PMN varies significantly across 1 metropolitan area. Pharmacies can be key collaborators within communities focused on improving population health.
Abstract
BACKGROUND AND OBJECTIVES:
Variability in primary medication nonadherence (PMN), or failure to fill a new prescription, influences disparities and widens equity gaps. This study sought to evaluate PMN across 1 metropolitan area and assess relationships with underlying zip code–level measures.
METHODS:
This was a retrospective observational study using data extracted from 1 regional community pharmacy market-share leader (October 2016–April 2017). Data included patient age, sex, payer, medication type, and home zip code. This zip code was connected to US census measures enumerating poverty and vehicle access, which were treated as continuous variables and within quintiles. The prescription-level outcome was whether prescriptions were not filled within 30 days of reaching the pharmacy. The ecological-level outcome was PMN calculated for each zip code (numerator, unfilled prescriptions; denominator, received prescriptions).
RESULTS:
There were 213 719 prescriptions received by 54 included pharmacies; 12.2% were unfilled. Older children, boys, and those with public insurance were more likely to have prescriptions not filled. Prescriptions originating from the highest poverty quintile were significantly more likely to not be filled than those from the lowest poverty quintile (adjusted odds ratio 1.60; 95% confidence interval 1.52–1.69); a similar pattern was noted for vehicle access (adjusted odds ratio 1.77; 95% confidence interval 1.68–1.87). At the ecological level, there were significant, graded relationships between PMN rates and poverty and vehicle access (both P < .0001); these gradients extended across all medication classes.
CONCLUSIONS:
Poverty and vehicle access are related to significant differences in prescription- and ecological-level PMN across 1 metropolitan area. Pharmacists and pharmacies can be key partners in population health efforts.
What’s Known on This Subject:
Primary medication nonadherence is the failure to fill a new prescription; rates are as high as 25% in some populations. Nonadherence can result from patient, health care system, and contextual factors that, if not effectively understood, may widen equity gaps.
What This Study Adds:
Nearly 1 in 8 prescriptions received by a large, regional, community pharmacy chain goes unfilled. Prescriptions from high-poverty (and low–vehicle-access) zip codes are significantly less likely to be filled. Similar gradients are seen across each medication class.
Prescription medications are components of high-quality, high-value health care services. Nonadherence to prescription medications remains high, although it can lead to poor health outcomes.1–3 Primary medication nonadherence (PMN) is defined as the failure to fill a newly prescribed medication.4,5 PMN rates are as high as 25% in certain populations.6 Failure to initiate newly prescribed medications can burden patients, families, and the broader health care system through potentially avoidable morbidity and cost.7,8
Nonadherence can result from health care system, patient, and contextual factors. Within the health care system, information exchange among providers, pharmacies, and patients may be insufficient. These challenges may be compounded by patient- or family-level competing priorities (eg, food or housing insecurity), a lack of trust in the health care system, or an incomplete understanding of a prescription’s indications.5,9–15 Contextual, place-based factors may limit the ability of certain individuals or populations to access prescriptions; they may live in “pharmacy deserts” or areas with limited transportation options.16,17 If pharmacies are difficult to access, risks often rooted in poverty may become more influential, which is in line with previously identified relationships between population-level PMN and socioeconomic factors.6,18–21
Electronic prescribing, defined as the “computerized ordering of specific medication regimens for individual patients,”22 allows prescribers to directly send medication orders to a pharmacy of their patient’s choice. When sent, an electronic record is generated, which is then linked to whether the prescribed medication gets dispensed. This electronic data trail has made studies of PMN more feasible. To date, studies have used data from pharmacy benefit management companies and claims data sets to define PMN rates across subpopulations and/or medication classes.1,2,6,18,19,23–26 Researchers in pediatric studies have calculated PMN rates between 7% and 25%.27–30 To our knowledge, however, an assessment of pediatric PMN rates across an entire region, regardless of clinical setting, medication class, or payer, has not been pursued. Thus, we sought to determine prescription-level and ecological determinants of PMN for children living in 1 metropolitan area using data from 1 large, community pharmacy chain. We hypothesized that prescriptions for patients from zip codes with more poverty and less vehicle access would be less likely to be filled. We also hypothesized that zip code–level PMN rates would be characterized by equity gaps that would extend across medication classes. A deeper understanding of such differences could lead to interventions that effectively overcome health care system, patient, and contextual barriers to achieving population health.
Methods
Study Design and Setting
We used a retrospective observational design to pursue prescription-level and ecological analyses of data extracted from 1 community pharmacy chain. The chain is among the regional leaders for pharmacy market share, capturing ∼40% of prescriptions within the study area. This area includes metropolitan Cincinnati, with 54 pharmacies located in 44 unique urban, suburban, and rural zip codes. Across these zip codes, the median poverty rate is 10.9% (range: 2.9%–61.0%); the median rate of households without access to a vehicle is 5.5% (range: 0.8%–37.4%).31
Measures
We extracted data for all prescriptions received by included pharmacies between October 2016 and April 2017 for patients aged 0 to 18 years. We analyzed only new prescriptions with unique prescription numbers. Refills of maintenance medications were only included if they were ordered as new prescriptions, although we were unable to determine how frequently this occurred. Each prescription was attached to deidentified information about the patient for which it was prescribed. Prescription-level data included patient age, sex, payer, and home zip code and the receiving pharmacy. Patient age was defined as a continuous variable, 0 to 18 years. Payer was defined as private, public, discount card, or unknown (including prescriptions paid for in cash). We were unable to assess the dollar amount of copays incurred by the patient and/or family. Given that data were deidentified, we were also unable to discern the number of unique prescriptions received per unique patient. Prescription-level data did include the name of the medication and the medication type. Medication types were grouped into medication classes for analytic purposes (Supplemental Table 4). When possible, classifications were guided by previous work.4,6,19,25
We defined prescriptions as “filled” if the patient picked the medication up and “not filled” if it was not picked up within 30 days of being received. There are no consistent temporal definitions for PMN within the literature; a recent systemic review found definitions for nonadherence ranging from 24 hours to 365 days.7 The data available to us allowed us to assess nonadherence at 14 days and 30 days. Given no substantive differences between these cut-points, we opted to pursue our analyses using 30 days, which was the more conservative of our 2 potential PMN markers. For prescription-level analyses, PMN was kept as a dichotomous, filled–not filled measure. For ecological analyses, we calculated a PMN rate for each zip code. The numerator was all not-filled prescriptions within a zip code; the denominator was the sum of filled and not-filled prescriptions within that zip code.
In parallel, we extracted area-based socioeconomic indicators for the 98 unique zip codes contributing prescriptions,32 pulling from the publicly available 2011–2015 US Census American Community Survey.31 A priori, we focused on zip code–level poverty and vehicle access. We expected that poverty would characterize a broad array of socioeconomic risk factors, whereas vehicle access would approximate one’s ability to access pharmacies (Greater Cincinnati’s public transportation system is limited).20,32 The poverty variable was defined as the percentage of individuals living at or below the federal poverty level. The vehicle access variable was defined as the percentage of households with no available vehicle. These area-based indicators were analyzed both as continuous measures and in quintiles.
Analytic Approach
We used descriptive statistics to outline prescription-level and ecological sample characteristics, enumerating proportions for dichotomous variables, medians, and interquartile ranges for continuous variables.
For prescription-level analyses, we compared sample characteristics with respect to PMN using the χ2 test or Kruskal-Wallis test. The Mantel-Haenszel χ2 test of trend was used in our assessment of associations between PMN and zip code quintiles of poverty and vehicle access. Next, we pursued a series of logistic regression models. Our outcome was whether a unique prescription was filled or not filled (prescription-level PMN). Our primary predictors were quintile measures of zip code poverty and vehicle access. Model 1 included patient age, sex, and payer. Model 2 added the zip code poverty variable. Model 3 replaced poverty with the vehicle access variable. Given the correlation between poverty and vehicle access (Spearman correlation coefficient of 0.82; P < .0001), we did not include them in the same model. Models provided adjusted odds ratios (aORs) and 95% confidence intervals (CIs).
For our ecological analyses, we visually observed the geospatial distribution of PMN rates across the region. We then used unadjusted linear regression to assess bivariate relationships between PMN rates and zip code–level predictors on a continuous scale. Given the roughly linear relationships, we looked at PMN rates within each quintile of poverty and vehicle access. We did this across medication classes, highlighting PMN rates for the entire sample and for those zip code quintiles with the lowest and highest rates of poverty and the lowest and highest rates of household vehicle access.
Finally, we estimated the potential opportunity for narrowing equity gaps, assuming causal relationships between PMN and our area-based indicators. To do so, we calculated the change in the absolute number of filled prescriptions if all zip codes had the PMN rate of the quintile with the lowest poverty (and highest vehicle access).
This study was completed as part of a larger set of analyses approved by the Cincinnati Children’s Institutional Review Board. Statistical analyses were pursued by using SAS version 9.3 (SAS Institute, Inc, Cary, NC). Mapping was pursued with ArcGIS version 10.5.1 (Esri, Redlands, CA).
Results
Sample Characteristics
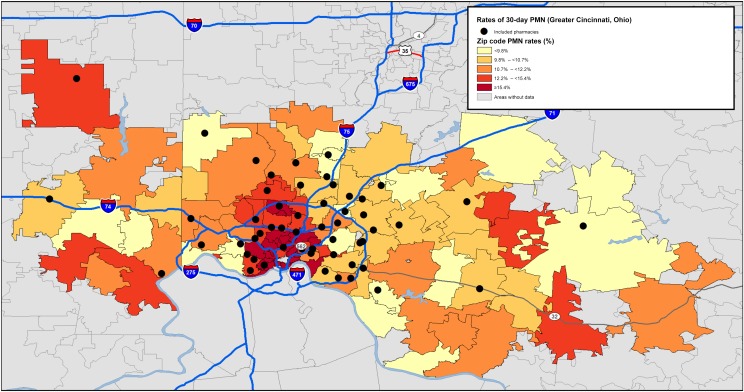
There were 213 719 prescriptions received by the 54 included pharmacies during the study period. Across the sample, 26 054 prescriptions (12.2%) were not filled within 30 days, constituting our PMN rate (Table 1). PMN was slightly higher among older compared with younger children, boys compared with girls, and those with public insurance (or a discount card) compared with those with private insurance (all P < .0001). PMN rates varied across included pharmacies (range 8.1%–22.6%). Although prescriptions were written for patients from 98 unique zip codes, the number written was not distributed equally (median per zip code: 1598 prescriptions; range: 204–8650). PMN rates also varied across zip codes (range: 6.8%–26.4%) (Fig 1).
TABLE 1.
Sociodemographic and Zip Code Characteristics for All Prescriptions Written to 1 Community Pharmacy Chain in Greater Cincinnati Between October 1, 2016, and April 1, 2017, Including a Bivariate Assessment of Whether Those Prescriptions Were Filled Within 30 Days
| Characteristic | All Prescriptions Written (n = 213 719) | Prescriptions Filled Within 30 d (n = 187 665) | Prescriptions Not Filled Within 30 d (n = 26 054) | Pa |
|---|---|---|---|---|
| Across entire sample, n (%) | 213 719 (100) | 187 665 (87.8) | 26 054 (12.2) | — |
| Age, y, median (IQR) | 9.0 (4.0–14.0) | 8.0 (4.0–14.0) | 10.0 (5.0–14.0) | <.0001 |
| Sex, n (%) | <.0001 | |||
| Female | 105 660 (49.4) | 93 133 (88.1) | 12 527 (11.9) | |
| Male | 108 059 (50.6) | 94 532 (87.5) | 13 527 (12.5) | |
| Payment mechanism, n (%) | <.0001 | |||
| Private | 107 999 (50.5) | 98 906 (91.6) | 9093 (8.4) | |
| Public | 83 983 (39.3) | 74 932 (89.2) | 9051 (10.8) | |
| Discount card | 2051 (1.0) | 1829 (89.2) | 222 (10.8) | |
| Unknown | 19 686 (9.2) | — | — | |
| Medication class, n (%) | <.0001 | |||
| Antiepileptic | 3248 (1.5) | 2781 (85.6) | 467 (14.4) | |
| Analgesic and/or antipyretic | 18 651 (8.7) | 16 062 (86.1) | 2589 (13.9) | |
| Asthma and/or allergy | 40 244 (18.8) | 33 094 (82.2) | 7150 (17.8) | |
| Contraceptive | 2058 (1.0) | 1693 (82.3) | 365 (17.7) | |
| Dermatology | 10 989 (5.1) | 8519 (77.5) | 2470 (22.5) | |
| Endocrine | 2793 (1.3) | 2282 (81.7) | 511 (18.3) | |
| Gastrointestinal | 10 017 (4.7) | 8363 (83.5) | 1654 (16.5) | |
| Antihypertensive and/or nephrology | 1823 (1.0) | 1517 (83.2) | 306 (16.8) | |
| Mental health | 19 818 (9.3) | 16 750 (84.5) | 3068 (15.5) | |
| Nutritional | 3472 (1.6) | 2455 (70.7) | 1017 (29.3) | |
| Oral anti-infective | 77 936 (36.5) | 74 680 (95.8) | 3256 (4.2) | |
| Topical anti-infective | 18 050 (8.5) | 15 768 (87.4) | 2282 (12.6) | |
| Other | 4620 (2.2) | 3701 (80.1) | 919 (19.9) | |
| Quintiles of zip code percentage below federal poverty level,b n (%) | <.0001 | |||
| Low | 45 804 (22.1) | 40 989 (89.5) | 4815 (10.5) | |
| Low-medium | 42 519 (20.6) | 38 103 (89.6) | 4416 (10.4) | |
| Medium | 37 195 (18.0) | 33 071 (88.9) | 4124 (11.1) | |
| Medium-high | 52 445 (25.4) | 45 743 (87.2) | 6702 (12.8) | |
| High | 28 922 (14.0) | 23 796 (82.3) | 5216 (17.7) | |
| Quintiles of zip code percentage of households without access to a vehicle,c n (%) | <.0001 | |||
| Low | 35 869 (17.3) | 32 203 (89.8) | 3666 (10.2) | |
| Low-medium | 48 956 (23.7) | 43 754 (89.4) | 5202 (10.6) | |
| Medium | 39 359 (19.0) | 34 831 (88.5) | 4528 (11.5) | |
| Medium-high | 54 014 (27.1) | 49 281 (88.0) | 6733 (12.0) | |
| High | 26 687 (12.9) | 21 633 (81.1) | 5054 (18.9) |
When numbers do not add up to the total prescription count, it is a result of missing data. IQR, interquartile range; —, not applicable.
For P values, we compared nonfilled prescriptions with filled prescriptions. Categorical variables were assessed by using the χ2 test and the Mantel-Haenszel χ2 test of trend; continuous variables were assessed by using the Wilcoxon rank test.
Zip code poverty cut-points were as follows: low (1.6%–6.8%), low-medium (7.1%–10.4%), medium (10.4%–14.3%), medium-high (14.9%–20.5%), and high (20.7%–61.0%).
Zip code vehicle access cut-points were as follows: low (0.4%–3.0%), low-medium (3.1%–4.5%), medium (4.5%–6.0%), medium-high (6.1%–11.8%), and high (12.8%–43.5%).
FIGURE 1.
Zip codes with prescriptions filled between October 1, 2016, and April 1, 2017, and corresponding rates of 30-day PMN.
There were ∼150 different medication types across 13 medication classes. Oral anti-infectives were the most commonly prescribed class (36.5% of all prescriptions). Contraceptives and antihypertensive and/or nephrology medications were the least commonly prescribed (both 1.0%). Oral anti-infectives had the lowest PMN rate (4.2%), whereas nutritional medications (ie, multivitamins and supplements) had the highest rate (29.3%).
Prescription-Level Analyses
Table 2 depicts prescription-level analyses. Model 1 included just those available patient-level variables. We found that older children, boys, and those with either public insurance or a discount card were at significantly higher odds of not filling their prescriptions. In Model 2, we found that those living in the zip code with the highest poverty quintile had a 60% increased likelihood of not filling their prescriptions when compared with those living in the low poverty quintile (aOR 1.60; 95% CI 1.52–1.69). In Model 3, we found that those living in the quintile with the highest rate of households without vehicle access were significantly more likely than those living in the quintile with the lowest rate to not fill their prescriptions (aOR 1.77; 95% CI 1.68–1.87).
TABLE 2.
Odds of Not Filling a Prescription by 30 Days by Sociodemographic and Area-Level Characteristics
| Characteristic | Model 1, OR (95% CI)a | Model 2, OR (95% CI)b | Model 3, OR (95% CI)c |
|---|---|---|---|
| Age, y | 1.04 (1.03–1.04) | 1.04 (1.03–1.04) | 1.04 (1.03–1.04) |
| Sex | |||
| Female | Reference | Reference | Reference |
| Male | 1.10 (1.07–1.14) | 1.10 (1.07–1.14) | 1.11 (1.07–1.14) |
| Payment mechanism | |||
| Private | Reference | Reference | Reference |
| Public | 1.36 (1.32–1.40) | 1.24 (1.20–1.28) | 1.22 (1.18–1.26) |
| Discount card | 1.28 (1.11–1.47) | 1.27 (1.10–1.47) | 1.27 (1.10–1.47) |
| Quintiles of zip code percentage below federal poverty level | |||
| Low | — | Reference | — |
| Low-medium | — | 0.93 (0.89–0.98) | — |
| Medium | — | 1.01 (0.96–1.06) | — |
| Medium-high | — | 1.120 (1.07–1.17) | — |
| High | — | 1.60 (1.52–1.69) | — |
| Quintiles of zip code percentage of households without access to a vehicle | |||
| Low | — | — | Reference |
| Low-medium | — | — | 0.99 (0.94–1.05) |
| Medium | — | — | 1.07 (1.01–1.13) |
| Medium-high | — | — | 1.13 (1.07–1.18) |
| High | — | — | 1.77 (1.68–1.87) |
OR, odds ratio; —, not applicable.
Model 1 included age, sex, and payer.
Model 2 included age, sex, payer, and quintiles as defined by percentage below the federal poverty level.
Model 3 included age, sex, payer, and quintiles as defined by zip code percentage of households without access to a vehicle.
Ecological Analyses
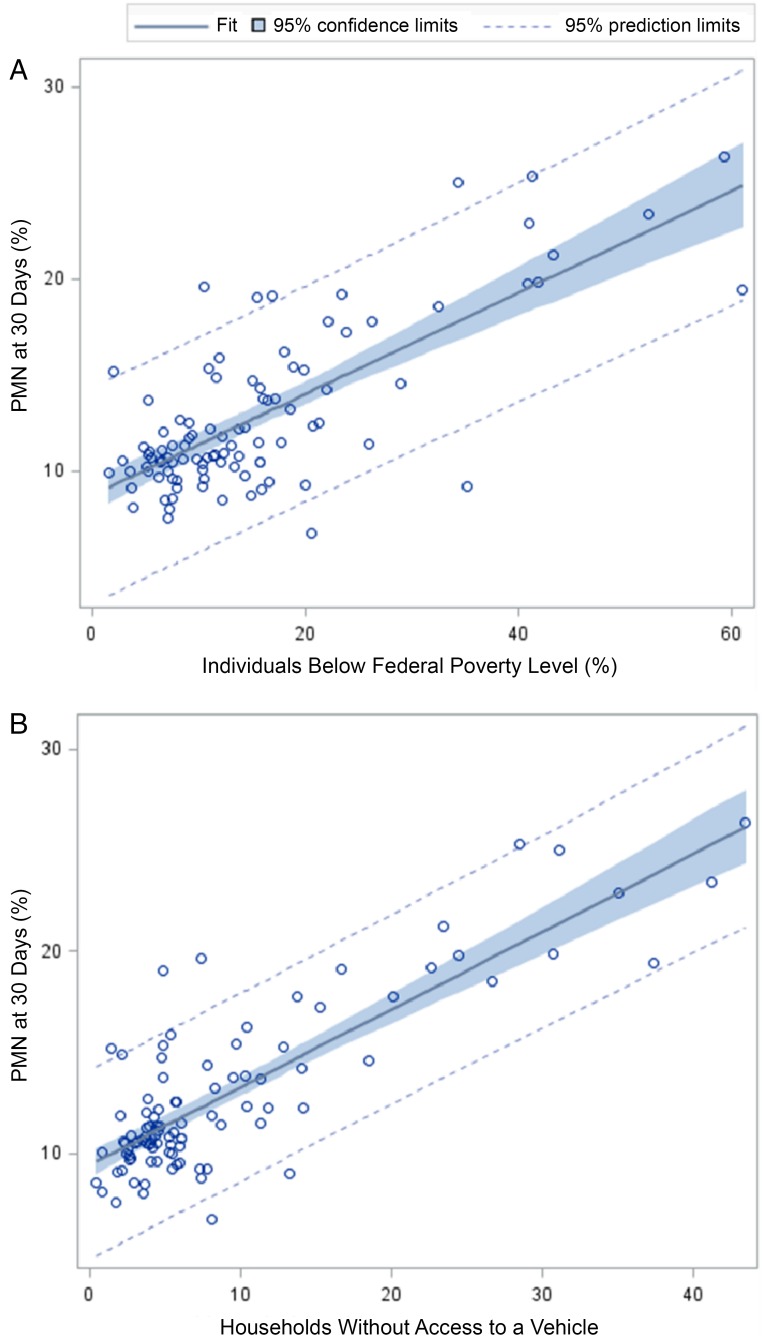
At the zip code level, PMN rates were significantly associated with both poverty and vehicle access on a continuous scale. In a bivariate linear regression model, the R2 for the relationship between PMN and poverty was 0.57 (P < .0001; Fig 2A). The R2 for the relationship between PMN and vehicle access was 0.69 (P < .0001; Fig 2B). Given the roughly linear relationships, we pursued additional analyses with the zip codes in quintiles. There was a significant, graded relationship between zip code–level PMN and poverty quintiles (Table 1). The PMN rate ranged from 10.5% in the low poverty quintile to 17.7% in the high poverty quintile (Mantel-Haenszel P < .0001). Similar trends were noted for our vehicle access variable; the PMN rate increased from 10.2% to 18.9% as the vehicle access rate decreased (P < .0001).
FIGURE 2.
A, Linear regression models used to assess associations between zip code PMN as measured at 30 days and the continuous measure of zip code percentage of individuals living below the federal poverty level. B, Linear regression models used to assess associations between zip code PMN as measured at 30 days and the percentage of households without access to a vehicle.
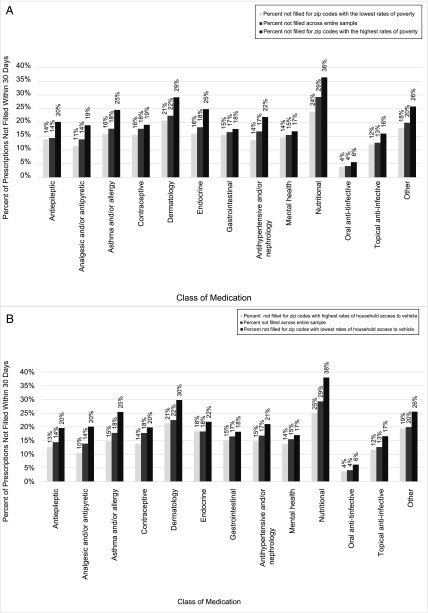
These gradients extended across all medication classes. Figure 3A depicts the PMN rate for each class within the lowest poverty quintile, the entire sample, and the highest poverty quintile. The largest absolute PMN rate differences between the high- and low-poverty zip codes were in the nutritional (difference of 12.5%), endocrine (8.9%), and asthma and/or allergy (8.8%) classes. The smallest difference was found in the oral anti-infective class (1.8%). Figure 3B highlights these same absolute differences by the vehicle access variable. The relative differences by poverty and vehicle access were generally consistent across all medication classes.
FIGURE 3.
A, PMN as measured at 30 days for medication classes across the entire sample and for zip code quintiles with the lowest and highest rates of poverty. B, PMN as measured at 30 days for medication classes across the entire sample and for zip code quintiles with the lowest and highest rates of household access to a vehicle.
Opportunity for Narrowing Nonadherence Equity Gaps
If the PMN rate across all zip codes was the same as the PMN rate in the low poverty quintile, 3438 more prescriptions would have been filled (Table 3). If the PMN rate was the same as the quintile with the highest rate of vehicle access, 4040 additional prescriptions would have been filled.
TABLE 3.
Number of Unfilled Prescriptions at 30 Days by Zip Code Poverty and Vehicle Quintiles With Estimates of How Many Fewer Medications Would Be Unfilled Should Fill Rates Be Less Inequitable Across Quintiles
| No. Filled Prescriptions at 30 d | No. Medications Filled at 30 d if the PMN Rate Was the Same as the First Quintile (Low Poverty, High Vehicle Access) | Change in No. Filled Prescriptions | |
|---|---|---|---|
| Quintiles of zip code percentage below federal poverty levela | |||
| Low | 40 989 | 40 989 | 0 |
| Low-medium | 38 103 | 38 050 | −53 |
| Medium | 33 071 | 33 286 | 215 |
| Medium-high | 45 743 | 46 933 | 1190 |
| High | 23 796 | 25 882 | 2086 |
| Total across all quintiles | 181 702 | 185 140 | 3438 |
| Quintiles of zip code percentage of households without access to a vehicleb | |||
| Low | 32 203 | 32 203 | 0 |
| Low-medium | 43 754 | 43 953 | 199 |
| Medium | 34 831 | 35 337 | 506 |
| Medium-high | 49 281 | 50 289 | 1008 |
| High | 21 633 | 23 960 | 2327 |
| Total across all quintiles | 181 702 | 185 742 | 4040 |
Zip code poverty cut-points were as follows: low (1.6%–6.8%), low-medium (7.1%–10.4%), medium (10.4%–14.3%), medium-high (14.9%–20.5%), high (20.7%–61.0%).
Zip code vehicle access cut-points were as follows: low (0.4%–3.0%), low-medium (3.1%–4.5%), medium (4.5%–6.0%), medium-high (6.1%–11.8%), high (12.8%–43.5%).
Discussion
In this study, nearly 1 in 8 prescriptions for children received by a large, regional chain of pharmacies were not filled, and 1 in 4 were within zip codes with high poverty and low vehicle access. Together, at-risk zip codes had PMN rates that were nearly double their less at-risk counterparts. Each instance in which a prescription goes unfilled represents a circumstance in which a patient may not be receiving a required treatment. When extrapolated to populations, such missed treatment opportunities may perpetuate health equity gaps. Our assessment was limited to new prescriptions. For many chronic medications, this is just the first step in the course of medication adherence. If a medication is not filled at the first opportunity, refills will not be obtained. These findings draw attention to the potential expanded, more proactive role pharmacists (and pharmacies) can play as key partners in the drive toward health equity,33 particularly given their frontline, in-community status.
The link between not-filled prescriptions and area-level poverty and vehicle access aligns with previous work supporting the relevance of place to health.21,32,34 Still, current health care delivery systems, including clinics and hospitals, are largely siloed from communities and community-based pharmacies. There is great potential for expanded communication and collaboration between clinicians and pharmacists, and the broader health care system and pharmacies, in support of medication adherence.35,36 The deployment and evaluation of medication delivery, community health workers, and altered incentives (for clinicians, pharmacists, and patients) may be warranted.21,37–41 An enhanced information technology backbone may further support collaboration, enabling more efficient alerting of PMN to all those on the care team.42
Zip code PMN hot spots could be a worthy starting point for interventions that promote equitable medication access.43 Our study, like others, revealed high rates of PMN and corresponding links with area-based socioeconomic variables.6,18,19,25,44 We expect that these links align with a real or perceived cost to adherence, or barriers that get in the way of patients and families picking up prescriptions from the pharmacy. The monetary cost of purchasing the medication, including the copayment and/or cost of transportation to and from the pharmacy, may affect a family’s ability to fill a prescription. This cost may extend to the time required to go and competing demands that may take precedence (eg, food and housing), placing potentially insurmountable barriers between the written prescription and the filled prescription.12
The relationship between PMN and contextual factors, including vehicle availability, suggests that pharmacy accessibility and the concept of pharmacy deserts16 may be important in our region. Although pharmacies were well distributed geographically across Greater Cincinnati, mere placement and proximity neglects to account for factors including household vehicle access or public transportation, walkability, and safety in moving from 1 place to another. Considering these and other place-based geomarkers32 may be relevant to future studies and, ultimately, to interventions or policies focused on enhancing resource accessibility. For example, policy changes that affect the social determinants of health, including public transportation, may be influential in reducing PMN rates in target neighborhoods or zip codes.34
There are also economic arguments for actions that support PMN reduction. Shrank et al19 note that every unfilled prescription that is returned to stock costs a pharmacy ∼$10. In the 6 months of our study, the restocking of 26 054 prescriptions would cost this pharmacy chain >$250 000, not accounting for the lost revenue of unfilled prescriptions. Our analyses suggest that if poverty or vehicle access were truly driving PMN disparities and if interventions and policies were applied to narrow gaps, thousands of additional prescriptions might be filled. Conceivably, this would reduce costs and increase revenue for pharmacies. This could also reduce morbidity that would be costly to patients and families, the health care delivery system, payers, and society at large. Thus, interventions and policies that align incentives with quality outcomes (eg, reduction in PMN) have the potential to reduce cost and improve health in economically meaningful ways.
We found differences in PMN rates across medication classes. Clearly, not all PMN will have equal clinical impacts. For instance, some clinicians may prescribe a medication for an acute illness but recommend that the patient fills it only if symptoms do not subside.45 It is also possible that clinicians may prescribe a medication even if it is available and may be less costly when purchased over the counter. We expect these are reasons why certain medication classes (eg, dermatology and nutritional) had high PMN rates. That said, there are other situations in which PMN can be clinically problematic, such as when a missed prescription can prompt potentially preventable morbidity. Classes of medications characterized by the need for chronic, long-term therapy with PMN rates of 15% to 20% included asthma and/or allergy, endocrine, and mental health. Patients with asthma without their controller medications, diabetes without their insulin, and a depressive disorder without their antidepressants are certainly all at risk for poor outcomes. We also uncovered absolute and relative equity gaps for each medication class, even for those with relatively low PMN rates (eg, oral anti-infectives). Given the gradient noted across zip codes for each class, it is inherently possible that PMN could influence and perhaps perpetuate known disparities.46
This study has certain limitations. First, our data set did not include prescriptions sent to other pharmacies. We expect patients choose their pharmacy on the basis of payer and/or convenience. The included chain had ∼40% of the regional market and spanned a diverse urban, suburban, and rural landscape with patients covered by many different payers. Second, we did not have information about whether prescriptions were transferred to another pharmacy. Thus, a not-filled prescription may not always reflect the circumstance of a prescription not being filled; they may occasionally represent a miscommunication between the patient and prescriber or a technical error. Third, our data set included information at the prescription level, not that of the patient or prescriber. As a result, we are unable to tell the breadth or severity of underlying conditions or barriers to accessing medical care for included patients. We are also unable to tell if certain prescriptions were written for back-up or rescue purposes (ie, anti-infectives with instructions to only fill should symptoms persist for a certain duration of time), situations in which not filling a medication may be an appropriate course of action.45 Similarly, medication refills were included if they were ordered as new prescriptions; we were unable to determine how frequently this occurred. Given our inability to trace a specific prescription to a specific patient, it is also impossible to determine if high or low PMN rates in certain zip codes are attributable to individuals with multiple prescriptions. With >200 000 prescriptions in the data set, we do not think that this would have significantly changed our results. Fourth, our data were only able to be aggregated to zip codes and not smaller, more homogeneous geographies such as census tracts.47 We expect this would bias our results toward the null. Finally, our study is limited to 1 large pharmacy chain in the Midwest; as a result, our findings may not generalize to other regions.
Conclusions
PMN varies considerably across 1 large, metropolitan area. High poverty and low vehicle access are likely influential. We suggest that clinics, hospitals, pharmacies, patients, and families align around the closure of equity gaps through intervention development, deployment, and evaluation.
Acknowledgments
We thank Drs Joseph Wedig and Meghan Hackerson for their assistance and collaboration. We also thank Dr Karen Jerardi, Naomi Beck, and Eli Beck for their guidance.
Glossary
- aOR
adjusted odds ratio
- CI
confidence interval
- PMN
primary medication nonadherence
Footnotes
Ms Hensley and Drs Heaton and Kahn participated in the study concept and design, analysis and interpretation, drafting and critical revision of the manuscript, statistical analysis, and study supervision; Drs Luder and Frede participated in the study concept and design, acquisition of data, analysis and interpretation, and drafting and critical revision of the manuscript; and Dr Beck participated in the study concept and design, acquisition of data, analysis and interpretation, drafting and critical revision of the manuscript, statistical analysis, and study supervision; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Heaton owns stock in The Kroger Company; The Kroger Company also provides funding to the University of Cincinnati’s James L. Winkle College of Pharmacy, but Dr Heaton received no funding from Kroger for her work on this project. Dr Frede works for Kroger Pharmacy but received no funding from Kroger for her work on this project. Dr Luder now works for Pfizer Inc, but at the time of this study, she was an employee of the University of Cincinnati’s James L. Winkle College of Pharmacy; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by the National Institutes of Health (NIH 1K23AI112916). Additional support was provided by the Academy Health Community Health Peer Learning Program (Office of the National Coordinator for Health Information Technology, Department of Health and Human Services, grant 90CL0001.01-00, subaward 375.90CL.006). The funders played no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the article. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Heaton owns stock in The Kroger Company; The Kroger Company also provides funding to the University of Cincinnati James L. Winkle College of Pharmacy, but Dr Heaton received no funding from Kroger for her work on this project. Dr Frede works for Kroger Pharmacy. Dr Luder now works for Pfizer Inc. None of those listed received any support from these entities for this work; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. [DOI] [PubMed] [Google Scholar]
- 2.Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet. 1996;348(9024):383–386 [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Schmitt B, Wallner E. Impact of medication nonadherence on coronary heart disease outcomes. A critical review. Arch Intern Med. 1997;157(17):1921–1929 [PubMed] [Google Scholar]
- 4.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574; discussion 575–577 [DOI] [PubMed] [Google Scholar]
- 5.Beardon PH, McGilchrist MM, McKendrick AD, McDevitt DG, MacDonald TM. Primary non-compliance with prescribed medication in primary care. BMJ. 1993;307(6908):846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer MA, Choudhry NK, Brill G, et al. . Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124(11):1081.e9–1081.e22 [DOI] [PubMed] [Google Scholar]
- 7.McGrady ME, Hommel KA. Medication adherence and health care utilization in pediatric chronic illness: a systematic review. Pediatrics. 2013;132(4):730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530 [DOI] [PubMed] [Google Scholar]
- 9.Coleman TJ. Non-redemption of prescriptions. Linked to poor consultations. BMJ. 1994;308(6921):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stange KC. The problem of fragmentation and the need for integrative solutions. Ann Fam Med. 2009;7(2):100–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zullig LL, Granger BB, Bosworth HB. A renewed Medication Adherence Alliance call to action: harnessing momentum to address medication nonadherence in the United States. Patient Prefer Adherence. 2016;10:1189–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith LA, Bokhour B, Hohman KH, et al. . Modifiable risk factors for suboptimal control and controller medication underuse among children with asthma. Pediatrics. 2008;122(4):760–769 [DOI] [PubMed] [Google Scholar]
- 13.Johnell K, Lindström M, Sundquist J, Eriksson C, Merlo J. Individual characteristics, area social participation, and primary non-concordance with medication: a multilevel analysis. BMC Public Health. 2006;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHorney CA, Spain CV. Frequency of and reasons for medication non-fulfillment and non-persistence among American adults with chronic disease in 2008. Health Expect. 2011;14(3):307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon MD, Goldman DP, Joyce GF, Escarce JJ. Cost sharing and the initiation of drug therapy for the chronically ill. Arch Intern Med. 2009;169(8):740–748; discussion 748–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qato DM, Daviglus ML, Wilder J, Lee T, Qato D, Lambert B. ‘Pharmacy deserts’ are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33(11):1958–1965 [DOI] [PubMed] [Google Scholar]
- 17.Amstislavski P, Matthews A, Sheffield S, Maroko AR, Weedon J. Medication deserts: survey of neighborhood disparities in availability of prescription medications. Int J Health Geogr. 2012;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson TH, Bentley JP, McCaffrey DJ III, Pace P, Holmes E, West-Strum D. Store and prescription characteristics associated with primary medication nonadherence. J Manag Care Spec Pharm. 2014;20(8):824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrank WH, Choudhry NK, Fischer MA, et al. . The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153(10):633–640 [DOI] [PubMed] [Google Scholar]
- 20.Beck AF, Bradley CL, Huang B, Simmons JM, Heaton PC, Kahn RS. The pharmacy-level asthma medication ratio and population health. Pediatrics. 2015;135(6):1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dankwa-Mullan I, Pérez-Stable EJ. Addressing health disparities is a place-based issue. Am J Public Health. 2016;106(4):637–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell DS, Cretin S, Marken RS, Landman AB. A conceptual framework for evaluating outpatient electronic prescribing systems based on their functional capabilities. J Am Med Inform Assoc. 2004;11(1):60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams AJ, Stolpe SF. Defining and measuring primary medication nonadherence: development of a quality measure. J Manag Care Spec Pharm. 2016;22(5):516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657–665 [DOI] [PubMed] [Google Scholar]
- 25.Fischer MA, Stedman MR, Lii J, et al. . Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25(4):284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. Am J Manag Care. 2011;17(suppl 5 developing):SP38–SP44 [PubMed] [Google Scholar]
- 27.Matsui D, Joubert GI, Dykxhoorn S, Rieder MJ. Compliance with prescription filling in the pediatric emergency department. Arch Pediatr Adolesc Med. 2000;154(2):195–198 [DOI] [PubMed] [Google Scholar]
- 28.Zweigoron RT, Binns HJ, Tanz RR. Unfilled prescriptions in pediatric primary care. Pediatrics. 2012;130(4):620–626 [DOI] [PubMed] [Google Scholar]
- 29.Wright H, Forbes D, Graham H. Primary compliance with medication prescribed for paediatric patients discharged from a regional hospital. J Paediatr Child Health. 2003;39(8):611–612 [DOI] [PubMed] [Google Scholar]
- 30.Campbell SG, McCarvill EM, Magee KD, Cajee I, Crawford M. The consent and prescription compliance (COPRECO) study: does obtaining consent in the emergency department affect study results in a telephone follow-up study of medication compliance? Acad Emerg Med. 2008;15(10):932–938 [DOI] [PubMed] [Google Scholar]
- 31.United States Census Bureau American FactFinder. 2017. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t. Accessed May 25, 2017
- 32.Beck AF, Sandel MT, Ryan PH, Kahn RS. Mapping neighborhood health geomarkers to clinical care decisions to promote equity in child health. Health Aff (Millwood). 2017;36(6):999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plough AL. Building a culture of health: a critical role for public health services and systems research [published correction appears in Am J Public Health. 2015;105(10):e11]. Am J Public Health. 2015;105(suppl 2):S150–S152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corburn J. Urban place and health equity: critical issues and practices. Int J Environ Res Public Health. 2017;14(2):E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley CL, Luder HR, Beck AF, et al. . Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455–460 [DOI] [PubMed] [Google Scholar]
- 36.Garg A, Sandel M, Dworkin PH, Kahn RS, Zuckerman B. From medical home to health neighborhood: transforming the medical home into a community-based health neighborhood. J Pediatr. 2012;160(4):535–536.e1 [DOI] [PubMed] [Google Scholar]
- 37.Acevedo-Garcia D, Osypuk TL, McArdle N, Williams DR. Toward a policy-relevant analysis of geographic and racial/ethnic disparities in child health. Health Aff (Millwood). 2008;27(2):321–333 [DOI] [PubMed] [Google Scholar]
- 38.Sequist TD, Taveras EM. Clinic-community linkages for high-value care. N Engl J Med. 2014;371(23):2148–2150 [DOI] [PubMed] [Google Scholar]
- 39.Fierman AH, Beck AF, Chung EK, et al. . Redesigning health care practices to address childhood poverty. Acad Pediatr. 2016;16(suppl 3):S136–S146 [DOI] [PubMed] [Google Scholar]
- 40.Lindau ST, Makelarski J, Abramsohn E, et al. . CommunityRx: a population health improvement innovation that connects clinics to communities [published correction appears in Health Aff (Millwood). 2017;36(2):384]. Health Aff (Millwood). 2016;35(11):2020–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzau VJ, McClellan MB, McGinnis JM, et al. . Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA. 2017;317(14):1461–1470 [DOI] [PubMed] [Google Scholar]
- 42.Nguyen OK, Chan CV, Makam A, Stieglitz H, Amarasingham R. Envisioning a social-health information exchange as a platform to support a patient-centered medical neighborhood: a feasibility study. J Gen Intern Med. 2015;30(1):60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong WF, LaVeist TA, Sharfstein JM. Achieving health equity by design. JAMA. 2015;313(14):1417–1418 [DOI] [PubMed] [Google Scholar]
- 44.Guagliardo MF, Huber WA, Quint DM, Teach SJ. Does spatial accessibility of pharmacy services predict compliance with long-term control medications? J Asthma. 2007;44(10):881–883 [DOI] [PubMed] [Google Scholar]
- 45.Siegel RM, Kiely M, Bien JP, et al. . Treatment of otitis media with observation and a safety-net antibiotic prescription. Pediatrics. 2003;112(3, pt 1):527–531 [DOI] [PubMed] [Google Scholar]
- 46.Beck AF, Moncrief T, Huang B, et al. . Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J Pediatr. 2013;163(2):574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krieger N, Waterman P, Chen JT, Soobader MJ, Subramanian SV, Carson R. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas–the Public Health Disparities Geocoding Project. Am J Public Health. 2002;92(7):1100–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]