Abstract
Background
Despite the wide array of treatments available for rheumatoid arthritis (RA), some patients continue to report unmet clinical needs. We investigated the extent of inadequate disease control in patients with RA.
Methods
Data were drawn from the Adelphi 2014 RA Disease-Specific Program in France, Germany, Italy, Spain and the UK. Rheumatologists provided patient demographics, comorbidities, satisfaction with RA control and other clinical details. Patients reported their level of satisfaction and completed the EuroQoL 5-Dimensions Health Questionnaire and Work Productivity and Activity Impairment Questionnaire. Patients had been on their current therapy ≥3 months and had 28-joint disease activity scores (DAS28) reported. Adequately controlled (DAS28 ≤3.2) and inadequately controlled (DAS28 >3.2) patient cohorts were compared using univariate tests.
Results
Of 1147 patients, 74% were women, the mean age was 52 years and the mean time since RA diagnosis was 7 years. Twenty-seven percent of patients had inadequately controlled RA, whereas 73% had adequately controlled RA. Inadequately controlled patients were more affected clinically versus adequately controlled patients; 69% vs 13% had moderate/severe RA, the current level of pain was 4.6 vs 2.3, and 67% vs 41% experienced flares, respectively (all p<0.0001). Inadequately controlled patients had higher rates of depression (16% vs 5%; p<0.0001), worse health state, greater work and activity impairment, and lower satisfaction rates among the patients and their physicians than the adequately controlled cohort.
Conclusion
RA was insufficiently controlled in over a quarter of patients despite their current therapy and this had a negative impact on the patients.
Keywords: dmards (biologic), patient perspective
Key messages.
What is already known about this subject?
Despite the availability of numerous treatments for rheumatoid arthritis (RA), some patients continue to experience pain, impaired physical/mental function and fatigue.
What does this study add?
This study demonstrates the extent of unmet clinical needs associated with both adequate and inadequate disease control in patients with RA.
How might this impact on clinical practice?
These findings highlight the need to assess patients’ physical and mental well-being alongside clinical measures of disease activity and may help to guide treatment decisions.
Introduction
Rheumatoid arthritis (RA) is a debilitating autoimmune disease affecting up to 1% of the world population.1 Progress in the last two decades has resulted in an armamentarium of available treatments, the majority of which are disease-modifying antirheumatic drugs (DMARDs). Current European League Against Rheumatism (EULAR) guidelines recommend starting therapy with the conventional synthetic DMARD (csDMARD), methotrexate, in combination with short-term glucocorticoids.2 If this treatment is not successful or methotrexate is contraindicated, in the absence of poor prognostic factors, other csDMARDS should be considered. The guidelines state that if the treatment target is not reached with the first csDMARD strategy, when poor prognostic factors are present, a biologic DMARD (bDMARD) should be added (current practice). Unlike the 2014 EULAR guidelines,3 the 2016 EULAR guidelines have expanded this recommendation to include targeted synthetic DMARDs (tsDMARDs), specifically Janus kinase (JAK) inhibitors, as second-line options (currently tofacitinib and baricitinib). The American College of Rheumatology (ACR) guidelines recommend treatment strategies based on disease activity, as well as differentiating between early RA, established RA and high-risk comorbidities.4 When using disease activity scores for 28 joints (DAS28), ACR defined remission as <2.6, low disease activity as ≥2.6–<3.2, moderate disease activity as ≥3.2–≤5.1 and high disease activity as >5.1. For moderate to high disease activity, ACR recommendations are similar to the EULAR recommendations but offer the choice of combination csDMARD therapy or adding a tumour necrosis factor inhibitor (TNFi) or non-TNFi biologic or tofacitinib if disease activity remains moderate or high despite csDMARD monotherapy.4
The phenotype and disease course of RA has evolved over the last two generations,5–7 such that fewer patients have severely deforming disease at presentation (due to a shorter referral time and early diagnosis) or very high acute phase responses, fewer patients develop severely deforming disease and severe comorbidities are less common. Despite moderate to severe levels of disease activity by composite scores, the majority of patients no longer exhibit progressive structural damage to joints. These changes are possibly due to earlier and more optimal use of DMARDs and/or environmental changes, which may have led to tighter control of RA. However, up to 30% of patients have an inadequate response or intolerance to methotrexate or have an inadequate response or loss of response to bDMARDs.8 9 Additionally, despite the wide array of available treatments for RA, there are unmet clinical needs across key domains such as pain, physical function, mental function and fatigue, which can affect social function, sexual function and the ability to work.10–13 Indeed, in the established phase of disease, real-world data show that remission, particularly as assessed by more stringent criteria such as the Clinical Disease Activity Index or the EULAR/ACR Boolean criteria, remains a largely aspirational goal.14–16 Current EULAR guidelines recommend targeting sustained remission or low disease activity for the management of RA as well as shared decision-making between rheumatologists and their patients.3
In order to understand the scope of unmet needs in RA, we examined the extent of inadequately controlled RA and the accompanying clinical and contextual characteristics in patients in five European countries.
Methods
Study design
This was an analysis of cross-sectional data drawn from the Adelphi RA Disease Specific Programme (DSP), a survey of rheumatologists and their consulting patients with RA. A DSP is a survey conducted to provide impartial observations of real-world clinical practice from a physician and matched patient viewpoint, irrespective of what guidelines are advocated. The DSP is not run to test any specific hypotheses, and it is not set up to demonstrate cause and effect, but is designed to provide a holistic, benchmark view of contemporary RA management via physician-reported and patient-reported measures.17 The survey was conducted in France, Germany, Italy, Spain and the UK from January 2014 to August 2014.
Physicians and patients
Physicians had to have seen more than eight patients with RA in a typical month, to have qualified as a physician between 1975 and 2010 and be actively involved in the management of RA to be eligible for inclusion. Physicians provided treatment histories for all patients, including use of csDMARDs, bDMARDs or other RA treatments (cyclo-oxygenase-2 (COX-2) inhibitors, non-steroidal anti-inflammatory drugs other than COX-2 inhibitors, non-opioid analgesics, opioid analgesics, oral steroids, locally injected steroids and gastroprotective agents). The sequence of bDMARD treatments received and reasons for not prescribing a bDMARD were also given, if applicable. Assessment of the disease severity (mild, moderate and severe) of patients with RA or of remission was based on the physician’s own perception of the disease status. Patients with a diagnosis of RA, aged ≥18 years and not currently in a clinical trial were eligible for inclusion in the DSP.
Data collection
Physicians were identified by the local fieldwork teams from public lists of rheumatologists. Candidate respondents who met the eligibility criteria were subsequently invited to participate in the DSP and were compensated to participate in this research according to fair market research rates consistent with the time involved. Participating physicians included the first eight consecutive patients who met the eligibility criteria in the survey. As the methodology required consecutively presenting patients with RA for each physician, the DSP sample is representative of the consulting population.
Physicians completed a detailed physician record form (PRF) on each patient who met the recruitment criteria. The PRF contained detailed questions on patient demographics, diagnoses, severity of condition and specific symptoms, acute episodes/flares, concomitant conditions, current treatment, drivers of therapy choice, compliance and general patient management (such as frequency of consultation with the treating and other physicians). Each patient with a completed PRF was invited to complete a patient self-completion (PSC) form; on agreement, these patients provided written informed consent for participation in the survey and use of their anonymised and aggregated data for research and publication in scientific journals. Patients completed their PSCs independently of their physician and returned them in a sealed envelope to ensure that responses were kept confidential from physicians. PSCs contained detailed questions on demographics, the patient’s current condition, level of satisfaction with their treatment, and compliance. PSCs also included validated quality of life instruments; the EuroQoL 5-Dimensions (EQ-5D) health questionnaire18 was used to assess the emotional and physical impact of RA, while the Work Productivity and Activity Impairment (WPAI) questionnaire19 assessed the impact on functioning. Patients could choose not to complete the PSC; however, each completed PSC could be matched to the physician-reported information on that patient.
A complete description of the methods of the survey has been previously published and validated.17 20 21 Using a check box, patients provided informed consent for use of their anonymised and aggregated data for research and publication in scientific journals. Data were collected in such a way that patients and physicians could not be identified directly; all data were aggregated and de-identified before receipt. Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines22 and as such it does not require ethics committee approval. The survey was performed in full accordance with relevant legislation at the time of data collection, including the Health Information Technology for Economic and Clinical Health Act legislation.23
Statistical analysis
Two patient cohorts were created based on the physician-reported DAS28: adequately controlled (DAS28 ≤3.2) and inadequately controlled (DAS28 >3.2). DAS28 was calculated by the physician when the patient was visiting them (thus the PGA was available and used in the calculation), and later reported by the physician in the PRF. Patients without a DAS28 score provided by their rheumatologist and those who had not been on their current therapy for ≥3 months were excluded from this analysis. Descriptive statistics were used to describe the two cohorts and statistical differences between the cohorts were assessed using Mann-Whitney U tests for numerical outcomes and Fisher exact tests for categorical data. Missing data were not imputed. Any patients with missing values for a particular variable were removed for all analyses where that variable was used, but remained eligible for inclusion in other analysis.
Results
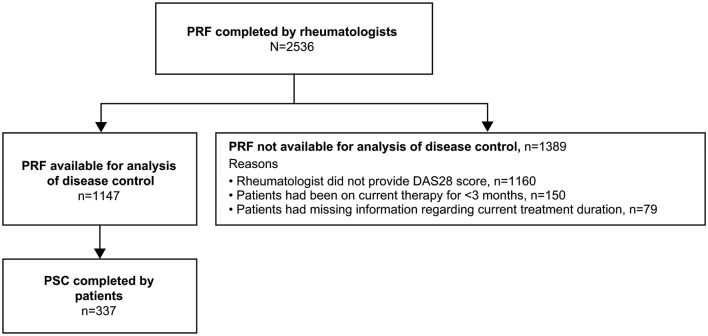
A total of 307 rheumatologists provided data for 2536 patients (France n=502; Germany n=491; Italy n=501; Spain n=486; UK n=556). Of these, 1147 PRFs and 337 PSCs were available for analysis in this study (figure 1). The remaining 1389 PRFs were not available for analysis as described in figure 1. A majority of the patients were women (74%), the mean age was 52 years, the mean time since RA diagnosis was 7 years and 76% were reported to be positive for anticyclic citrullinated peptide antibodies by their rheumatologist at their most recent assessment (table 1). All patients who had ever received bDMARDs were receiving bDMARDs at the time of the survey.
Figure 1.
Flow of participants. DAS28, disease activity score in 28 joints; PRF, patient record form; PSC, patient self-completion form.
Table 1.
Demographics and physician-reported disease characteristics of the adequately and not adequately controlled population
| Overall (n=1147) |
Inadequate Control (DAS28 >3.2) (n=308) |
Adequate Control (DAS28 ≤3.2) (n=839) |
P value | |
| Age (years), mean (SD) | 51.6 (13.7) | 53.0 (13.7) | 51.1 (13.7) | 0.0366 (MW) |
| Gender (female), n (%) | 851 (74) | 230 (75) | 621 (74) | 0.8790 (FE) |
| BMI (kg/m2), mean (SD) | 25.2 (4.4) | 25.8 (4.8) | 24.9 (4.2) | 0.0038 (MW) |
| Positive for anticyclic citrullinated peptide antibodies, n (%) | 670 (76) | 184 (78) | 486 (75) | 0.3763 (FE) |
| Most recent ESR (mm/hour), mean (SD) | 18.2 (14.3) | 26.6 (17.2) | 15.2 (11.8) | <0.0001 (MW) |
| Most recent CRP (mg/L), mean (SD) | 5.4 (4.7) | 7.5 (6.4) | 4.7 (3.7) | <0.0001 (MW) |
| RF positive, n (%) | 775 (82) | 211 (81) | 564 (83) | 0.4489 (FE) |
| Time since diagnosis (years), mean (SD) | 7.0 (6.8) | 7.1 (6.9) | 7.0 (6.8) | 0.8961 (MW) |
| Currently in remission, n (%) | 614 (54) | 42 (14) | 572 (68) | <0.0001 (FE) |
| Current severity, n (%) | < 0.0001 (MW) | |||
| Mild | 829 (72) | 96 (31) | 733 (87) | |
| Moderate | 279 (24) | 179 (58) | 100 (12) | |
| Severe | 39 (3) | 33 (11) | 6 (1) | |
| Current disease status, n (%) | < 0.0001 (MW) | |||
| Improving | 327 (29) | 67 (22) | 260 (31) | |
| Stable | 661 (58) | 116 (38) | 545 (65) | |
| Deteriorating slowly | 97 (9) | 78 (26) | 19 (2) | |
| Deteriorating rapidly | 22 (2) | 21 (7) | 1 (0.1) | |
| Unstable | 31 (3) | 21 (7) | 10 (1) | |
| Current level of pain (1=none; 10=worst), mean (SD) | 2.9 (1.8) | 4.6 (1.9) | 2.3 (1.2) | <0.0001 (MW) |
| Patients who had ever experienced flares*, n (%) | 550 (48) | 204 (67) | 346 (41) | <0.0001 (FE) |
| Comorbidities, n (%)† | ||||
| Depression | 93 (8) | 48 (16) | 45 (5) | <0.0001 (FE) |
| None | 584 (51) | 107 (35) | 477 (57) | <0.0001 (FE) |
| Ever received bDMARD, n (%) | 526 (46) | 157 (51) | 369 (44) | 0.0382 (FE) |
| Current/most recent bDMARD, n (%) | 0.0178 (PC) | |||
| TNF inhibitor | 344 (65) | 91 (58) | 253 (69) | |
| Non-TNF inhibitor | 182 (35) | 66 (42) | 116 (31) | |
| Missing | 621 | 151 | 470 |
*Based on the physician’s own definition of flare.
†Only those that were significantly different between the two groups are listed.
bDMARD, biological disease-modifying antirheumatic drug; BMI, body mass index; CRP, C-reactive protein; DAS28, disease activity score in 28 joints; ESR, erythrocyte sedimentation rate; FE, Fisher exact; MW, Mann-Whitney; PC, Pearson’s χ2; RF, rheumatoid factor; TNF, tumour necrosis factor.
Approximately a quarter of the patients (27%, 308/1147) had inadequately controlled RA compared with 73% (839/1147) who had adequately controlled RA. As shown in table 1, the inadequately controlled cohort had more patients with moderate/severe RA than those with adequately controlled RA (69% vs 13%, respectively; p<0.0001) and fewer patients with stable disease status (38% vs 65%; p<0.0001). Further analyses revealed that mean DAS28 scores associated with mild, moderate and severe disease status were 2.47, 3.67 and 5.02, respectively.
Patients in the inadequately controlled cohort had a higher level of pain compared with the adequately controlled cohort (4.6 vs 2.3; p<0.0001), were more likely to ever have experienced flares (67% vs 41%; p<0.0001) and had higher rates of depression (16% vs 5%; p<0.0001). Of note, 14% of patients in the inadequately controlled cohort were considered to be in remission by their physician despite their DAS28 score being >3.2.
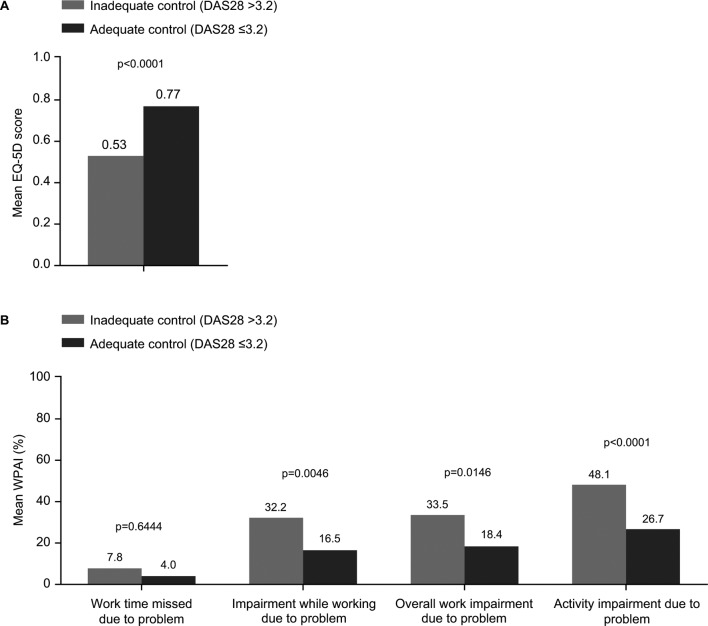
A difference in quality of life was also observed between the two cohorts, with a mean EQ-5D of 0.53 for the inadequately controlled patients compared with 0.77 for the adequately controlled patients (p<0.0001; figure 2A). As would be expected, the WPAI scores indicated greater work and activity impairment in the inadequately controlled patients than in the adequately controlled cohort; however, some impairment also persisted in the adequately controlled cohort (figure 2B).
Figure 2.
Patient-reported outcomes. (A) EQ-5D (B) WPAI. P values were calculated using the Mann-Whitney U test. DAS28, disease activity score in 28 joints; EQ-5D, EuroQoL 5-Dimensions; WPAI, Work Productivity and Activity Impairment.
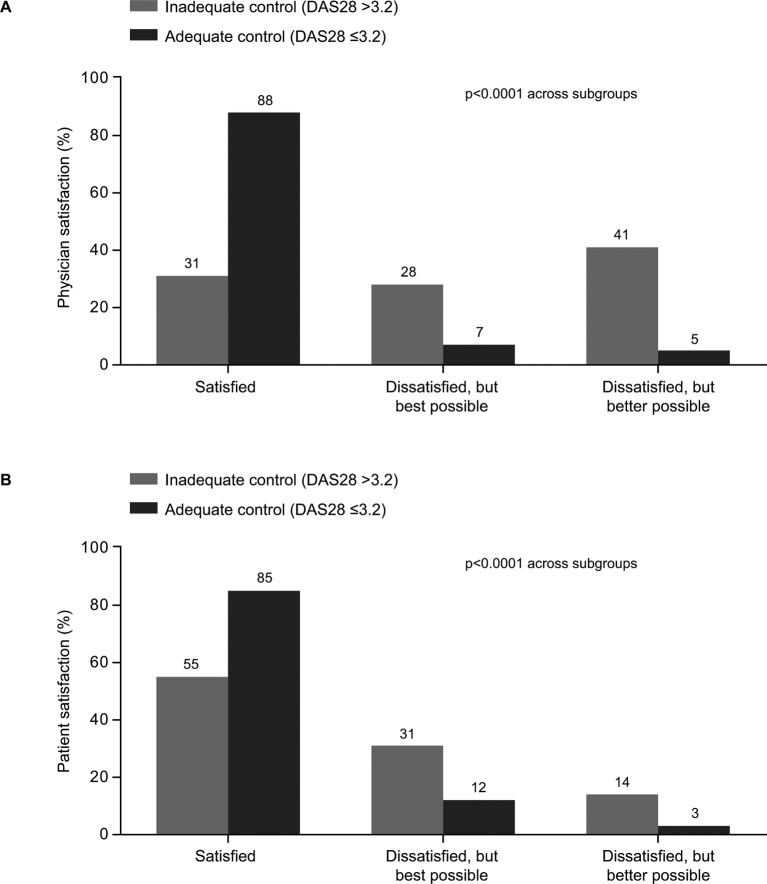
Fewer physicians were satisfied with control of RA in the inadequately controlled patient group compared with the adequately controlled group (31% vs 88%, respectively; p<0.0001; figure 3A), and this was mirrored by the satisfaction levels reported by patients (55% vs 85%, respectively; p<0.0001; figure 3B). Interestingly, even in the adequately controlled cohort, 7% of physicians and 12% of patients stated that they were dissatisfied with the level of RA control but thought it was the best possible, while 5% of physicians and 3% of patients were dissatisfied but thought it would be possible to achieve better RA control.
Figure 3.
Satisfaction with control of RA. (A) Physician-reported satisfaction, (B) Patient-reported satisfaction. p values were calculated using the Mann-Whitney U test. DAS28, disease activity score in 28 joints; RA, rheumatoid arthritis.
Discussion
This cross-sectional study was performed to define key ‘real-world’ unmet needs in the treatment of patients with RA in the era of bDMARDs, by identifying areas where guideline-defined aspirations and the realities of patient experiences and clinical practice do not match. The study showed that almost a quarter of patients with RA have insufficiently controlled disease (DAS28 >3.2) despite current therapy, which could be related to the long average duration of disease (7 years). Our findings are consistent with other reports from observational studies. For example, in 2013, based on standardised monitoring of patients in an ordinary outpatient clinic in southern Norway, 26.6% of patients had DAS28 >3.2.24
Even though patients with inadequately controlled RA are more affected clinically, more impacted in their daily lives and less satisfied overall, in some cases physicians may perceive these patients to be adequately controlled. This was demonstrated in this study as 14% of physicians reported that patients were in remission despite separately reporting DAS28 >3.2 scores, that is, in the inadequate control cohort. In the PRF, the physician was asked if the patient was currently in remission in one section, without any guidance that the response should be based on any particular criteria. The physician was asked to report the DAS28 score as assessed at the consultation in a separate section, thus it is quite likely that physicians reported a subjective view for the question about remission, which resulted in discordance between disease status and DAS28 score. Similar differences in physician-reported and DAS28-based assessment of disease remission have been reported previously among patients with RA in clinical practices in the USA, with physicians subjectively reporting that 50% of patients were in remission although only 32% were in remission by DAS28 criteria.25 Physicians may also have considered a patient to have achieved the lowest level of disease activity attainable by that individual, as demonstrated in this study by physicians reporting that they were dissatisfied with RA control in 28% of patients with inadequate control by DAS28, but that this was the best possible in those patients. The discordance between physician perception and DAS28 scores may also have been driven by the number of bDMARDs received by some patients, with patients who had received more bDMARDs being perceived to have attained the best control possible. Consequently, discordance between physicians’ perceptions and the objective DAS28 may result in less than optimal therapeutic management in some patients. In this study, over half of patients with inadequately controlled disease reported being satisfied with the control that their RA therapy provided, suggesting that they too may be accepting suboptimal outcomes. A study on the degree of discordance between patient and physician assessment of RA severity reported that nearly a third of patients differed from their physicians, with physicians recording less severe disease compared with the patients.26 The same study also highlighted that greater depressive symptoms in patients were associated with discordance in patient-reported versus physician-reported RA severity measured by DAS28 scores. High rates of depression were also reported in patients with inadequately controlled RA in the current study.
In terms of limitations, this study was performed in Germany, Spain, France, Italy and the UK, all of which are countries with relatively advanced healthcare systems and broad access to treatment and disease management programmes, although access to bDMARDs is restricted to patients with DAS28 >5.1 in the UK. Generalisation of this study’s findings beyond these countries warrants caution; there is a discrepancy in access to treatment/early diagnosis, patient perceptions are different in more affluent countries, cost often restricts access to the full range of available treatments, and subsets of patients can have reduced access.27–29 Additionally, the sample is not entirely representative of the practising population of rheumatologists (the physicians participating in the DSP will be skewed towards those with a higher workload due to the screening criteria) and infrequently consulting patients may be under-represented due to the sampling approach. There could also be an element of measurement bias in some of the responses provided by the physician/patient; however, they have to be relied on to provide the most accurate information possible. Furthermore, data for a large number of patients (1160/2536) could not be included because of failure to record DAS28. In a further 229 patients, PRF forms were not included as treatment duration was either not available or was not considered sufficiently long for it to be effective (figure 1). In relation to this, results from the CAPEA study indicated that treatment was not changed in response to disease activity in 60% of the patients who did not reach remission within 3 months and 54% of patients who did not reach remission by 6 months.30 Taken together, these studies reflect a common limitation of current practice and suggest that further training on the role of DAS28 is needed for rheumatologists, to reinforce the importance of using objective measures of disease to make informed treatment decisions for patients with RA.
The nature of unmet needs in RA has changed over the last few decades; although the mean disease activity may be lower than before, psychological and other aspects that affect patients’ well-being, such as depression, fatigue and comorbidities, constitute contemporary unmet needs that should be addressed with a multidisciplinary approach.31–33 In this context, a structured literature review from 2004 to 2014 identified that patients continued to experience pain, morning stiffness, physical disability, mental health problems and unacceptable levels of fatigue despite ongoing treatment.13 A large longitudinal study carried out over 8 years also demonstrated recently that improved treatment strategies did not result in less severe fatigue in patients with RA.34 Experts at the Targeted Therapies 2016 meeting acknowledged the progress in treatment of RA but recognised that a significant proportion of patients continued to suffer with moderate to high RA, despite ongoing treatment and adoption of a treat to target strategy.35 Identification of patients in remission who would be candidates for dose reduction and development of biomarkers or imaging programmes to identify these patients, as well as development of therapeutics that could repair the damage caused by RA or increase remission rates, were also identified as current unmet needs in RA.35 Of note, there is increasing focus on involving patients in the decision-making process from an early stage as this has been associated with higher patient satisfaction with care,36 and the hope is that increased communication between patients and physicians will enable amelioration of these remaining unmet needs in RA. DMARDs that meet patient preferences, for example, the route of administration or monotherapy versus combination therapy with methotrexate, may increase compliance and adherence.37 Furthermore, new drugs currently being developed and tested, such as small molecule tsDMARDs, may improve the efficacy of treatment in patients who are non-responsive or intolerant to the currently available RA treatments.38 39
Conclusions
This study documents the continued existence of unmet needs in patients with RA, despite the advances in treatments and strategies. These unmet needs exist not only for the inadequately controlled patients (DAS28 >3.2), as might be expected, but also, to a lesser extent, for those considered to be adequately controlled (DAS28 ≤3.2). This may be associated with discordance between patients’ and physicians’ perceptions of RA severity as well as a lack of shared decision-making.
Acknowledgments
The authors wish to thank all patients and rheumatologists who participated in the survey.
Footnotes
Contributors: ES and JP contributed to study concept, design and data collection. RW contributed to study concept and design, and provided statistical support. PCT, RA, JJG-R, RC and PB contributed to interpretation of data for the work. All authors read, critically revised and approved the final manuscript.
Funding: This study was sponsored by Pfizer. ES, RW and JP are employees of Adelphi Real World and were contracted by Pfizer to provide data, input into design of data collection and statistical support for the development of this paper. Medical writing support was provided by Rina Vekaria Passmore of Engage Scientific Solutions and was funded by Pfizer.
Competing interests: PCT has received fees from AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Merck, Pfizer, Sandoz, Biogen and UCB Pharma. RA has received fees from Pfizer. JJGR has received fees from AbbVie, Biogen, Bristol-Myers Squibb, Hospira, Janssen, Merck, Pfizer, Regeneron and UCB Pharma. RC has received fees from AbbVie, MSD, Pfizer, Roche and UCB Pharma. PB has received fees from MSD, Pfizer, Reckitt Benckiser and Roche. ES, RW and JP are employees of Adelphi Real World and were contracted by Pfizer to provide data, input into design of data collection and statistical support for the development of this paper. RV, DS, JA and MT are employees of Pfizer.
Patient consent: Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data supporting the conclusions of this article are included within the article.
References
- 1.Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care 2012;18(Suppl):S295–302. [PubMed] [Google Scholar]
- 2.Smolen JS, Landewé R, Bijlsma J, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé R, Breedveld FC, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh JA, Saag KG, Bridges SL, et al. . 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 5.Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum 2005;52:1009–19. 10.1002/art.20941 [DOI] [PubMed] [Google Scholar]
- 6.Abelson B, Sokka T, Pincus T. Declines in erythrocyte sedimentation rates in patients with rheumatoid arthritis over the second half of the 20th century. J Rheumatol 2009;36:1596–9. 10.3899/jrheum.081255 [DOI] [PubMed] [Google Scholar]
- 7.Rahman MU, Buchanan J, Doyle MK, et al. . Changes in patient characteristics in anti-tumour necrosis factor clinical trials for rheumatoid arthritis: results of an analysis of the literature over the past 16 years. Ann Rheum Dis 2011;70:1631–40. 10.1136/ard.2010.146043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 9.Emery P. Optimizing outcomes in patients with rheumatoid arthritis and an inadequate response to anti-TNF treatment. Rheumatology 2012;51(Suppl 5):v22–30. 10.1093/rheumatology/kes115 [DOI] [PubMed] [Google Scholar]
- 10.Ryan S. Psychological effects of living with rheumatoid arthritis. Nurs Stand 2014;29:52–9. 10.7748/ns.29.13.52.e9484 [DOI] [PubMed] [Google Scholar]
- 11.Smolen JS, Strand V, Koenig AS, et al. . Discordance between patient and physician assessments of global disease activity in rheumatoid arthritis and association with work productivity. Arthritis Res Ther 2016;18:114 10.1186/s13075-016-1004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tristano AG. Impact of rheumatoid arthritis on sexual function. World J Orthop 2014;5:107–11. 10.5312/wjo.v5.i2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor PC, Moore A, Vasilescu R, et al. . A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int 2016;36:685–95. 10.1007/s00296-015-3415-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Punder YM, Fransen J, Kievit W, et al. . The prevalence of clinical remission in RA patients treated with anti-TNF: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Rheumatology 2012;51:1610–7. 10.1093/rheumatology/kes078 [DOI] [PubMed] [Google Scholar]
- 15.Khan NA, Spencer HJ, Abda E, et al. . Determinants of discordance in patients' and physicians' rating of rheumatoid arthritis disease activity. Arthritis Care Res 2012;64:206–14. 10.1002/acr.20685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuriya B, Sun Y, Boire G, et al. . Remission in early rheumatoid arthritis–a comparison of new ACR/EULAR remission criteria to established criteria. J Rheumatol 2012;39:1155–8. 10.3899/jrheum.111341 [DOI] [PubMed] [Google Scholar]
- 17.Anderson P, Benford M, Harris N, et al. . Real-world physician and patient behaviour across countries: Disease-Specific Programmes - a means to understand. Curr Med Res Opin 2008;24:3063–72. 10.1185/03007990802457040 [DOI] [PubMed] [Google Scholar]
- 18.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 19.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 20.Babineaux SM, Curtis B, Holbrook T, et al. . Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open 2016;6:e010352 10.1136/bmjopen-2015-010352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins V, Piercy J, Roughley A, et al. . Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes 2016;9:371–80. 10.2147/DMSO.S120101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Pharmaceutical Market Research Association (EphMRA). Code of conduct: EphMRA. 2017. http://www.ephmra.org/Code-of-Conduct-Support (accessed 28 Jun 2017).
- 23.Senate and House of Representatives of the United States. Health information technology for economic and clinical health act. Washington, DC: US Government, 2009. [Google Scholar]
- 24.Haugeberg G, Hansen IJ, Soldal DM, et al. . Ten years of change in clinical disease status and treatment in rheumatoid arthritis: results based on standardized monitoring of patients in an ordinary outpatient clinic in southern Norway. Arthritis Res Ther 2015;17:219 10.1186/s13075-015-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei W, Chen C, Sullivan E, et al. . OP0133 Differences in Physician-Reported and DAS28-Based Assessment of Disease Remission Among Patients with Rheumatoid Arthritis (RA) in Clinical Practices. Ann Rheum Dis 2015;74:118.2–9. 10.1136/annrheumdis-2015-eular.4207 [DOI] [Google Scholar]
- 26.Barton JL, Imboden J, Graf J, et al. . Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res 2010;62:857–64. 10.1002/acr.20132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putrik P, Ramiro S, Keszei AP, et al. . Lower education and living in countries with lower wealth are associated with higher disease activity in rheumatoid arthritis: results from the multinational COMORA study. Ann Rheum Dis 2016;75:540–6. 10.1136/annrheumdis-2014-206737 [DOI] [PubMed] [Google Scholar]
- 28.Putrik P, Ramiro S, Kvien TK, et al. . Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis 2014;73:198–206. 10.1136/annrheumdis-2012-202603 [DOI] [PubMed] [Google Scholar]
- 29.Putrik P, Ramiro S, Lie E, et al. . Less educated and older patients have reduced access to biologic DMARDs even in a country with highly developed social welfare (Norway): results from Norwegian cohort study NOR-DMARD. Rheumatology 2016;55:1217–24. 10.1093/rheumatology/kew048 [DOI] [PubMed] [Google Scholar]
- 30.Albrecht K, Callhoff J, Edelmann E, et al. . [Clinical remission in rheumatoid arthritis. Data from the early arthritis cohort study CAPEA]. Z Rheumatol 2016;75:90–6. 10.1007/s00393-015-0019-5 [DOI] [PubMed] [Google Scholar]
- 31.Dougados M, Soubrier M, Antunez A, et al. . Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 2014;73:62–8. 10.1136/annrheumdis-2013-204223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe F, Michaud K. Predicting depression in rheumatoid arthritis: the signal importance of pain extent and fatigue, and comorbidity. Arthritis Rheum 2009;61:667–73. 10.1002/art.24428 [DOI] [PubMed] [Google Scholar]
- 33.Hallert E, Björk M, Dahlström O, et al. . Disease activity and disability in women and men with early rheumatoid arthritis (RA): an 8-year followup of a Swedish early RA project. Arthritis Care Res 2012;64:1101–7. 10.1002/acr.21662 [DOI] [PubMed] [Google Scholar]
- 34.van Steenbergen HW, Tsonaka R, Huizinga TW, et al. . Fatigue in rheumatoid arthritis; a persistent problem: a large longitudinal study. RMD Open 2015;1:e000041 10.1136/rmdopen-2014-000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winthrop KL, Strand V, van der Heijde DM, et al. . The unmet need in rheumatology: reports from the Targeted Therapies meeting 2016. Clin Exp Rheumatol 2016;34:69–76. [PubMed] [Google Scholar]
- 36.Nota I, Drossaert CH, Taal E, et al. . Patient participation in decisions about disease modifying anti-rheumatic drugs: a cross-sectional survey. BMC Musculoskelet Disord 2014;15:333 10.1186/1471-2474-15-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alten R, Krüger K, Rellecke J, et al. . Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence 2016;10:2217–28. 10.2147/PPA.S117774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalden JR. Emerging Therapies for Rheumatoid Arthritis. Rheumatol Ther 2016;3:31–42. 10.1007/s40744-016-0032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs 2014;23:1067–77. 10.1517/13543784.2014.918604 [DOI] [PubMed] [Google Scholar]