The delivery of automated compliance-linked mobile phone incentives via a cloud-based, biometric-linked record and reminder software platform improves childhood immunizations in rural India.
Abstract
OBJECTIVES:
Young children in resource-poor settings remain inadequately immunized. We evaluated the role of compliance-linked incentives versus mobile phone messaging to improve childhood immunizations.
METHODS:
Children aged ≤24 months from a rural community in India were randomly assigned to either a control group or 1 of 2 study groups. A cloud-based, biometric-linked software platform was used for positive identification, record keeping for all groups, and delivery of automated mobile phone reminders with or without compliance-linked incentives (Indian rupee Rs30 or US dollar $0.50 of phone talk time) for the study groups. Immunization coverage was analyzed by using multivariable Poisson regression.
RESULTS:
Between July 11, 2016, and July 20, 2017, 608 children were randomly assigned to the study groups. Five hundred and forty-nine (90.3%) children fulfilled eligibility criteria, with a median age of 5 months; 51.4% were girls, 83.6% of their mothers had no schooling, and they were in the study for a median duration of 292 days. Median immunization coverage at enrollment was 33% in all groups and increased to 41.7% (interquartile range [IQR]: 23.1%–69.2%), 40.1% (IQR: 30.8%–69.2%), and 50.0% (IQR: 30.8%–76.9%) by the end of the study in the control group, the group with mobile phone reminders, and the compliance-linked incentives group, respectively. The administration of compliance-linked incentives was independently associated with improvement in immunization coverage and a modest increase in timeliness of immunizations.
CONCLUSIONS:
Compliance-linked incentives are an important intervention for improving the coverage and timeliness of immunizations in young children in resource-poor settings.
What’s Known on This Subject:
In previous studies, researchers have established the role of mobile phone messaging in improving health outcomes. However, there are few studies in which researchers have evaluated the role of compliance-linked incentives versus mobile phone messaging to improve childhood immunization coverage.
What This Study Adds:
A biometric-linked, cloud-based immunization record platform was used for positive identification and tamper-proof delivery of automated compliance-linked incentives. We demonstrate that incentives are an important intervention for improving the timeliness and coverage of childhood immunizations in a resource-poor setting.
Although coverage of primary childhood immunizations has improved over the last decade, a significant proportion of young children remain inadequately immunized. According to the World Health Organization, 12.9 million infants (nearly 1 in 10) did not receive any immunizations in 2016.1 Although many young children receive some primary immunizations, many are never fully immunized because of inaccessibility to adequate health care and follow-up. For example, in 2016, an estimated 6.6 million infants who received their first dose of the diphtheria, pertussis, and tetanus (DPT) vaccine did not complete the full 3-dose DPT immunization series.1 Furthermore, the lack of reliable immunization records linked to positive identification of individual children as well as the lack of real-time assessments of the immunization status of communities remain major hindrances to current immunization programs.2
Mobile and Web connectivity have expanded exponentially in several countries in both rural and urban settings,3–5 with a dramatic decrease in costs for messaging and connectivity.6,7 Novel approaches that integrate these modern technologies with existing resources in low- and middle-income countries could cost-effectively improve immunization coverage of young children. In several studies, researchers have assessed the role of mobile messaging and/or compliance-linked incentives to improve health indices8 and immunizations in young children.9–12 Gibson et al11 recently reported that mobile phone reminders coupled with mobile phone–delivered monetary incentives significantly improved immunization coverage and timeliness in rural western Kenya, a community with high baseline (86%) immunization coverage.
In this study, we used a cloud-based, biometric-linked immunization record and message reminder platform to improve and streamline record keeping. We also tested the role of automated mobile phone message reminders and compliance-linked incentives (as mobile phone talk time) in improving immunization coverage in children 24 months and younger in a rural community in India with low literacy and poor baseline immunization coverage.
Methods
Study Design and Participants
We prospectively enrolled children 24 months and younger and pregnant women from a rural community in the Mewat region that is located within the state of Haryana, India, from July 11, 2016, to July 20, 2017. Recruitment and follow-up were performed concurrently, although the recruitment target was met on February 11, 2017. The study was administered by Bal Umang Drishya Sanstha (BUDS), a New Delhi–based nonprofit organization with a focus on child health. This study was approved by the research ethics committees of both BUDS and Johns Hopkins University. This trial is registered at clinicaltrials.gov (identifier NCT03180138).
Study Procedures
An encrypted, cloud-based software platform developed by Royal Datamatics Pvt Ltd (New Delhi, India) was used for record keeping and delivery of automated mobile phone reminders and compliance-linked incentives (Supplemental Fig 4). Any children 24 months or younger at the time of enrollment with a mobile phone in the household and the ability of their caregiver to provide written informed consent were considered eligible for this study. After obtaining written informed consent from the caregiver, basic demographic information, Global Positioning System (GPS) location, and the caregiver’s finger biometrics were collected. Although the standard of care in this setting involves the use of written immunization records (cards and records at the local primary health center) and verbal notification of follow-up visits, record keeping for all groups in this study, including the control group, was performed by using the software platform. Eligible subjects were randomly assigned to 1 of the 3 following study groups: self-returns with the software platform for record keeping (control), automated mobile phone reminders alone, or automated reminders with compliance-linked incentives in the form of mobile phone talk time. At the time of enrollment, data on previous immunizations received by the child were obtained from government-issued immunization cards provided by the families. If cards were unavailable or lost, immunization records were verified by using written records at the local primary health center. The government-run National Immunization program (Supplemental Table 6) provided and administered the immunizations. The date for the next scheduled immunization visit was provided at each follow-up by the government staff and entered into the software platform by the field workers. Each follow-up visit was also correlated with the scheduled return date (if recorded in the system) of the administered immunization. Because finger identification is unreliable in young children,13,14 the child’s name along with his or her caregiver’s fingerprint was used to ensure positive identification of individual children at every visit. The GPS coordinates (longitude and latitude) were also recorded at recruitment and at each follow-up visit. All users (field staff and study team) were identified and logged onto the software platform by using biometric identification. For the study groups receiving automated reminders, Short Message Service (SMS) in the local language (Hindi) was used. At follow-up visits, mobile phone minutes equivalent to Indian rupee (INR) Rs30 (approximately US dollar [USD] $0.50) per completed immunization were provided in the compliance-linked incentives group. The amount of the incentive was determined after input was received from the local investigators as well as the community workers. All data transfers and compliance-linked incentive transactions were automated and occurred in real time.
Randomization and Masking
Randomization was stratified by group (young children or pregnant) by using a block randomization algorithm (block size of 6), but in the current article, only data for children are presented. All caregivers were informed of their child’s group assignment, and study field staff had access to group assignments for individual patients at the time of recruitment or follow-up visits; however, all other study team members were blinded to the group assignments until the end of the study and after analysis of the blinded groups.
End Points
Immunization coverage was the primary outcome of the study and was calculated for each child as the proportion of the total number of immunizations received divided by the total number of immunizations required at the time of measurement (ie, at enrollment, end of study, etc). We also calculated the cost of mobile phone messaging and compliance-linked incentives. Timeliness of immunizations was a secondary outcome measure and was defined as the proportion of immunizations administered before or within 14 days after the scheduled date recorded during the study.
Statistical Analysis
Baseline data across the 3 study groups were summarized by using either median and interquartile range (IQR) or frequencies and percentages. Multivariable Poisson regression analysis was used to estimate adjusted immunization rates in the 2 test study groups, with the control group as a reference.
Results
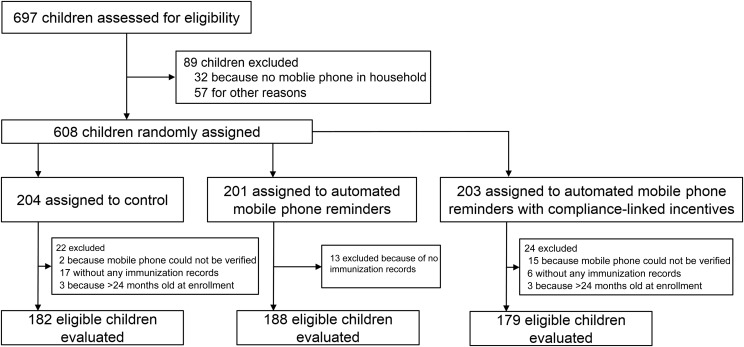
We assessed 697 children for eligibility and randomly assigned 608 children to the 3 study groups. The amount of children in a household with access to a mobile phone was high at the study site (>95%) and was consistent with the national data.3 We excluded 59 children because they had no previous immunization records (n = 36), their caregivers’ mobile phones could not receive automated SMS messages and/or compliance-linked incentives (n = 17), or they were found to be older than 24 months after random assignment (n = 6) (Fig 1). The number of children excluded was similar among the 3 study groups.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram.
The median age was 5 months (IQR: 1–11), with a median duration of 292 days in the study. Girls constituted 282 out of 549 children (51.4%). Overall, this community had low literacy rates and family income; 83.6% (459 out of 549) of mothers had no schooling, and 85.8% (471 out of 549) had family incomes less than INR Rs25 000 (approximately USD $375) per month (or approximately USD $4500 per year). There were no differences in the baseline characteristics of children among the 3 study groups (Table 1).
TABLE 1.
Baseline Demographic Characteristics at Enrollment
| Overall (n = 549) | Control (n = 182) | Automated Reminders (n = 188) | Automated Reminders With Compliance-Linked Incentives (n = 179) | |
|---|---|---|---|---|
| Median age (IQR), mo | 5 (1–11) | 5 (1–11) | 5 (1–11) | 4 (1–11) |
| Days in study, median (IQR) | 292 (238–334) | 291 (236–332) | 295 (245–351) | 289 (238–331) |
| Sex, n (%) | ||||
| Female | 282 (51) | 95 (52) | 97 (52) | 90 (50) |
| Male | 267 (49) | 87 (48) | 91 (48) | 89 (50) |
| No. children in family, n (%) | ||||
| 1–2 | 459 (84) | 146 (80) | 165 (88) | 148 (83) |
| 3–5 | 90 (16) | 36 (20) | 23 (12) | 31 (17) |
| Maternal education, n (%) | ||||
| No school | 459 (84) | 150 (82) | 159 (85) | 150 (84) |
| Primary | 72 (13) | 28 (15) | 23 (12) | 21 (12) |
| Secondary | 6 (1) | 1 (0.6) | 3 (2) | 2 (1) |
| High | 11 (2) | 3 (2) | 3 (2) | 5 (3) |
| Graduate | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.6) |
| Family income in INR, n (%) | ||||
| 0–25 000 | 471 (86) | 154 (85) | 161 (86) | 156 (87) |
| 25 000–50 000 | 78 (14) | 28 (15) | 27 (14) | 23 (13) |
| Place of birth, n (%) | ||||
| Hospital | 490 (89) | 167 (92) | 165 (88) | 158 (88) |
| Other | 59 (11) | 15 (8) | 23 (12) | 21 (12) |
| Baseline immunization coverage per 100 person-immunizations, % (CI) | 40 (38–42) | 39 (36–42) | 40 (37–43) | 40 (37–43) |
1 INR is approximately USD $0.015. CI, 95% confidence interval.
Immunization Coverage
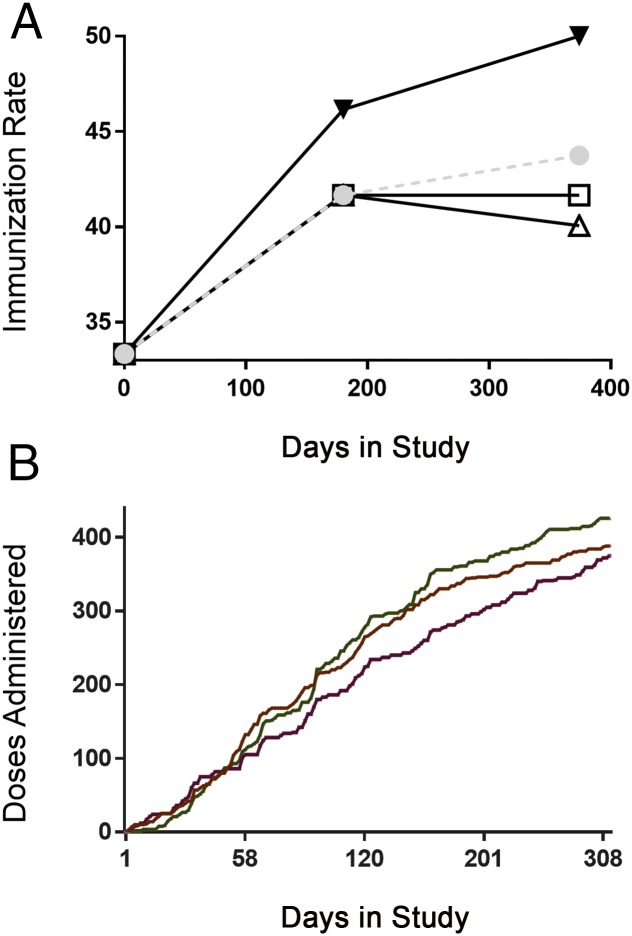
The median immunization coverage at enrollment was 33% across all groups and was not statistically different among the 3 study groups (Table 2). There was a progressive and time-dependent increase in the immunization coverage in all groups (Fig 2), with median rates of 41.7%, 40.1%, and 50.0% at the end of the study for the control group, those receiving automated mobile phone reminders alone, and those receiving automated reminders with compliance-linked incentives, respectively (Table 2). Accounting for the number of days in the study, we found that subjects receiving automated reminders with compliance-linked incentives had a significant increase (incidence rate ratio: 1.46; P = .02) compared with nonsignificant increases in the control group (incidence rate ratio: 1.32; P = .09) in their baseline immunization coverages, respectively. After adjustment for age, baseline immunization coverage, maternal education, and child’s place of birth, only the administration of compliance-linked incentives was independently associated with improvement in immunization coverage, with an adjusted risk ratio of 1.09 (P = .04) compared with the control group (Table 3). No other baseline characteristic was independently associated with improvement in immunization coverage (Table 4), although children born in the hospital benefited significantly more from compliance-linked incentives than those not born in the hospital (P = .02). Although the study was not powered to allow for measurement of differences among rates for individual immunizations, an increase was noted for all immunizations in the compliance-linked incentive group (Supplemental Table 7, Supplemental Fig 5). In the group receiving automated reminders with compliance-linked incentives, 574 immunizations were recorded during the study and were associated with 7894 mobile phone reminders. Because automated reminders were sent to multiple caregivers for each child, an average of 13.75 messages (cumulative of all messages sent to all caregivers) were sent per successful immunization. Each additional immunization administered during the study in the group receiving automated reminders with compliance-linked incentives cost INR Rs29.89 (approximately USD $0.48). This averaged INR 241·66 (∼USD 3·87) for each additional immunization administered over the control group.
TABLE 2.
Immunization Coverage at Enrollment and End of Study
| Baseline (Enrollment) | End of Study | |
|---|---|---|
| Median (IQR), % | ||
| Control (n = 182) | 33.3 (0–66.7) | 41.7 (23.1–69.2) |
| Automated reminders (n = 188) | 33.3 (0–58.3) | 40.1 (30.8–69.2) |
| Automated reminders with compliance-linked incentives (n = 179) | 33.3 (0–58.3) | 50.0 (30.8–76.9) |
| Overall (n = 549) | 33.3 (0–58.3) | 43.8 (25.0–75.0) |
Immunization coverage is defined as the proportion of the total number of immunizations received by a child divided by the total number of immunizations required for the child for their age at the time of measurement.
FIGURE 2.
Immunization coverage and number of doses administered over the course of the study. A, Median immunization coverage over the course of the study period in the control (white square), automated mobile phone reminders alone (white triangle), and automated reminders with compliance-linked incentives (black triangle) groups are shown. The dotted line represents the overall rates. Immunization coverage is defined as the proportion of the total number of immunizations received divided by the total number of immunizations required for each child at the time of measurement. B, We show the cumulative number of doses administered from the time of enrollment in the control (brown), automated mobile phone reminders alone (purple), and automated reminders with compliance-linked incentives (green) groups.
TABLE 3.
Poisson Regression Analysis and Risk Ratios
| Unadjusted Risk Ratio (CI) | P | Adjusted Risk Ratio (CI)a | P | |
|---|---|---|---|---|
| Control (n = 182) | Reference | — | Reference | — |
| Automated reminders (n = 188) | 1.01 (0.93–1.09) | .84 | 1.02 (0.94–1.11) | .64 |
| Automated reminders with compliance-linked incentives (n = 179) | 1.07 (0.99–1.16) | .09 | 1.09 (1.002–1.18) | .04 |
Immunization coverage is defined as the proportion of the total number of immunizations received by a child divided by the total number of immunizations required for the child for their age at the time of measurement. CI, 95% confidence interval; —, not applicable.
Adjusted risk ratio was calculated by adjusting for age, baseline immunization coverage, maternal education, and place of birth.
TABLE 4.
Immunization Coverage at the End of the Study by Baseline Characteristics and Treatment Groups
| Overall (n = 549) | Control (n = 182) | Automated Reminders (n = 188) | Automated Reminders With Compliance-Linked Incentives (n = 179) | Pa | |
|---|---|---|---|---|---|
| Mean (95% Exact Poisson Confidence Intervals) | |||||
| Sex | |||||
| Female | 47 (45–49) | 46 (42–49) | 47 (43–51) | 48 (44–52) | .40 |
| Male | 52 (50–54) | 51 (47–55) | 50 (46–54) | 55 (51–60) | .13 |
| No. children in family | |||||
| 1–2 | 49 (47–51) | 49 (46–52) | 47 (44–50) | 52 (49–55) | .15 |
| 3–5 | 49 (45–54) | 45 (39–52) | 56 (48–65) | 49 (43–57) | .36 |
| Maternal education | |||||
| Any education | 53 (49–57) | 48 (42–55) | 55 (48–63) | 55 (48–63) | .17 |
| No school | 49 (47–50) | 48 (45–51) | 47 (44–50) | 51 (48–54) | .20 |
| Family income in INR | |||||
| 0–25 000 | 49 (47–50) | 49 (46–52) | 46 (43–49) | 51 (48–54) | .25 |
| 25 000–50 000 | 53 (48–57) | 45 (39–52) | 60 (52–68) | 54 (46–63) | .09 |
| Place of birth | |||||
| Hospital | 49 (47–51) | 47 (44–50) | 48 (45–51) | 52 (49–56) | .02 |
| Other | 50 (46–56) | 58 (48–70) | 50 (43–59) | 45 (38–54) | .06 |
1 INR is approximately USD $0.015.
P values are for the comparison between the control group and the automated reminders with compliance-linked incentives group.
Timeliness of Immunizations
Children in the compliance-linked incentive group were significantly more likely to have received timely immunizations (40.8%; P < .03) compared with children in the control (31.3%) or automated mobile phone reminder groups (26.7%) (Table 5).
TABLE 5.
Timeliness of Immunizations
| Overall | Control | Automated Reminders | Automated Reminders With Compliance-Linked Incentives | P | |
|---|---|---|---|---|---|
| Children receiving timely immunizations, % (n out of n) | 32.7 (253 out of 773) | 31.3 (76 out of 243) | 24.7 (60 out of 243) | 40.8 (117 out of 287) | <.03a |
Timeliness is defined as an immunization administered before or within 14 d after the scheduled date.
Two-tailed Fisher's exact test.
Real-Time Information on Immunization Status
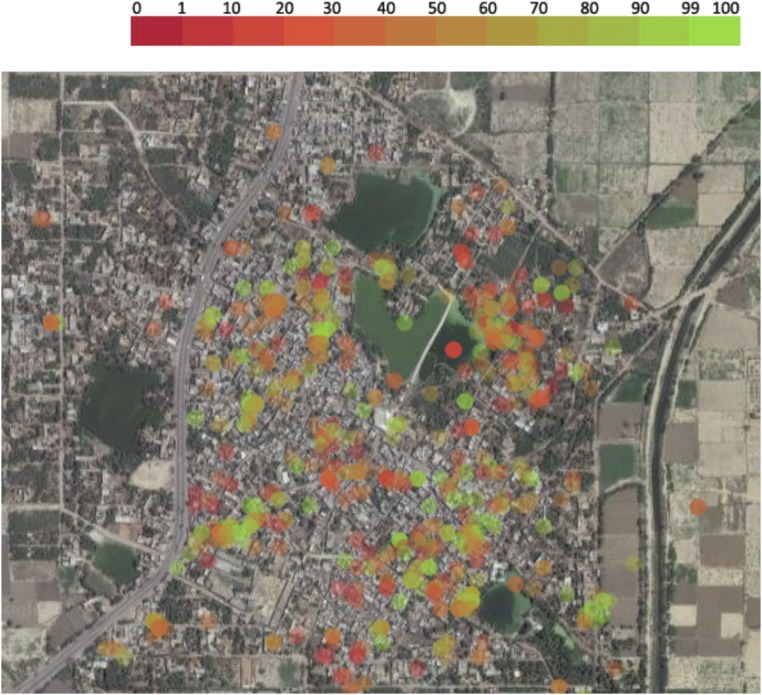
We also collected GPS locations of the subjects and were thus able to provide a real-time (overall or vaccine-specific) immunization status of the community (Fig 3).
FIGURE 3.
Real-time information on immunization status. Overall or vaccine-specific immunization of the community can be obtained in real time. Each dot on this satellite map of the community represents a single subject’s GPS location at the time of enrollment. The color represents the immunization status of each subject: red represents no immunization, green represents full immunization.
Discussion
Our overall goals with this study were to evaluate whether a cloud-based, biometric-linked immunization platform could improve immunization coverage in young children 24 months and younger in a resource-poor setting with poor baseline immunization coverage. In addition to providing automated mobile phone reminders and compliance-linked incentives, the platform also served as a standardized, cloud-based immunization record system that linked to positive identification (biometrics) of individual children. Unlike previous studies in which researchers restricted recruitment to young infants (≤6 weeks old),10,11 we recruited all eligible children 24 months and younger to measure improvement in primary immunizations in young infants recruited early in their lives as well as partially immunized toddlers.
This study was performed in a rural, low-income area with low literacy rates; 83.6% of mothers had no schooling. However, consistent with the national data,3 there were high rates of children in households with access to a mobile phone. Baseline immunization coverage was similar among the study groups and was generally low in this community. During the course of the study, there was a progressive increase in the immunization coverage for all groups. Although there was a trend toward higher rates in those receiving automated mobile phone reminders, only the administration of compliance-linked incentives was independently and significantly associated with improvement in immunization coverage. Although there have been major improvements in primary immunization coverage in many countries, many children, even in resource-rich settings, do not receive timely immunizations.15 Moreover, delays in immunization have potentially serious health consequences or can lead to disease outbreaks.16,17 Therefore, we also evaluated the effect of the interventions on timeliness of immunizations and noted that, similar to the primary outcome, only the group receiving compliance-linked incentives showed significant, albeit modest, improvement in the timely administration of immunizations. With these data, we suggest that compliance-linked incentives are an important intervention that is needed for significant, albeit modest, change in behavior, as has been shown for other health interventions.8,18–20
The delivery of compliance-linked incentives was robust and tamper proof because of the biometric validation of all caregivers combined with the use of automated mobile phone minutes rather than cash-based incentives. Correct identification of study subjects is of vital importance in clinical trials,21 and we consider the use of biometrics to be an important strength of the current study. Finally, it should be noted that this approach is highly scalable and pertinent to several low-resource settings. The collection of biometric data specifically for health interventions (as performed in the current study) is highly feasible, and commercial hardware for up to 10-finger biometrics (to ensure accuracy for scale-up) is easily available. The Indian government has enrolled >1 billion citizens in a biometric-based identification system (Aadhaar) for the targeted delivery of financial and other subsidies,22 which could facilitate the implementation of positive identification for immunization programs nationally. Other systems including unique identification numbers and/or cards or wrist bands could also be used for positive identification. Finally, mobile and Web connectivity have expanded globally,5 allowing large-scale implementation of such platforms. Geographic information systems have previously been used to improve health resource allocation in resource-limited settings.23 Therefore, we believe that the use of GPS-linked records could further improve immunization coverage of communities and also assist in response to disease outbreaks. For example, missed immunization visits or incomplete immunizations for each subject could be tracked, and real-time alerts (via mobile phones) could be provided to health care workers for home visits. Given that each encounter automatically records the GPS location and time, the system would have the ability to verify these home visits. Future applications that could map the most efficient travel route for health care workers or unmanned aerial vehicles for immunization and vaccine delivery could also be envisioned.
Although immunization coverage increased significantly in the intervention groups, a nonsignificant increase was also observed in the control group, consistent with observations made in similar studies.11 The institution of electronic records reduces errors and reveals improvements in immunization coverage.24 A recent study in a resource-limited setting also revealed that in comparison with records from written health logbooks, the direct capture of data from the source documents by using mobile devices led to higher reported immunization coverage among children.25 Thus, the use of electronic records, direct data capture, and biometric validation during recruitment and follow-up visits may have contributed to the nonsignificant increase (8%) in the immunization coverage observed in the control group merely from better record keeping. This is supported by the fact that 18% of the subjects in this study did not have any written immunization record at the time of recruitment. Although we were able to obtain written records for several children from the local primary health center, records for 6% of children could not be obtained. Each additional immunization administered in the group receiving automated reminders with compliance-linked incentives had a cost of approximately USD $0.50 (equivalent to the compliance-linked incentive), but the cost of each additional immunization administered in the control group was INR Rs241.66 (approximately USD $3.87). The latter was due to improved immunization rates observed in the control group. However, improved record keeping, attributable to the study’s intervention (software platform), likely led to improved rates in the control group, above and beyond the current standard of care. Although overall savings from immunizations would be region specific, the economic benefits even in low- and middle-income countries are likely to outweigh these costs.26,27
Our study has some limitations. We used a small incentive (approximately USD $0.50) in this study to improve compliance, which was <5% of the daily family income of the poorest in this population. Although we kept the value of the incentive low enough to not be considered coercive, it is possible that higher-value incentives could have even more significant benefit in the immunization coverage. All study and subject data, including biometric information, were stored on an encrypted, cloud-based server. Although privacy concerns can be an issue, the use of cloud-based servers for protected data are common nowadays. In addition, there were no concerns in the community regarding the use of mobile phone reminders or finger biometrics for validation, both of which were well accepted. Given that the subjects from the intervention and control groups were residing in the same geographical region, it is possible that the Hawthorne effect,28 by which individuals modify an aspect of their behavior in response to their awareness of being observed, could have contributed to a lower observed effect of the intervention. Although this may be true, the administration of compliance-linked incentives did lead to significantly higher immunization coverage than in the control group. Moreover, after adjustment for age, baseline immunization coverage, maternal education, and child’s place of birth, only the administration of compliance-linked incentives was independently associated with improvement in immunization coverage, suggesting that this is an important component in behavior modification for such programs. For cultural acceptability, to enable follow-up visits in situations in which the primary caregiver was unavailable and to overcome the issue of mobile phone accessibility, we established one-to-many (a single child associated with more than 1 caregiver) as well as many-to-one (more than 1 child associated with a single caregiver) relationships between the children and their caregivers (eg, mother, father, grandparent, uncle, neighbor, etc) in this study. However, it is possible that mobile phone reminders could still have been ignored or not correctly interpreted. For example, although the SMS messages were in the local language, the low literacy rate could have precluded the correct interpretation of these messages. Prerecorded voice messages in the local language or the use of graphical messages could potentially overcome these issues in future studies. Access to a mobile phone by the family was high in this study (>95%), consistent with the national data,3 and was therefore unlikely to bias recruitment. Finally, we used the automated delivery of mobile phone talk time as a compliance-linked incentive, which is advantageous because delivery of talk time is widely available and also tamper proof. In other recent studies, researchers have used mobile money as an incentive,11 which may be considered more akin to a cash transfer than phone minutes. However, mobile phone talk time is easily transferable between mobile phones and is often used as a pseudocurrency in resource-limited settings.
Although we engaged the community by meeting with village elders before the start of the study and recruiting health care workers from within the community, barriers such as cultural beliefs, lack of knowledge, etc, also need to be addressed for effective immunization campaigns. For example, automated and targeted (age, immunization status, geographical location) messaging could be used to educate the communities on the benefits of immunizations and plan or advertise upcoming camps, thereby efficiently connecting the target subjects with available resources. Holistic programs that can incorporate modern technologies with programs to educate, engage, and empower the communities are therefore needed for sustained changes and improvements.
Conclusions
We demonstrated the successful implementation of a cloud-based, biometrically linked immunization record and reminder platform in a pragmatic randomized control study. Although automated reminders may be useful in some settings, we show that small compliance-linked incentives delivered through an automated system are important for improving coverage as well as the timeliness of immunizations in young children in a resource-poor setting with poor baseline immunization coverage. Additional research in which robust platforms with positive identification of subjects are used is needed to assess the role of mobile phone reminders and compliance-linked incentives in other settings.
Acknowledgments
We thank all members of the BUDS health team, local community leaders, and the study participants.
Glossary
- BUDS
Bal Umang Drishya Sanstha
- DPT
diphtheria, pertussis, and tetanus
- GPS
Global Positioning System
- INR
Indian rupee
- IQR
interquartile range
- SMS
Short Message Service
- USD
US dollar
Footnotes
Dr Seth designed the study, provided oversight, supervised the study site, analyzed and interpreted the data, and obtained funding for the study; Dr Akinboyo analyzed the data; Mr Chhabra and Mr Qaiyum analyzed the data and supervised the study site; Dr Shet analyzed and interpreted the data; Dr Gupte was the primary biostatistician, generated the randomization sequences, analyzed and interpreted the data, and performed the statistical analyses; Dr Ajay K. Jain conceived and designed the study, provided oversight, analyzed and interpreted the data, performed the literature searches, and wrote the initial draft; Dr Sanjay K. Jain conceived and designed the study, provided oversight, analyzed and interpreted the data, performed the literature searches, wrote the initial draft, and obtained funding for the study; and all authors were involved with drafting or revising the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
This study has been registered at www.clinicaltrials.gov (identifier NCT03180138).
FINANCIAL DISCLOSURE: Dr Ajay K. Jain reports grants and personal fees from Alexion Pharmaceuticals outside the submitted work; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Sanjay K. Jain was supported by the Indo-US Collaborative Program on Low-Cost Medical Devices through the National Institutes of Health (grant R03-EB015955), and Dr Seth was supported by awards from the Department of Biotechnology in India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Ajay K. Jain reports grants and personal fees from Alexion Pharmaceuticals outside the submitted work; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.World Health Organization; United Nations Children’s Fund 1 in 10 infants worldwide did not receive any vaccinations in 2016. Available at: www.who.int/mediacentre/news/releases/2017/infants-worldwide-vaccinations/en/. Accessed November 30, 2017
- 2.Bhatnagar P, Gupta S, Kumar R, Haldar P, Sethi R, Bahl S. Estimation of child vaccination coverage at state and national levels in India. Bull World Health Organ. 2016;94(10):728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PRICE 88% of households in India have a mobile phone. Available at: www.ice360.in/en/projects/homepageservey/88-of-households-in-india-have-a-mobile-phone. Accessed September 25, 2017
- 4.Rai S. India just crossed 1 billion mobile subscribers milestone and the excitement’s just beginning. Forbes January 6, 2016. Available at: https://www.forbes.com/sites/saritharai/2016/01/06/india-just-crossed-1-billion-mobile-subscribers-milestone-and-the-excitements-just-beginning/#1e6c34d47db0. Accessed September 25, 2017
- 5.International Telecommunication Union ICT facts and figures 2017. Available at: www.itu.int/en/ITU-D/Statistics/Documents/facts/ICTFactsFigures2017.pdf. Accessed September 25, 2017
- 6.Bhushan R, Mukherjee W Rural India cuts down on discretionary spends to save for internet and mobile talk-time packs. The Economic Times July 12, 2016. Available at: http://economictimes.indiatimes.com/tech/hardware/rural-india-cuts-down-on-discretionary-spends-to-save-for-internet-and-mobile-talk-time-packs/articleshow/53163098.cms. Accessed November 30, 2017
- 7.Rai S. High-end devices launch at mid-range prices as global phone makers inundate India market with budget smartphones. Forbes July 16, 2014. Available at: https://www.forbes.com/sites/saritharai/2014/07/16/highend-devices-launch-at-midend-prices-as-global-phonemakers-inundate-india-market-with-budget-smartphones/#4d3c6f8cae9c. Accessed September 25, 2017
- 8.Lester RT, Ritvo P, Mills EJ, et al. . Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–1845 [DOI] [PubMed] [Google Scholar]
- 9.Banerjee AV, Duflo E, Glennerster R, Kothari D. Improving immunisation coverage in rural India: clustered randomised controlled evaluation of immunisation campaigns with and without incentives. BMJ. 2010;340:c2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domek GJ, Contreras-Roldan IL, O’Leary ST, et al. . SMS text message reminders to improve infant vaccination coverage in Guatemala: a pilot randomized controlled trial. Vaccine. 2016;34(21):2437–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson DG, Ochieng B, Kagucia EW, et al. . Mobile phone-delivered reminders and incentives to improve childhood immunisation coverage and timeliness in Kenya (M-SIMU): a cluster randomised controlled trial. Lancet Glob Health. 2017;5(4):e428–e438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen NT, Vu HM, Dao SD, Tran HT, Nguyen TXC. Digital immunization registry: evidence for the impact of mHealth on enhancing the immunization system and improving immunization coverage for children under one year old in Vietnam. mHealth. 2017;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serwaa-Bonsu A, Herbst AJ, Reniers G, et al. . First experiences in the implementation of biometric technology to link data from Health and Demographic Surveillance Systems with health facility data. Glob Health Action. 2010;3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weibel D, Schelling E, Bonfoh B, et al. . Demographic and health surveillance of mobile pastoralists in Chad: integration of biometric fingerprint identification into a geographical information system. Geospat Health. 2008;3(1):113–124 [DOI] [PubMed] [Google Scholar]
- 15.Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66(7):e14. [DOI] [PubMed] [Google Scholar]
- 16.Guerra FA. Delays in immunization have potentially serious health consequences. Paediatr Drugs. 2007;9(3):143–148 [DOI] [PubMed] [Google Scholar]
- 17.Hutchins SS, Escolan J, Markowitz LE, et al. . Measles outbreak among unvaccinated preschool-aged children: opportunities missed by health care providers to administer measles vaccine. Pediatrics. 1989;83(3):369–374 [PubMed] [Google Scholar]
- 18.Giles EL, Robalino S, McColl E, Sniehotta FF, Adams J. The effectiveness of financial incentives for health behaviour change: systematic review and meta-analysis. PLoS One. 2014;9(3):e90347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marteau TM, Ashcroft RE, Oliver A. Using financial incentives to achieve healthy behaviour. BMJ. 2009;338:b1415. [DOI] [PubMed] [Google Scholar]
- 20.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SonLa Study Group Using a fingerprint recognition system in a vaccine trial to avoid misclassification. Bull World Health Organ. 2007;85(1):64–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unique Identification Authority of India (UIDAI); Government of India Aadhaar. 2016. Available at: https://uidai.gov.in/. Accessed August 29, 2017
- 23.Sudhof L, Amoroso C, Barebwanuwe P, et al. . Local use of geographic information systems to improve data utilisation and health services: mapping caesarean section coverage in rural Rwanda. Trop Med Int Health. 2013;18(1):18–26 [DOI] [PubMed] [Google Scholar]
- 24.Au L, Oster A, Yeh GH, Magno J, Paek HM. Utilizing an electronic health record system to improve vaccination coverage in children. Appl Clin Inform. 2010;1(3):221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jandee K, Kaewkungwal J, Khamsiriwatchara A, Lawpoolsri S, Wongwit W, Wansatid P. Effectiveness of using mobile phone image capture for collecting secondary data: a case study on immunization history data among children in remote areas of Thailand. JMIR Mhealth Uhealth. 2015;3(3):e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, Shefer A, Wenger J, et al. . Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics. 2014;133(4):577–585 [DOI] [PubMed] [Google Scholar]
- 27.Ozawa S, Mirelman A, Stack ML, Walker DG, Levine OS. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: a systematic review. Vaccine. 2012;31(1):96–108 [DOI] [PubMed] [Google Scholar]
- 28.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]