In this study, we develop and validate a new score that can be used to accurately quantify risk for appendicitis.
Abstract
OBJECTIVES:
We sought to develop and validate a clinical calculator that can be used to quantify risk for appendicitis on a continuous scale for patients with acute abdominal pain.
METHODS:
The pediatric appendicitis risk calculator (pARC) was developed and validated through secondary analyses of 3 distinct cohorts. The derivation sample included visits to 9 pediatric emergency departments between March 2009 and April 2010. The validation sample included visits to a single pediatric emergency department from 2003 to 2004 and 2013 to 2015. Variables evaluated were as follows: age, sex, temperature, nausea and/or vomiting, pain duration, pain location, pain with walking, pain migration, guarding, white blood cell count, and absolute neutrophil count. We used stepwise regression to develop and select the best model. Test performance of the pARC was compared with the Pediatric Appendicitis Score (PAS).
RESULTS:
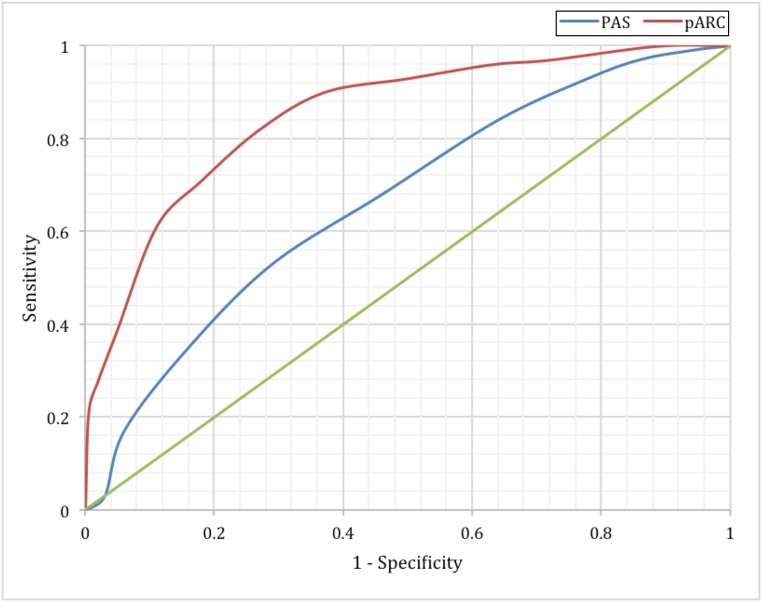
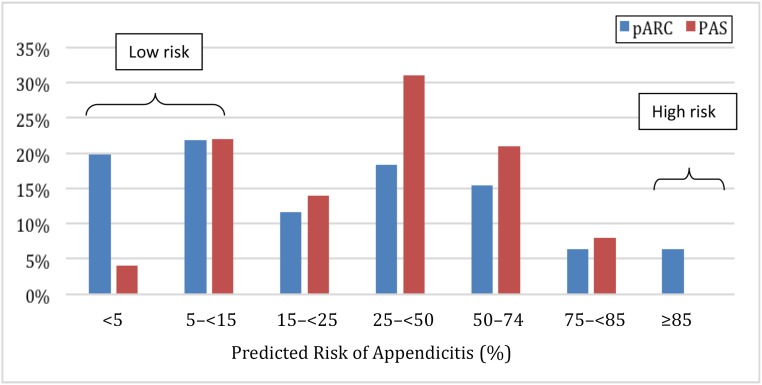
The derivation sample included 2423 children, 40% of whom had appendicitis. The validation sample included 1426 children, 35% of whom had appendicitis. The final pARC model included the following variables: sex, age, duration of pain, guarding, pain migration, maximal tenderness in the right-lower quadrant, and absolute neutrophil count. In the validation sample, the pARC exhibited near perfect calibration and a high degree of discrimination (area under the curve: 0.85; 95% confidence interval: 0.83 to 0.87) and outperformed the PAS (area under the curve: 0.77; 95% confidence interval: 0.75 to 0.80). By using the pARC, almost half of patients in the validation cohort could be accurately classified as at <15% risk or ≥85% risk for appendicitis, whereas only 23% would be identified as having a comparable PAS of <3 or >8.
CONCLUSIONS:
In our validation cohort of patients with acute abdominal pain, the pARC accurately quantified risk for appendicitis.
What’s Known on This Subject:
Available clinical scores are designed for ease of calculation but have had variable validity and clinical use on external validation. A score that is used to capture more complex interactions between variables may have improved accuracy for predicting appendicitis risk.
What This Study Adds:
In this derivation and validation study, the pediatric appendicitis risk calculator was used to accurately quantify the risk of appendicitis among children and adolescents presenting to the pediatric emergency department with acute abdominal pain, providing clinically actionable classifications.
Appendicitis remains a common pediatric surgical emergency, with more than 75 000 children diagnosed annually in the United States.1 Recently, there have been incremental improvements in the evaluation of pediatric patients with possible appendicitis. For example, clinical pathways for acute abdominal pain have revealed the feasibility and effectiveness2–5 of using ultrasound as first-line imaging without increasing missed diagnoses or negative appendectomy results.4,5 Reductions in computed tomography (CT) for appendicitis have also been observed in a national sample of 35 pediatric institutions.6 However, rates for appendiceal perforation have remained unchanged, and variations in care persist.6
Clinical scores have been developed to standardize care and limit imaging for patients with possible appendicitis.7,8 Previous scores were developed with an emphasis on simplicity, to be calculated by hand. Both the Pediatric Appendicitis Score (PAS) and the Alvarado score appeared promising in derivation samples,7,8 but have revealed variable accuracy and limited clinical use on external validation.9–11 For example, many patients receive a score signifying intermediate risk (ie, a PAS score of 4–6), encouraging clinicians to seek surgical consultation or advanced diagnostic imaging.12
More recently, authors of large observational studies have described variability in the clinical presentation of appendicitis and have emphasized the importance of subgroup analyses and interactions among covariates. For example, age, sex, and duration of symptoms can impact laboratory findings and accuracy of diagnostic imaging in children with acute abdominal pain.13,14 Age- and sex-specific scores, or scores to identify low-risk patients have been developed by our group and others,15–17 but these may have limited use in an emergency department (ED) because they cannot be applied to the full range of patients with acute abdominal pain. Widespread adoption of electronic health records18,19 along with increased use of risk prediction in other domains of medicine20 provided the impetus to develop a more sophisticated prediction tool for patients with possible appendicitis. Our aim in this investigation was to develop and validate a new pediatric Appendicitis Risk Calculator (pARC), quantifying risk for appendicitis on a continuous scale. Using rigorous methodology for score development and allowing for complex calculations, we aimed for the pARC to have improved accuracy and clinical use over the PAS.
Methods
Derivation Cohort
We derived the pARC from an existing, deidentified cohort of children with suspected appendicitis.17 Although the parent study included children 3 to 18.9 years old, given the low risk for appendicitis in children <5 years old and increased likelihood of atypical clinical presentations,21 the pARC score was derived and validated in patients 5 to 18 years old. In the previous prospective study, conducted from March 2009 through April 2010, ED clinicians collected clinical data from patients with suspected appendicitis at 9 pediatric emergency departments (PEDs). Treating clinicians enrolled children and adolescents who presented to the PED with <96 hours of abdominal pain and who were under evaluation for suspected appendicitis. “Suspected appendicitis” was defined as undergoing laboratory testing, diagnostic imaging, or a surgical consultation for possible appendicitis. Patients with the following conditions were excluded: pregnancy, previous abdominal surgery, inflammatory bowel disease, chronic pancreatitis, sickle cell anemia, cystic fibrosis, a medical condition affecting the ability to obtain an accurate history, or a history of abdominal trauma within the previous 7 days. Study procedures related to training of site staff, patient enrollment, data collection, and data management have been described previously.17
Validation Cohort
We validated the pARC using deidentified data from 2 independent cohorts of patients 5 to 18 years old with visits to the Boston Children’s Hospital PED from 2003 to 2004 and from 2013 to 2015. These cohorts were chosen as the validation sample because their criteria for cohort entry, data collection, cleaning and quality control were similar to those used in the derivation sample. Consistent with the recommendations of Altman, the validation population did not overlap with the derivation population.22 Clinical data were collected as part of distinct research23 and quality improvement projects. For both cohorts, children and adolescents with possible appendicitis were prospectively identified by trained coordinators who screened patients in the PED 10 hours per day. Subjects were included in the cohort when their treating emergency physician ordered advanced imaging or a surgical consult with concern for appendicitis. Parental consent and patient assent were obtained before data collection. Historical, physical examination, and laboratory data were collected in real time. The final diagnosis was based on pathology and surgical reports.
Outcome Measures
In both the derivation and validation cohorts, the primary outcome was appendicitis. For those who underwent an appendectomy, appendicitis was confirmed through the pathology report. To identify missed cases of appendicitis, families were contacted within 2 to 3 weeks of PED discharge to assess for visits to other sites of care and whether their child had an appendectomy in the interim. For families who could not be contacted, medical records were reviewed for 3 months after the index PED visit. Further description of these methods has been previously published.15,17,23,24
Data collection for the parent studies was approved by all participating institutional review boards, with parents or legal guardians consenting to study participation. The current analyses were conducted by using deidentified data sets and were exempt from additional institutional review.
Score Development
Patient history, physical examination, and laboratory variables were collected by using standardized processes in the parent studies.17,23 We first reviewed distributions, means, medians, ranges, and proportion of variables with missing values in the derivation and validation cohorts.7,8,17 Predictors evaluated for inclusion in the pARC7,8,17 were coded as binary variables unless otherwise indicated and included the following: sex, age (5–7.9 years old, 8–11.9 years old, or 12–18 years old for girls and 5–7.9 years old, 8–13.9 years old, or 14–18 years old for boys, accounting for variability in appendicitis risk and alternate causes for acute abdominal pain by age and sex subgroups), a fever in the ED >38°C, duration of pain (<24 hours, 24–47 hours, or 48–96 hours), a history of nausea, a history of emesis, migration of pain to the right-lower quadrant (RLQ), maximum tenderness in the RLQ, abdominal guarding, and pain with walking, coughing, or hopping. For these analyses, “unsure,” “don’t know,” and “missing” responses were coded as not having the sign or symptom. We evaluated the white blood cell (WBC) count and absolute neutrophil count (ANC) as continuous measures (103/mL); we assessed for normality and nonlinear associations with appendicitis using a generalized additive model.25
For consideration in the pARC, we included only predictors with <10% missing data and at least moderate interrater reliability (κ > 0.35).24 Because clinical decisions are often made in the context of a patient’s age and sex, we evaluated the interactions between age and sex. Following the prognostic model development approach recommend by Royston et al,26 we selected all potential predictors for the multivariable model on the basis of the following rules: (1) variables associated with appendicitis had a P value <.05 in the age- and sex-adjusted models; (2) associations between variables and appendicitis were in the expected direction; (3) for binary predictors, the β coefficient was >.4; (4) transformation of the laboratory values to a normal scale and shape of the association was informed by graphical exploration; (5) if only the WBC count was available, but not the ANC, the ANC was imputed as ANC = (−0.8783 + 1.1008 × sqrt(WBC))^2; (6) if neither the WBC count nor the ANC was available, the ANC was imputed as 7 × 103/µL, corresponding to the mean ANC in our derivation cohort; and (7) interactions between age and sex and each additional predictor with appendicitis were evaluated as potential terms in the model.
Validation
The newly derived pARC was then applied to the validation cohort. On the basis of input from investigators providing clinical care for this population (A.B.K., D.W.B., D.R.V.), we stratified subjects into 7 risk strata as clinically actionable categories: <5%, 5% to 14%, 15% to 24%, 25% to 49%, 50% to 74%, 75% to 84%, and ≥85%. We evaluated calibration of the pARC in the validation cohort by plotting observed and predicted risks and used the Hosmer and Lemeshow27 goodness of fit test on the basis of a decile partition. We evaluated the discriminatory performance using the area under the curve (AUC) plot and AUC statistic.
For the validation cohort, we compared the calibration and discrimination performance of the pARC versus the previously published PAS. We also evaluated clinical use of the PAS and pARC, comparing the proportion of subjects in the validation cohort classified as high risk or low risk for appendicitis.
Exploratory Analyses
We conducted additional exploratory analyses related to potential clinical applications of the pARC score. Using the larger derivation sample, we evaluated positive, negative, and equivocal ultrasound findings, stratified by pARC score.
Results
Study Population
The derivation cohort included 2423 children and adolescents, 40% of whom had appendicitis. The validation cohort had 1426 patients, 35% of whom had appendicitis. Clinical characteristics of the 2 cohorts are presented in Table 1. Derivation and validation subjects were similar in age and sex. Reported rates of nausea or vomiting, pain with walking or hopping, and migration of pain to the RLQ were all higher in the derivation cohort. Similarly, on physical examination, maximal tenderness in the RLQ and guarding were more common in the derivation cohort.
TABLE 1.
Characteristics of Derivation and Validation Cohorts
| Derivation Cohort, N = 2423 | Validation Cohort, N = 1426 | |
|---|---|---|
| No. PEDs | 9 | 1 |
| Years included | 2009–2010 | 2003–2004, 2013–2015 |
| Median age, y (IQR) | 11 (8–14) | 11 (8–14) |
| Sex and age, n (%) | ||
| Male | 1217 (50) | 686 (48) |
| 5–7.9 y | 256 (11) | 139 (10) |
| 8–13.9 y | 708 (29) | 319 (22) |
| 14–18 y | 253 (10) | 228(16) |
| Female | 1206 (50) | 740 (52) |
| 5–7.9 y | 205 (8) | 128 (9) |
| 8–11.9 y | 419 (17) | 283 (20) |
| 12–18.9 y | 582 (24) | 329 (23) |
| Reported clinical presentation, n (%) | ||
| Duration of abdominal pain, h | ||
| <24 | 1344 (55) | 724 (51) |
| 24–47 | 587 (24) | 438 (31) |
| 48–96 | 492 (20) | 264 (19) |
| Fever in ED | 440 (18) | 275 (19) |
| Nausea or vomiting | 1689 (70) | 845 (59) |
| Pain with walking or hopping | 1694 (70) | 785 (55) |
| Migration of pain to RLQ | 1035 (43) | 474 (33) |
| Findings on examination, n (%) | ||
| Maximal tenderness in RLQ | 1691 (70) | 838 (59) |
| Abdominal guarding | 1339 (55) | 592 (42) |
| Median laboratory results (IQR) | ||
| WBC (×103/µL) | 11.2 (7.4–15.9) | 10.0 (6.9–14.6) |
| ANC (×103/µL) | 7.9 (3.9–12.6) | 7.0 (3.7–11.8) |
| Appendicitis confirmed, n (%) | 968 (40) | 493 (35) |
Missing values are set as not having the clinical characteristic. N (%) is for the clinical characteristic being present. IQR, interquartile range.
Score Development
All predictors evaluated in the derivation set were significantly associated with appendicitis risk (P value < .05), adjusted by age and sex (Table 2). Fever was missing for 18% in the derivation cohort and so was not entered in the pARC model. The relationship between WBC count or ANC and appendicitis risk did not vary across 6 predefined age and sex subgroups. However, the relationship between WBC count, ANC and risk for appendicitis was linear up to a threshold of 20 × 103/µL and 14 × 103/µL, respectively. Beyond these thresholds, increases in WBC count or ANC were not associated with increases in appendicitis risk. We used this information to model WBC count and ANC as a 2-step linear function (Supplemental Fig 4).
TABLE 2.
Analysis of Individual Predictors of Appendicitis (Derivation Set: n = 2423)
| Clinical Characteristic | Missing Values, n (%) | PPV (P) | βa (95% CI) | P |
|---|---|---|---|---|
| Duration of pain, 24–47 h | 0 (0) | 0.47 (<.0001) | .30 (0.10 to 0.50); | .003 |
| Reference <24 h | ||||
| Fever | 440 (18) | 0.45 (.02) | .26 (0.04 to 0.48) | .02 |
| Nausea or vomiting | 51 (2) | 0.44 (<.0001) | .59 (0.41 to 0.78) | <.0001 |
| Pain with walking, hopping, or coughing | 177 (7) | 0.48 (<.0001) | 1.3 (1.0 to 1.8) | <.0001 |
| Migration of pain to RLQ | 152 (6) | 0.51 (<.0001) | 1.3 (1.1 to 1.5) | <.0001 |
| Maximal tenderness in RLQ | 96 (4) | 0.48 (<.0001) | 1.2 (1.0 to 1.4) | <.0001 |
| Abdominal guarding | 22 (1) | 0.51 (<.0001) | 1.05 (0.87 to 1.2) | <.0001 |
| WBC count (×103/µL) | 172 (7) | — | — | — |
| ANC (×103/µL) | 216 (9) | — | — | — |
—, not applicable.
The model included the clinical characteristic and sex-age covariates; for PPV and β coefficient, missing values were set as not having the clinical characteristic.
An initial appendicitis risk model was developed with the remaining categorical predictors (duration of pain, combination of nausea or vomiting, pain with walking, migration of pain to RLQ, maximal tenderness in the RLQ, guarding) and with the ANC as a linear function up to a threshold of 14 × 103/µL and a constant function for higher values. In the initial model, the combination of nausea or vomiting was not significant (β coefficient .16; P value = .18), so it was not included in the final model. In Table 3, we present the final pARC model with the associated β coefficients. The concordance statistic for the final model was 0.86 (95% confidence interval [CI]: 0.85 to 0.88).
TABLE 3.
Risk Score Equation From Logistic Regression Analysis
| Clinical Characteristic | Final Model, β Coefficient (95% CI) | P |
|---|---|---|
| Intercept | −8.7 | NA |
| Male sex | 1.28 (0.89 to 1.66) | <.0001 |
| Age and sex | ||
| Age 5–7.9 y | .38 (−0.04 to 0.80) | .08 |
| Male 5–7.9 y | −1.05 (−1.65 to −0.44) | .001 |
| Age 8–13.9 y | −.72 (−1.21 to −0.23) | .004 |
| Male 14–18 y, Female 12–18 y | Reference | NA |
| Duration of pain, h | ||
| <24 | Reference | NA |
| 24– <48 | .47 (0.22 to 0.72) | .001 |
| 48–96 | .10 (−0.18 to 0.38) | .49 |
| Presence of pain with walking | 1.05 (0.80 to 1.30) | <.0001 |
| History of migration of pain to RLQ | .46 (0.24 to 0.67) | <.0001 |
| Maximal tenderness in RLQ | 1.14 (0.89 to 1.40) | <.0001 |
| Abdominal guarding | .67 (0.46 to 0.89) | <.0001 |
| For ANC <14 × 103/µL, √ANC | 1.77 (1.56 to 1.99) | <.0001 |
| For ANC ≥14 × 103/µL, constant | 6.62 (5.94 to 7.29) | <.0001 |
The concordance statistic for the final model was 0.864. NA, not applicable.
Score Validation
Complete data for validation of the pARC were available for 1426 patients. Across 7 clinically actionable risk categories (<5%, 5%–14%, 15%–24%, 25%–49%, 50%–74%, 75%–84%, and ≥85%), the pARC score provided valid risk prediction. The AUC for the pARC was 0.85 (95% CI: 0.83 to 0.87) (Table 4).
TABLE 4.
Predicted and Observed Rate of Appendicitis and Performance of the pARC at Each Score Cut Point in the Validation Sample
| Predicted Risk for Appendicitis | Observed Rate of Appendicitis, % | Proportion of Patients Identified, n (%) | PPV, % | Sensitivity, % | Specificity, % | Missed Appendicitis Rate, % | Negative Appendectomy Results Rate, % | |
|---|---|---|---|---|---|---|---|---|
| Low | <5% | 5 | 282 (20) | 34.6 | 100.0 | 0.0 | 0.4 | 8.8 |
| 5–14% | 10 | 313 (22) | 41.9 | 97.2 | 28.7 | 0.4 | 7.7 | |
| Intermediate | 15–24% | 23 | 167 (12) | 53.7 | 90.7 | 58.7 | 0.6 | 6.8 |
| 25–49% | 42 | 262 (18) | 61.4 | 82.8 | 72.5 | 0.8 | 5.2 | |
| 50–74% | 62 | 221 (16) | 73.7 | 60.2 | 88.8 | 1.2 | 5.5 | |
| 75–84% | 78 | 91 (6) | 87.4 | 32.3 | 97.5 | 1.1 | 2.6 | |
| High | ≥85% | 97 | 91 (6) | 96.7 | 17.8 | 99.7 | 0 | 1.2 |
Validation sample (n = 1426; 35% with appendicitis); test result was positive if the score was greater than or equal to the cut point.
Score Comparison
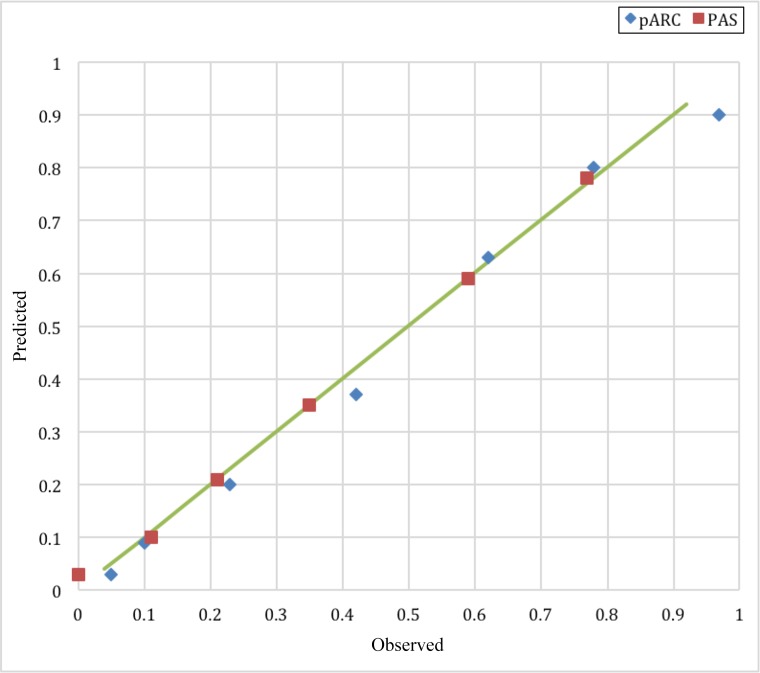
To compare the PAS and pARC, we developed PAS to pARC conversions using appendicitis rates by individual PAS from our validation cohort and compared results to those in previous studies4,9,11,12: <2: <5%; 2: 5% to 14%; 3: 15% to 24%; 4–5: 25% to 49%; 6: 50% to 74%; 7–8: 75% to 84%; 9–10: ≥85%. The calibration plot for the pARC and PAS is demonstrated in Fig 1. Both scores predicted appendicitis with high accuracy. In Fig 2, we demonstrate that in the validation cohort, the pARC score had an AUC greater than that for the PAS (0.85 [95% CI: 0.83 to 0.87] versus 0.77 [95% CI: 0.75 to 0.80], respectively). The use of the pARC also allowed more subjects to be classified as ≥85% risk or <15% risk for appendicitis, as compared with PAS (Fig 3). Even in patients with a PAS >8, the maximum positive predictive value (PPV) was 81%. Full test performance of the PAS is provided in Supplemental Table 6.
FIGURE 1.
Calibration plot of the pARC and PAS comparison (n = 1426).
FIGURE 2.
Comparison of the pARC and PAS in the validation sample (n = 1426). The AUC for the PAS was 0.77 (95% CI: 0.75 to 0.80). The AUC for the pARC was 0.85 (95% CI: 0.83 to 0.87).
FIGURE 3.
Comparison of the pARC and PAS based on predicted risk of appendicitis in the validation sample (n = 1426).
The pARC and Ultrasound
In Table 5, we present the relationship between the pARC strata, ultrasound use, and performance of ultrasound. Of 2423 subjects in our derivation sample, 905 (37%) had an ultrasound, with use being the highest in the 2 lowest pARC strata (45% for pARC scores of <5% risk and 46% for pARC scores of 5% to 14% risk). Overall, 443 (49%) of patients who underwent an ultrasound had an equivocal study, and the rate of appendicitis after an equivocal ultrasound was 18%. The PPV of ultrasound was ≤70% for patients with a pARC score of <25%. For pARC risk strata of 25% or higher, the PPV of ultrasound increased to ≥94%.
TABLE 5.
Application of the pARC for Evaluating Ultrasound Use Within the Derivation Sample
| pARC Score Strata, % | Ultrasound Performed, n (%) | Appendicitis in Those With Ultrasound Performed, n (%) | Equivocal Ultrasound, n (%) | Equivocal Ultrasound With Appendicitis, n (%) | NPV of Ultrasound, % | PPV of Ultrasound, % |
|---|---|---|---|---|---|---|
| <5 | 148 (45) | 9 (6.1) | 89 (60) | 5 (6) | 100 | 67 |
| 5–14 | 188 (46) | 18 (9.6) | 109 (58) | 3 (3) | 97 | 68 |
| 15–24 | 102 (38) | 12 (11.8) | 59 (58) | 4 (7) | 97 | 70 |
| 25–49 | 174 (36) | 59 (33.9) | 85 (49) | 15 (18) | 98 | 94 |
| 50–74 | 153 (34) | 98 (64.1) | 65 (42) | 33 (51) | 100 | 97 |
| 75–84 | 85 (35) | 66 (77.6) | 21(25) | 11 (52) | 67 | 98 |
| ≥85 | 55 (23) | 49 (89.1) | 15 (27) | 9 (60) | — | 100 |
| Overall | 905 (37) | 311 (34.4) | 443 (49) | 80 (18) | 96 | 93 |
The derivation sample total was 2423. Ultrasounds were performed for 905 patients, and appendicitis occurred in 311 patients. NPV was used to indicate a negative test result for appendicitis and/or a negative ultrasound test result. PPV was used to indicate a positive test result for appendicitis and/or a positive ultrasound test result. Equivocal ultrasounds were not included in the calculation of NPV and PPV. NPV, negative predictive value; —, not applicable.
Discussion
In this large multicenter study, we have demonstrated that the newly derived and validated pARC can be used to provide an accurate and discrete assessment of a patient’s risk for appendicitis, with improved accuracy and clinical use as compared with a previously published appendicitis score. Importantly, in our validation cohort with a background risk of appendicitis of 35%, the pARC score was able to classify half of patients as at ≥85% risk or <15% risk for appendicitis, thresholds where surgical evaluation or observation, respectively, may be recommended over immediate diagnostic imaging.
Advanced diagnostic imaging remains common for patients with suspected appendicitis,28 with upwards of 80% undergoing ultrasound, CT, or MRI.29 Kotagal et al30 recently reported that among 2538 children with an appendectomy in the Washington State surgical database from 2008 to 2012, 99.7% had a CT or ultrasound before surgery. Equally concerning, among patients with undifferentiated abdominal pain, Fahimi et al31 described that within the National Hospital Ambulatory Medical Center Survey, 52% underwent imaging with CT and 25% underwent imaging with CT or ultrasound. Despite the high use, the type of diagnostic imaging has shifted dramatically over the past decade, with Bachur et al6 reporting a 48% decline in CT use from 2010 to 2013 for patients with appendicitis across 35 children’s hospitals. During this period, the use of ultrasound increased 46%, and overall imaging rates remained unchanged.6
The decline in use of CT is significant because fewer children are exposed to ionizing radiation.32 However, the high use of ultrasound raises the potential for overuse. The unintended consequences of increased use of ultrasound may be increases in ED length of stay, hospital expenditures, and false-positive or indeterminate study results.29 The most impactful consequence of the increased ultrasound may be the likelihood of nonvisualization of the appendix and thus an equivocal interpretation. In some centers, half of ultrasounds are reported as equivocal33; these equivocal ultrasounds may compel providers to order a CT, MRI, or admit for observation.34 This is especially concerning in cases in which the a priori risk for appendicitis is low and highlights the need for judicious, risk-stratified use of any diagnostic imaging.
In previous studies, appendicitis scores were touted as mechanisms to decrease the use of CT and standardize care for patients with acute abdominal pain. In the 2 most commonly cited scores, derived by Alvarado8 and Samuel,7 the authors assign point values to patient history, physical examination, and laboratory findings. Points are summed and cutoffs applied to define low, intermediate, and high risk groups. These scores are easy to calculate, but upwards of 70% of patients may be assigned scores that do not aid in diagnostic assessment (ie, risk scores of 4, 5, or 6).9,12,35,36 The high proportion of patients assigned scores in which appendicitis can neither be ruled in nor ruled out has also been demonstrated in several real world implementation studies.3,37 For example, Depinet et al4 found that 61% of 489 pediatric patients with acute abdominal pain at a single center received a PAS score between 3 and 6, which the authors classified as equivocal or medium risk.
The use of diagnostic imaging for patients with acute abdominal pain may be magnified when patients are assigned intermediate appendicitis risk scores. It is in this context that the pARC may be most impactful on clinical care. In our study, up to 40% of the pediatric patients who presented with acute abdominal pain would have been assigned a low risk pARC score by which the clinician could defer diagnostic imaging. Furthermore, in the subset assigned a pARC score of 15% to 24%, the high rate of equivocal ultrasound readings and ultimate low risk for appendicitis suggests that these patients may be managed with observation rather than ultrasound. Finally, among patients with pARC scores ≥85%, it would be reasonable and safe to encourage surgical evaluation with selective use of diagnostic imaging. The potential implications of this approach merit discussion among multidisciplinary care teams. Suggested in the data presented here is that broad use of the pARC score at the point of care could facilitate a reduction in the use of ultrasound, CT, and MRI.
One strength of our study lies in the approach used to develop the pARC, because it differed substantially from methods used in the derivation of previous appendicitis risk scores. As outlined by Royston et al,26 we selected clinically relevant candidate predictors, evaluated the quality of the data, developed a strategy to consistently model continuous variables, identified the influence of outliers, and considered multiple potential interactions between predictors and impact on overall model performance. Next, for rule validation, we followed the guidance outlined by Altman et al,22 in that we validated our model on a similar but not contemporaneous patient population. Because the aim of most authors of prognostic studies is to create clinically valuable risk scores or indexes, the definition of risk groups should be driven by clinical rather than statistical criteria. Strengths of the pARC include its validity in predicting appendicitis risk and its classification of patients into clinically actionable risk groupings. Finally, we selected our model to favor a minimal number of predictors, applying a priori knowledge regarding reproducibility of predictors.24 More complex models are prone to overfitting data, with little practical gain.26
Several limitations should be noted. First, a few variables we considered for inclusion had substantial missing data and could not be incorporated in the model. Second, the pARC was derived and validated by using data from patients at children’s hospitals. Furthermore, for the validation cohort, we aggregated data from different time periods from a single children’s hospital. As such, our results require validation in new populations before widespread dissemination. Similarly, our derivation and validation cohorts had appendicitis at rates of 40% and 35%, respectively. The discriminative power of the pARC may be diminished if applied in populations with higher or lower appendicitis rates. In addition, the pARC is not intuitive and requires sophisticated calculations. Nevertheless, the pARC can be easily programmed and integrated within the electronic health record, promoting meaningful use of available clinical and laboratory data.
Conclusions
In this derivation and validation study, the pARC was used to accurately quantify the risk of appendicitis among children and adolescents presenting to the PED with acute abdominal pain. Next steps include a prospective validation of the pARC and an evaluation of how the pARC may impact the care delivered.
Acknowledgments
We thank the investigators who assisted in data collection at each of the enrolling sites from which the pARC was developed and validated.
Glossary
- ANC
absolute neutrophil count
- AUC
area under the curve
- CI
confidence interval
- CT
computed tomography
- ED
emergency department
- pARC
pediatric Appendicitis Risk Calculator
- PAS
Pediatric Appendicitis Score
- PED
pediatric emergency department
- PPV
positive predictive value
- RLQ
right-lower quadrant
- WBC
white blood cell
Footnotes
Dr Anupam B. Kharbanda conceptualized and designed the study, interpreted the data, drafted the initial manuscript, and revised the manuscript critically for important intellectual content; Drs Vazquez-Benitez and Dehmer analyzed and interpreted the data and reviewed and revised the manuscript critically for important intellectual content; Drs Ballard, Vinson, Chettipally, Kene, Bachur, Dayan, Kuppermann, and O’Connor were involved in the design of the study, participated in the interpretation of the data, and reviewed and revised the manuscript; Dr Elyse O. Kharbanda conceptualized and designed the study, interpreted the data, and revised the manuscript critically for important intellectual content; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health (R01 HD079463 [Kharbanda]). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose. The funding agencies took no part in data analysis, interpretation, or manuscript preparation. No person received any honorarium or other payment to produce this manuscript. This article was written by Dr Anupam Kharbanda, and all authors take full responsibility for the integrity of the data and the accuracy of data analysis.
References
- 1.Barrett ML, Hines AL, Andrews RM. Trends in Rates of Perforated Appendix, 2001-2010: Healthcare Cost and Utilization Project (HCUP) Statistical Brief #159. Rockville, MD: Agency for Healthcare Research and Quality; 2013 [Google Scholar]
- 2.Shah SR, Sinclair KA, Theut SB, Johnson KM, Holcomb GW III, St Peter SD. Computed tomography utilization for the diagnosis of acute appendicitis in children decreases with a diagnostic algorithm. Ann Surg. 2016;264(3):474–481 [DOI] [PubMed] [Google Scholar]
- 3.Santillanes G, Simms S, Gausche-Hill M, et al. Prospective evaluation of a clinical practice guideline for diagnosis of appendicitis in children. Acad Emerg Med. 2012;19(8):886–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Depinet H, von Allmen D, Towbin A, Hornung R, Ho M, Alessandrini E. Risk stratification to decrease unnecessary diagnostic imaging for acute appendicitis. Pediatrics. 2016;138(3):e20154031. [DOI] [PubMed] [Google Scholar]
- 5.Kharbanda AB, Madhok M, Krause E, et al. Implementation of electronic clinical decision support for pediatric appendicitis. Pediatrics. 2016;137(5):e20151745. [DOI] [PubMed] [Google Scholar]
- 6.Bachur RG, Levy JA, Callahan MJ, Rangel SJ, Monuteaux MC. Effect of reduction in the use of computed tomography on clinical outcomes of appendicitis. JAMA Pediatr. 2015;169(8):755–760 [DOI] [PubMed] [Google Scholar]
- 7.Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37(6):877–881 [DOI] [PubMed] [Google Scholar]
- 8.Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15(5):557–564 [DOI] [PubMed] [Google Scholar]
- 9.Schneider C, Kharbanda A, Bachur R. Evaluating appendicitis scoring systems using a prospective pediatric cohort. Ann Emerg Med. 2007;49(6):778–784, 784.e1 [DOI] [PubMed] [Google Scholar]
- 10.Kulik DM, Uleryk EM, Maguire JL. Does this child have appendicitis? A systematic review of clinical prediction rules for children with acute abdominal pain. J Clin Epidemiol. 2013;66(1):95–104 [DOI] [PubMed] [Google Scholar]
- 11.Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and pediatric appendicitis scores? A systematic review. Ann Emerg Med. 2014;64(4):365– 372.e2 [DOI] [PubMed] [Google Scholar]
- 12.Bhatt M, Joseph L, Ducharme FM, Dougherty G, McGillivray D. Prospective validation of the pediatric appendicitis score in a Canadian pediatric emergency department. Acad Emerg Med. 2009;16(7):591–596 [DOI] [PubMed] [Google Scholar]
- 13.Bachur RG, Dayan PS, Dudley NC, et al. The influence of age on the diagnostic performance of white blood cell count and absolute neutrophil count in suspected pediatric appendicitis. Acad Emerg Med. 2016;23(11):1235–1242 [DOI] [PubMed] [Google Scholar]
- 14.Bachur RG, Dayan PS, Bajaj L, et al. ; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics . The effect of abdominal pain duration on the accuracy of diagnostic imaging for pediatric appendicitis. Ann Emerg Med. 2012;60(5):582–590.e3 [DOI] [PubMed] [Google Scholar]
- 15.Kharbanda AB, Monuteaux MC, Bachur RG, et al. ; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics . A clinical score to predict appendicitis in older male children. Acad Pediatr. 2017;17(3):261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheller RL, Depinet HE, Ho ML, Hornung RW, Reed JL. Utility of pediatric appendicitis score in female adolescent patients. Acad Emerg Med. 2016;23(5):610–615 [DOI] [PubMed] [Google Scholar]
- 17.Kharbanda AB, Dudley NC, Bajaj L, et al. ; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics . Validation and refinement of a prediction rule to identify children at low risk for acute appendicitis. Arch Pediatr Adolesc Med. 2012;166(8):738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura MM, Harper MB, Jha AK. Change in adoption of electronic health records by US children’s hospitals. Pediatrics. 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1563 [DOI] [PubMed] [Google Scholar]
- 19.Jamoom E, Hing E. Progress with electronic health record adoption among emergency and outpatient departments: United States, 2006-2011. NCHS Data Brief. 2015;(187):1–8 [PubMed] [Google Scholar]
- 20.Dziadzko MA, Gajic O, Pickering BW, Herasevich V. Clinical calculators in hospital medicine: availability, classification, and needs. Comput Methods Programs Biomed. 2016;133:1–6 [DOI] [PubMed] [Google Scholar]
- 21.Becker T, Kharbanda A, Bachur R. Atypical clinical features of pediatric appendicitis. Acad Emerg Med. 2007;14(2):124–129 [DOI] [PubMed] [Google Scholar]
- 22.Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda AB, Taylor GA, Fishman SJ, Bachur RG. A clinical decision rule to identify children at low risk for appendicitis. Pediatrics. 2005;116(3):709–716 [DOI] [PubMed] [Google Scholar]
- 24.Kharbanda AB, Stevenson MD, Macias CG, et al. ; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics . Interrater reliability of clinical findings in children with possible appendicitis. Pediatrics. 2012;129(4):695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hastie TJ, Tibshirani RJ. Monographs on Statistics and Applied Probability 43: Generalized Additive Models. Boca Raton, FL: Chapman & Hall/CRC; 1990 [Google Scholar]
- 26.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: John Wiley & Sons, Inc; 2000 [Google Scholar]
- 28.Parker MW, Shah SS, Hall M, Fieldston ES, Coley BD, Morse RB. Computed tomography and shifts to alternate imaging modalities in hospitalized children. Pediatrics. 2015;136(3). Available at: www.pediatrics.org/cgi/content/full/136/3/e573 [DOI] [PubMed] [Google Scholar]
- 29.Bachur RG, Hennelly K, Callahan MJ, Monuteaux MC. Advanced radiologic imaging for pediatric appendicitis, 2005-2009: trends and outcomes. J Pediatr. 2012;160(6):1034–1038 [DOI] [PubMed] [Google Scholar]
- 30.Kotagal M, Richards MK, Flum DR, Acierno SP, Weinsheimer RL, Goldin AB. Use and accuracy of diagnostic imaging in the evaluation of pediatric appendicitis. J Pediatr Surg. 2015;50(4):642–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahimi J, Herring A, Harries A, Gonzales R, Alter H. Computed tomography use among children presenting to emergency departments with abdominal pain. Pediatrics. 2012;130(5). Available at: www.pediatrics.org/cgi/content/full/130/5/e1069 [DOI] [PubMed] [Google Scholar]
- 32.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson DB, Trout AT, Fierke SR, Towbin AJ. Improvement in diagnostic accuracy of ultrasound of the pediatric appendix through the use of equivocal interpretive categories. AJR Am J Roentgenol. 2015;204(4):849–856 [DOI] [PubMed] [Google Scholar]
- 34.Bachur RG, Rangel SJ. The threat of diagnostic uncertainty in the medical management of uncomplicated appendicitis. JAMA Pediatr. 2017;171(6):505–506 [DOI] [PubMed] [Google Scholar]
- 35.Meltzer AC, Baumann BM, Chen EH, Shofer FS, Mills AM. Poor sensitivity of a modified Alvarado score in adults with suspected appendicitis. Ann Emerg Med. 2013;62(2):126–131 [DOI] [PubMed] [Google Scholar]
- 36.Goldman RD, Carter S, Stephens D, Antoon R, Mounstephen W, Langer JC. Prospective validation of the pediatric appendicitis score. J Pediatr. 2008;153(2):278–282 [DOI] [PubMed] [Google Scholar]
- 37.Saucier A, Huang EY, Emeremni CA, Pershad J. Prospective evaluation of a clinical pathway for suspected appendicitis. Pediatrics. 2014;133(1). Available at: www.pediatrics.org/cgi/content/full/133/1/e88 [DOI] [PubMed] [Google Scholar]