Early-life transient dysbiosis has long-lasting effects on human health, suggesting a role of the microbiome in the DOHaD.
Abstract
Although the prominent role of the microbiome in human health has been established, the early-life microbiome is now being recognized as a major influence on long-term human health and development. Variations in the composition and functional potential of the early-life microbiome are the result of lifestyle factors, such as mode of birth, breastfeeding, diet, and antibiotic usage. In addition, variations in the composition of the early-life microbiome have been associated with specific disease outcomes, such as asthma, obesity, and neurodevelopmental disorders. This points toward this bacterial consortium as a mediator between early lifestyle factors and health and disease. In addition, variations in the microbial intrauterine environment may predispose neonates to specific health outcomes later in life. A role of the microbiome in the Developmental Origins of Health and Disease is supported in this collective research. Highlighting the early-life critical window of susceptibility associated with microbiome development, we discuss infant microbial colonization, beginning with the maternal-to-fetal exchange of microbes in utero and up through the influence of breastfeeding in the first year of life. In addition, we review the available disease-specific evidence pointing toward the microbiome as a mechanistic mediator in the Developmental Origins of Health and Disease.
During the past decade, the microbiome has emerged as a major contributor to human health.1 It has been suggested in current studies that the early-life microbiome is a crucial factor for proper immune development and long-term health.2,3 Transient microbial dysbiosis during this time period has been associated with the development of immune-mediated, metabolic, and neurodevelopmental disorders.4–7 In addition, increasing evidence has been used to support a vital role of the maternal and intrauterine microbiomes in childhood health and development.8 Collectively, these findings have been used to support the microbiome as a key participant in the Developmental Origins of Health and Disease (DOHaD). In this review, we will discuss the current research surrounding the maturation of the early-life microbiome and how transient variations in this bacterial consortium can have long-term consequences for human health.
The DOHaD: Where Does the Microbiome Fit?
The developmental origins hypothesis proposes variations in fetal and infant programming through environmental exposures during a critical window of early life.9 Termed originally as the Barker hypothesis,10,11 in which the association between fetal malnutrition and hypertension later in life was a focus, this theory has since expanded to account for many types of early-life exposures and birth outcomes associated with long-term health and development. For example, high birth weight is associated with an increase in breast and colon cancer risk,12,13 and the underlying mechanism of this association may be related to intrauterine exposure to high levels of growth hormones.9
Early-life infections and microbial exposures were not originally associated with DOHaD but were proposed as significant environmental influences on infant immune development in the hygiene hypothesis of allergic disease.14 With the advancement of human microbiome research, a modern extension of the hygiene hypothesis has since been proposed, known as the microflora hypothesis.15 In the microflora hypothesis, it is suggested that early-life environmental exposures alter the development of the human microbiome.15 Shifts in the composition of the microbiome are thought to bias maturation of the immune system toward a hypersensitive and/or hyperinflammatory state.15 For example, the innate and adaptive branches of the immune system are both highly involved in promoting an inflammatory response. However, exposure to microbes typically evokes a T-helper 1–mediated response, which suppresses the T-helper 2 activity often associated with immune-mediated and hypersensitivity reactions.3 Discussion of the mechanisms regulating the interface between the immune system and the microbiota is beyond the scope of this review; please see Tamburini et al3 for a recent in-depth discussion on this topic.
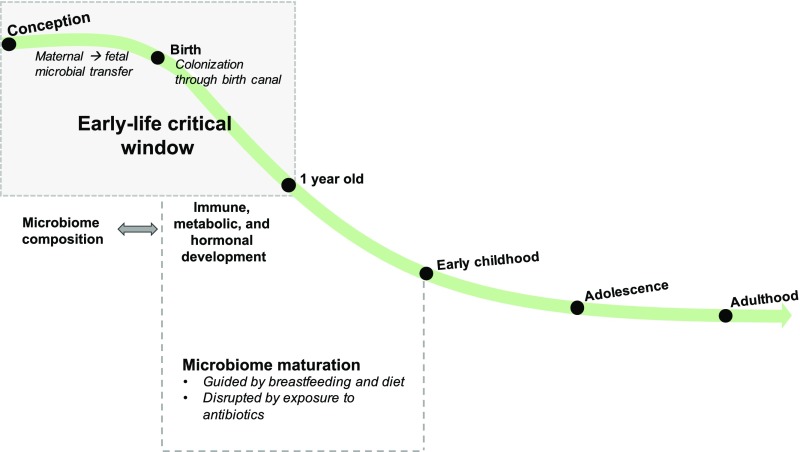
Analogous to DOHaD, an early-life critical window of development has also been proposed for the microbiome. Transient microbial dysbiosis (unhealthy microbial state) during this time frame has been associated with long-term immune and metabolic health issues,2 meriting its exploration within the developmental origins field (Fig 1).
FIGURE 1.
The infant microbiome is most vulnerable to environmental influences in early life. Maternal to fetal microbial transfer, mode of birth, antibiotics, and diet can alter the colonization and maturation of the early-life microbiome. These lifestyle-induced variations in microbiome composition and function can have prolonged influences on human health and may lead to the development of disease later in life.
Early-Life Development of the Microbiome
The human microbiota is a composite organism, composed of 10 trillion to 100 trillion microbial cells (bacteria, archaea, and microbial eukaryotes) and viruses.16,17 To highlight the impressive functional potential of the microbiota, the genomic catalog of this super organism, the microbiome, is composed of ∼3.3 million nonredundant genes.16 Although the terms “microbiota” and “microbiome” are descriptive of the microbial composition and genomic catalog, respectively, they are used interchangeably within this research field. The following sections are devoted to delineating the initial colonization and establishment of the human bacterial microbiota in infancy, from conception through the first year of life.
Maternal-to-Fetal Microbial Transfer
Until recently, the intrauterine environment was perceived as sterile.18 However, nonpathogenic bacteria have since been detected by molecular techniques in the amniotic fluid and placentas of healthy infants,19,20 suggesting a maternal-to-fetal exchange of microbes. In addition, through comparisons of the amniotic, placental, and meconium microbiotas, Collado et al21 report that the meconium microbiota of infants delivered via cesarean delivery shares ∼55% of its bacterial taxa with the placenta and amniotic fluid microbiotas. The prenatal maternal microbiome may also modulate the infant immune system. For example, gestation-only colonization with Escherichia coli HA107 was reported to modify the intestinal mucosal innate immune system and transcriptome of the offspring.22
In humans, variations in the placental microbiome composition have been associated with maternal pregnancy-related (stress and gestational diabetes) and neonatal health outcomes (birth weight, preterm birth).7,23–25 The placental microbiome of infants born preterm was also reported to differ in composition according to gestational weight gain,26 suggesting that this bacterial consortium may mediate fetal development depending on the health status of the mother.
Isolation of bacteria from the placenta is often associated with a pathophysiological state, which threatens the health of the mother and child. Because of the lack of culture-based analyses of the placental microbiome, there is some controversy surrounding its validity.27 In addition, inclusion of appropriate controls to address background contamination is also lacking in many molecular-based studies characterizing the intrauterine microbiome.28
It is also possible that the placenta does not harbor any viable bacteria but rather is composed of phagocytosed microbial by-products or cell wall components.8,22,29 The lack of viable bacteria does not negate the capacity of the placental microbiome to modulate fetal development because interactions with pathogen-associated molecular patterns may still regulate cell differentiation and proliferation.8,22,29 In addition, upon implementation of stringent validation strategies for placental metagenomic analyses, the placental microbiome might be referenced as a biomarker for maternal and fetal health and disease. Few studies have been conducted to explore the function of the placental microbiome,23,25 emphasizing an opportunity for future research in this area. Analysis of microbial metabolites and the application of metatranscriptomic approaches to characterize the functional capacity of the placental microbiome will be key in defining its role in DOHaD.
Vaginal Birth: The First Step in Postnatal Microbial Colonization
Postnatal bacterial colonization of the infant begins during birth, at which time neonates are exposed to the maternal fecal and vaginal microbiotas.30 Within 24 hours of delivery, the microbiotas across various body sites (oral, skin, meconium, etc) of cesarean delivered infants are initially populated with bacteria residing on the mother’s skin (eg, Staphylococcus spp.), whereas vaginally delivered infants are populated with typical vaginal bacteria (eg, Prevotella, Atopobium spp.).30 In a recent study, Chu et al31 suggest this finding may be specific to the neonatal gut microbiome. In their study of 81 mother and infant dyads, the microbiomes of other body sites (nares, skin, etc) from infants delivered vaginally revealed a bimodal pattern of maternal origin, populated by both the mothers’ vaginal and skin bacteria rather than by one or the other.31 However, it is suggested in both studies that the microbiotas of infants are homogeneously distributed across body sites (eg, meconium, skin, nares, etc) immediately after birth.30
Profiling of the neonatal intestinal microbiome immediately after birth and up to age 2 years suggests that birth mode can result in prolonged infant gut microbial dysbiosis.32 In a study of 43 mother and infant dyads, infants delivered via cesarean delivery exhibited increased phylogenetic diversity immediately after birth.32 However, after 1 month of age, the phylogenetic diversity of infants delivered via cesarean delivery declined below that of vaginally delivered infants.32 Chu et al31 challenge this finding slightly. In their recent study, birth mode was associated with variations in the microbiomes of the nares, skin, and oral cavity immediately after birth but not with variations in the infant meconium microbiome. Researchers of both studies do support the influence of birth mode on neonatal colonization in general; however, more research is needed to determine the influence of this early-life factor on the microbiomes of specific body sites.
Because of the health benefits associated with vaginal birth,33 Dominguez-bello et al34 conducted the first study aimed at recolonizing infants delivered via cesarean delivery with vaginal bacteria. After swabbing neonates with maternal vaginal fluids within 2 minutes of birth, the authors report partial restoration of the microbiota of infants delivered via cesarean delivery to that of vaginally delivered infants.34 However, the long-term health effects and composition of the infant microbiome are not yet known.34 Future analysis of these cesarean delivered infants exposed to the vaginal microbiome will be extremely valuable in determining the benefits of vaginally derived bacteria in long-term human health. In addition, the establishment of prospective human studies and animal models attempting similar colonization with vaginal bacteria among offspring delivered via cesarean delivery will be crucial in elucidating the role of vaginal microbes in the development of disease.
Breastfeeding Furthers Microbiota Maturation in Early Life
As the neonate grows, the homogeneous microbiome populating his or her body diverges into microbe-specific body niches.18,31 The maturation of the total infant microbiome has been studied for the first year of life, but beyond this time point, most researchers focus specifically on the gut microbiome. Driven in large part by breastfeeding and infant diet, the human gut microbiome continues to mature until the child reaches 2 to 3 years of age, after which its composition stabilizes.35
Breastfeeding seeds the infant gut microbiome through contact with maternal areolar and breast milk microbes and provides key energy sources for many bacteria (human milk oligosaccharides).36–39 In a study of 107 mother and infant dyads, infants who were breastfed during the first 30 to 40 days of life received a mean of ∼28% of their bacteria from breast milk and ∼10% from maternal areolar skin.38 The authors also report a dose-dependent association between the infant gut microbiome composition and the proportion of daily breastfeeding.38
There is a clear compositional distinction between breastfed and formula-fed infants, with breastfed infants being populated with higher proportions of Bifidobacteria and Lactobacillus spp. and formula-fed infants being populated with a greater prevalence of clostridiales and proteobacteria.32,40 In addition, formula-fed infants exhibit decreased diversity and bacterial richness even after the first year of life (12–24 months of age).32 In a study of 30 preterm infants, the effect of breastfeeding (versus formula feeding) was also reported to mask the influence of birth weight on the infant microbiome, highlighting breastfeeding as being potentially protective (at least with regard to the infant microbiome) against a traditional DOHaD risk factor.41 Epidemiologic evidence provides further support for the beneficial roles of breastfeeding in promoting infant health. Formula feeding has been associated with an increased risk of various hyperinflammatory and immune-mediated diseases.42,43 In addition, researchers of a recent epidemiologic study reported that breastfeeding protects against wheezing during the first year of life among infants born to mothers with asthma.43 With the work discussed in this section, we suggest that the microbiome may be a mediator between these associations.
Gestation-Associated Maternal Diet Shapes the Developing Infant Microbiome
Recent evidence has been used to support a significant role of gestation-associated maternal diet in shaping the microbiome of infancy. A maternal high-fat diet during pregnancy and breastfeeding was reported to induce dysbiosis in the offspring microbiome of Japanese macaques.44 These maternal diet–induced microbial variations persisted in juvenile macaques.44 In addition, a postweaning, low-fat control diet was unable to correct this maternal high-fat diet–induced dysbiosis.44 In a prospective cohort of 26 mother and infant dyads, a high-fat maternal gestational diet was associated with distinct variations in the neonatal gut microbial composition (meconium), which persisted to 4 to 6 weeks of age.45 In a mouse model, a maternal high-fiber diet was associated with increased short-chain fatty acid (SCFA) production in the offspring.46 Emphasizing the immune-modulatory capacity of the maternal diet–driven microbiome during pregnancy, higher frequencies of thymic T-regulatory cells were also found in these pups.46 Finally, demonstrating the effect of maternal diet on the functional capacity of the infant microbiome, piglets born of sows that were fed a western diet (high-energy, high-fat, fructose-based diet) during pregnancy displayed decreased SCFA production.47 In these studies, the importance of the microbiome as a mediator linking gestation-associated maternal diet to infant health is supported, substantiating the role of the microbiome in DOHaD. Future studies may shed more light on the prolonged health and developmental effects of gestation-associated maternal diet.
Antibiotics Alter Infant Colonization and Decrease Microbiota Maturation
Perhaps unsurprisingly, pre- and postnatal antibiotic exposure is a major early-life environmental stressor on the infant microbiome. Prenatal maternal antibiotic exposure has been reported to alter the diversity of both the neonatal48–50 and maternal51 microbiotas. Intrapartum antibiotic exposure was also reported to alter the infant oral microbiome composition.52 Specifically, the bacterial families streptococcaceae and gemellaceae and the order lactobacillales were decreased, whereas bacterial families in the proteobacteria phylum were enriched among infants whose mothers received intrapartum antibiotics.52 In addition, among infants whose mothers received antibiotics, the infant oral microbiome composition was further differentiated depending on whether the mother received a cocktail of antibiotics compared with if she received only 1 antibiotic.52
Postnatal antibiotic courses given to the infant in the first 3 to 9 months of life were reported to alter abundances of specific gut bacterial taxa (namely, Ruminococcus and clostridiales).32 In addition, antibiotic use in the first 6 to 12 months of life has been associated with decreased maturation of the infant microbiota.32 This suggests antibiotics may stunt the development of the microbiota if administered during this time period, which could potentially predispose infants to microbiome-associated diseases later in life.32
In epidemiologic studies, it has been suggested that antibiotic-induced dysbiosis in infancy promotes the development of many noncommunicable diseases in later childhood and adulthood (ie, obesity, asthma, inflammatory bowel disease [IBD]).53–56 However, these epidemiologic analyses do not address whether these diseases are causally related to early-life antibiotic use or whether they are indicative of early-life immune deficiencies or propensity for infection. Nevertheless, there are some promising animal models and prospective human cohort studies that attempt to address this quandary and provide additional support for the microbiome as a key developmental mediator in DOHaD.
Dysbiosis-Associated Noncommunicable Diseases Provide Additional Support for the Microbiome in DOHaD
The Critical Window in Early Life
As mentioned at the beginning of this review, DOHaD emphasizes a critical window of susceptibility from conception to early life in which environmental stressors most profoundly affect long-term human health. A similar critical window has emerged for the development of the microbiome. Scientists have narrowed the microbiome early-life critical window to the time period between conception and the first year of life (Fig 1).1 Though more research is needed to confirm this theory, the microbiome’s vulnerability to environmental influences appears to be time varying, more substantial earlier in life and lessening as it matures toward that of an adult (Fig 1). In the following sections, we will discuss disease-specific research (specifically, necrotizing enterocolitis [NEC], asthma and atopic disease, obesity, and neurodevelopmental disorders) that points toward this early-life critical window and further supports the role of the microbiome in DOHaD (summarized in Table 1).
TABLE 1.
Studies in Which Researchers Relate the Early-Life Microbiome With Health Outcomes in Later Childhood and Adulthood
| Research Objective | Study Population or Animal Model | Early-Life Factors and Timing of Exposure | Timing of Microbiome Analysis or Intervention | Health Outcome and Timing of Assessment | Summary of Findings | Ref. No. |
|---|---|---|---|---|---|---|
| NEC | ||||||
| To assess microbial dysbiosis before NEC in a systematic review and meta-analysis | Systematic review and meta-analysis of 14 human fecal microbiome studies of NEC | None | Variations in the microbiome before NEC development | NEC at ∼30 wk postconception | Increased proteobacteria and decreased firmicutes and bacteroidetes preceded NEC onset. Antibiotics, diet, and mode of delivery do contribute to microbial dysbiosis associated with NEC. However, causality related to these factors cannot be determined | 57 |
| To determine if 1 or more gut bacterial taxa differ between cases of NEC and controls | Prospective human cohort analysis (primary cohort, n = 122; secondary cohorts, n = 44) | None | Variations in the microbiome before NEC development | NEC in very low birth wt infants | Increases in gammaproteobacteria and decreases in negativicutes and clostridia-negativicutes classes over time preceded NEC development | 58 |
| To enhance strain-level resolution of NEC-associated pathogens by using deep shotgun metagenomics sequencing | Prospective human cohort analysis (n = 166) | None | Variations in the microbiome before NEC development | NEC in infancy | Variations in the microbiome were detected before NEC development. However, at 17–22 d postpartum, infants with high antibiotic treatment were enriched for E coli. The group later identified uropathogenic E coli as a major risk factor for NEC and associated death | 59 |
| To compare the efficacy and safety of enteral probiotic administration in preventing NEC | Systematic review and meta-analysis of 24 randomized or quasi-randomized controlled trials in humans | None | Enteral probiotic supplementation before NEC development | Stage II and stage III NEC in infancy | Enteral probiotic supplementation significantly reduced the incidence of NEC and mortality in infants | 60 |
| To assess the intestinal microbiota composition before NEC development in infants who developed NEC and controls | Prospective human cohort analysis (n = 38) | None | Variations in the microbiome before NEC development | NEC in infancy | An average of 7 samples were collected per subject and the temporal changes in microbiome composition were assessed. Throughout early life, before the development of NEC, different microbial populations dominate the gut and are associated with NEC development. In addition, the microbiome compositional progression appears to be associated with the timing of NEC onset | 61 |
| To identify microbial and metabolic biomarkers of NEC | Nested case-control design (n = 35) | None | Variations in the microbiome before NEC development | NEC in infancy | Lower α diversity 4–9 d postbirth was associated with NEC development. Microbiomes of subjects tended to cluster according to NEC status. These microbial variations were associated with shifts in urine metabolites, namely alanine and histidine | 62 |
| To determine if gut microbiome composition can be used to predict NEC severity | Prospective human cohort analysis (n = 30) | None | Variations in the microbiome before NEC development | NEC in infancy | Variations in the microbiome were not associated with NEC severity. There were also no differences in the microbiome post-NEC compared with controls | 63 |
| To assess whether fecal microbiota transplantation is an effective treatment of NEC | Wild-type and Grx1−/− mouse models of NEC | None | Fecal microbiota transplant from 1 to 4 d postbirth | NEC 5 d postbirth | Fecal microbiota transplant from healthy 6–8-wk-old mice to mouse pups conditioned for NEC reduced NEC incidence and severity compared with controls. This was dependent on Grx1. The mechanism of action is potentially through TLR-mediated inflammation and gut permeability | 64 |
| To determine if patients at risk for NEC can be identified by their meconium and early postnatal microbiota | Prospective human cohort analysis (n = 33) | Early enteral feeding and breast milk | Variations in meconium microbiome and neonatal microbiome before NEC | NEC in infancy | Clostridium perfringens and Bacteroides dorei were increased in the meconium of infants who developed NEC. C perfringens abundance persisted in neonatal stool samples. The amount of breast milk before NEC and earlier enteral feeding was negatively associated with NEC and associated with increased lactate-producing bacilli | 65 |
| To compare enteral versus parenteral antibiotics in preventing formula-induced NEC lesions in pigs | Piglet model of NEC | Antibiotic-induced | Enteral and parenteral antibiotic treatment (for 5 d post birth) | NEC in preterm piglets | Enteral antibiotics prevented NEC lesions, whereas lesions in piglets that were treated with parenteral antibiotics were increased. Enteral antibiotics decreased bacterial load and abundances of Gram-positive bacteria in the intestine. It is suggested that delayed colonization (particularly with Gram-positive bacteria) may prevent NEC. However, although microbiome variations correlate with NEC, they do not necessarily precede NEC | 66 |
| To determine if total parenteral nutrition, before the start of enteral feeding, can prevent NEC-associated gut dysfunction and inflammation | Piglet model of NEC | Enteral and total parenteral nutrition | Variations in the microbiome after feeding methods | NEC in preterm piglets | Enteral feeding increased microbial diversity and the abundance of Clostridium species. Density of C perfringens was associated with NEC severity. Microbiome variations correlate with NEC but do not necessarily precede NEC | 67 |
| To identify microbial profiles before NEC diagnosis | Prospective human cohort analysis (n = 369) | None | Variations in the microbiome before NEC diagnosis | NEC in infancy | Identification of 2 fecal microbiota profiles associated with NEC development (C perfringens type A dominant and Klebsiella dominant) | 68 |
| To characterize epigenome to microbiome crosstalk at critical neonatal stages of development | Tissue-based (immature enterocytes) and mouse models (dexamethasone or 5-azacytidine to induce epigenetic changes) | Prenatal dexamethasone or 5-azacytidine treatment | Microbiome to epigenome crosstalk in perinatal life | TLR and tight junction-signaling pathways in offspring | Prenatal dexamethasone and azacytidine treatment alters DNA methylation of tight junction and TLR genes and associated inflammatory pathways in fetuses and guts of 2-wk-old offspring. Both prenatal exposures also altered the offspring microbiome. Azacytidine treatment induces global demethylation, suggesting that the pre- and neonatal epigenome influences neonatal microbial colonization | 69 |
| Asthma and atopic disease | ||||||
| To analyze the microbiome of infants before atopic disease development at 1 y of age | Nested case-control design (n = 319) and Ovalbumin-challenged mouse models of asthma | None | Variations in the 3-mo-old gut microbiome | Atopy and wheezing at 1 y of age | Four bacterial taxa (Faecalibacterium, Lachnospira, Veillonella, and Rothia) were decreased among infants with atopy and wheezing at 1 y of age. Supplementation of asthma-induced mice with these 4 bacteria ameliorated airway inflammation | 4 |
| To analyze the microbiota of infants before asthma development by 4 y of age | Nested case-control design (n = 319) | None | Variations in the 3-mo-old gut microbiome | Asthma by 4 y of age | Decreased Lachnospira/Clostridium neonatale ratio at 3 mo of age was associated with asthma by 4 y of age | 5 |
| To characterize the bacterial and fungal microbiomes in neonates before asthma development at 4 y of age | Prospective human cohort analysis (n = 168) | None | Variations in gut microbiome 35 d postbirth | Asthma at 4 y of age | Variations in bacterial and fungal taxa at 35 d postbirth associated with highest relative risk of asthma. Sterile fecal water from the highest-risk group induces CD4+ T-cell dysfunction | 70 |
| To characterize the early-life critical window associated with exacerbated allergic airways responses in mice | Ovalbumin-challenged mouse model | Antibiotic-induced | Perinatal (in utero and through weaning) | Asthma induced later in life | Perinatal vancomycin exposure promotes expansion of firmicutes and exacerbates airway inflammation in mice | 71 |
| To analyze the effects of perinatal antibiotic treatment on development of hypersensitivity pneumonitis | Th1/Th17-mediated mouse model | Antibiotic-induced | Perinatal antibiotic exposure | Hypersensitivity pneumonitis in adulthood | Perinatal streptomycin promotes expansion of bacteroidetes and results in exaggerated hypersensitivity pneumonitis | 72 |
| To characterize the cellular mechanisms associated with diet or microbiota-mediated immune regulation | Wild-type, Gpr43−/−, and HDAC9−/− house dust mite mouse models of AAD; nested case-control design (n = 40) | Maternal acetate or high-fiber diet; maternal serum levels of acetate | Prenatal antibiotic exposure; prenatal acetate exposure | AAD induced in adulthood; coughing and wheeze by 1 y of age | High-fiber diet or acetate feeding of dams in pregnancy prevents robust AAD in adult offspring. Maternal serum levels of acetate were inversely associated with general practitioner visits for coughing and wheezing in the first 12 mo of life | 73 |
| To analyze the infant microbiota in association with food sensitization | Nested case-control design (n = 166) | None | Variations in the gut microbiome at 3 mo of age | Food sensitivity at 1 y of age | Decreased α diversity and increased enterobacteriaceae/bacteroidaceae ratio associated with food sensitization to at least 1 food allergen (milk, egg, peanut, soy) at 1 y of age. | 74 |
| To analyze the early-life microbiota in association with resolution of cow’s milk allergy | Nested case-control design (n = 226 subjects with milk allergy) | None | Variations in the gut microbiome at 3–6 mo of age | Milk allergy resolution by 8 y of age | Firmicutes and clostridia were enriched in microbiomes of subjects whose milk allergy resolved by 8 y of age. Bacteroidetes and Enterobacter were enriched among those whose milk allergy did not resolve by 8 y of age | 75 |
| To investigate age-dependent microbial modulation of iNKTs in mouse models of IBD and asthma | Ovalbumin-challenged mouse model | None | Exposure to maternal gut microbiome at birth | Asthma induced later in life | Neonatal exposure to a conventional microbiota (compared with GF conditions) increased iNKT in the lungs and protected against allergic asthma induced in adulthood. In addition, hypomethylation of CXCL16, driven by the microbiome, was associated with iNKT induction, implicating the microbiome in gene regulation | 76 |
| To analyze the role of the early-life lung microbiota in allergen-induced airway inflammation | Mouse model of house dust mite–induced airway inflammation | None | 2-wk window of susceptibility (lung microbiome) | Asthma induced later in life | Variations in the lung microbiome (shift from gammaproteobacteria and firmicutes to bacteroidetes) associated with decreased aeroallergen responsiveness and increased Helios− T-regulatory cells. This is mediated by PD-L1. Blockage of PD-L1 in the first 2 wk of life results in enhanced allergic airway inflammation | 77 |
| To analyze the nasopharyngeal microbiome in infancy in association with respiratory disease later in life | Prospective human cohort study (n = 234) | None | Variations in the nasopharyngeal microbiome 7–9 wk postbirth | Chronic wheezing at 5–10 y of age | Children who developed chronic wheezing at 5–7 y of age and were atopic by age 2 were twice as likely to have been colonized with asymptomatic Streptococcus | 78 |
| To determine if variations in the skin microbiome in early life are associated with atopic dermatitis | Nested case-control design (n = 20) | None | Variations in the skin (antecubital fossa) microbiome at 2 mo of age | Atopic dermatitis at 1 y of age | Colonization of antecubital fossa at 2 mo of age with Staphylococcus was associated with decreased incidence of atopic dermatitis at 1 y of age | 79 |
| To analyze associations between neonatal gut Bifidobacterium species and eczema or atopy development in the first year of life | Nested case-control design (n = 117) | Colonization patterns influenced by household pets, number of siblings, and maternal allergic status | Variations in Bifidobacterium species at 1 wk and 3 mo of age | Atopic dermatitis at 1 y of age | Variations in Bifidobacterium species at 1 wk and 3 mo of age were associated with risk of eczema at 1 y of age. However, the microbiome was not analyzed after 3 mo of age | 80 |
| To analyze associations between proportions of IgA coating and bacteria bound to IgA in infancy and allergy development later in life | Nested case-control design (n = 48) | None | IgA and total bacterial load measured at 1 wk and 1 y of age | Asthma, allergic rhinoconjunctivitis, allergic urticaria, and eczema by 7 y of age | At 12 mo of age, children with allergic disease (particularly asthma) displayed a lower proportion of IgA bound to fecal bacteria. IgA recognition patterns for the microbiota varied between children with allergies and healthy children at 1 wk of age | 81 |
| To analyze fecal microbial diversity and bacterial abundances in the first year of life in association with asthma and allergies later in life | Nested case-control design (n = 47) | None | Microbial diversity at 1 mo of age | Asthma at 7 y of age | Children with asthma displayed lower overall microbial diversity than children without asthma | 82 |
| To investigate the infant intestinal microbiota composition in association with maternal prenatal stress and infant health | Nested case-control design (n = 56) | Prenatal stress | Variations in the microbiome at postnatal days 7, 14, 28, 80, and 110 | GI symptoms and allergic response by 3 mo of age | Infants exposed to prenatal stress displayed more GI symptoms (38% compared with 22%) and allergic reactions (43% compared with 0%), which were associated with variations in the microbiome. This microbiome was characterized by less lactic acid bacteria and Akkermansia and greater Escherichia, Enterobacter, and Serratia | 83 |
| Obesity and metabolism | ||||||
| To analyze if the early-life microbiota composition is associated with childhood BMI and if antibiotic use modifies this association | Nested case-control design from 2 cohorts (Bibo cohort, n = 87; Flora cohort, n = 75) | Antibiotic treatment | Variations in the gut microbiome at 3 mo of age | BMI at 5–6 y | In the 3-mo-old microbiome, relative abundance of streptococci was positively associated with BMI at 5–6 y of age, and relative abundance of bifidobacteria was negatively associated with BMI at 5–6 y of age. Among children with a history of multiple antibiotic courses, the firmicutes phylum was significantly associated with BMI. However, microbiome composition was not measured at any other time point | 84 |
| To analyze the impact of diet on the early-life microbiome in a primate model | Primate model (high-fat versus standard diet) | Maternal high-fat diet during pregnancy and breastfeeding | Variations in the offspring microbiome composition | Shifts in microbial metabolic pathways | In this study, a link to a specific health outcome was not established. However, a maternal high-fat diet did alter the microbiome composition of offspring macaques, which persisted in juvenile macaques. Offspring displayed altered metabolic pathways on the basis of maternal diet. Additionally, these functional pathways (amino acid, carbohydrate, and lipid metabolism) correlated with abundances of specific gut bacteria | 44 |
| To determine if the placental microbiome varies in association with birth wt | Prospective human cohort analysis (n = 24) | None | Variations in the placental microbiome composition | Birth wt | Low birth wt infants displayed lower gut microbiome richness and variations in the abundances of specific bacterial taxa compared with normal birth wt infants. Lactobacillus percentage was positively correlated with birth wt | 24 |
| To analyze the effect of early-life microbial perturbation with antibiotic treatment on host metabolism and adiposity | Mouse model (high-fat versus standard diet) | Antibiotic-induced | LDP exposure from birth through weaning | Body composition in adulthood | Compared with controls and mice exposed to long-term LDP, mice exposed to LDP for 4 or 8 wk after birth displayed elevated caloric intake and faster total mass and fat mass accumulation. The authors also report that the penicillin-selected microbiota can induce metabolic changes when transferred to GF mice | 6 |
| To better understand how early-life antibiotic use alters the gut microbiome composition and metabolic development | Mouse model (high fat versus standard diet) | Antibiotic-induced | Pulsed antibiotic treatment completed shortly after weaning | Body composition from 3–6 wk | Early-life pulsed antibiotic treatment accelerates total mass and bone growth. The authors also report that response to high-fat diet is altered depending on the particular antibiotic and number of courses used to perturb the microbiota | 85 |
| To analyze the impact of maternal prepregnancy BMI on the infant gut microbiome composition and functional potential | Nested case-control design (n = 39) | Maternal prepregnancy obesity | Variations in infant microbiome composition and function | Infant metabolism at 18 mo of age | Firmicutes were reported enriched in children born to mothers at a normal wt, whereas bacteroidetes were enriched in infants born to women who were obese. In this study, a link to an infant health outcome was not established, but differential microbiome metabolic functions that were based on whether infants were born to mothers at a normal wt or to women who were obese was identified | 86 |
| To investigate the effect of cadmium exposure on the early-life gut microbiota and metabolism in adulthood | SPF mouse model | Cadmium exposure 1 wk before parental mating | Variations in the microbiome composition observed at 8 wk and at adulthood (20 wk) | Body composition measured in adulthood | Cadmium exposure in parental mice resulted in increased fat accumulation in male offspring. Alterations in microbiome composition occurred before measurements of body composition. In addition, through microbiota transfer experiments, the group reported that fat accumulation was driven by the cadmium-exposed microbiome | 87 |
| To determine if the early-life gut microbiota composition is associated with wt development in early childhood | Nested case-control design (n = 49) | None | Variations in the microbiome composition between 6 and 12 mo of age | BMI measured at 7 y of age | Greater abundance of Staphylococcus aureus in children who were obese in infancy. Greater abundances of bifidobacteria in children at a normal wt in infancy | 88 |
| To investigate the effects of early-life factors on the trajectory of gut microbial development and childhood adiposity | Prospective human cohort analysis (n = 75) | Gestational age and delivery mode | Variations in the microbiome before 6 mo of age | Adiposity at 18 mo of age | Infants with high Bifidobacterium and Collinsella at a later age displayed lower adiposity at 18 mo of age. Infants who acquired these taxa at 6 mo showed the lowest adiposity at 18 mo. In addition, acquisition of these bacterial taxa was influenced by length of gestation and delivery mode | 89 |
| To analyze the effect of subtherapeutic antibiotic administration on the gut microbiome and host metabolism | Mouse model of antibiotic-induced adiposity | Antibiotic-induced | Variations in the microbiome measured before sacrifice | Body composition measured in adulthood (16–20 wk) | The authors generated a mouse model of adiposity by exposing mice in early life to antibiotics. Variations in microbial composition before adiposity measurement was not assessed. However, the antibiotics did alter the microbiome composition, SCFA metabolism, and hepatic metabolism of fatty acids and lipids | 90 |
| To analyze the association between the early-life gut microbiome composition and BMI in childhood | Prospective human cohort analysis (n = 138) | None | Variations in the microbiome composition within the first year of age | BMI SD score between 1 and 3 y of age | Abundance of Bacteroides fragilis at 3 and 26 wk of age is associated with BMI SD score between 1 and 3 y of age. Abundance of Staphylococcus at 3 and 52 wk is inversely associated with BMI SD score between 1 and 3 y | 91 |
| Neurodevelopment | ||||||
| To determine if limited nesting stress alters offspring microbiota, corticosterone levels, and intestinal permeability | Rat model of limited nesting stress | Limited nesting stress | Variations in the gut microbiome composition 21 d post birth (at weaning) | Limited nesting stress from postnatal days 2–10 | Limited-nesting pups had hypercorticosteronemia, enhanced intestinal permeability, decreased microbial diversity, and variations in specific microbial taxa | 92 |
| To determine if maternal high-fat-diet-induced obesity is associated with social behavioral deficits and altered microbiota in the offspring | High-fat diet mouse model | Maternal high-fat diet | Variations in the gut microbiome composition in offspring | Behavior exams on 7–12-wk-old offspring mice | A maternal high-fat diet induces compositional variations in the gut microbiome of offspring mice. Offspring mice of mothers on a high-fat diet cohoused with mice born of mothers raised on a regular diet displayed normal social behavior. In addition, reintroduction of L reuteri (lacking in offspring mice of a mother on a high-fat diet) restored normal social behavior in these mice | 93 |
| To determine if cognitive ability is associated with particular infant gut microbiota profiles | Prospective human cohort analysis (n = 89) | Group 2 was more likely to have been breastfed, less likely to have been born by cesarean delivery, and associated with white ethnicity. Having older siblings was associated with increased α diversity | 3 groups of subjects were identified on the basis of their 1-y microbiome analysis | Cognitive outcomes at 1 and 2 y of age | Three groups of subjects were identified on the basis of their microbiome. Group 1 displayed high abundance of Faecalibacterium, group 2 displayed high abundance of Bacteroides, and group 3 displayed high abundance of ruminococcaceae. Individual Mullen scales differed between groups. α diversity was negatively associated with individual Mullen scales at 2 y of age (expressive language and visual reception) | 94 |
| To determine if maternal prenatal stress alters the microbial intrauterine environment and behavior in offspring | Mouse model of prenatal stress | Prenatal stress | Variations in placental microbiome and fecal microbiome of offspring | Anxiety-like behavior in offspring | Prenatal maternal stress was associated with variations in the microbiome of dams, offspring, and in the placenta. Additionally, prenatal stress is associated with increased IL-1β in the placenta and reduced brain-derived neurotrophic factor in placenta and adult offspring amygdala | 7 |
| To determine if probiotic administration in early life modifies maternal separation-induced gut dysfunction | Rat model (stress induced by maternal separation) | Maternal separation in early life (day 4 to day 19) | Variations in microbiome and gut function in offspring | Hypothalamus-pituitary-adrenal axis activity | Increased corticosterone levels and altered colonic mucosal barrier function in maternally separated rat pups. Early-life administration of probiotics (composed of Lactobacillus rhamnosus and Lactobacillus helveticus strains) to rat pups ameliorates these findings and persists to adulthood | 95 |
| To determine if early-life stress alters the gut-brain axis | Rat model (stress induced by maternal separation) | Maternal separation in early life | Variations in microbiome composition in rat pups | Symptoms of psychiatric disorders and irritable bowel syndrome | Increased plasma corticosterone and increased tumor necrosis factor-α and interferon-γ. Also, the microbiome composition varied in the maternally separated group compared with the rats that were not maternally separated | 96 |
| To examine the effects of prenatal and early-life exposure to propionic acid and LPS on offspring gut microbial metabolism, locomotor activity, and anxiety-like behavior | Rat model | None | Pre- and postnatal LPS or propionic acid exposure | Behavior traits in offspring | Prenatal propionic acid increased anxiety-like behavior in male and female adolescent offspring. Postnatal propionic acid increased anxiety-like behavior in female offspring only. Prenatal propionic acid and LPS induced developmental delays (including delays in eye opening) | 97 |
| To determine if colonization by gut microbiota in early life impacts brain development and adult behavior | GF and SPF mouse models | None | Offspring of previously GF mice colonized with SPF microbiota | Behavior in adulthood | Adult colonized offspring displayed similar behaviors compared with SPF mice. These mice spent less time exploring the open arms in the maze (less locomotor activity). Additionally, colonized mice expressed less synaptophysin and PSD-95 in the striatum compared with GF mice, suggesting the microbiome is involved in programming brain development | 98 |
| To determine if a maternal high-fat-diet-altered microbiome can modify offspring behavior | Mouse model colonized with maternal high-fat-diet-shaped microbiota | Maternal high-fat diet | Female mice transplanted with high-fat diet microbiome | Behavior traits in offspring | Female mice were transplanted with a high-fat-diet- or control low-fat-diet-associated gut microbiome. Offspring of these mice displayed altered behavior in a sex-dependent manner. Offspring mice displayed less stress after maternal separation. Male offspring displayed decreased exploratory and cognitive behaviors, which is indicative of increased anxiety. Female mice displayed increases in adiposity and body wt | 99 |
| To analyze the microbial and molecular mechanisms that underlie the gut-brain axis | GF and conventionally raised mouse models | None | GF mice colonized after weaning | Neuronal activity in the amygdala | Absence of a microbiota in early life results in differential gene expression, exon usage, RNA editing, and upstream gene regulation in the amygdala. This was similar to mice who were raised GF for their entire lives but varied when compared with conventionalized mice | 100 |
| To examine whether variations in the vaginal microbiome are associated with varied offspring programming | Mouse model of prenatal stress | Prenatal stress | Variations in the maternal vaginal and neonatal gut microbiome | Metabolic and neurologic programming and in offspring | Lactobacillus abundance is decreased in the vaginal microbiome and in neonates born to dams exposed to early prenatal stress. Other bacterial population abundances also varied in offspring exposed to early prenatal stress. Early prenatal stress altered metabolic profiles and amino acid availability in the brain | 101 |
| Immune-mediated diseases (IBD, T1D, etc) | ||||||
| To investigate age-dependent microbial modulation of iNKTs in mouse models of IBD and asthma | Mouse model of oxazolone-induced colitis | None | SPF-colonization during pregnancy lead to SPF-colonized offspring | Colitis induced later in life | Neonatal exposure to a conventional microbiota compared with GF conditions protected mice from oxazolone-induced colitis | 76 |
| To determine if and how early-life exposure to antibiotics changes susceptibility to IBD | Mouse model DSS-induced colitis | Antibiotic-induced | LDP after weaning | Colitis induced later in life | LDP-treated mice displayed transient gut microbial compositional alterations, including eradication of segmented filamentous bacteria. In addition, after DSS-induced colitis, LDP mice displayed reduced colitis symptoms, Il-17 expression, and ileal Th17 differentiation compared with mice exposed to metronidazole, enrofloxacin, and controls. Finally, the authors report penicillin’s effects are dependent on eradiation of segmented filamentous bacteria, implicating the microbiome as the mediator between this early life exposure and colitis development | 102 |
| To explore the influence of gut dysbiosis in the progression of T1D | NOD mouse model | Antibiotic-induced | Antibiotic treatment in early life (conception until 40 wk postnatal) | Spontaneous diabetes later in life | Antibiotics were administered to NOD mice from conception until 40 wk postnatal development. Treatment with antibiotics increased incidence of T1D in male mice. Antibiotic treatment also resulted in near ablation of the gut microbiome at 8 wk of age, which may partially explain the increased T1D incidence in male mice | 103 |
| To determine if exposure to prenatal antibiotics can protect offspring from T1D | NOD mouse model | Antibiotic-induced | Prenatal antibiotic treatment induced variations in offspring and maternal microbiomes | Spontaneous diabetes later in life | Prenatal neomycin and vancomycin treatment resulted in differential shifts in the offspring and maternal microbiomes. Offspring treated prenatally with neomycin were protected from T1D development, whereas offspring treated prenatally with vancomycin displayed accelerated T1D development. The antibiotic treatment also resulted in altered immune profiles, such as increased T-cell–mediated inflammation in mice treated with vancomycin and altered antigen-presenting cell phenotypes in mice treated with neomycin | 104 |
| To determine the impact of targeting Gram-negative gut bacteria at various time points in early life on T1D development | NOD mouse model | Antibiotic-induced | Prenatal antibiotic treatment induced variations in offspring microbiome | Spontaneous diabetes later in life | Pregnant, NOD mice treated with an antibiotic mixture (neomycin, polymyxin B, and streptomycin) were protected from T1D compared with mice treated postnatally. Microbiota transfer from these mice to untreated mice resulted in protection from T1D | 105 |
| To compare the effects of pulsed therapeutic antibiotics or continuous low-dose antibiotics in early life on T1D development | NOD mouse model | Antibiotic-induced | Pulsed antibiotic treatment induced variations in 6-wk-old microbiome | Spontaneous diabetes later in life | Pulsed postnatal treatment with tylosin altered the mouse microbiome and accelerated T1D development compared with mice treated with subtherapeutic penicillin from pregnancy to week 12 | 106 |
| To analyze the association between the infant gut microbiome and T1D development | Prospective human cohort analysis (n = 33) | None | Variations in the microbiome before diagnosis with T1D | T1D diagnosis at ∼3 y of age | T1D disease state was distinguishable by the gut microbiome composition. Seroconverted subjects diagnosed with T1D displayed a marked decrease in α diversity before diagnosis when compared with seroconverted subjects not diagnosed with T1D and nonseroconverted subjects | 107 |
| To analyze the effect of peripartum cefoperazone administration on the maternal and offspring microbiota and IBD in the offspring | SPF IL-10 knock-out mouse model combined with DSS-induced colitis | Antibiotic-induced | Peripartum antibiotic treatment induced gut dysbiosis in offspring that persists to adulthood | Spontaneous colitis later in life | Peripartum exposure to cefoperazone increases risk of spontaneous colitis in offspring. Antibiotics also contribute to immune skewing and promote gut dysbiosis that persists to adulthood. Additionally, as demonstrated by fecal transplant to GF IL-10 knock-out dams, immune skewing is mediated by the antibiotic-induced dysbiosis | 108 |
| To analyze the impact of a maternal high-fiber diet on T-regulatory cell differentiation in the offspring | SPF GPR41−/− mouse model | Maternal high-fiber diet during pregnancy and breastfeeding | Increased plasma SCFAs | Increased thymic and peripheral T-regulatory cells | Compared with offspring from maternal mice fed a normal diet, high-fiber diet during pregnancy and breastfeeding resulted in increased plasma SCFAs in the offspring. These offspring also displayed higher frequencies of thymic and peripheral T-regulatory cells, which may be prompted by increased SCFA levels | 46 |
AAD, allergic airways disease; CD4+, cluster of differentiation 4; CXCL16, chemokine ligand 16; DSS, dextran sodium sulfate; GI, gastrointestinal; GPR41, G protein–coupled receptor 41; GPR43, G protein–coupled receptor 43; Grx1, Glutaredoxin-1; HDAC9, histone deacetylase 9; IgA, immunoglobulin A; IL-1β, interleukin 1 beta; IL-10, interleukin 10; IL-17, interleukin 17; iNKT, invariant natural killer T cell; LPS, lipopolysaccharide; NOD, nonobese diabetic; PD-L1, programmed death ligand-1; PSD-95, postsynaptic density protein 95; Ref., reference; Th1, T-helper 1; Th17, T-helper 17; TLR, Toll-like receptor; T1D, type 1 diabetes.
NEC
In addition to the health risks associated with preterm birth, preterm infants display marked neonatal microbial dysbiosis. This dysbiosis enhances their susceptibility to disease, particularly NEC, which may be modified by exposure to antibiotics during this critical time period.57 Through analysis of existing 16S ribosomal RNA gene sequence data, the authors identified differential abundances of proteobacteria, firmicutes, and bacteroidetes, which preceded the onset of NEC.57 Gut dysbiosis characterized by similar variations in bacterial taxa was also reported to precede NEC in a study of 122 extremely low birth weight neonates.58 The increase in proteobacteria along with increased enterocyte Toll-like receptor 4 activity in these neonates with NEC suggest a hyperinflammatory response to a dysbiotic microbiome.59 However, a recent study was conducted in which researchers identified uropathogenic E coli colonization as a significant risk marker for NEC.59 This suggests an invasive microbial species may be synergistic in driving this dysbiosis-associated disorder. In addition, infants treated with antibiotics for 7–14 days (regardless of NEC status) were enriched for E coli relative to infants treated with antibiotics for 0–6 days.59 This supports a role of neonatal antibiotic-induced dysbiosis in NEC, which may enhance the vulnerability of the neonatal gut microbiome to pathogen invasion. Because of the apparent link between microbial dysbiosis and NEC, probiotic supplementation of preterm neonates is becoming a prominent research area for NEC prevention and treatment.109,110 In a systematic review and meta-analysis of 26 probiotic intervention studies for NEC, it is suggested that probiotic intervention does prevent NEC.60 However, the particular strains to be used and the effects on high-risk populations (extremely low birth weight infants) requires further study.60 Nonetheless, it may be possible in the near future to optimize the neonatal microbiome to combat development of this disease and prevent associated infant mortality.
Asthma and Atopic Disease
NEC studies highlight the dynamic relation between the gut microbiome and the developing immune system. However, although the neonatal immune system is on high alert for pathogenic invaders, researchers who have conducted studies of asthma and atopic diseases (food allergies, atopic dermatitis, etc) suggest its development is strongly influenced by commensal bacteria.
In a recent assessment of the microbiome in asthma development, a more substantial role of the maternal microbiota in fetal development is supported than what was previously thought. In a mouse model of allergic airway inflammation, high-fiber (which has been reported to stimulate production of SCFAs by the microbiome111) or acetate (a SCFA) feeding of pregnant mice modulated the maternal microbiota and protected the subsequent offspring from developing allergic airway disease.73 The authors of this study also provided preliminary evidence of this association in humans; high acetate levels in the serum of pregnant individuals correlated with reduced general practitioner visits for coughing and wheezing in the offspring during the first 12 months of life.73
Postnatal early-life dysbiosis in both the human gut2,4,5,70 and airway78 microbiomes is also associated with asthma and atopic disease development in children. This dysbiosis is characterized by shifts in the prevalence of specific bacterial4,5 and fungal taxa,70 which are transient and most prominent between birth and 3 months of life. The majority of human studies in this research area reveal only correlative evidence to link the early-life dysbiosis with asthma. However, Arrieta et al4 provide preliminary evidence of causality related to early-life transient dysbiosis and immune development in the context of asthma. In this study, inoculation of previously germ-free (GF) mice with 4 bacterial species, which were reduced in infants at high risk of asthma, ameliorated allergic airway inflammation in these mice.4
There is also evidence to support a role of the early-life microbiome in food allergies. Azad et al74 report a decrease in microbial diversity at 3 months of age and an increased enterobacteriaceae/bacteroidaceae ratio at 3 months and 1 year of age, which was associated with increased food sensitization among 1-year-old children. Variations in the gut microbiome at 3 and 6 months of age have also been associated with the resolution of a milk allergy by 8 years of age.75 Thus, authors of the current microbiome research for asthma and allergies suggest the time period between birth and 1 year of age as the developmental origins window for this disease. These immune hypersensitivities persist into later childhood and adulthood despite the transient nature of microbial dysbiosis.
Obesity
Similar to asthma and atopic disease, prospective studies reveal early-life gut microbiome compositional variations, which precede the development of obesity.88,91 A recent study reveals temporal and compositional microbiome associations with early childhood adiposity.89 Specifically, infants with high Streptococcus abundance at 6 months of age displayed increased adiposity at 18 months of age.89 This emphasizes the importance of both microbiome composition and timing of microbiome maturation in the development of childhood obesity.
Mouse model studies have been used to mechanistically support the potential metabolic effects of early-life microbial dysbiosis and emphasize the obesity-inducing abilities of antibiotics. In a study by Cho et al,90 antibiotics administered to mice in early life resulted in subsequent increases in adiposity and metabolic hormones. Cox et al6 furthered this research by manipulating the early-life microbiota using low-dose penicillin (LDP). Here, they report LDP exposure during early life increased the effect of a high-fat diet on the microbiota, which could be transferred to GF mice to induce obesity.6 It will be important for future researchers to incorporate both prospective human studies and animal models to determine the microbiome-associated effects of early-life antibiotic exposure on human health.
Neurodevelopmental Disorders
A less intuitive role of the gut microbiome in human health and development is the impact of early-life microbial dysbiosis in neurodevelopment (ie, the gut-brain axis). There is increasing evidence to support early-life microbial compositional variations in stress response and anxiety. In animal models, it is suggested that neonatal stress after maternal separation results in long-term compositional changes to the intestinal microbiota,95,96 and treating rat pups with probiotics can counter the resulting elevated corticosterone levels.95 Prenatal exposure to the SCFA, propionic acid, was also reported to increase anxiety-like behavior in mice.97 In a recent study, Gur et al7 report the influence of prenatal maternal stress in mice and its ability to alter both the maternal and neonatal intestinal microbiomes. Adult offspring exposed to prenatal maternal stress also displayed increased anxiety-like behavior.7 Additionally, prenatal stress also induced variations in the placental microbiome.7 This suggests that the intrauterine microbial environment is a potential mediator of maternal prenatal stress and resulting stress in the offspring.
Variations in the gut microbiome have also been associated with many facets of social behavior. Adult offspring of conventionalized dams (previously GF but colonized with the microbiome of specific pathogen–free [SPF] mice) were reported to display decreased motor activity compared with offspring from GF dams.98 Buffington et al93 report impaired social behavior (similar to that observed in autism spectrum disorder) in the offspring of dams that were fed a high-fat diet, which is mediated by variations in the offspring microbiome. In addition, using shotgun metagenomic sequencing, this group identified a significant reduction in Lactobacillus reuteri among the maternal high-fat diet–fed offspring.93 Supplementation of drinking water with L reuteri resulted in restoration of offspring social deficits.93 Notably, the majority of studies with researchers focusing on the early-life critical window in relation to neurodevelopment have been conducted in animal models. However, there is recent evidence to support a role of the infant gut microbiome in cognitive development in humans. Carlson et al94 reported variations in gut microbiome α diversity in 1-year-old children, which correlated with their cognitive abilities measured at age 2. Additional studies in humans will substantiate this evidence. However, it is interesting to note that researchers consistently identify the time period between pregnancy and the first year of life as the most influential in human development.
Collectively, this disease-specific research is used to support a role of the early-life microbiome in fetal and childhood development in a variety of contexts. However, prediagnostic variations in the early-life microbiome composition have been associated with other chronic noncommunicable diseases in later childhood and adulthood (ie, IBD and type 1 diabetes, Table 1).1 In addition, Harris et al112 identified an association between adolescent diet and breast cancer diagnosed in adulthood, suggesting that the timing of susceptibility for the microbiome may also be disease specific. The future of research surrounding the microbiome in DOHaD is thus ripe with opportunity.
Conclusions and Future Directions
In this review, we summarize evidence supporting the role of the microbiome as a fundamental part of human physiology and key to our understanding of DOHaD. Pre- and postbirth exposures alter the microbiome composition and functional potential in the neonate. In turn, this dysbiosis results in a number of health and disease outcomes later in life. Collaborative translational efforts using both animal models and human samples will mechanistically define the extent to which the microbiome plays a causal role during this critical window of developmental plasticity. Multiomics approaches, combining epigenetic, transcriptome, and microbiome analyses in large, prospective, human birth cohorts will enhance our current understanding of how the microbiome may interact with host components to drive aspects of infant development. In addition, as it is becoming clearer that the microbiota (both maternal and placental) can influence fetal development, more research surrounding the vaginal and intrauterine microbiomes is needed to paint a fuller picture of the fetal and neonatal origins of disease. We propose revisiting DOHaD and incorporating the microbiome into our current understanding of the many aspects of early human development associated with long-term health.
Glossary
- DOHaD
Developmental Origins of Health and Disease
- GF
germ free
- IBD
inflammatory bowel disease
- LDP
low-dose penicillin
- NEC
necrotizing enterocolitis
- SCFA
short-chain fatty acid
- SPF
specific pathogen free
Footnotes
Dr Stiemsma conceptualized and outlined the review, conducted the literature search, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Michels supervised the project and critically reviewed and edited the manuscript; and all authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Stiemsma is supported by T32 training grant 5T32CA009142-37 from the National Cancer Institute (NCI), National Institutes of Health (NIH). Funded by the National Institues of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Stiemsma LT, Reynolds LA, Turvey SE, Finlay BB. The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther. 2015;4:143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiemsma LT, Turvey SE. Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin Immunol. 2017;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22(7):713–722 [DOI] [PubMed] [Google Scholar]
- 4.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. ; CHILD Study Investigators . Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. [DOI] [PubMed] [Google Scholar]
- 5.Stiemsma LT, Arrieta MC, Dimitriu PA, et al. ; Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators . Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci (Lond). 2016;130(23):2199–2207 [DOI] [PubMed] [Google Scholar]
- 6.Cox LM, Yamanishi S, Sohn J, et al. . Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gur TL, Shay L, Palkar AV, et al. . Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav Immun. 2017;64:50–58 [DOI] [PubMed] [Google Scholar]
- 8.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388 [DOI] [PubMed] [Google Scholar]
- 10.Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307(6918):1519–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–1081 [DOI] [PubMed] [Google Scholar]
- 12.Hjalgrim LL, Westergaard T, Rostgaard K, et al. . Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am J Epidemiol. 2003;158(8):724–735 [DOI] [PubMed] [Google Scholar]
- 13.Trichopoulos D. Hypothesis: does breast cancer originate in utero? Lancet. 1990;335(8695):939–940 [DOI] [PubMed] [Google Scholar]
- 14.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shreiner A, Huffnagle GB, Noverr MC. The “Microflora Hypothesis” of allergic disease. Adv Exp Med Biol. 2008;635:113–134 [DOI] [PubMed] [Google Scholar]
- 16.Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70(suppl 1):S38–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson CM, Pfeiffer JK. Viruses and the microbiota. Annu Rev Virol. 2014;1:55–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steel JH, Malatos S, Kennea N, et al. . Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57(3):404–411 [DOI] [PubMed] [Google Scholar]
- 20.Satokari R, Grönroos T, Laitinen K, Salminen S, Isolauri E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol. 2009;48(1):8–12 [DOI] [PubMed] [Google Scholar]
- 21.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, et al. . The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302 [DOI] [PubMed] [Google Scholar]
- 23.Bassols J, Serino M, Carreras-Badosa G, et al. . Gestational diabetes is associated with changes in placental microbiota and microbiome. Pediatr Res. 2016;80(6):777–784 [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Xiao X, Zhang Q, Mao L, Yu M, Xu J. The placental microbiome varies in association with low birth weight in full-term neonates. Nutrients. 2015;7(8):6924–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince AL, Ma J, Kannan PS, et al. . The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol. 2016;214(5):627.e1–627.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol. 2015;212(5):653.e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauder AP, Roche AM, Sherrill-Mix S, et al. . Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boro P, Kumaresan A, Singh AK, et al. . Expression of short chain fatty acid receptors and pro-inflammatory cytokines in utero-placental tissues is altered in cows developing retention of fetal membranes. Placenta. 2014;35(7):455–460 [DOI] [PubMed] [Google Scholar]
- 30.Dominguez-Bello MG, Costello EK, Contreras M, et al. . Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107(26):11971–11975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokulich NA, Chung J, Battaglia T, et al. . Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1). Available at: www.pediatrics.org/cgi/content/full/135/1/e92 [DOI] [PubMed] [Google Scholar]
- 34.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, et al. . Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22(3):250–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yatsunenko T, Rey FE, Manary MJ, et al. . Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96(3):544–551 [DOI] [PubMed] [Google Scholar]
- 37.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, et al. . Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30(1):61–67 [DOI] [PubMed] [Google Scholar]
- 38.Pannaraj PS, Li F, Cerini C, et al. . Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171(7):647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcobal A, Barboza M, Froehlich JW, et al. . Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. 2010;58(9):5334–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe. 2011;17(6):478–482 [DOI] [PubMed] [Google Scholar]
- 41.Gregory KE, Samuel BS, Houghteling P, et al. . Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016;4(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brugman S, Visser JT, Hillebrands JL, Bos NA, Rozing J. Prolonged exclusive breastfeeding reduces autoimmune diabetes incidence and increases regulatory T-cell frequency in bio-breeding diabetes-prone rats. Diabetes Metab Res Rev. 2009;25(4):380–387 [DOI] [PubMed] [Google Scholar]
- 43.Azad MB, Vehling L, Lu Z, et al. ; CHILD Study Investigators . Breastfeeding, maternal asthma and wheezing in the first year of life: a longitudinal birth cohort study. Eur Respir J. 2017;49(5):1602019. [DOI] [PubMed] [Google Scholar]
- 44.Ma J, Prince AL, Bader D, et al. . High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu DM, Antony KM, Ma J, et al. . The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakajima A, Kaga N, Nakanishi Y, et al. . Maternal high fiber diet during pregnancy and lactation influences regulatory T cell differentiation in offspring in mice. J Immunol. 2017;199(10):3516–3524 [DOI] [PubMed] [Google Scholar]
- 47.Val-Laillet D, Besson M, Guérin S, et al. . A maternal Western diet during gestation and lactation modifies offspring’s microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in Yucatan pigs. FASEB J. 2017;31(5):2037–2049 [DOI] [PubMed] [Google Scholar]
- 48.Tormo-Badia N, Håkansson Å, Vasudevan K, Molin G, Ahrné S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol. 2014;80(4):250–260 [DOI] [PubMed] [Google Scholar]
- 49.Corvaglia L, Tonti G, Martini S, et al. . Influence of intrapartum antibiotic prophylaxis for Group B Streptococcus on gut microbiota in the first month of life. J Pediatr Gastroenterol Nutr. 2016;62(2):304–308 [DOI] [PubMed] [Google Scholar]
- 50.Keski-Nisula L, Kyynäräinen HR, Kärkkäinen U, Karhukorpi J, Heinonen S, Pekkanen J. Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr. 2013;102(5):480–485 [DOI] [PubMed] [Google Scholar]
- 51.Khan I, Azhar EI, Abbas AT, et al. . Metagenomic analysis of antibiotic-induced changes in gut microbiota in a pregnant rat model. Front Pharmacol. 2016;7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M. Antibiotic treatment at delivery shapes the initial oral microbiome in neonates. Sci Rep. 2017:7:43481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott FI, Horton DB, Mamtani R, et al. . Administration of antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology. 2016;151(1):120–129.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hviid A, Svanström H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60(1):49–54 [DOI] [PubMed] [Google Scholar]
- 55.Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2687–2692 [DOI] [PubMed] [Google Scholar]
- 56.Hoskin-Parr L, Teyhan A, Blocker A, Henderson AJ. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatr Allergy Immunol. 2013;24(8):762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pammi M, Cope J, Tarr PI, et al. . Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warner BB, Deych E, Zhou Y, et al. . Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387(10031):1928–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward DV, Scholz M, Zolfo M, et al. . Metagenomic sequencing with strain-level resolution implicates uropathogenic E. coli in necrotizing enterocolitis and mortality in preterm infants. Cell Reports. 2016;14(12):2912–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aceti A, Gori D, Barone G, et al. ; Italian Society of Neonatology . Probiotics for prevention of necrotizing enterocolitis in preterm infants: systematic review and meta-analysis. Ital J Pediatr. 2015;41:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Y, Shan G, Sodergren E, Weinstock G, Walker WA, Gregory KE. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case-control study. PLoS One. 2015;10(3):e0118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrow AL, Lagomarcino AJ, Schibler KR, et al. . Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barron LK, Warner BB, Tarr PI, Shannon WD, Deych E, Warner BW. Independence of gut bacterial content and neonatal necrotizing enterocolitis severity. J Pediatr Surg. 2017;52(6):993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Li X, Shang Q, et al. . Fecal microbiota transplantation (FMT) could reverse the severity of experimental necrotizing enterocolitis (NEC) via oxidative stress modulation. Free Radic Biol Med. 2017;108:32–43 [DOI] [PubMed] [Google Scholar]
- 65.Heida FH, van Zoonen AGJF, Hulscher JBF, et al. . A necrotizing enterocolitis-associated gut microbiota is present in the meconium: results of a prospective study. Clin Infect Dis. 2016;62(7):863–870 [DOI] [PubMed] [Google Scholar]
- 66.Birck MM, Nguyen DN, Cilieborg MS, et al. . Enteral but not parenteral antibiotics enhance gut function and prevent necrotizing enterocolitis in formula-fed newborn preterm pigs. Am J Physiol Gastrointest Liver Physiol. 2016;310(5):G323–G333 [DOI] [PubMed] [Google Scholar]
- 67.Bjornvad CR, Thymann T, Deutz NE, et al. . Enteral feeding induces diet-dependent mucosal dysfunction, bacterial proliferation, and necrotizing enterocolitis in preterm pigs on parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1092–G1103 [DOI] [PubMed] [Google Scholar]
- 68.Sim K, Shaw AG, Randell P, et al. . Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin Infect Dis. 2015;60(3):389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cortese R, Lu L, Yu Y, Ruden D, Claud EC. Epigenome-microbiome crosstalk: a potential new paradigm influencing neonatal susceptibility to disease. Epigenetics. 2016;11(3):205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujimura KE, Sitarik AR, Havstad S, et al. . Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russell SL, Gold MJ, Willing BP, Thorson L, McNagny KM, Finlay BB. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes. 2013;4(2):158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russell SL, Gold MJ, Reynolds LA, et al. . Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol. 2015;135(1):100–109 [DOI] [PubMed] [Google Scholar]
- 73.Thorburn AN, McKenzie CI, Shen S, et al. . Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. [DOI] [PubMed] [Google Scholar]
- 74.Azad MB, Konya T, Guttman DS, et al. ; CHILD Study Investigators . Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45(3):632–643 [DOI] [PubMed] [Google Scholar]
- 75.Bunyavanich S, Shen N, Grishin A, et al. . Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol. 2016;138(4):1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olszak T, An D, Zeissig S, et al. . Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gollwitzer ES, Saglani S, Trompette A, et al. . Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20(6):642–647 [DOI] [PubMed] [Google Scholar]
- 78.Teo SM, Mok D, Pham K, et al. . The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kennedy EA, Connolly J, Hourihane JO, et al. . Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol. 2017;139(1):166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ismail IH, Boyle RJ, Licciardi PV, et al. . Early gut colonization by Bifidobacterium breve and B. catenulatum differentially modulates eczema risk in children at high risk of developing allergic disease. Pediatr Allergy Immunol. 2016;27(8):838–846 [DOI] [PubMed] [Google Scholar]
- 81.Dzidic M, Abrahamsson TR, Artacho A, et al. . Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J Allergy Clin Immunol. 2017;139(3):1017–1025.e14 [DOI] [PubMed] [Google Scholar]
- 82.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–850 [DOI] [PubMed] [Google Scholar]
- 83.Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245 [DOI] [PubMed] [Google Scholar]
- 84.Korpela K, Zijlmans MA, Kuitunen M, et al. . Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nobel YR, Cox LM, Kirigin FF, et al. . Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. 2015;6:7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cerdó T, Ruiz A, Jáuregui R, et al. . Maternal obesity is associated with gut microbial metabolic potential in offspring during infancy [published online ahead of print August 17, 2017]. J Physiol Biochem. doi: 10.1007/s13105-017-0577-x [DOI] [PubMed] [Google Scholar]
- 87.Ba Q, Li M, Chen P, et al. . Sex-dependent effects of cadmium exposure in early life on gut microbiota and fat accumulation in mice. Environ Health Perspect. 2017;125(3):437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–538 [DOI] [PubMed] [Google Scholar]
- 89.Dogra S, Sakwinska O, Soh SE, et al. ; GUSTO Study Group . Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. MBio. 2015;6(1):e02419–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cho I, Yamanishi S, Cox L, et al. . Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vael C, Verhulst SL, Nelen V, Goossens H, Desager KN. Intestinal microflora and body mass index during the first three years of life: an observational study. Gut Pathog. 2011;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moussaoui N, Jacobs JP, Larauche M, et al. . Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: influence of sex. J Neurogastroenterol Motil. 2017;23(1):135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carlson AL, Xia K, Azcarate-Peril MA, et al. . Infant gut microbiome associated with cognitive development. Biol Psychiatry. 2018;83(2):148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56(11):1522–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Mahony SM, Marchesi JR, Scully P, et al. . Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65(3):263–267 [DOI] [PubMed] [Google Scholar]
- 97.Foley KA, Ossenkopp KP, Kavaliers M, Macfabe DF. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One. 2014;9(1):e87072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diaz Heijtz R, Wang S, Anuar F, et al. . Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108(7):3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruce-Keller AJ, Fernandez-Kim SO, Townsend RL, et al. . Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One. 2017;12(4):e0175577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stilling RM, Ryan FJ, Hoban AE, et al. . Microbes & neurodevelopment—absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015;50:209–220 [DOI] [PubMed] [Google Scholar]
- 101.Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156(9):3265–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin S, Zhao D, Cai C, et al. . Low-dose penicillin exposure in early life decreases Th17 and the susceptibility to DSS colitis in mice through gut microbiota modification. Sci Rep. 2017;7:43662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Candon S, Perez-Arroyo A, Marquet C, et al. . Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes [published correction appears in PLoS One. 2016;11(1):e0147888]. PLoS One. 2015;10(5):e0125448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu Y, Jin P, Peng J, Zhang X, Wong FS, Wen L. Different immunological responses to early-life antibiotic exposure affecting autoimmune diabetes development in NOD mice. J Autoimmun. 2016;72:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu Y, Peng J, Tai N, et al. . Maternal antibiotic treatment protects offspring from diabetes development in nonobese diabetic mice by generation of tolerogenic APCs. J Immunol. 2015;195(9):4176–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Livanos AE, Greiner TU, Vangay P, et al. . Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol. 2016;1(11):16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kostic AD, Gevers D, Siljander H, et al. ; DIABIMMUNE Study Group . The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17(2):260–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyoshi J, Bobe AM, Miyoshi S, et al. . Peripartum antibiotics promote gut dysbiosis, loss of immune tolerance, and inflammatory bowel disease in genetically prone offspring. Cell Reports. 2017;20(2):491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Millar M, Seale J, Greenland M, et al. . The microbiome of infants recruited to a randomised placebo-controlled probiotic trial (PiPS trial). EBioMedicine. 2017;20:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strunk T, Hibbert J, Doherty D, et al. . Probiotics and antimicrobial protein and peptide levels in preterm infants. Acta Paediatr. 2017;106(11):1747–1753 [DOI] [PubMed] [Google Scholar]
- 111.Trompette A, Gollwitzer ES, Yadava K, et al. . Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166 [DOI] [PubMed] [Google Scholar]
- 112.Harris HR, Willett WC, Vaidya RL, Michels KB. An adolescent and early adulthood dietary pattern associated with inflammation and the incidence of breast cancer. Cancer Res. 2017;77(5):1179–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]