Abstract
Translocation of the tRNA–mRNA complex is a fundamental step in the elongation cycle of protein synthesis. Our studies show that the ribosome can translocate a P-site-bound tRNAMet with a break in the phosphodiester backbone between positions 56 and 57 in the TΨC-loop. We have used this fragmented P-site-bound tRNAMet to identify two 2′-hydroxyl groups at positions 71 and 76 in the 3′-acceptor arm that are essential for translocation. Crystallographic data show that the 2′-hydroxyl group at positions 71 and 76 contacts the backbone of 23S rRNA residues 1892 and 2433–2434, respectively, in the ribosomal E site. These results establish a set of functional interactions between P-site tRNA and 23S rRNA that are essential for translocation.
Ribosomes are the macromolecular complexes responsible for protein synthesis. Escherichia coli ribosomes are made up of a large 50S subunit and a small 30S subunit that contain three tRNA-binding sites (A, aminoacyl; P, peptidyl; E, exit). One of the fundamental steps in the elongation cycle of protein synthesis is the step-wise movement—called translocation—of tRNAs from one site to the next within the ribosome. Very little is known about how this highly precise and complex process occurs on the ribosome. Biochemical (1, 2) and biophysical (3) studies revealed that translocation occurs in two steps. In the first step, after peptide-bond formation, the acceptor ends of deacylated tRNA and peptidyl-tRNA move from P to E, and from A to P sites, respectively, in the 50S subunit, whereas their anticodon ends still interact with the 30S subunit P and A sites, respectively. This movement occurs spontaneously after peptide-bond formation, which results in tRNAs occupying A/P and P/E hybrid states. In the second step, elongation factor G (EF-G) catalyzes the movement of the anticodon ends of both tRNAs relative to the 30S subunit, translocating deacylated tRNA from P/E to E state and peptidyl-tRNA from A/P to P/P state. Thus, movement of the acceptor end of tRNAs occurs independently of the anticodon ends, relative to the two ribosomal subunits.
EF-G uses the chemical energy of GTP hydrolysis to accelerate translocation of the tRNA–mRNA complex (4). Interestingly, ribosomes can perform spontaneous translocation in the absence of EF-G⋅GTP (5, 6), suggesting that the mechanism of translocation is inherent to the ribosome. Additionally, translocation can occur in the absence of mRNA (7, 8), demonstrating that neither codon–anticodon interactions nor mRNA–ribosome interactions are essential for translocation. Thus, the only components indispensable for translocation are the A- and P-site tRNAs and the ribosome.
The requirement for peptidyl-tRNA in the A site can be satisfied by a 15-nucleotide anticodon stem–loop analog (ASL)-4 of tRNA (9). The ASL, consisting of a seven-nucleotide loop and a four base-pair stem, translocates, indicating that the D stem, T stem, and acceptor arm of the A-site tRNA are not essential for translocation. In contrast to the ribosomal A site, an ASL bound to the ribosomal P site is not translocated (9), suggesting that interactions involving the elbow and/or acceptor end of the P-site tRNA with the ribosomal large subunit are required for translocation.
To identify specific molecular interactions between the acceptor arm of P-site tRNA and the ribosome that are important for translocation, we developed a fragmented tRNA approach. Previous studies have demonstrated that tRNAs with breaks at certain positions in the phosphodiester backbone have the ability to fold into an active structure (10–13). Because several tRNAs with limited sequence conservation interact with the ribosome, it is likely that the translocation machinery utilizes interactions involving the sugar–phosphate backbone of tRNAs. Therefore, in this study, we examined the importance of 2′-hydroxyl groups within the 3′-acceptor end of P-site tRNA for EF-G-dependent translocation.
Materials and Methods
Synthesis and Assembly of Fragmented tRNAMet.
The large 5′ tRNAMet fragment corresponding to positions 1 through 56 was transcribed in vitro from a partially duplex synthetic DNA template by using T7 RNA polymerase (14). RNA transcripts were purified on a 10% denaturing polyacrylamide gel and recovered by passive elution and ethanol precipitation. The smaller 3′ tRNAMet fragments corresponding to positions 57 through 76 were chemically synthesized on an ABI 392 DNA Synthesizer (Applied Biosystems) by using an RNA phosphoramidite method (15). Site-specific incorporation of 2′-O-methyl, 2′-deoxy, 2′-fluoro, and 2′-amino groups (Glen Research, Sterling, VA) were performed during solid-phase synthesis. The synthetic oligoribonucleotides were deprotected as described (16) and purified on a 20% denaturing polyacrylamide gel. The 5′ large fragment and the 3′ small fragment were annealed essentially as described in (13). Briefly, 100 picomoles of the 5′ large fragment was mixed with 150 picomoles of the 3′ small fragment in 50 mM Hepes (final volume of 5 μl) and heated at 60°C for 3 min. MgCl2 was added to a final concentration of 10 mM, and the mixture was slowly cooled to room temperature and placed on ice.
Nondenaturing Polyacrylamide Gel Analysis.
E. coli tRNAMet, the 5′ fragment, and the smaller 3′ fragments were 5′ end-labeled using [γ-32P]-ATP and T4 polynucleotide kinase (New England Biolabs). The labeled RNAs were purified on denaturing polyacrylamide gels and cpm were determined with a liquid scintillation counter (Beckman Coulter). A 32P-labeled 3′ fragment (50,000 cpm) was combined with 150 picomoles of unlabeled 3′ fragment and 100 picomoles of the 5′ large fragment and annealed as described above in 10 μl of final volume. The native tRNAs (50,000 cpm) also were folded by heating and slow cooling. Equal volumes of loading dye (40% glycerol, 0.2% bromophenol blue, and 0.2% xylene cyanol; final concentration) were added and 4 μl (10,000 cpm per lane) of sample was analyzed on a nondenaturing 15% polyacrylamide (acrylamide/bisacrylamide, 19:1) gel [40 mM Tris acetate (pH 7.5), 12 mM Mg(OAc)2] (17). The gels were run at 11W overnight in a 4°C cold room and visualized by autoradiography.
Aminoacylation of Fragmented tRNAMet.
E. coli tRNAfMet, tRNAMet transcript, and fragmented tRNAMet were annealed as described above and used for aminoacylation assays at 37°C for 45 min in 20 mM Hepes (pH 7.5), 150 mM NH4Cl, 10 mM MgCl2, 4 mM ATP (pH 7.0), 100 μM EDTA, 20 μM methionine, 0.04–0.05 μM [35S]methionine (specific activity = 5,000 cpm per picomoles of methionine), and 5 μl of tRNA-free E. coli S-100 extract (3.3 mg/ml, stock concentration) in a final reaction volume of 100 μl. Aliquots of 50 μl of the reaction mixture were removed and mixed with 50 μl of carrier (1 μg/ml BSA/1 M sodium acetate, pH 5.2) and 1 ml of precipitation mix (2.5% trichloroacetic acid/50% ethanol). The mixes were placed at −0°C for 20 min and then filtered through Whatman GF/C filters and washed five times with 5% trichloroacetic acid, followed by five washes with 95% ethanol. Filters were dried and the amount of filter-bound [35S]methionine was quantitated by liquid scintillation. Background values were determined by performing the reactions either in the absence of tRNA or S-100 extract. The specific activity of the reaction mix was determined by spotting 2 μl of the reaction mixture onto filters without washing.
Formation of Pretranslocation Complexes and the Translocation Reaction.
Pretranslocation complexes were formed by using ribosomes programmed with gene 32 mRNA and containing tRNAMet in the P site and ASL4Phe in the A site, as described (9). The final concentration of the components were 0.4 μM tight-couple ribosomes, 0.8 μM gene 32 mRNA, 2 μM tRNAMet (or annealed-fragmented tRNAMet), 6 μM ASL4Phe in binding buffer [80 mM K-cacodylate, pH 7.2/20 mM Mg(OAc)2/150 mM NH4Cl at final concentration]. Translocation reactions were performed by the addition of 0.4 μM EF-G and 300 μM GTP (final concentrations) to the complexes and incubating at room temperature for 15 min. Except in Fig. 2A, the translocation reaction was performed at 37°C for 30 min with 2 μM EF-G. For time-course experiments, aliquots of the reaction mixture were taken at defined time points and placed on ice. The extent of translocation was determined by toeprinting assays, all as described (9). The gels were quantified with a Molecular Dynamics PhosphorImager. Rectangles were used to quantify the toeprint bands corresponding to pre- and posttranslocation for each reaction time by using the volume integrate function of IMAGEQUANT software (Molecular Dynamics). Total counts for each time point are the sums of pre- and posttranslocation bands. The extent of translocation was calculated by dividing the posttranslocation counts by the total counts for each reaction. The translocation of modified tRNAs were normalized with respect to translocation of unfragmented tRNAMet (set at 100% for the 60-min time point). The apparent rate of translocation (k) was obtained from plots of fraction translocated vs. time. The data were fit by least squares fitting to the single exponential equation Y = Ymax[1−exp(−kx)], where Ymax is the maximum extent of translocation, Y is the fraction translocated at time x, and k is the observed rate constant (GRAPHPAD PRISM; GraphPad, San Diego). The observed endpoints for each reaction were used to fit the data. The rates were derived from the average of at least three independent experiments and are within the 95% confidence intervals. Errors in the rate were ≤30%. The relative inhibition was calculated as the ratio of k values.
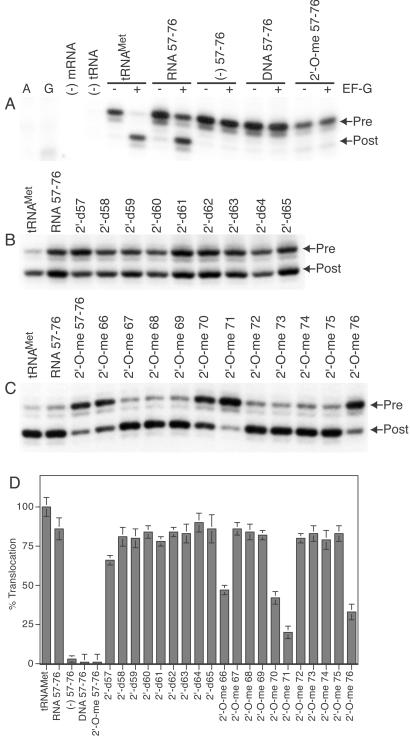
Figure 2.
Translocation of fragmented tRNAMet bound to the ribosomal P site. (A–C) Arrows indicate toeprints before (Pre) and after (Post) translocation. (A) Translocation of fragmented tRNAMet having either unsubstituted or fully 2′-deoxy or 2′-O-methyl substituted small fragment. A and G, respective dideoxy sequencing lanes; (−) mRNA, reaction in the absence of mRNA; (−) tRNA, reaction in the absence of A- and P-site tRNAs. (B) Translocation of fragmented tRNAMet having single 2′-deoxy substitutions at positions 57–65. (C) Translocation of fragmented tRNAMet having single 2′-O-methyl substitutions at positions 66–76. (D) Extent of translocation of tRNAMet having single 2′-deoxy substitutions at positions 57–65 and single 2′-O-methyl substitutions at positions 66–76. Lanes: tRNAMet, E. coli tRNAMet; RNA 57–76, tRNAMet fragment 1–56 having smaller RNA fragment 57–76; (−) 57–76, tRNAMet fragment 1–56 lacking the small fragment 57–76; DNA 57–76, tRNAMet fragment 1–56 having the fully 2′-deoxy substituted small fragment; 2′-O-me 57–76, tRNAMet fragment 1–56 having fully 2′-O-methyl substituted small fragment. The single 2′-deoxy (2′-d) and 2′-O-methyl (2′-O-me) substitutions from position 57 through 76 are as indicated. Values are normalized with respect to translocation of control tRNAMet, which was set to 100%. Gels were quantified with a Molecular Dynamics PhophorImager. Results are the average of at least three experiments.
Ligation of Fragmented tRNAMet.
Ligation of the 3′ small fragment to the 5′ large fragment was accomplished with T4 DNA ligase and a deoxyoligonucleotide complementary to positions 34–74 of E. coli tRNAMet, essentially as described (18).
Results and Discussion
Folding of Fragmented tRNAMet.
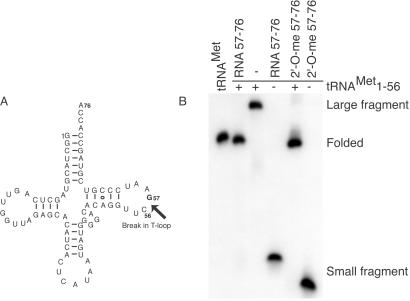
E. coli tRNAMet with a break in the TΨC-loop between positions 56 and 57 was assembled from two separate fragments (Fig. 1A). Nondenaturing PAGE showed that the two fragments anneal together and fold into a conformation that resembles the full-length tRNAMet (Fig. 1B). Additionally, 3′ fragments incorporating 2′-O-methyl substitutions at positions 57–76 also anneal to the 5′ fragment and fold into the tRNA tertiary structure. Thus, 2′-hydroxyl groups from positions 57–76 are not required for the proper folding of the tRNA.
Figure 1.
Folding of fragmented E. coli tRNAMet. (A) Secondary structure of tRNAMet with a break between positions 56 and 57 in the phosphodiester backbone is indicated by the arrow. (B) Folding of fragmented tRNAMet is monitored with nondenaturing PAGE. Lanes: tRNAMet, E. coli tRNAMet; RNA 57–76, unsubstituted smaller RNA fragment; 2′-O-me 57–76, fully 2′-O-methyl substituted smaller fragment. (+) and (−) indicate presence and absence, respectively, of the 5′ large fragment (positions 1–56). Mobility of the folded tRNAMet, 5′ large fragment, and 3′ small fragment are indicated on the right.
The functional integrity of the fragmented tRNAMet was analyzed further by its ability to be aminoacylated by methionyl-tRNA synthetase. The aminoacylation efficiency of the fragmented tRNAMet was about 2-fold lower compared with tRNAMet transcript (data not shown), indicating that the nick in the tRNA backbone between positions 56 and 57 have minor effects on the overall tRNA tertiary structure.
Fragmented tRNAMet Translocates from the Ribosomal P Site.
We constructed pretranslocation complexes with ribosomes programmed with a fragment of phage T4 gene 32 mRNA having either deacylated tRNAMet or fragmented tRNAMet in the P site and an ASL4Phe in the A site. ASL4Phe was used because translocation of tRNAPhe is very rapid, and the linear range cannot be resolved reliably by using our assay system. Additionally, we used a buffer system that further reduces the rate of translocation. This strategy permitted us to distinguish the kinetic behavior of the fragmented tRNAMet bound to the ribosomal P site. Translocation was initiated by the addition of EF-G⋅GTP to the pretranslocation complex. The movement of mRNA was detected by using the toeprinting assay (9, 19, 20). Consistent with previous studies (21–23), the large 5′ fragment 1–56 that contains the sequence corresponding to the anticodon arm of tRNAMet is capable of binding to the 30S P site even in the absence of the 3′ fragment, but does not translocate (Fig. 2A). Remarkably, tRNAMet assembled from a 5′ large fragment and a synthetic 3′ small fragment translocates from the ribosomal P site. Replacement of the small fragment with a DNA analog or 2′-O-methyl analog inhibits translocation, indicating that one or more 2′-hydroxyl groups from positions 57–76 within P tRNAMet is required for translocation.
Identification of 2′-Hydroxyl Groups Essential for Translocation.
To determine the precise locations of critical 2′-hydroxyl groups, we systematically tested single 2′-deoxy substitutions at positions 57 through 65. No inhibition of translocation was observed at these positions (Fig. 2 B and D). We next analyzed single 2′-O-methyl substitutions at positions 66 through 76. Interestingly, inhibition of translocation was observed at positions 66, 70, 71, and 76 (Fig. 2 C and D), indicating that the 2′-hydroxyl groups at these positions may play an important role in translocation.
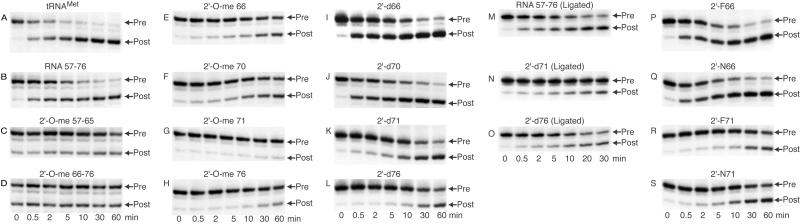
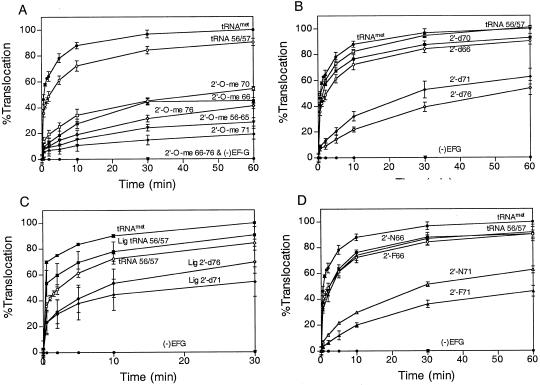
We studied the kinetic behavior of P-site-bound tRNAMet incorporating a single 2′-O-methyl substitution at positions 66, 70, 71, and 76. Our results showed that 2′-O-methyl substitution at positions 66, 70, 71, and 76 results in at least a 7-fold inhibition in the rate of translocation (Fig. 3 E–H, Fig. 4A, and Table 1). Because the 2′-O-methyl group is bulkier than the 2′-hydroxyl group, inhibition of translocation could be caused by steric interference. To rule out this possibility, we analyzed the 2′-deoxy substitution at positions 66, 70, 71, and 76. 2′-deoxyribose favors a C2′-endo sugar conformation compared with a C3′-endo sugar conformation in RNA (24). Again, we observed a greater than 10-fold inhibition with 2′-d71 and 2′-d76, suggesting that these 2′-hydroxyl groups are important for translocation (Fig. 3 I–L, Fig. 4B, and Table 1). In contrast, 2′-d66 and 2′-d70 showed no inhibition, indicating that neither the 2′-hydroxyl group nor the sugar conformation at positions 66 and 70 are critical for translocation. Therefore, the inhibition observed with 2′-O-methyl substitutions at positions 66 and 70 may be caused by steric interference with ribosomal components during translocation. The importance of a 2′-hydroxyl group at positions 71 and 76 was further confirmed by ligating the 3′ small fragment incorporating these substitutions to the 5′ large fragment and testing for their ability to translocate. We again observed at least a 7-fold reduction in translocation rate with the ligated 2′-d71 and 2′-d76 fragments (Fig. 3 M–O, Fig. 4C, and Table 1).
Figure 3.
Time course of translocation of fragmented tRNAMet incorporating 2′-hydroxyl group substitutions within the small fragment. Lanes are as described above in Fig. 2. Ligated, tRNAMet fragment 1–56 ligated to the respective small fragment 57–76. 2′-F66 and 2′-F71 are 2′-fluoro substitutions at positions 66 and 71, respectively; 2′-N66 and 2′-N71 are 2′-amino substitutions at positions 66 and 71, respectively.
Figure 4.
Kinetic analysis of translocation of fragmented tRNAMet incorporating 2′-hydroxyl group substitutions. (A) Translocation of 2′-O-methyl substituted tRNAMet. (B) Translocation of 2′-deoxy substituted tRNAMet. (C) Translocation of ligated tRNAMet incorporating 2′-deoxy substitutions. (D) Translocation of 2′-fluoro and 2′-amino substituted tRNAMet. Labels are as described above in Figs. 2 and 3. Values are normalized with respect to translocation of control tRNAMet done in parallel. Results are the average of at least three experiments.
Table 1.
Apparent translocation rates of 2′-hydroxyl group substituted fragmented tRNAMet
| Position | k, min−1 | Relative inhibition* |
|---|---|---|
| tRNAMet | 0.7 | 1.0 |
| Fragmented tRNAMet | 0.4 | 1.8 |
| Ligated tRNA 56/57 | 0.3 | 2.3 |
| Ligated tRNA 2′-d71 | 0.1 | 7.0 |
| Ligated tRNA 2′-d76 | 0.07 | 10.3 |
| 2′-O-me 57–65 | 0.1 | 7.0 |
| 2′-O-me 66–76 | <0.001 | – |
| 2′-O-me 66 | 0.1 | 7.0 |
| 2′-O-me 70 | 0.1 | 7.0 |
| 2′-O-me 71 | 0.1 | 7.0 |
| 2′-O-me 76 | 0.07 | 10.0 |
| 2′-d66 | 0.5 | 1.4 |
| 2′-d70 | 0.5 | 1.4 |
| 2′-d71 | 0.07 | 10.0 |
| 2′-d76 | 0.05 | 14.0 |
| 2′-F66 | 0.3 | 2.3 |
| 2′-N66 | 0.3 | 2.3 |
| 2′-F71 | 0.06 | 11.7 |
| 2′-N71 | 0.07 | 10.0 |
Relative inhibition was calculated as the ratio of K values.
To dissect further the role of the 2′-hydroxyl group at position 71, we tested 2′-fluoro and 2′-amino substitutions at position 71. Both 2′-fluoro and 2′-amino groups are similar in size but differ in their potential for forming hydrogen bonds and in their sugar conformation. The 2′-fluoro group can act as a weak hydrogen-bond acceptor and favors the C3′-endo form, whereas the 2′-amino group retains the hydrogen-bonding properties of the 2′-hydroxyl group but adopts the C2′-endo pucker (24–26). As a control, we also analyzed similar substitutions at position 66. We were unable to test 2′-fluoro and 2′-amino substitutions at positions G70 and A76, because their phosphoramidite monomers are not commercially available at the present time. Both 2′-fluoro and 2′-amino group substitutions at position 66 have only modest effects on translocation (Fig. 3 P and Q, Fig. 4D, and Table 1). Hence, the 2′-hydroxyl group at position 66 is not critical for translocation. Interestingly, the 2′-fluoro and 2′-amino substitutions at position 71 inhibit translocation by about 10-fold (Fig. 3 R and S, Fig. 4D, and Table 1). These results demonstrate that both the 2′-hydroxyl and the sugar conformation at position 71 are critical for translocation.
Elegant experiments by Wintermeyer and coworkers (27) showed that deletion or mutation of A76 in P-site tRNA results in a 30- to 40-fold inhibition in the rate of translocation. Especially relevant is the 2′-deoxy substitution at position 76 that results in a 15-fold reduction in E-site affinity and causes a 14-fold inhibition in the rate of translocation. Thus, both the nucleotide base and the ribose 2′-hydroxyl group at position 76 are critical for E-site binding (28, 29) and translocation. These results suggest that interactions between the acceptor end of P-site tRNA and the 50S subunit E site are a prerequisite for EF-G-dependent translocation in the 30S subunit.
Functional Interactions Between P tRNA and the Ribosome.
Three-dimensional cryo-electron microscopy (30) and directed hydroxyl-radical probing (31) were used to position EF-G on the ribosome at low resolution. EF-G binds at the base of the L7/L12 stalk of the 50S subunit, a location that is distant from the acceptor end of P-site tRNA. Therefore, contact between ribose 2′-hydroxyl groups at positions 71 and 76 of P-site tRNA and EF-G can be excluded, and it is more likely that they interact directly with the ribosome. Recently, the structure of the ribosome with tRNAs bound to the A, P, and E sites has been solved at 5.5-Å resolution (32). We used this crystal structure to identify ribosomal components that specifically interact with these 2′-hydroxyl groups in tRNA (Fig. 5). Position 66 is located in the acceptor helix and projects toward the anticodon arm of the tRNA. We observed inhibition with the bulky 2′-O-methyl substitution at position 66; however, no inhibition was detected with the 2′-deoxy, 2′-fluoro, and 2′-amino substitutions, suggesting that the 2′-hydroxyl at this position is not essential for translocation. Furthermore, the x-ray structure does not show any contact between position 66 within P or E-site tRNA and the ribosome (32). Positions 70 and 71 are located on the opposite surface of the acceptor helix facing the 50S subunit E site. Although they are exposed and are available for potential interactions with the ribosome, there is no interaction between position 70 and the ribosomal P or E sites in the crystal structure. This finding is consistent with our results that show full restoration of activity with the 2′-deoxy substitution at position 70 in the P-site tRNA.
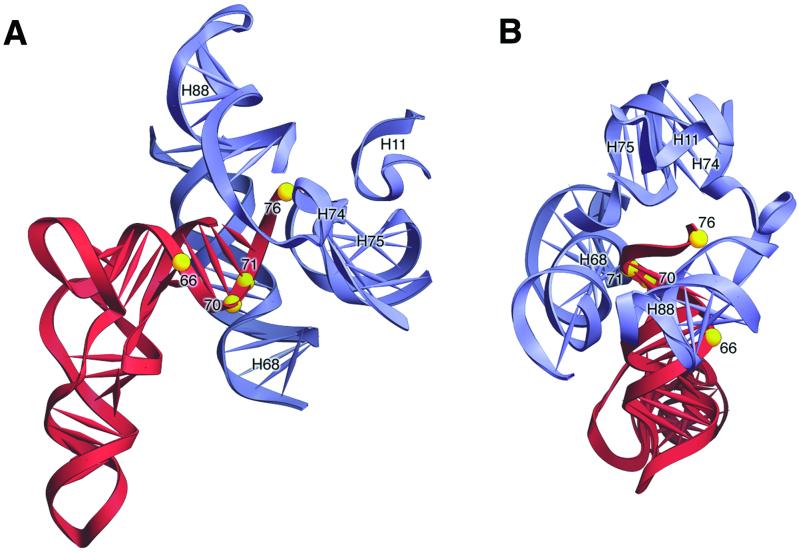
Figure 5.
Interaction of E-site tRNA with the 50S subunit. (A) View from the side with TΨC-loop in front and the D-loop behind. In this orientation, positions 66 and 76 are located in front, and positions 70 and 71 are located behind (yellow spheres). (B) View from the top of the acceptor stem-TΨC helix with the anticodon stem pointing into the paper. Positions 66 and 76 are located on the surface to the right, and positions 70 and 71 are located to the left. The interaction of E-site tRNA (red) with 23S rRNA (blue) is based on the 5.5-Å resolution ribosome structure (32). H11, H68, H74, H75, and H88 correspond to the helices in 23S rRNA.
Remarkably, the x-ray crystal structure of the ribosome shows a minor grove–minor grove interaction between position 71 of E-site tRNA and residue 1892 in helix 68 of 23S rRNA (32). Results presented here show that this interaction is essential for translocation. Position 76 is located in the 3′ single-stranded terminus of the tRNA. This region of the tRNA is very flexible and is available for interaction with ribosomal components that are located within a radius of 20 Å. In the crystal structure, the backbone of position 76 in E-site tRNA interacts with 23S rRNA residues 2433 and 2434 in helix 74 and the base-contacts residue 199 in helix 11 of 23S rRNA (32). Our study shows that interactions involving the 2′-hydroxyl groups at position 71 and 76 in the acceptor arm of P-site tRNA (in the P/E hybrid state) with 23S rRNA elements in the 50S subunit E site are of functional significance, because substitutions that disrupt these contacts inhibit translocation. These results establish an active role for 23S rRNA in the molecular mechanism of translocation.
Translocation of the tRNA–mRNA complex involves disruption of existing interactions in one site and the establishment of new interactions in the next site within the ribosome. Specific hydrogen-bonding interactions between the acceptor arm of P-site tRNA and the 50S subunit E site may facilitate the first step of translocation by permitting the acceptor end of the tRNAs to move relative to the 50S subunit (in to the P/E and A/P hybrid states) followed by movement of the anticodon arms relative to the 30S subunit. Such a step-wise movement will lower the energy barrier required for translocation and may be essential for EF-G-dependent translocation in the 30S subunit. Alternatively, interactions between the acceptor end of P-site tRNA and the 50S subunit E site may act as a signal for the ribosome to undergo conformational changes essential for 30S translocation. Presently, it is not possible to distinguish between these two mechanisms. Finally, our study demonstrates that the fragmented-tRNA approach is a powerful method for identifying functional groups within tRNA that are important for translocation. Future biochemical experiments addressing such functional interactions within the ribosome will complement high resolution x-ray structure analysis of the ribosome in elucidating the molecular basis of translocation.
Acknowledgments
We thank David Pecchia and John M. Burke for synthesizing most of the oligoribonucleotides used in this work. We thank Harry F. Noller for discussions of x-ray crystallographic data before publication, Albion Baucom for preparing Fig. 5, Scott A. Strobel for the His-tagged T4 DNA ligase clone, Kevin Wilson for the E. coli S-100 extract, Andrew McCammon for use of the Silicon Graphics workstation, Joseph Adams for discussions on curve fitting, and G. Ghosh, P. Ghosh, P. Jennings, and the anonymous reviewers for comments on the manuscript. J.S.F. also thanks G. W. Feinberg for insightful discussions. This work was supported by Grant MCB-0078322 from the National Science Foundation, the Hellman Fellows Program, and the Academic Senate, University of California, San Diego (to S.J.).
Abbreviations
- A site
aminoacyl site
- P site
peptidyl site
- E site
exit site
- EF-G
elongation factor G
- ASL
anticodon stem–loop analog
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Moazed D, Noller H F. Nature (London) 1989;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- 2.Borowski C, Rodnina M V, Wintermeyer W. Proc Natl Acad Sci USA. 1996;93:4202–4206. doi: 10.1073/pnas.93.9.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odom O W, Picking W D, Hardesty B. Biochemistry. 1990;29:10734–10744. doi: 10.1021/bi00500a004. [DOI] [PubMed] [Google Scholar]
- 4.Rodnina M V, Savelsbergh A, Katunin V I, Wintermeyer W. Nature (London) 1997;385:37–41. doi: 10.1038/385037a0. [DOI] [PubMed] [Google Scholar]
- 5.Pestka S. J Biol Chem. 1968;243:2810–2820. [PubMed] [Google Scholar]
- 6.Gavrilova L P, Spirin A S. FEBS Lett. 1971;17:324–326. doi: 10.1016/0014-5793(71)80177-1. [DOI] [PubMed] [Google Scholar]
- 7.Belitsina N V, Tnalina G Z, Spirin A S. FEBS Lett. 1981;131:289–292. doi: 10.1016/0014-5793(81)80387-0. [DOI] [PubMed] [Google Scholar]
- 8.Belitsina N V, Tnalina G Z, Spirin A S. BioSystems. 1982;15:233–241. doi: 10.1016/0303-2647(82)90008-9. [DOI] [PubMed] [Google Scholar]
- 9.Joseph S, Noller H F. EMBO J. 1998;17:3478–3483. doi: 10.1093/emboj/17.12.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imura N, Weiss G B, Chambers R W. Nature (London) 1969;222:1147–1148. doi: 10.1038/2221147a0. [DOI] [PubMed] [Google Scholar]
- 11.Thiebe R, Zachau H G. Biochem Biophys Res Commun. 1969;36:1024–1031. doi: 10.1016/0006-291x(69)90307-6. [DOI] [PubMed] [Google Scholar]
- 12.Pan T, Gutell R R, Uhlenbeck O C. Science. 1991;254:1361–1364. doi: 10.1126/science.1720569. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Musier-Forsyth K. Biochemistry. 1994;33:12708–12714. doi: 10.1021/bi00208a023. [DOI] [PubMed] [Google Scholar]
- 14.Milligan J F, Uhlenbeck O C. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- 15.Scaringe S A, Francklyn C, Usman N. Nucleic Acids Res. 1990;18:5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wincott F, DiRenzo A, Shaffer C, Grimm S, Tracz D, Workman C, Sweedler D, Gonzalez C, Scaringe S, Usman N. Nucleic Acids Res. 1995;23:2677–2684. doi: 10.1093/nar/23.14.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedor M J, Uhlenbeck O C. Proc Natl Acad Sci USA. 1990;87:1668–1672. doi: 10.1073/pnas.87.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore M J, Query C C. Methods Enzymol. 2000;317:109–123. doi: 10.1016/s0076-6879(00)17009-0. [DOI] [PubMed] [Google Scholar]
- 19.Hartz D, McPheeters D S, Gold L. Genes Dev. 1989;3:1899–1912. doi: 10.1101/gad.3.12a.1899. [DOI] [PubMed] [Google Scholar]
- 20.Hartz D, McPheeters D S, Traut R, Gold L. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- 21.Rose S J, III, Lowary P T, Uhlenbeck O C. J Mol Biol. 1983;167:103–117. doi: 10.1016/s0022-2836(83)80036-9. [DOI] [PubMed] [Google Scholar]
- 22.Moazed D, Noller H F. Cell. 1986;47:985–994. doi: 10.1016/0092-8674(86)90813-5. [DOI] [PubMed] [Google Scholar]
- 23.Moazed D, Noller H F. J Mol Biol. 1990;211:135–145. doi: 10.1016/0022-2836(90)90016-F. [DOI] [PubMed] [Google Scholar]
- 24.Saenger W. Principles of Nucleic Acid Structure. New York: Springer; 1984. [Google Scholar]
- 25.Withers S G, Street I P, Percival M D. Fluorinated Carbohydrates as Probes of Enzyme Specificity and Mechanism. Washington, DC: Am. Chem. Soc.; 1988. [Google Scholar]
- 26.Baidya N, Uhlenbeck O C. Biochemistry. 1995;34:12363–12368. doi: 10.1021/bi00038a033. [DOI] [PubMed] [Google Scholar]
- 27.Lill R, Robertson J M, Wintermeyer W. EMBO J. 1989;8:3933–3938. doi: 10.1002/j.1460-2075.1989.tb08574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grajevskaja R A, Ivanov Y V, Saminsky E M. Eur J Biochem. 1982;128:47–52. doi: 10.1111/j.1432-1033.1982.tb06929.x. [DOI] [PubMed] [Google Scholar]
- 29.Lill R, Lepier A, Schwagele F, Sprinzl M, Vogt H, Wintermeyer W. J Mol Biol. 1988;203:699–705. doi: 10.1016/0022-2836(88)90203-3. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal R K, Penczek P, Grassucci R A, Frank J. Proc Natl Acad Sci USA. 1998;95:6134–6138. doi: 10.1073/pnas.95.11.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson K S, Noller H F. Cell. 1998;92:131–139. doi: 10.1016/s0092-8674(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 32.Yusupov M M, Yusupova G Z, Baucom A, Lieberman K, Earnest T N, Cate J H D, Noller H F. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]