A central role of inflammation in the pathophysiology of sickle cell disease (SCD) is supported by clinical observations. Both an elevated leucocyte count and C-reactive protein (CRP) are associated with early death in SCD (Platt et al, 1994; van Beers et al, 2015). We have shown that iron-regulated gene expression is associated with striking upregulation of inflammasome pathway gene expression, including a 200-fold increase in Toll-like receptor 4 (TLR4) expression in peripheral blood mononuclear cells, suggesting a cross-talk between iron and inflammation pathways (van Beers et al, 2015). Haem, a form of iron, augments pro-inflammatory TLR4 signaling in sickle cell mice, with subsequent inflammation, vaso-occlusion, organ damage and death (Ghosh et al, 2013; Belcher et al, 2014; Vinchi et al, 2016). TLR4 is highly expressed on macrophages and peripheral blood monocytes, and its ligand, lipopolysaccharide (LPS), induces expression of the pro-inflammatory cytokine interleukin 6 (IL6). We hypothesized that intracellular iron is involved in this LPS induction of IL6.
Subjects were recruited under a protocol approved by the National Institutes of Health (NIH) Institutional Review Board (ClinicalTrials.gov identifier NCT00081523). Blood was obtained from 18 patients with homozygous sickle cell anaemia (HbSS) in steady state and from 10 healthy controls. See Supplemental Table SI for baseline characteristics.
We aliquoted 1 ml of heparinized fresh whole blood from SCD patients or controls, blocked cytokine secretion with Brefeldin A, added iron chelator [0·1 mmol/l deferasirox, haem (20 µmol/l) and/or LPS (1, 10 or 100 ng/ml)] and incubated the tubes at 37°C. After a 3-h incubation, samples were put on ice and the percentage monocytes expressing intracellular IL6 were quantified using intracellular staining by flow cytometry.
Surprisingly, monocytes of patients and controls had a comparable number of IL6 positive monocytes after stimulation with LPS, haem or iron chelation and comparable intracellular iron. This report therefore presents the pooled analysis of these experiments.
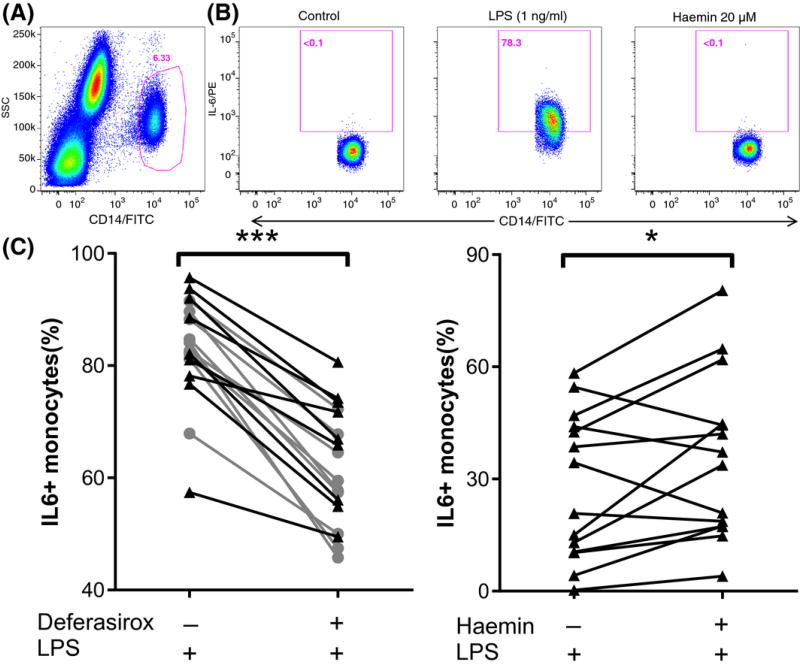
After 3 h of stimulation with LPS, the median percentage of monocytes expressing IL6 was 83·4% (81·0–89·6). Iron chelation diminished this percentage significantly, to 62·0 (54·9–71·8, P = 0·004; Fig 1C). The TLR4 inhibitor TAK-242 inhibited the response to LPS. In the absence of LPS, haem was insufficient to induce IL6 producing monocytes (Fig 1B). In contrast, haem potentiated the effect of LPS (1 ng/ml) with a median of 5·7% (−3·3 to 19·7, P = 0·046) compared to LPS alone (Fig 1D).
Figure 1. Haem increases and iron chelation decreases TLR4 signaling.
Fresh whole blood from patients (n = 10) and controls (n = 10) was incubated with combinations of vehicle, a Toll-like receptor-4 (TLR4) agonist [lipopolysaccharide (LPS)] or 20 µmol/l haem. After 3 h, the percentage of monocytes with detectable levels of intracellular interleukin 6 (IL6) was quantified by flowcytometry. (A) Monocytes were identified using side scatter (SSC) and CD14-positivity. (B) Representative results of monocytes from sickle cell disease patients that had been treated with vehicle, LPS or Haem. (C) Compared to incubation with high dose LPS (100 ng/ml) alone, co-incubation with the iron chelator deferasirox significantly decreased the absolute percentage of IL6-producing monocytes by 20·4% (15·2–26·3) (P = 0·004) (D) In contrast, compared to incubation with low dose LPS (1 ng/ml or 10 ng/ml) alone, co-incubation with LPS and haem increased the absolute percentage of monocytes producing IL6 with a median 5·7% (interquartile range −3·3 to 19·7, P = 0·046). ***P < 0·005, *P < 0·05. Black triangles denote patients. Grey spheres denote healthy controls.
Finally, we evaluated the intracellular free or chelatable iron content of the monocytes (as quantified by the calcein assay; Epsztejn et al, 1997) and the plasma level of CRP, a marker of in vivo inflammation. The monocyte intracellular chelatable iron [expressed as delta mean fluorescence intensity (ΔMFI) (arbitrary units)] in patients and controls was comparable [median (interquartile range) 192 (160–333) vs. 218 (161–271)]. The individual monocyte chelatable iron pool correlated positively with individual plasma levels of CRP (Spearman R = 0·454, P = 0·044), supporting a relationship of intracellular iron to inflammation.
Our results in samples from SCD patients and healthy controls are consistent with previous results obtained in animals (Fernandez et al, 2010; Belcher et al, 2014). Figueiredo et al (2007) had previously shown that the addition of haem but not protoporphyrin IX (porphyrin without iron) to thioglycollate-activated macrophages increased TLR4-mediated inflammation, and that haem alone did not induce inflammation (Fernandez et al, 2010). Importantly, Fernandez et al (2010) showed that this non-specific pro-inflammatory effect of haem can be mimicked by the addition of paraquat, a strong oxidant, and can be inhibited either by iron chelation or anti-oxidants. All of these results suggest that haem alone cannot induce TLR4 signaling, but rather that the pro-oxidant effect of haem-bound iron amplifies the activity of TLR4 ligands. Although this pro-inflammatory effect of haem is thus not specific to SCD monocytes, its downstream effect in SCD pathophysiology is profound in SCD mice, resulting in cell adhesion, organ damage and death (Ghosh et al, 2013; Belcher et al, 2014).
Given that SCD monocytes are exposed to intravascular haemolysis and free haem in vivo, we expected a higher intracellular iron and higher response to LPS in monocytes from SCD patients compared to healthy controls. We speculate that we did not find this because chronic exposure of monocytes to haem leads to its rapid export, involving upregulation of the iron exporter ferroportin (Theurl et al, 2016), probably maintaining normal levels of intracellular free iron in monocytes and subsequent normal response to LPS. It is also possible that intracellular iron level is mitigated via the induction of ferroxidase activity by ferritin heavy chain, as documented in SCD mice (Vercellotti et al, 2014). IL6 induction might also be dampened by anti-inflammatory IL10, which we found to be associated with iron-regulated genes in monocytes of SCD patients (van Beers et al, 2015).
In the light of present literature, our human data strongly support animal data indicating that haem iron is able to augment ligand (LPS)-induced TLR4 signaling. There is increasing evidence that inflammation plays a central role in SCD pathophysiology. Our results show that the iron chelator deferasirox ameliorates the proinflammatory effect of haem iron ex vivo. Deferasirox, widely used in patients with transfusional iron overload, readily achieves plasma levels comparable to our assay concentration of 0·1 mmol/l. Therefore, iron chelation could provide an interesting, readily available alternative therapeutic option to reduce inflammatory burden in SCD. This hypothesis merits further evaluation.
In conclusion, we suggest that haem-bound iron, which is released during intravascular haemolysis and scavenged by monocytes, can contribute to activation and pro-inflammatory state in human SCD monocytes, by augmenting TLR4 signaling, consistent with SCD mice models.
Supplementary Material
Acknowledgments
This research was funded by the Division of Intramural Research of the National Heart, Lung and Blood Institute (ZIA HL006013-05).
Footnotes
Author contributions
All authors critically reviewed and approved the submitted and final versions of the paper. P.D., E.B. and L.M. performed the research. J.N., L.M. and C.S. contributed to acquisition of data and the inclusion of patients. P.D., E.B. J.M. and G.K. designed the study and interpreted the data. P.D. and E.B. analysed the data and drafted the paper.
References
- van Beers EJ, Yang Y, Raghavachari N, Tian X, Allen DT, Nichols JS, Mendelsohn L, Nekhai S, Gordeuk VR, Taylor JG, Kato GJ. Iron, inflammation, and early death in adults with sickle cell disease. Circulation Research. 2015;116:298–306. doi: 10.1161/CIRCRESAHA.116.304577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I. Fluorescence analysis of the labile iron pool of mammalian cells. Analytical Biochemistry. 1997;248:31–40. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- Fernandez PL, Dutra FF, Alves L, Figueiredo RT, Mourão-Sa D, Fortes GB, Bergstrand S, Lönn D, Cevallos RR, Pereira RMS, Lopes UG, Travassos LH, Paiva CN, Bozza MT. Heme amplifies the innate immune response to microbial molecules through Syk-dependent ROS generation. The Journal of Biological Chemistry. 2010;285:32844–32851. doi: 10.1074/jbc.M110.146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graça-Souza AV, Bozza MT. Characterization of heme as activator of Toll-like receptor 4. The Journal of Biological Chemistry. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Adisa OA, Chappa P, Tan F, Jackson KA, Archer DR, Ofori-Acquah SF. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. The Journal of Clinical Investigation. 2013;123:4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. The New England Journal of Medicine. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M, He S, Gerhardt LMS, Holderried TAW, Seifert M, Sopper S, Fenn AM, Anzai A, Rattik S, McAlpine C, Theurl M, Wieghofer P, Iwamoto Y, Weber GF, Harder NK, Chousterman BG, Arvedson TL, McKee M, Wang F, Lutz OMD, Rezoagli E, Babitt JL, Berra L, Prinz M, Nahrendorf M, Weiss G, Weissleder R, Lin HY, Swirski FK. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nature Medicine. 2016;22:945–951. doi: 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti GM, Khan FB, Nguyen J, Chen C, Bruzzone CM, Bechtel H, Brown G, Nath KA, Steer CJ, Hebbel RP, Belcher JD. H-ferritin ferroxidase induces cytoprotective pathways and inhibits microvascular stasis in transgenic sickle mice. Frontiers in Pharmacology. 2014;5:79. doi: 10.3389/fphar.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinchi F, Costa da Silva M, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, Cerwenka A, Tolosano E, Muckenthaler MU. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016;127:473–486. doi: 10.1182/blood-2015-08-663245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.