Abstract
Introduction
Nivolumab is a promising treatment in patients with advanced malignancies. Among immune-related adverse events, autoimmune thyroid disorders are frequently reported.
Patient
A 61-year-old male patient had no history of familial or personal thyroid disease. In 2012, this patient, a heavy smoker, presented with non-small-cell lung cancer that was treated with radiotherapy and chemotherapy. In 2015, the cancer progressed with cervical compressive symptoms, and the patient was treated with nivolumab.
Results
After 3 infusions, bilateral eyelid ptosis and bilateral conjunctival redness with chemosis were observed. Ophthalmologic examination revealed severe proptosis with complete ophthalmoplegia but with normal vision, color test, and optic disk. Thyroid function tests were normal (TSH = 0.65 mU/L, free T4 = 15.4 pmol/L) without anti-thyroperoxidase or anti-TSH receptor antibodies. CT scan of the orbits confirmed marked bilateral proptosis with expansion of the orbital adipose tissue without significant thickening of extraocular muscles. T2-weighted MRI showed inflammation of orbital adipose tissue. Nivolumab treatment was withdrawn, and the patient received weekly intravenous high-dose methylprednisolone (1 g for 2 weeks, 500 mg for 4 weeks, and 250 mg for 5 weeks). After the first 3 cycles, significant improvement of left chemosis was observed whereas bilateral ptosis and ophthalmoplegia were unchanged. The patient was euthyroid without thyroid autoimmunity 1 week prior to his death due to massive hemoptysis.
Conclusion
We report severe inflammatory ophthalmopathy in a euthyroid patient with non-small-cell lung cancer during nivolumab therapy. The occurrence of such ophthalmic adverse events is likely to increase during nivolumab therapy in patients with advanced malignancies.
Keywords: Nivolumab, Ophthalmopathy, Non-small-cell lung cancer, Cigarette smoking, Pathogenesis
What Is Known about This Topic?
Anti-PD-1 monoclonal antibody therapy is associated with immune-related adverse events, and the most frequent is inflammatory thyroiditis.
During immunotherapy, immune-related ocular toxicities are rare, and the most common are uveitis and dry eyes.
During cytotoxic T-lymphocytic-associated antigen-4 (ipilimumab) treatment, thyroid-like orbitopathy was reported in patients with absent, slightly elevated, and high anti-TSH receptor antibodies.
What Does This Case Report Add?
We report severe inflammatory ophthalmopathy in a patient with a non-small-cell lung cancer treated with anti-PD-1 monoclonal antibody (nivolumab).
The euthyroid patient had no detectable anti-thyroid antibodies (anti-TPO, anti-TSH receptor) during the course of the inflammatory ophthalmopathy.
During nivolumab treatment, orbital changes of inflammatory ophthalmopathy are not secondary to thyroid autoimmunity.
Introduction
Immunotherapy has recently emerged as a promising therapy for cancer patients. Nivolumab, a fully human IgG4 subtype antibody to human programmed death-1 (PD-1), has demonstrated in vitro activity on T cells specific for tumor antigens. It has been used successfully in the treatment of patients with melanoma and non-small-cell lung cancer, with prolonged survival time in some patients [1, 2]. However, therapeutic results are obtained at the expense of immune-related adverse events involving many organs, in particular the endocrine system, and thyroid disorders (thyrotoxicosis, hypothyroidism, inflammatory thyroiditis) are more frequent than hypophysitis and adrenal insufficiency. On the other hand, immune-related ocular toxicities are rare, and the most common are uveitis and dry eyes [3].
We report the case of a male patient, an active smoker who developed severe inflammatory ophthalmopathy without thyroid autoimmunity during treatment with nivolumab for non-small-cell lung cancer.
Case Report
A 61-year-old male patient was seen in the multidisciplinary thyroid eye consultation of our endocrinology department for right eyelid ptosis and bilateral proptosis.
In his personal history, the patient was a heavy smoker (40–45 pack-years). He had severe obstructive sleep apnea syndrome, chronic obstructive pulmonary disease, hypertension, type-2 diabetes mellitus, and moderate chronic kidney insufficiency.
In November 2012, a non-small-cell lung cancer was diagnosed, and the patient was treated with radiotherapy and chemotherapy (carboplatin, vinorelbine) for 6 months. Then, between August and November 2015 the patient received 5 cycles of cisplatin and navelbine. The lung cancer progressed with cervical compressive symptoms, and the patient was included in the IMMUNO-PREDICT study after informed consent (ClinicalTrials.gov ID: NCT02827344). After 3 infusions of nivolumab (Bristol-Myers Squibb Laboratory), the patient developed eyelid ptosis and bilateral proptosis without ocular pain.
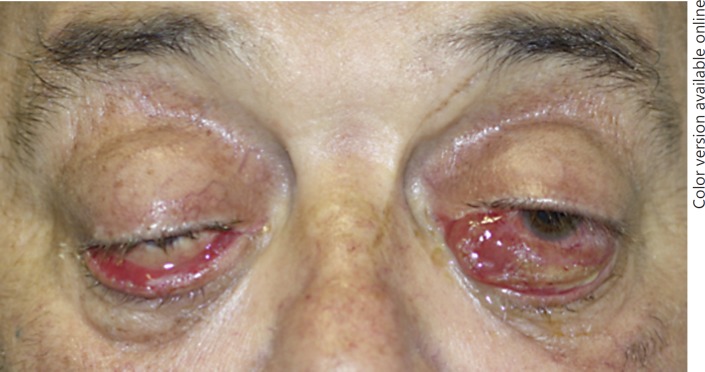
The patient had no history of familial or personal thyroid disease, and he was euthyroid prior to nivolumab therapy. Physical examination showed bilateral eyelid ptosis and bilateral conjunctival redness with chemosis, which was more severe in the left eye, attesting inflammatory ophthalmopathy (Fig. 1). Hertel exophthalmometry measured 28 mm bilaterally indicating severe proptosis, with complete ophthalmoplegia but with normal vision, color test, and optic disk.
Fig. 1.
Initial presentation of the patient with nivolumab-associated inflammatory ophthalmopathy.
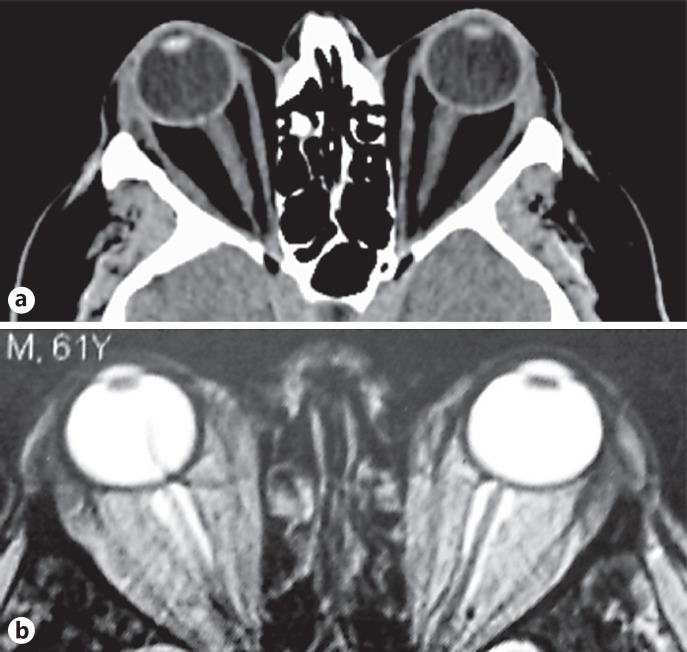
Physical examination of the neck was normal. Thyroid function tests remained in the normal range: TSH = 0.65 mU/L (normal range 0.27–4.2 mU/L), free T4 = 15.4 pmol/L (normal range 12.0–21.9 pmol/L) and free T3 = 4.3 pmol/L (normal range 3.1–6.6 pmol/L), without anti-thyroperoxidase (ECL Cobas 8000; Roche Diagnostics, USA) or anti-TSH receptor antibodies (RIA; CisBio International, France). Ultrasonography of the neck showed a normal-sized thyroid gland with a 12-mm nodule. CT scan of the orbits showed marked bilateral proptosis with expansion of the orbital adipose tissue without significant thickening of extraocular muscles (Fig. 2a). Magnetic resonance imaging confirmed orbital fat expansion (Fig. 2b) and showed inflammation on T2-weighted images similar to thyroid-like ophthalmopathy. Clinical workup found no evidence for myasthenia gravis (absence of anti-acetylcholine receptor antibody), orbital inflammatory processes, IgG4-related orbitopathy (IgG4 = 0.67 g/L, normal range 0.03–1.31 g/L), orbital cellulitis, or metastases.
Fig. 2.
a Axial CT scan showing severe exophthalmos after nivolumab treatment, with severe proptosis of both orbits with fat tissue augmentation and no extra-ocular muscle involvement. b Axial T2-weighted MR images confirmed exophthalmos with inflammatory edema of the retro-ocular fat tissue.
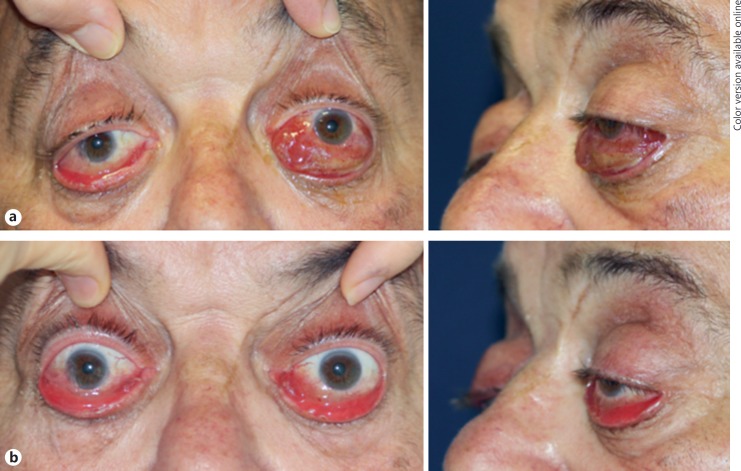
Nivolumab treatment was withdrawn, and the patient received weekly intravenous high-dose glucocorticoids (methylprednisolone 1 g for 2 weeks, 500 mg for 4 weeks, and 250 mg for 5 weeks). After the first 3 cycles, bilateral Hertel exophthalmometry was 27 mm with significant improvement of chemosis of the left eye (Fig. 3), whereas bilateral ptosis and ophthalmoplegia were unchanged. The tolerance of steroid therapy was good on arterial blood pressure and metabolic parameters. The patient was euthyroid (TSH = 0.61 mU/L) without anti-thyroid antibodies at the last visit, 1 week prior to his death due to massive hemoptysis.
Fig. 3.
Severe ophthalmopathy at diagnosis (a) and after 3 weeks of treatment (b) with weekly high-dose glucocorticoids (methylprednisolone 1 g × 2 and 500 mg × 1).
Discussion
During immunotherapy with cytotoxic T-lymphocytic-associated antigen-4 and anti-PD-1 specific monoclonal antibodies in patients with malignant melanoma and other advanced malignancies, clinical studies have reported several immune-related adverse events. Anti-cytotoxic T-lymphocytic-associated antigen-4 antibodies (ipilimumab, tremelimumab) preferentially exhibited pituitary toxicity with hypophysitis as the primary consequence. The most frequent endocrine disorders related to anti-PD-1 monoclonal antibodies (nivolumab, pembrolizumab) are thyroid diseases, particularly thyroiditis. During immune checkpoint inhibitor therapy, ophthalmic toxicity was uncommon [1, 2], and the most common ocular toxicities reported included uveitis and dry eyes [3]. Rare cases of orbital myositis [4] and orbital inflammation resembling thyroid eye disease [5, 6, 7, 8] have been associated with ipilimumab. During such treatment, thyroid-like orbitopathy was reported in euthyroid patients with absent [7], slightly elevated [5], or high [6] anti-TSH receptor antibodies. Using sensitive assays, auto-antibodies directed against the TSH receptor can be detected in the majority of patients with Graves' ophthalmopathy [9], but up to 5% of patients with thyroid orbitopathy are euthyroid or hypothyroid, and have low titers of anti-TSH receptor antibodies at the time of diagnosis. Interestingly, one euthyroid patient with thyroid autoimmunity at diagnosis of ipilimumab-related orbitopathy subsequently developed hyperthyroidism after 24 months of continued exposure to ipilimumab [10]. We report severe inflammatory ophthalmopathy in a patient during nivolumab treatment for advanced non-small-cell lung cancer. He had no history of autoimmune thyroid disease, was euthyroid during nivolumab treatment, and had no detectable anti-thyroid antibody at diagnosis or during steroid therapy, suggesting that immune changes secondary to nivolumab therapy did not play a major role in the course of inflammatory ophthalmopathy.
Smoking is the main environmental factor linked to the development or worsening of thyroid orbitopathy. The patient we report had a long history of active cigarette smoking and presented severe obstructive sleep apnea syndrome and chronic obstructive pulmonary disease leading to chronic hypoxia. Indeed, in patients without thyroid autoimmunity during nivolumab therapy, the role of cigarette smoking in the pathogenesis of thyroid-like inflammatory orbitopathy should be taken into consideration in order to optimize their management.
In patients with ipilimumab-related orbitopathy, treatment with oral or intravenous high-dose glucocorticoids resulted in complete [4, 8] or partial [5, 7, 11] recovery with possible recurrence during follow-up [5, 8]. Due to the severity of the inflammatory ophthalmopathy, the patient initially received 1 g of methylprednisolone for 2 weeks with significant improvement of chemosis at the 3-week evaluation. However, such treatment in cancer patients treated with nivolumab can be challenging since anti-inflammatory agents such as high-dose steroids can theoretically reverse the anti-cancer benefit of nivolumab treatment. Clinical research on treatment modalities of inflammatory thyroid-like orbitopathy, based on reported patients of clinical studies, is needed to optimize medical treatment of nivolumab-associated ophthalmopathy.
Finally, based on the recent studies reporting the efficacy of immune checkpoint inhibitors, it is likely that more patients will receive immunotherapy for advanced malignancies, and the incidence of thyroid-like inflammatory ophthalmopathy associated with immune modulators is likely to increase in the near future.
Disclosure Statement
The authors have no competing financial interest relevant to this paper.
Supplementary Material
Supplementary data
References
- 1.Barbee MS, Ogunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49:907–937. doi: 10.1177/1060028015586218. [DOI] [PubMed] [Google Scholar]
- 2.Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-LI antibodies. Int Immunol. 2015;27:39–46. doi: 10.1093/intimm/dxu095. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Rahman O, Oweira H, Tetrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, Schöb O, Giryes A. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther. 2017;17:387–394. doi: 10.1080/14737140.2017.1296765. [DOI] [PubMed] [Google Scholar]
- 4.Lecouflet M, Verschoore M, Giard C, Gohier P, Le Corre Y, Milea D, Martin L. Orbital myositis associated with ipilimumab. Ann Dermatol Venereol. 2013;140:448–451. doi: 10.1016/j.annder.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164:303–307. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borodic G, Hinkle DM, Cia Y. Drug-induced Graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg. 2011;27:e87–e88. doi: 10.1097/IOP.0b013e3181ef72a1. [DOI] [PubMed] [Google Scholar]
- 7.McElnea E, Ní Mhéalóid A, Moran S, Kelly R, Fulcher T. Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab. Orbit. 2014;33:424–427. doi: 10.3109/01676830.2014.949792. [DOI] [PubMed] [Google Scholar]
- 8.Sohrab MA, Desai RU, Chambers CB, Lissner GS. Re: “Drug-induced Graves disease from CTLA-4 receptor suppression.”. Ophthal Plast Reconstr Surg. 2013;29:239–240. doi: 10.1097/IOP.0b013e3182895795. [DOI] [PubMed] [Google Scholar]
- 9.Khoo DH, Eng PH, HO SC, Tai ES, Morgenthaler NG, Seah LL, Fong KS, Chee SP, Choo CT, Aw SE. Graves' ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid. 2000;10:1093–1100. doi: 10.1089/thy.2000.10.1093. [DOI] [PubMed] [Google Scholar]
- 10.Borodic GE, Hinkle D. Ipilimumab-induced orbital inflammation resembling Graves disease with subsequent development of systemic hyperthyroidism from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg. 2014;30:83. doi: 10.1097/IOP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 11.Borodic GE, Hinkle DM. Reply Re: “Drug-induced Graves disease from CTLA-4 receptor suppression.”. Ophthal Plast Reconstr Surg. 2013;29:241. doi: 10.1097/IOP.0b013e31828957c3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data