzev AS. Diabetes after Transplantation and Sirolimus: What’s the Connection? J. Am. Soc. Nephrol. 2008;19:1255–1256. doi: 10.1681/ASN.2008050474. [DOI] [PubMed] [Google Scholar]
- Apelo SIA, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME, Lamming DW. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. 2016;15:28–38. doi: 10.1111/acel.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurez V, Dao V, Liu A, Pandeswara S, Gelfond J, Sun L, Bergman M, Orihuela C, Galvan V, Padro I, Drerup J, Liu Y, Hasty P, Sharp Z, Curiel T. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune- deficient mice. Aging cell. 2015;14:945–956. doi: 10.1111/acel.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D, Ye L, Astle C, Baur J, Sabatini D, Harrison D. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12:712–718. doi: 10.1111/acel.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J. Amer. Geriatr. Soc. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Tariq SH, Tumosa N, Chibnall JT, Perry MH, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder-a pilot study. Am. J. Geriatr. Psychiatry. 2006;14:900–910. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- Grosch MC, Parikh MR, Graham LL, Hynan LS, Weiner MF, Cullum C. A New, Quick and Cost Effective Coding Test: The Texas Assessment of Processing Speed (TAPS) International Neuropsychological Society Final Program Fortieth Annual Meeting. 2012;71 [Google Scholar]
- Tardif S, Ross C, Bergman P, Fernandez E, Marty Javors, Salmon A, Spross J, Strong R, Richardson A. Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. Journals of Gerontology: Biological Sciences. 2014;70:577–588. doi: 10.1093/gerona/glu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana B, Napolia K, Kellya P, Podbielskia J, Husseina I, Urbauerb D, Katza S, Burena CV. Therapeutic drug monitoring of sirolimus: correlations with efficacy and toxicity. Clin Transplantation. 2000;14:97–109. doi: 10.1034/j.1399-0012.2000.140201.x. [DOI] [PubMed] [Google Scholar]
- Moes DJAR, Guchelaar H-J, Fijter JWd. Sirolimus and everolimus in kidney transplantation. Drug Discovery Today. 2015;20:1243–1249. doi: 10.1016/j.drudis.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Trepanier D, Gallant H, Leggatt D, Yatscoff R. Rapamycin: Distribution, Pharmacokinetics and Therapeutic Range Investigations: An Update. Clinical Biochemistry. 1998;31:345–351. doi: 10.1016/s0009-9120(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Roekel SV, Winkleman L, Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buron F, Malvezzi P, Villar E, Chauvet C, Janbon B, Denis L, Brunet M, Daoud S, Cahen R, Pouteil-Noble C, Gagnieu M-C, Bienvenu J, Bayle F, Morelon E, Thaunat O. Profiling Sirolimus-Induced Inflammatory Syndrome: A Prospective Tricentric Observational Study. PLoS ONE. 2013;8:e53078. doi: 10.1371/journal.pone.0053078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luna-Preitschopf A, Zwickl H, Nehrer S, Hengstschläge rM, Mikula M. Rapamycin Maintains the Chondrocytic Phenotype and Interferes with Inflammatory Cytokine Induced Processes. Int J Mol Sci. 2017;18:1494–1509. doi: 10.3390/ijms18071494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen P, Wang J, Almlöf M, Solberg R, Okkenhaug C, Scholz T, Thiemermann C, Foster S, Aasen A. Sirolimus interferes with the innate response to bacterial products in human whole blood by attenuation of IL-10 production. Scand. J. Immunol. 2001;53:184–191. doi: 10.1046/j.1365-3083.2001.00862.x. [DOI] [PubMed] [Google Scholar]
- Salehi M, Bagherpour B, Shayghannejad V, Mohebi F, Jafari R. Th1, Th2 and Th17 Cytokine Profile in Patients with Multiple Sclerosis Following Treatment with Rapamycin. Iranian J. Immunol. 2016;13:141–147. [PubMed] [Google Scholar]
- Thomson A, Turnquist H, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–347. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC, Donato AJ. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017;16:17–26. doi: 10.1111/acel.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yard B, Pancham R, Paape M, Daha M, van Es L, van der Woude F. CsA, FK506, corticosteroids and rapamycin inhibit TNFα production by cultured PTEC. Kidney International. 1993;44:352–358. doi: 10.1038/ki.1993.251. [DOI] [PubMed] [Google Scholar]
- Park J, Jeon Y, Lee J, Ahn S, Ha S, Bang S, Park E, Yi S, Lee M, Han J. Destabilization of TNF-α mRNA by Rapamycin. Biomol Ther. 2012;20:43–49. doi: 10.4062/biomolther.2012.20.1.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.