Abstract
The aim of this study was to investigate the effect and mechanisms of short term hyperthermia on a series of proinflammatory genes in type-B-synoviocytes (fibroblast like synoviocytes - FLS). In vitro experiments demonstrate that exposure of FLS to elevated temperatures for the duration of 30 minutes prevents activation of a series of genes with proinflammatory properties. Exposure to hyperthermia reduces IL-1f3 induced PGE2 release, suppresses activation of the adhesion molecules VCAM-1, ICAM-1, the cytokines TNFa, IL-1a, IL-1p, IL-8 as well as COX-2 protein synthesis. Real time RT-PCR showed that hyperthermia altered gene expression at the transcriptional level. As to the mechanism of inhibition, EMSA experiments demonstrated that exposure of FLS to hyperthermia prevents IL-1f3 induced NF-κB translocation and subsequent DNA binding. Many mechanisms have been shown to be involved in hyperthermia mediated effects on NF-κB-DNA interactions. We demonstrated by Western blot experiments that in FLS, hyperthermia prevents the phosphorylation and subsequent degradation of IκBCC, therefore retaining the NF-κB complex in the cytoplasm. Such data might, at least in part, explain and provide a rationale for treating inflammation e.g. associated with rheumatoid arthritis by balneological means.
Introduction
The transcription factor nuclear factor-KB (NF-κB) is involved in most (if not all) inflammatory processes e.g. in the pathogenesis of rheumatoid arthritis. It is thought that hyperthermia might exert beneficial effects by interfering with the pathway leading to the activation and translocation of NF-κB (1). Heat shock proteins (HSPs) are intracellular proteins with housekeeping and cytoprotective functions. Their levels increase significantly when cells are exposed to heat shock or other types of stress (2). An important prerequisite for utilizing hyperthermia as an effective anti-inflammatory regimen is a better understanding of the mechanisms involved.
The aim of this investigation was to determine whether short-term hyperthermia could be utilized to dampen inflammatory events in fibroblast-like synoviocytes (FLS), a cell type that plays an important role in the genesis of rheumatoid arthritis. Furthermore, we investigated the time requirements of HSPs transcription and translation in fibroblast-like synoviocytes induced by short term hyperthermia.
Materials and Methods
FLS were exposed to short-term hyperthermia (41°C, 30 minutes) and subsequently stimulated with interleukin-1β (IL-1β(3). FLS challenged with this cytokine upregulate a number of proinflammatory genes, most of which are regulated by the transcription factor nuclear factor-kappaB (NF-κB). Gene translation was monitored by Western blotting or ELISA experiments. Steady state mRNA levels were investigated by real-time RT-PCR, nuclear translocation of NF-κB by EMSA, and IκB phosphorylation and degradation by Western blotting experiments.
Results
We demonstrate that short-term hyperthermia suppresses IL-1β induced activation of the adhesion molecules ICAM-1 and VCAM-1, the cytokines/chemokines TNFα, IL-1α, IL-1β, IL-8 and MCP1, but also prevents the release of PGE2. The effect of such treatment is specific in that certain genes e.g. COX1 are not affected. Furthermore, while effects of hyperthermia on ICAM-1 can be observed for more than 20 hours, effects on other genes subside significantly earlier.
Our Western blotting experiments show that FLS exposed to hyperthermia readily respond with Hsp70 protein synthesis. However, such experiments also demonstrate high basal levels of Hsp70 protein in unstimulated FLS. Also of importance, hyperthermia induced HSP70 translation requires at least 1 hour, since in this cell type a substantial increase of Hsp70 proteins could only be observed after a recovery period of at least 60 minutes at 37°C. Furthermore, highest levels of Hsp70 proteins were seen at 5 hours - the latest time point monitored.
Steady state mRNA levels of HSPs were investigated by real-time RT-PCR. Hsp32 (HO-1) mRNA levels did increase substantially in response to hyperthermia, however, no significant increase could be observed within the first 6 hours after exposure to hyperthermia. In stark contrast, steady state Hsp70 mRNA levels increased significantly within a very short time. Within 10 minutes of exposure to hyperthermia, HSP70 mRNA levels reached values that were 9 times higher than those in cells kept at 37°C while maximal levels were seen approximately 3 hours afterwards. After 8 hours, concentration of Hsp70 mRNA was again reduced to basal levels.
As to the mechanisms of hyperthermia-mediated protection, we show that nuclear translocation of NF-κB is suppressed in FLS that were exposed to hyperthermia prior to activation with IL-1β. Furthermore, phosphorylation and degradation of IκBα, induced by IL-1β and a prerequisite for the activation of NF-κB, is blocked as well (Fig 1).
(HT – hyperthermia treatment, REC – duration of recovery period prior to IL-1β addition, LC – loading control indicates equal loading as well as transfer of proteins)
Discussion
Short-term hyperthermia prevents the activation of a series of genes associated with inflammation by blocking the activation of the transcription factor NF-κB. The presented data seem to exclude the possibility that newly synthesized Hsps e.g. Hsp70 are essential for the early suppressive effects of hyperthermia on NF-κB dependent genes. Such an assumption is based on our observation that (i) IL-1β induced degradation of the Inhibitor-κB (IκB), a prerequisite for the activation of NF-κB, is already blocked in FLS that were exposed to hyperthermia for no more than 15 minutes and, (ii) that IL-1β induced phosphorylation of IκB, an event that occurs prior to IκB degradation, is prevented as well. In addition, as shown by Western blot experiments, Hsp70 protein levels are still increasing in cells long after the effects of hyperthermia on the activation of NF-κB have subsided (3).
The presented data might, at least in part, explain and provide a rationale for treating inflammation e.g. associated with rheumatoid arthritis by balneological means. Only carefully controlled and executed in vivo studies will clarify whether it is indeed viable to make use of the stress response to ameliorate inflammation in the joints
Figure 1.
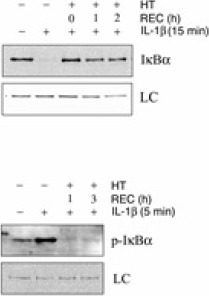
Hyperthermia prevents IL-1f3 induced IκBCC phosphorylation and degradation. Shown in the upper section of this figure are data demonstrating that stimulation of FLS results in complete degradation of IκBCC within 15 minutes. However, prior exposure to heat shock for 30 minutes prevents this event irrespective of a recovery period of up to 2 hours at 37°C prior to addition of IL-1f3 for 15 minutes. As shown in the lower section, IκBCC phosphorylation is readily detectable in non heat-treated cells but absent in cells that were exposed to hyperthermia prior to IL-1p treatment. As indicated, IκBα phosphorylation was monitored after 1 and 3 hours of recovery (REC) at 37°C.
Acknowledgement
I wanted to express my gratitude for being given the opportunity to participate in the IFCC sponsored “Professional Scientific Exchange Program” for training in cell- and molecular biological techniques at the LBI, Vienna, Austria. Currently, I am in the process of finishing my PhD thesis at the Faculty of Pharmacy, University of Belgrade. This thesis is based on experimental work performed during a one-year stay at the LBI in Vienna, Austria as a scholar of the IFCC. I also want to thank Dr. K.M. Stuhlmaier for professional guidance and for training in the methodologies run in his laboratory. So far the scientific output of my experimental work in Vienna is one publication and 4 appearances at congresses.
Marica Markovic, MSc visited Ludwig Boltzmann Institute for Rheumatology and Balneology (LBI), Vienna, Austria for one year (October 2005 – September 2006) and participated in the project “Investigation of the adaptive stress response in Type-B-synoviocytes at the level of gene transcription and gene translation” under the supervision of Univ.-Doz. Dr. Karl M. Stuhlmeier.
References
- 1.Tytell M, Hooper PL. Heat shock proteins: new keys to the development of cytoprotective therapies. Expert Opin Ther Targets 2001; 52: 267-287. [DOI] [PubMed] [Google Scholar]
- 2.Siebenlist US, Brown K, Franzoso G. NF-kB: A Mediator of Pahtogen and Stress Responses. In: Bauerle PA, editor. Inducible Gene Expression, Environemental Stresses and Nutrients. Boston: Birkaeuser, 1995. p. 93-141. [Google Scholar]
- 3.Marković M, Stuhlmeier KM. Short term hyperthermia prevents activation of proinflammatory genes fibroblast-like-synoviocyes by blocking the activation of the transcription factor NF-κB. J Mol Med 2006; 84: 821-832. [DOI] [PubMed] [Google Scholar]


