Abstract
Cytochrome P450s (CYPs), heme-containing monooxygenases, play important roles in a wide variety of metabolic processes important for development as well as biotic/trophic interactions in most living organisms. Functions of some CYP enzymes are similar across organisms, but some are organism-specific; they are involved in the biosynthesis of structural components, signaling networks, secondary metabolisms, and xenobiotic/drug detoxification. Fungi possess more diverse CYP families than plants, animals, or bacteria. Various fungal CYPs are involved in not only ergosterol synthesis and virulence but also in the production of a wide array of secondary metabolites, which exert toxic effects on humans and other animals. Although few studies have investigated the functions of fungal CYPs, a recent systematic functional analysis of CYP genes in the plant pathogen Fusarium graminearum identified several novel CYPs specifically involved in virulence, asexual and sexual development, and degradation of xenobiotics. This review provides fundamental information on fungal CYPs and a new platform for further metabolomic and biochemical studies of CYPs in toxigenic fungi.
Keywords: cytochrome P450, secondary metabolism, xenobiotics, Fusarium graminearum
1. Introduction
Cytochrome P450s (CYPs), which are heme-containing proteins, represent one of the largest protein families; they are present in all biological kingdoms [1,2]. CYPs commonly act as terminal monooxygenases in a range of biochemical reactions including hydroxylation, dealkylation, epoxidation, deamination, desulfuration, dehalogenation, sulfoxidation, and N-oxide reduction, by catalyzing the transfer of molecular oxygen to various cellular substrates [3,4,5]. This diversity of catalytic capabilities and the ability to manipulate them means that CYPs have been under the spotlight for biotechnological applications, such as biosynthesis of useful chemical compounds [6].
Organisms vary in the number of CYP genes they possess. The reference human genome contains 57 CYP genes and 58 pseudogenes, which are distributed into 18 families [7]. The function of human CYPs has been extensively studied since 1960, particularly with respect to xenobiotic and drug metabolism [8]. Insect genomes contain various numbers of CYPs, for example, 76–91 in Drosophila spp. (fruit flies), 87 in Bombyx mori (silkworm), and 46 in Apis mellifera (honey bee). In insects, CYPs are involved in the production of defence toxins and pheromones [9,10]. CYP-mediated detoxification of plant compounds, as well as insecticides, has also been reported [11]. Plant genomes tend to contain many more CYP genes than animal genomes, e.g., 334 in Oryza sativa (rice), 245 in Arabidopsis thaliana (thale cress), and 318 in Zea mays (corn), possibly because plants produce a wide variety of secondary metabolites [9]. Plant CYPs are mainly involved in biosynthesis of protective toxins and repellent molecules, as well as various signalling molecules [12].
The number of CYP genes in fungal species varies depending on their lifestyle. Whereas yeast-like fungi possess relatively few CYPs (three in Saccharomyces cerevisiae, six in Cryptococcus neoformans, and 10 in Candida albicans), filamentous fungi tend to possess more CYP genes. Plant pathogenic fungi tend to possess larger numbers of CYP genes; for example, Magnaporthe oryzae and Cryphonectria parasitica harbor 107 and 121 CYPs, respectively [13,14]. Fungal CYPs are involved in diverse biological processes, including production of primary and secondary metabolites and denitrification. However, compared to plants and animals, few fungal CYPs have been functionally characterized. Investigation of fungal CYPs will improve our understanding of fungal biology and metabolism, and may offer opportunities to exploit their catalytic functions.
In this review, we provide an overview of the fungal metabolic systems in which CYPs play roles, such as primary and secondary metabolism, degradation of xenobiotics, and other fungal traits. We also present a phenome-based functional analysis of whole CYP genes in the plant pathogenic fungus Fusarium graminearum, which illuminates the functional diversity and potential applications of fungal CYPs.
2. Fungal CYPs
Fungi represent a large kingdom of lower eukaryotic organisms, which are ubiquitous in ecological niches such as soil, living plants and animals, and decaying organic materials [15]. To rapidly adapt to environmental stresses and new niches, fungi have evolved extraordinary cellular defense systems, including CYP-mediated mechanisms for detoxification of exogenous toxic compounds. In particular, filamentous fungi have an outstanding ability to degrade a variety of toxic substances (e.g., environmental pollutants, xenobiotics, and plant-derived toxins) [16,17,18,19], and some filamentous fungi are well known for production of characteristic toxins via CYPs. Recent genetic evidence suggests that CYP enzyme reactions are closely involved in fungal developmental processes and pathogenesis [20,21].
CYP nomenclature is mainly based on amino acid sequence identity; 40% identity or greater places CYPs in the same family and greater than 55% identity places CYPs in the same subfamily [7]. As mentioned above, CYPs are key enzymes in many fungal processes, and classifiable into multigene families, CYP51–CYP69, CYP501–CYP699, and CYP5001–CYP6999 [15,22,23] (Table 1). CYP51, CYP56, CYP61, and many other known fungal CYPs are involved in biosynthesis of primary and secondary metabolites, as well as detoxification/degradation of xenobiotics. Many studies have predicted the functions of CYPs of individual fungi using bioinformatics tools [24,25,26,27,28]. Fungal CYPs are grouped in 15 clades based on their phylogenetic relationships [14]. Clade 8 is composed of the most family members including CYP59, CYP60, and CYP65 (Table 1). Recently, 14,896 CYPs were identified from 157 fungal and oomycete species [29]. However, the precise biological functions of most fungal CYPs remain undefined. For example, the CYPome of the white rot fungus Phanerochaete chrysosporium comprises ~150 CYPs, mostly arranged in gene clusters. In this fungus, except for the structurally and functionally conserved fungal CYP families, CYP51, CYP61, and CYP53, the roles of the other CYPs are still largely unknown and await functional characterization [30,31].
Table 1.
Cytochrome P450 monooxygenases in fungi.
| Clade 1 | Family | Class 2 | Organism | Function | Reference |
|---|---|---|---|---|---|
| 1 | CYP51 | E, group I, IV | S. cerevisiae, C. albicans, C. kefyr, C. glabrata, C. guilliermondii, C. parapsilosis, C. tropicalis, C. krusei, Ustilago maydis, Schizosaccharomyces pombe, Kluyveromyces marxianus, Penicillium italicum, Fusarium graminearum | Demethylation of eburicol/lanosterol at 14α position | [21,23,32,33,34,35,36,37,38,39,40,41,42] |
| 2 | CYP52 | E, group II | C. maltose, C. tropicalis, C. apicola | n-alkane and fatty acid assimilation | [3,42,43,44,45,46,47,48,49,50] |
| 2 | CYP53 | E, group I | Aspergillus niger, Beauveria bassiana, Cochliobolus lunatus, P. chrysosporium, Rhombophryne minuta | Degradation or detoxification benzoate and its derivatives | [51,52,53,54,55,56] |
| 2 | CYP54 | E, group I | Neurospora crassa | Cycloheximide inducible, but function is unknown | [57] |
| 3 | CYP55 | E, group I | F. oxysporum, Cylindrocarpon tonkinensis, A. oryzae, Trichosporon cutaneum | Denitrification process | [58,59,60,61,62,63] |
| 4 | CYP56 | E, group IV | S. cerevisiae, C. albicans | Formation of dityrosine | [64,65,66] |
| 6 | CYP57 | E, group I | Nectria haematococca | Pisatin detoxification | [67,68,69] |
| 6 | CYP58 | E, group I | F. sporotrichioides, F. graminearum | Trichothecene biosynthesis (TRI4) | [70,71] |
| 7 | CYP58 | B | A. flavus, A. parasiticus | Aflatoxin biosynthesis | [72,73,74] |
| 8 | CYP59 | E, group I | A. nidulans | Sterigmatocystin biosynthesis (stcS/verA) | [75,76] |
| 8 | CYP60 | E, group I | A. parasiticus, A. nidulans | o-methylsterigmatocystin to aflatoxin (ord1), sterigmatocystin biosynthesis (stcF and stcL) | [76,77] |
| 8 | CYP61 | E, group I | S. cerevisiae, C. glabrata | Sterol D22-desaturase in ergosterol biosynthesis (erg5) | [22,78] |
| 8 | CYP62 | E, group I | A. nidulans | Sterigmatocystin biosynthesis (stcB) | [76] |
| 8 | CYP63 | E, group I | P. chrysosporium | Unknown function | [79] |
| 8 | CYP64 | E, group I | A. flavus | Conversion of o-methylsterigmatocystin to aflatoxin (ord1) | [80] |
| 8 | CYP65 | E, group I | F. sporotrichioides | Trichothecene biosynthesis (TRI11) | [71] |
| 9 | CYP66 | E, group IV | Agaricus bisporus | Developmental regulation of mushroom | [81] |
| 10 | CYP68, CYP69, CYP503 | E, group I | F. fujikuroi | Gibberellin biosynthesis | [82,83] |
| 10 | CYP504 | E, group I | A. nidulans | Catalyzing phenylacetate 2-hydroxylation | [84,85,86] |
| 14 | CYP505 | E, group IV | F. oxysporum | ω-1 to ω-3 carbon hydroxylation of fatty acids | [86,87] |
| 15 | CYP505 | E, group IV | F. verticillioides | Fumonisin biosynthesis | [88,89] |
| 15 | CYP526 | E, group IV | F. sporotrichioides | Trichothecene biosynthesis | [71] |
3. CYPs Related to Secondary Metabolite Biosynthesis
Fungi produce a variety of secondary metabolites. In limited environmental niches, some fungi utilize secondary metabolites as weapons to compete against other organisms including bacteria, plants, animals, and even other fungi, and some toxins of pathogenic fungi function as important virulence factors in host-microbe interactions [91,92,93,94]. The functions of many fungal secondary metabolites remain obscure or unknown, but are predicted to play roles in interactions with other organisms. Secondary metabolites carry out a broad range of useful antibiotic, immunosuppressant, and mycotoxic activities [95,96,97]. Mycotoxins are toxic secondary metabolites produced by fungi, usually found in contaminated crops, and have severe effects on both humans and animals (Table 2). Structures of major mycotoxins are shown in Figure 1. Many fungal CYPs are known to be involved in mycotoxin biosynthesis [15,98].
Table 2.
Mycotoxins produced by Cytochrome P450 (CYP)-mediated reactions.
| Mycotoxin | Organism | Characteristics | Reference |
|---|---|---|---|
| Aflatoxin | A. flavus, A. parasiticus, etc. | Carcinogenic compounds posing a potential risk to livestock and human health | [99] |
| Ak-toxin | Alternaria alternata | Host-selective toxin, virulence factor to infect Japanese pear | [100] |
| Af-toxin | A. alternata | Host-selective toxin, virulence factor to infect strawberry | [101] |
| Botridial | Botrytis cinerea | Induction of chlorosis and cell collapse in plant | [102,103,104] |
| Depudecin | A. brassicicola | An inhibitor of histone deacetylase (HDAC) | [105] |
| Dothistromin | Dothistroma septosporum | A broad-spectrum toxin that generates oxygen radicals by reductive oxygen activation | [106] |
| Ergot alkaloid | Claviceps, Penicillium, and Aspergillus spp. | A complex family of indole derivatives with diverse structures and biological activities | [107,108] |
| Fumonisin | F. verticillioides | Induction of several animal diseases, including leukoencephalomalacia, pulmonary edema, and cancer | [109] |
| Hc-toxin | Cochliobolus carbonum | An inhibitor of histone deacetylases (HDACs) in many organisms, including plants, insects, and mammals | [110,111] |
| Ochratoxin | Aspergillus, and Penicillium spp. | Possible carcinogenic | [112] |
| Paxilline | P. paxilli | A potassium channel blocker | [113,114] |
| PR-toxin | P. roqueforti | Liver toxicity and abortions in cows | [115] |
| Sterigmatocystin | A. nidulans, A. versicolor | A toxic metabolite structurally closely related to the aflatoxins | [75,76] |
| Trichothecene | F. sporotrichioides, F. graminearum | Inhibition of protein synthesis and highly cytotoxic to many eukaryotes | [71,95,116] |
Figure 1.
Chemical structures of mycotoxins. Chemical structures were obtained from PubChem (https://pubchem.ncbi.clm.cih.gov) [117].
3.1. Aflatoxins and Sterigmatocystin
Aflatoxins B1 (AFB1), B2 (AFB2), G1 (AFG1), and G2 (AFG2) and sterigmatocystin are polyketides derived from the same secondary metabolite biosynthetic pathway [118]. They are produced by several fungi, primarily by Aspergillus spp., which grow in soil, decaying vegetation, hay, and grain [119]. Although sterigmatocystin, the precursor of aflatoxin B1, has lower toxicity than other aflatoxins, both mycotoxins are potent carcinogens that cause liver cancer in mammals [120]. Among the 26 genes located in the aflatoxin biosynthetic gene cluster, aflG, aflQ, aflU, and aflV encode CYPs [76,99]. aflG is involved in the monooxygenase step that converts averantin to hydroxyaverantin, a precursor of aflatoxins [121]. aflQ encodes an oxidoreductase that is responsible for conversion of dihydro-O-methylsterigmatocystin to AFB1 and AFG1 and of O-methylsterigmatocystin to AFB2 and AFG2 [122,123]. aflV is involved in the reaction from averufin to 1′-hydroxyversicolorone, the first step in dihydrobisfuran formation during aflatoxin biosynthesis [74]. The pathway involvement of aflU remains unclear [99].
3.2. Fumonisins
Fumonisins are polyketide mycotoxins that cause severe animal diseases, including leukoencephalomalacia in horses, pulmonary edema in swine, and kidney and liver cancers in mice [124]. In addition, the consumption of fumonisin-contaminated maize induces high incidences of esophageal cancer in humans [125]. These mycotoxins are mainly produced by the maize pathogen F. verticillioides and several other Fusarium spp. [109]. At least 8 genes involved in biosynthesis of this toxin have been identified, one of which, FUM6, encodes a CYP [88]. In the fumonisin backbone, C-14 and C-15 are probably the direct substrate for Fum6-catalyzed hydroxylation [109,126].
3.3. Host-Selective Toxins
Host-specific toxins (also known as host-selective toxins; HSTs), which are produced by a number of plant pathogenic fungi, are a class of low-molecular-mass secondary metabolites [127]. They are called HSTs because these toxins are critical determinants of pathogenicity in specific plant–disease interactions [127]. The genera Alternaria and Cochliobolus are well known to produce HSTs [128]. A. alternata, a pathogenic fungus on a number of plants, produces several structurally diverse HSTs; AF-, AK-, ACT-, and AAL-toxins are produced by the strawberry, Japanese pear, tangerine, and tomato pathotypes of A. alternata, respectively [129]. In the Japanese pear pathotype, AKT7 encodes a CYP that suppresses AK-toxin production [100]. There are 13 ALT genes involved in the biosynthesis of AAL-toxin, and ALT2, a homolog of FUM6, also encodes a CYP [129].
The cyclic tetrapeptide HC-toxin is an inhibitor of histone deacetylases in several organisms, including plants and animals [130]. It is also a well-known host-selective toxin produced by C. carbonum that selectively affects maize lines of genotype hm1/hm1 [127]. One of the genes for HC-toxin biosynthesis encodes a CYP, which is involved in generation of the epoxide group [111].
3.4. Dothistromin
Dothistromin (DOTH), a polyketide-derived mycotoxin produced by D. septosporum, has broad-spectrum toxicity to plants, animals, and microbial cells via generation of oxygen radicals [131]. D. septosporum is an important forest pathogen that causes red band needle blight disease of pine trees [132], and DOTH is a virulence factor that affects the severity of disease, even though it is not required to cause disease [133]. The structure of DOTH is similar to that of versicolorin B, a precursor of aflatoxin, and the two DOTH biosynthetic CYP genes, CypX and AvnA, are orthologs of aflV and aflG, respectively. AvnA catalyzes the conversion of averantin to hydroxyaverantin in the early part of the DOTH biosynthetic pathway [106].
3.5. Botridial
B. cinerea is the causal agent of gray mold disease in more than 200 crop species worldwide [134]. B. cinerea, which has a necrotrophic lifestyle, secretes diverse cell wall–degrading enzymes and toxins to kill host cells [135]. Botridial is the best-studied toxin produced by this fungus, and CYP BcBot1 is responsible for hydroxylation of the C-15 carbon of 10-hydroxyprobotryane during botridial biosynthesis [103].
3.6. Ochratoxin A
Ochratoxin A (OTA) is a naturally occurring mycotoxin produced by a few of Aspergillus and Penicillium spp., e.g., Aspergillus ochraceus, A. carbonarius, A. westerdijkiae, A. steynii and Penicillium verrucosum, P. nordicum, and P. thymicola, on improperly stored food products [112,136]. OTA is the most toxic and frequently found among several forms of ochratoxins. The International Agency for Research on Cancer (IARC) has classified OTA as a possible human carcinogen, based on demonstrated carcinogenicity in animal studies [137]. Unlike other well-characterized mycotoxins, ochratoxin biosynthetic pathway has not yet been unraveled in detail. In addition to polyketide synthases (PKSs) and non-ribosomal peptide synthases (NRPSs), several key genes required for biosynthesis of OTA including putative CYPs were identified in a few Aspergillus species. Moreover, conserved OTA biosynthetic genes have been recently identified using a comparative genome analysis in several Aspergillus and Penicillium species [138].
4. Xenobiotic-Metabolizing CYPs
The environment contains a great number of substances and their mixtures, and more than 135 million organic and inorganic chemicals have been registered in the CAS RegistrySM collection to date [139]. Chemical compounds found within an organism or ecosystem that are not produced by that organism or ecosystem are called xenobiotics. Many xenobiotics are usually synthesized for industrial and agricultural purposes, i.e., aromatics, pesticides, and hydrocarbons; some of them are harmful to living organisms. Organisms have evolved efficient systems to prevent absorption of xenobiotics, to eliminate them, and to repair or adapt to damage caused by xenobiotics. Among xenobiotic-metabolizing enzymes, CYPs are the most abundant and versatile [140,141].
Studies of mammalian xenobiotic-metabolizing CYPs have led to the discovery and characterization of the xenobiotic metabolism pathways [8,140,141]. In humans, drug-metabolizing CYPs mostly presenting in the liver are responsible for oxidative metabolism of xenobiotics [140]. Many insect CYPs also play roles in detoxification of xenobiotics. Insect CYPs are inducible in response to botanical insecticides and/or plant secondary metabolites [11,142,143]. Also, some plant CYPs involved in the detoxification of xenobiotics such as herbicides [9]. In fungi, the wood-rotting basidiomycetes, particularly the white rot fungus P. chrysosporium and the brown rot fungus Postia placenta, are the most well-known fungi involved in the biodegradation of various xenobiotic compounds [144,145]. In particular, in P. chrysosporium 33 CYP families are involved in the hydroxylation of polycyclic aromatic hydrocarbons, and the genes belonging to the CYP63 family are predicted to be involved in degradation of various xenobiotics [144].
One of the best-characterized fungal CYPs is a pisatin demethylase (PDA, CYP57A1), which was first identified in N. haematococca [146]. PDA is the enzyme responsible for detoxifying pisatin, one of the isoflavonoid phytoalexins produced by Pisum sativum L. (garden pea). N. haematococca isolates with pisatin demethylating activity are tolerant to pisatin and highly virulent on pea, suggesting that PDA is a host-specific virulence factor in this fungus. F. oxysporum f. sp. pisi, another pea pathogen, possesses orthologs of PDA that play a major role in pisatin detoxification [147].
5. CYPs Required for Fungal Development and Virulence
One particularly well-studied fungal CYPs is CYP51, which mediates primary metabolism of ergosterol biosynthesis [148]. Ergosterol is a fungal-specific membrane sterol required for regulation of membrane fluidity and permeability [148,149]. It is essential for fungal growth and therefore is a primary target of antifungal compounds. Therefore, CYP51 has been exploited as a disease control target of fungal pathogens [139,150]. Sterol 14α-demethylase, a member of the CYP51 family, is the main target for therapeutic azole antifungal drugs and agricultural azole fungicides. Although the precise biochemical mechanism remains unclear, CYP51 orthologs are responsible for sensitivity to different azole fungicides, as these compounds specifically bind and inhibit sterol 14α-demethylase paralogs. Most fungi, including A. nidulans, A. fumigatus, C. albicans, C. neoformans, as well as F. graminearum, possess one or multiple CYP51 genes [139,151,152].
Oxylipins are oxygenated metabolites of linoleic or oleic acids that have hormone-like functions in sexual and asexual reproduction in many fungi [153]. The precocious sexual inducer (psi) factor-producing oxygenases (Ppos), natural fusion proteins of a heme peroxygenase at N-terminus and a P450 domain at C-terminus, are required for the production of oxylipins in Aspergillus spp., and CYP52-mediated oxygenation of hydrocarbons or fatty acids is important for lipid metabolism and pathogenesis in the human pathogen C. albicans. However, their exact biochemical mechanisms remain unclear.
Along with CYP51, which encodes sterol 14α-demethylase, CYP56 of S. cerevisiae and C. albicans are necessary for production of N, N′-bisformyl dityrosine, a component of the outer spore wall [65]. However, a homolog of CYP56 in C. albicans was found not to be essential for cell viability under culture conditions [66].
Fungal denitrification is the major pathway of nitrogen cycle in nature. CYP55, which encodes nitric oxide reductase (P450nor), is considered essential for most fungal denitrifying systems [154]. The multifunctional detoxifying enzyme CYP55 catalyzes the co-denitrification reaction and additionally exhibits NADH-peroxidase activity. Homologs of the CYP55 gene (P450nor) are distributed in many fungal genomes.
Significant regulation of CYPs has been observed during host-pathogen interactions via gene/transcriptome profiling analyses of several fungi, e.g., F. graminearum, Curvularia lunata, and Heterobasidion annosum, although the mechanisms are not yet known [155,156,157]. Recently, a pathogenicity-related CYP gene has been identified in Verticillium dahliae via transfer DNA (T-DNA) random insertional mutagenesis [158].
6. CYPs of F. graminearum
Several fungal CYPs have been known to play critical roles in primary and secondary metabolism and degradation of xenobiotics [159]. Besides, some CYPs in pathogenic fungi has emerged as important enzymes in virulence [155]. However, the involvement of CYPs in fungal development and other functions, such as virulence or xenobiotic detoxification, has rarely been elucidated, and there has been no systematic approach to the investigation of CYPs in filamentous fungi, including plant pathogens. The plant pathogenic fungus F. graminearum is an economically important pathogen that causes Fusarium head blight in major cereal crops such as wheat, barley, and rice, and Fusarium ear and stalk rot in maize worldwide [160]. In addition to yield losses, the fungus contaminates grains with mycotoxins such as trichothecenes (nivalenol (NIV) and deoxynivalenol (DON)) and zearalenone (ZEA), which pose serious threats to human and animal health [95]. F. graminearum is a good model organism, as its genome has been sequenced and targeted genetic modification is relatively easy [161]. The genome of F. graminearum is predicted to contain 119 putative CYP genes, which constitute 0.9% of the total predicted genes (Figure 2) [13].
Figure 2.
Classification of putative CYPs in F. graminearum. Total CYPs were categorized into six classes based on InterPro terms. These data were reproduced from [20]. Copyright 2017, John Wiley & Sons.
To date, only nine F. graminearum CYPs have been functionally characterized. Three CYP genes are paralogs of CYP51, which encodes an ergosterol biosynthetic enzyme [21,139]. Four CYPs (TRI1, TRI4, TRI13, and TRI11) are involved in trichothecene biosynthesis [71,162], and Fg08079 and CLM2 are required for the biosynthesis of butenolide [163] and culmorin [164], respectively. To systematically characterize the CYPome of F. graminearum, CYP gene knockout mutants were generated, and a comprehensive phenome of 102 CYP mutant library of F. graminearum was tested in 38 traits (Figure 3) [20]. Notably, specific CYP genes have been identified that are required for virulence (five CYPs), conidiation (one CYP), and sexual development (two CYPs) in this fungus.
Figure 3.
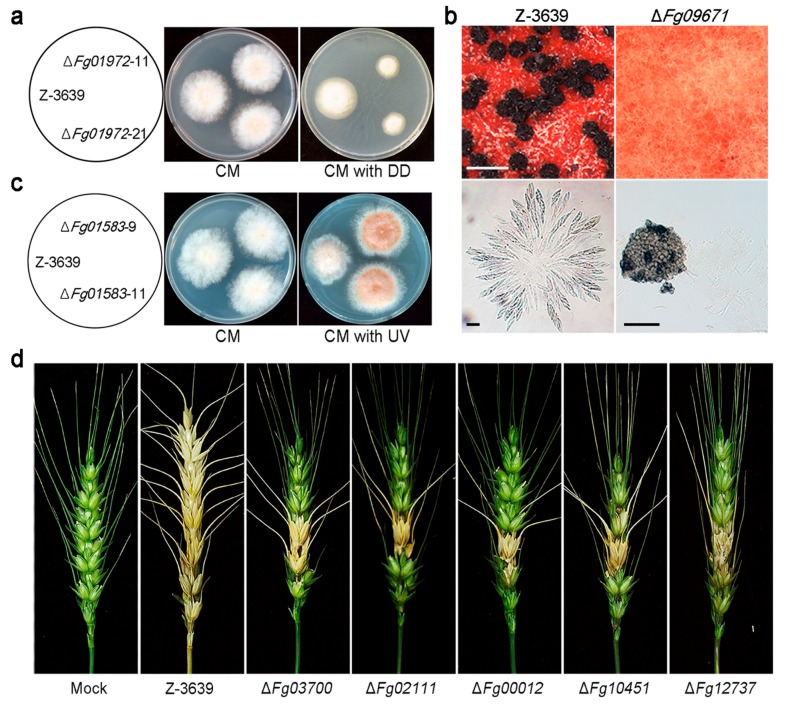
Phenotypic analyses of CYP deletion mutants of F. graminearum. (a) Altered xenobiotic stress response of wild type (Z-3639) and one CYP deletion mutant. DD, 1-dodecanol; (b) Development of perithecia (upper panel) and formation of asci rosettes (lower panel); A CYP gene deletion strain (right panel) showed defects in perithecia and ascospore formation whereas the parent strain (left panel) displayed normal perithecia and ascospores. Scale bar = 500 μm (upper), 20 μm (lower left), and 200 μm (lower right); (c) Altered ultra-violet (UV) stress response of wild type and CYP mutant strain; (d) Virulence of wild-type and CYP deletion strains on wheat heads. Five mutants showed reduced virulence compared to the wild-type strain (Z-3639). These data have been reproduced from [21] with slight modifications, Copyright 2013, John Wiley & Sons.
6.1. Trichothecenes
Trichothecenes are a large family of sesquiterpenoid secondary metabolites mainly produced by Fusarium spp. [165]. They are potent inhibitors of protein translation in eukaryotes via ribosomal binding [166]. DON and NIV are not only phytotoxins that contribute to the virulence of F. graminearum, but also mycotoxins that cause moldy-grain toxicosis in animals. In experimental animals, acute high-dose exposure induces a radiomimetic effect, with symptoms such as diarrhea, vomiting, leukocytosis, and gastrointestinal hemorrhage. At extremely high doses, these effects can cause circulatory shock, reduced cardiac output, and ultimately result in death [167]. NIV is significantly more toxic than DON [168]. Four CYPs, Tri1, Tri4, Tri11, and Tri13, are involved in biosynthesis of trichothecenes (Figure 4). Among the four CYPs in the trichothecene biosynthetic gene cluster, Tri13, 3-acetyltrichothecene C-4 hydroxylase, is responsible for hydroxylation of trichothecenes at C-4 [169]; the gene is therefore a determinant of the DON- and NIV-producing chemotypes in F. graminearum [170]. TRI4 encodes a multifunctional oxygenase that is responsible for the conversion of trichodiene to isotrichotriol. TRI4 mutants do not produce trichothecenes and highly accumulate trichodiene [70,171]. TRI1 and TRI11 encode 3-acetyltrichothecen C-8 hydroxylase and isotrichodermin C-15 hydroxylase, respectively [71].
Figure 4.
Fusarium trichothecene biosynthetic pathway. CYP enzymes, Tri1, Tri4, Tri11, Tri13, are involved in trichothecene biosynthesis. This scheme has been derived from [71] with slight modifications, Copyright 2007, Japan Society for Bioscience, Biotechnology, and Agrochemistry.
6.2. Xenobiotic-Metabolizing CYPs in F. graminearum
CYPs have been extensively studied in many organisms because of their abilities for xenobiotic decomposition. Likewise, some putative xenobiotic-metabolizing CYPs in F. graminearum have been characterized based on genetic evidence [20]. Fg01972 (CYP505A7) mutant strains showed reduced growth on 1-dodecanol-amended medium compared to the wild type (Figure 3a), and transcription of Fg01972 was highly up-regulated in response to exogenous treatment with 1-dodecanol. The Fg01972 enzyme may play an important role in detoxification and/or mineralization of this compound. Moreover, most CYP genes (93 of 102) were markedly induced in at least one xenobiotic condition [20], demonstrating that many are closely related to xenobiotic metabolism, with redundant functions. Further studies are required to identify the substrates of these CYP enzymes.
In F. oxysporum, CYP505 members are fatty acid hydroxylases that perform subterminal omega hydroxylation of fatty acids [86]. Both the CYP505 (Fg07596 and Fg01972) and CYP540 (Fg02138 and Fg02929) families in F. graminearum are highly induced by aliphatics such as n-dodecane and 1-dodecanol [20]. In addition, Fg10451 belongs to the CYP53A subfamily hydroxylates benzoic acid and other monosubstituted benzoate derivatives, forming para-hydroxylated products in the ascomycete fungi A. niger, A. nidulans, and C. lunatus, as well as in the basidiomycete fungus P. chrysosporium [51,52,55,56]. Fg02458 and Fg00012 are members of the CYP63 family, which is predicted to take part in the degradation of fatty acids, and members of the subfamily CYP537A2 (Fg12737) are known to be related to benzoate 4-monooxygenase cytochrome P450 [14,29].
6.3. CYPs Required for Fungal Development and Virulence in F. graminearum
Phenotype-based screens of mutant libraries have provided a powerful approach for functional genomic studies in F. graminearum [161,172]. A comprehensive phenome set illuminated the molecular mechanisms underpinning sexual and asexual development, mycotoxin production, stress responses, and pathogenicity in this fungus [161]. Our previous study on the systematic functional characterization of CYP genes in F. graminearum revealed that many novel CYPs are closely involved in fungal developmental processes including virulence [20]. Using this phenotypic dataset, we could gain insight into the cryptic functions of CYPs in fungi.
In A. nidulans, the psi factor, hormone-like fatty acid-derived oxylipins, serves as a signal molecule that modulates sexual and asexual sporulation by affecting the timing and balance of asexual and sexual spore development [173]. Fg09671 (CYP616A1) deletion mutants of F. graminearum showed defective sexual reproduction (Figure 3b) [20]. This indicates that Fg09671-mediated signalling might be related to regulation of the initial stages of sexual development, such as switching from mycelial growth to fruiting body development or differentiation of hyphae into fruiting body tissues.
The Fg01583 (CYP642A1) deletion mutant produced orange pigment in mycelia and grew faster than the wild-type strain under ultra-violet (UV)-B conditions (Figure 3c). Most plants produce flavonoids as the major red, blue, and purple pigments, and these pigments play key roles in defense, as antimicrobial agents and UV protectants [174]. The exact biochemical function of Fg01583 is not known yet, but this CYP642A1-type CYP enzyme seems to play a role in secondary metabolite biosynthesis, which is important for UV protection.
In infection assays with flowering wheat heads, deletion mutants of five CYPs, Fg03700 (CYP620B1), Fg02111 (CYP636A1), Fg00012 (CYP630A1), Fg10451 (CYP53A8), and Fg12737 (CYP537A2), showed reduced virulence compared to the wild type (Figure 3d). Plants synthesize and accumulate secondary metabolites such as phytoalexins that are toxic to most fungi and are suspected of being involved in plant defence mechanisms [175]. However, fungal pathogens frequently possess the ability to detoxify host phytoalexins. For instance, PDA, a CYP enzyme produced by N. haematococca, detoxifies the pisatin produced by its host [16]. We suspect that these CYPs, which displayed reduced virulence when deleted, are involved in degradation of plant-derived metabolites or plant tissues for successful infection. Recently, comparative in planta transcriptome analyses revealed that more than 40 CYPs may be involved in host–pathogen interactions of F. graminearum [176].
7. Conclusions
Fungal CYPs play essential roles for survival, and several azole fungicides that are mainly targeted at the CYPs have been commercially used for control of animal and plant pathogenic fungi [177]. Decades of studies on fungal genetics and biochemistry have established the involvement of CYPs in many bioconversion processes related to degradation of foreign compounds and biosynthesis of secondary metabolites in fungi. However, scant information is available on CYPs and their involvement in fungal developmental processes, including virulence, due to the lack of CYP mutants defective in those phenotypes and available up-to-date molecular techniques. Using data from a systematic functional genomic study of F. graminearum, we identified some clues to the novel functions of CYPs in toxigenic fungi. Further studies should focus on the identification of the substrate specificity of CYPs using metabolomic and/or biochemical approaches. Moreover, the complex regulatory genetic networks governing CYP enzyme reactions will be uncovered by applying advanced molecular genetics techniques and multi-omics approaches. These multidisciplinary studies on CYPs will provide fundamental information on fungal-specific CYPs compared to those of other organisms and new insights into fungal biology and virulence.
Acknowledgments
This work was supported by the Research Resettlement Fund for the new faculty of Seoul National University, the National Research Foundation of Korea (2013R1A6A3A04059121), and Next-Generation BioGreen 21 Program (No. PJ013121012018), Rural Development Administration, Republic of Korea.
Author Contributions
J.S., J.-E.K., Y.-W.L. and H.S. conceived and designed the review contents, and wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Fungal CYPs are involved in diverse biological processes, including production of primary and secondary metabolites, detoxification, and virulence. Systematic functional genomic study of F. graminearum has revealed some clues to the novel functions of CYPs in toxigenic fungi.
References
- 1.Nelson D.R., Koymans L., Kamataki T., Stegeman J.J., Feyereisen R., Waxman D.J., Waterman M.R., Gotoh O., Coon M.J., Estabrook R.W. P450 superfamily: Update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Lamb D.C., Lei L., Warrilow A.G., Lepesheva G.I., Mullins J.G., Waterman M.R., Kelly S.L. The first virally encoded cytochrome P450. J. Virol. 2009;83:8266–8269. doi: 10.1128/JVI.00289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanglard D., Loper J.C. Characterization of the alkane-inducible cytochrome P450 (P450alk) gene from the yeast Candida tropicalis: Identification of a new P450 gene family. Gene. 1989;76:121–136. doi: 10.1016/0378-1119(89)90014-0. [DOI] [PubMed] [Google Scholar]
- 4.Mansuy D. The great diversity of reactions catalyzed by cytochromes P450. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998;121:5–14. doi: 10.1016/S0742-8413(98)10026-9. [DOI] [PubMed] [Google Scholar]
- 5.Sono M., Roach M.P., Coulter E.D., Dawson J.H. Heme-containing oxygenases. Chem. Rev. 1996;96:2841–2888. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 6.Urlacher V.B., Girhard M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends Biotechnol. 2012;30:26–36. doi: 10.1016/j.tibtech.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Nelson D.R., Nebert D.W. eLS. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2001. Cytochrome P450 (CYP) gene superfamily. [Google Scholar]
- 8.Guengerich F.P. Cytochrome P450. Springer; Cham, Switzerland: 2005. Human cytochrome P450 enzymes; pp. 377–530. [Google Scholar]
- 9.Schuler M.A. P450s in plants, insects, and their fungal pathogens. In: Ortiz de Montellano P.R., editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. Springer; Cham, Switzerland: 2015. pp. 409–449. [Google Scholar]
- 10.Jensen N.B., Zagrobelny M., Hjernø K., Olsen C.E., Houghton-Larsen J., Borch J., Møller B.L., Bak S. Convergent evolution in biosynthesis of cyanogenic defence compounds in plants and insects. Nat. Commun. 2011;2:273. doi: 10.1038/ncomms1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui S., Wang L., Ma L., Geng X. P450-mediated detoxification of botanicals in insects. Phytoparasitica. 2016;44:585–599. doi: 10.1007/s12600-016-0550-1. [DOI] [Google Scholar]
- 12.Bolwell G.P., Bozak K., Zimmerlin A. Plant cytochrome P450. Phytochemistry. 1994;37:1491–1506. doi: 10.1016/S0031-9422(00)89567-9. [DOI] [PubMed] [Google Scholar]
- 13.Park J., Lee S., Choi J., Ahn K., Park B., Park J., Kang S., Lee Y.H. Fungal cytochrome P450 database. BMC Genom. 2008;9:402. doi: 10.1186/1471-2164-9-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W., Lee M.-K., Jefcoate C., Kim S.-C., Chen F., Yu J.-H. Fungal cytochrome P450 monooxygenases: Their distribution, structure, functions, family expansion, and evolutionary origin. Genome Biol. Evol. 2014;6:1620–1634. doi: 10.1093/gbe/evu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Brink H.J.M., van Gorcom R.F.M., van den Hondel C.A.M.J.J., Punt P.J. Cytochrome P450 enzyme systems in fungi. Fungal Genet. Biol. 1998;23:1–17. doi: 10.1006/fgbi.1997.1021. [DOI] [PubMed] [Google Scholar]
- 16.George H.L., Hirschi K.D., VanEtten H.D. Biochemical properties of the products of cytochrome P450 genes (PDA) encoding pisatin demethylase activity in Nectria haematococca. Arch. Microbiol. 1998;170:147–154. doi: 10.1007/s002030050627. [DOI] [PubMed] [Google Scholar]
- 17.Cerniglia C.E., Hebert R.L., Szaniszlo P.J., Gibson D.T. Fungal transformation of naphthalene. Arch. Microbiol. 1978;117:135–143. doi: 10.1007/BF00402301. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland J.B. Detoxification of polycyclic aromatic hydrocarbons by fungi. J. Ind. Microbiol. 1992;9:53–61. doi: 10.1007/BF01576368. [DOI] [PubMed] [Google Scholar]
- 19.Bezalel L., Hadar Y., Cerniglia C.E. Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 1997;63:2495–2501. doi: 10.1128/aem.63.7.2495-2501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin J.Y., Bui D.-C., Lee Y., Nam H., Jung S., Fang M., Kim J.-C., Lee T., Kim H., Choi G.J., et al. Functional characterization of cytochrome P450 monooxygenases in the cereal head blight fungus Fusarium graminearum. Environ. Microbiol. 2017;19:2053–2067. doi: 10.1111/1462-2920.13730. [DOI] [PubMed] [Google Scholar]
- 21.Fan J., Urban M., Parker J.E., Brewer H.C., Kelly S.L., Hammond-Kosack K.E., Fraaije B.A., Liu X., Cools H.J. Characterization of the sterol 14α-demethylases of Fusarium graminearum identifies a novel genus-specific CYP51 function. New Phytol. 2013;198:821–835. doi: 10.1111/nph.12193. [DOI] [PubMed] [Google Scholar]
- 22.Skaggs B., Alexander J., Pierson C., Schweitzer K., Chun K., Koegel C., Barbuch R., Bard M. Cloning and characterization of the Saccharomyces cerevisiae C-22 sterol desaturase gene, encoding a second cytochrome P-450 involved in ergosterol biosynthesis. Gene. 1996;169:105–109. doi: 10.1016/0378-1119(95)00770-9. [DOI] [PubMed] [Google Scholar]
- 23.Yun Y., Yin D., Dawood D.H., Liu X., Chen Y., Ma Z. Functional characterization of FgERG3 and FgERG5 associated with ergosterol biosynthesis, vegetative differentiation and virulence of Fusarium graminearum. Fungal Genet. Biol. 2014;68:60–70. doi: 10.1016/j.fgb.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Newsome A.W., Nelson D., Corran A., Kelly S.L., Kelly D.E. The cytochrome P450 complement (CYPome) of Mycosphaerella graminicola. Biotechnol. Appl. Biochem. 2013;60:52–64. doi: 10.1002/bab.1062. [DOI] [PubMed] [Google Scholar]
- 25.Lamb D.C., Skaug T., Song H.-L., Jackson C.J., Podust L.M., Waterman M.R., Kell D.B., Kelly D.E., Kelly S.L. The cytochrome P450 complement (CYPome) of Streptomyces coelicolor A3(2) J. Biol. Chem. 2002;277:24000–24005. doi: 10.1074/jbc.M111109200. [DOI] [PubMed] [Google Scholar]
- 26.Lamb D.C., Ikeda H., Nelson D.R., Ishikawa J., Skaug T., Jackson C., Omura S., Waterman M.R., Kelly S.L. Cytochrome P450 complement (CYPome) of the avermectin-producer Streptomyces avermitilis and comparison to that of Streptomyces coelicolor A3(2) Biochem. Biophys. Res. Commun. 2003;307:610–619. doi: 10.1016/S0006-291X(03)01231-2. [DOI] [PubMed] [Google Scholar]
- 27.Kelly D.E., Kraševec N., Mullins J., Nelson D.R. The CYPome (cytochrome P450 complement) of Aspergillus nidulans. Fungal Genet. Biol. 2009;46:S53–S61. doi: 10.1016/j.fgb.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Dejong C.A., Wilson J.Y. The cytochrome P450 superfamily Complement (CYPome) in the annelid Capitella teleta. PLoS ONE. 2014;9:e107728. doi: 10.1371/journal.pone.0107728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moktali V., Park J., Fedorova-Abrams N.D., Park B., Choi J., Lee Y.-H., Kang S. Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes. BMC Genom. 2012;13:525. doi: 10.1186/1471-2164-13-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirosue S., Tazaki M., Hiratsuka N., Yanai S., Kabumoto H., Shinkyo R., Arisawa A., Sakaki T., Tsunekawa H., Johdo O., et al. Insight into functional diversity of cytochrome P450 in the white-rot basidiomycete Phanerochaete chrysosporium: Involvement of versatile monooxygenase. Biochem. Biophys. Res. Commun. 2011;407:118–123. doi: 10.1016/j.bbrc.2011.02.121. [DOI] [PubMed] [Google Scholar]
- 31.Olicón-Hernández D.R., González-López J., Aranda E. Overview on the biochemical potential of filamentous fungi to degrade pharmaceutical compounds. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warrilow A., Ugochukwu C., Lamb D., Kelly D., Kelly S. Expression and characterization of CYP51, the ancient sterol 14-demethylase activity for cytochromes P450 (CYP), in the white-rot fungus Phanerochaete chrysosporium. Lipids. 2008;43:1143–1153. doi: 10.1007/s11745-008-3239-5. [DOI] [PubMed] [Google Scholar]
- 33.Aoyama Y., Yoshida Y., Sonoda Y., Sato Y. Deformylation of 32-oxo-24,25-dihydrolanosterol by the purified cytochrome P-45014DM (lanosterol 14α-demethylase) from yeast evidence confirming the intermediate step of lanosterol 14a-demethylation. J. Biol. Chem. 1989;264:18502–18505. [PubMed] [Google Scholar]
- 34.van Nistelrooy J.G.M., van den Brink J.M., van Kan J.A.L., van Gorcom R.F.M., de Waard M.A. Isolation and molecular characterisation of the gene encoding eburicol 14α-demethylase (CYP51) from Penicillium italicum. Mol. Gen. Genet. 1996;250:725–733. doi: 10.1007/BF02172984. [DOI] [PubMed] [Google Scholar]
- 35.Kalb V., Loper J., Dey C., Woods C., Sutter T. Isolation of a cytochrome P-450 structural gene from Saccharomyces cerevisiae. Gene. 1986;45:237–245. doi: 10.1016/0378-1119(86)90021-1. [DOI] [PubMed] [Google Scholar]
- 36.Kalb V.F., Woods C.W., Turi T.G., Dey C.R., Sutter T.R., Loper J.C. Primary structure of the P450 lanosterol demethylase gene from Saccharomyces cerevisiae. DNA. 1987;6:529–537. doi: 10.1089/dna.1987.6.529. [DOI] [PubMed] [Google Scholar]
- 37.Lai M., Kirsch D. Nucleotide sequence of cytochrome P450 L1A1 (lanosterol 14α-demethylase) from Candida albicans. Nucleic Acids Res. 1989;17:804. doi: 10.1093/nar/17.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morace G., Sanguinetti M., Posteraro B., Cascio G.L., Fadda G. Identification of various medically important Candida species in clinical specimens by PCR-restriction enzyme analysis. J. Clin. Microbiol. 1997;35:667–672. doi: 10.1128/jcm.35.3.667-672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgener-Kairuz P., Zuber J., Jaunin P., Buchman T., Bille J., Rossier M. Rapid detection and identification of Candida albicans and Torulopsis (Candida) glabrata in clinical specimens by species-specific nested PCR amplification of a cytochrome P-450 lanosterol-α-demethylase (L1A1) gene fragment. J. Clin. Microbiol. 1994;32:1902–1907. doi: 10.1128/jcm.32.8.1902-1907.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoyama Y., Yoshida Y., Sato R. Yeast cytochrome P-450 catalyzing lanosterol 14α-demethylation. J. Biol. Chem. 1984;259:1661–1666. [PubMed] [Google Scholar]
- 41.Sheehan D.J., Hitchcock C.A., Sibley C.M. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C., Turi T.G., Sanglard D., Loper J.C. Isolation of the Candida tropicalis gene for P450 lanosterol demethylase and its expression in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1987;146:1311–1317. doi: 10.1016/0006-291X(87)90792-3. [DOI] [PubMed] [Google Scholar]
- 43.Ohkuma M., Masuda Y., Mee Park S., Ohtomo R., Ohta A., Takagi M. Evidence that the expression of the gene for NADPH-cytochrome P-450 reductase is n-alkane-inducible in Candida maltosa. Biosci. Biotechnol. Biochem. 1995;59:1328–1330. doi: 10.1271/bbb.59.1328. [DOI] [PubMed] [Google Scholar]
- 44.Schunck W., Vogel F., Gross B., Kärgel E., Mauersberger S., Köpke K., Gengnagel C., Müller H. Comparison of two cytochromes P-450 from Candida maltosa: Primary structures, substrate specificities and effects of their expression in Saccharomyces cerevisiae on the proliferation of the endoplasmic reticulum. Eur. J. Cell Biol. 1991;55:336–345. [PubMed] [Google Scholar]
- 45.Seghezzi W., Sanglard D., Fiechter A. Characterization of a second alkane-inducible cytochrome P450-encoding gene, CYP52A2, from Candida tropicalis. Gene. 1991;106:51–60. doi: 10.1016/0378-1119(91)90565-S. [DOI] [PubMed] [Google Scholar]
- 46.Seghezzi W., Meili C., Ruffiner R., Kuenzi R., Sanglard D., Fiechter A. Identification and characterization of additional members of the cytochrome P450 multigene family CYP52 of Candida tropicalis. DNA Cell Biol. 1992;11:767–780. doi: 10.1089/dna.1992.11.767. [DOI] [PubMed] [Google Scholar]
- 47.Lottermoser K., Schunck W.H., Asperger O. Cytochromes P450 of the sophorose lipid-producing yeast Candida apicola: Heterogeneity and polymerase chain reaction-mediated cloning of two genes. Yeast. 1996;12:565–575. doi: 10.1002/(SICI)1097-0061(199605)12:6<565::AID-YEA951>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Eschenfeldt W.H., Zhang Y., Samaha H., Stols L., Eirich L.D., Wilson C.R., Donnelly M.I. Transformation of fatty acids catalyzed by cytochrome P450 monooxygenase enzymes of Candida tropicalis. Appl. Environ. Microbiol. 2003;69:5992–5999. doi: 10.1128/AEM.69.10.5992-5999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheller U., Zimmer T., Kärgel E., Schunck W.-H. Characterization of the n-alkane and fatty acid hydroxylating cytochrome P450 forms 52A3 and 52A4. Arch. Biochem. Biophys. 1996;328:245–254. doi: 10.1006/abbi.1996.0170. [DOI] [PubMed] [Google Scholar]
- 50.Iida T., Sumita T., Ohta A., Takagi M. The cytochrome P450ALK multigene family of an n-alkane-assimilating yeast, Yarrowia lipolytica: Cloning and characterization of genes coding for new CYP52 family members. Yeast. 2000;16:1077–1087. doi: 10.1002/1097-0061(20000915)16:12<1077::AID-YEA601>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 51.Faber B.W., van Gorcom R.F., Duine J.A. Purification and characterization of benzoate-para-hydroxylase, a cytochrome P450 (CYP53A1), from Aspergillus niger. Arch. Biochem. Biophys. 2001;394:245–254. doi: 10.1006/abbi.2001.2534. [DOI] [PubMed] [Google Scholar]
- 52.Fraser J.A., Davis M.A., Hynes M.J. The genes gmdA, encoding an amidase, and bzuA, encoding a cytochrome P450, are required for benzamide utilization in Aspergillus nidulans. Fungal Genet. Biol. 2002;35:135–146. doi: 10.1006/fgbi.2001.1307. [DOI] [PubMed] [Google Scholar]
- 53.Fukuda H., Fujii T., Sukita E., Tazaki M., Nagahama S., Ogawa T. Reconstitution of the isobutene-forming reaction catalyzed by cytochrome P450 and P450 reductase from Rhodotorula minuta: Decarboxylation with the formation of isobutene. Biochem. Biophys. Res. Commun. 1994;201:516–522. doi: 10.1006/bbrc.1994.1732. [DOI] [PubMed] [Google Scholar]
- 54.van Gorcom R.F., Boschloo J.G., Kuijvenhoven A., Lange J., van Vark A.J., Bos C.J., van Balken J.A., Pouwels P.H., van den Hondel C.A. Isolation and molecular characterisation of the benzoate-para-hydroxylase gene (bphA) of Aspergillus niger: A member of a new gene family of the cytochrome P450 superfamily. Mol. Gen. Genet. 1990;223:192–197. doi: 10.1007/BF00265053. [DOI] [PubMed] [Google Scholar]
- 55.Podobnik B., Stojan J., Lah L., Krasevec N., Seliskar M., Rizner T.L., Rozman D., Komel R. CYP53A15 of Cochliobolus lunatus, a target for natural antifungal compounds. J. Med. Chem. 2008;51:3480–3486. doi: 10.1021/jm800030e. [DOI] [PubMed] [Google Scholar]
- 56.Matsuzaki F., Wariishi H. Molecular characterization of cytochrome P450 catalyzing hydroxylation of benzoates from the white-rot fungus Phanerochaete chrysosporium. Biochem. Biophys. Res. Commun. 2005;334:1184–1190. doi: 10.1016/j.bbrc.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Attar R., Grotewold E., Taccioli G., Aisemberg G., Torres H., Judewicz N. A cycloheximide-inducible gene of Neurospora crassa belongs to the cytochrome P-450 superfamily. Nucleic Acids Res. 1989;17:7535–7536. doi: 10.1093/nar/17.18.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kizawa H., Tomura D., Oda M., Fukamizu A., Hoshino T., Gotoh O., Yasui T., Shoun H. Nucleotide sequence of the unique nitrate/nitrite-inducible cytochrome P-450 cDNA from Fusarium oxysporum. J. Biol. Chem. 1991;266:10632–10637. [PubMed] [Google Scholar]
- 59.Kudo T., Tomura D., Liu D., Dai X., Shoun H. Two isozymes of P450nor of Cylindrocarpon tonkinense: Molecular cloning of the cDNAs and genes, expressions in the yeast, and the putative NAD(P)H-binding site. Biochimie. 1996;78:792–799. doi: 10.1016/S0300-9084(97)82538-2. [DOI] [PubMed] [Google Scholar]
- 60.Usuda K., Toritsuka N., Matsuo Y., Kim D.-H., Shoun H. Denitrification by the fungus Cylindrocarpon tonkinense: Anaerobic cell growth and two isozyme forms of cytochrome P-450nor. Appl. Environ. Microbiol. 1995;61:883–889. doi: 10.1128/aem.61.3.883-889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaya M., Matsumura K., Higashida K., Hata Y., Kawato A., Abe Y., Akita O., Takaya N., Shoun H. Cloning and enhanced expression of the cytochrome P450nor gene (nicA; CYP55A5) encoding nitric oxide reductase from Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2004;68:2040–2049. doi: 10.1271/bbb.68.2040. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L., Takaya N., Kitazume T., Kondo T., Shoun H. Purification and cDNA cloning of nitric oxide reductase cytochrome P450nor (CYP55A4) from Trichosporon cutaneum. Eur. J. Biochem. 2001;268:3198–3204. doi: 10.1046/j.1432-1327.2001.02206.x. [DOI] [PubMed] [Google Scholar]
- 63.Shiro Y., Fujii M., Iizuka T., Adachi S.-I., Tsukamoto K., Nakahara K., Shoun H. Spectroscopic and kinetic studies on reaction of cytochrome P450nor with nitric oxide implication for its nitric oxide reduction mechanism. J. Biol. Chem. 1995;270:1617–1623. doi: 10.1074/jbc.270.4.1617. [DOI] [PubMed] [Google Scholar]
- 64.Briza P., Breitenbach M., Ellinger A., Segall J. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev. 1990;4:1775–1789. doi: 10.1101/gad.4.10.1775. [DOI] [PubMed] [Google Scholar]
- 65.Briza P., Eckerstorfer M., Breitenbach M. The sporulation-specific enzymes encoded by the DIT1 and DIT2 genes catalyze a two-step reaction leading to a soluble LL-dityrosine-containing precursor of the yeast spore wall. Proc. Natl. Acad. Sci. USA. 1994;91:4524–4528. doi: 10.1073/pnas.91.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melo N., Moran G., Warrilow A., Dudley E., Smith S., Sullivan D., Lamb D., Kelly D., Coleman D., Kelly S. CYP56 (Dit2p) in Candida albicans: Characterization and investigation of its role in growth and antifungal drug susceptibility. Antimicrob. Agents Chemother. 2008;52:3718–3724. doi: 10.1128/AAC.00446-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weltring K.-M., Turgeon B.G., Yoder O., VanEtten H.D. Isolation of a phytoalexin-detoxification gene from the plant pathogenic fungus Nectria haematococca by detecting its expression in Aspergillus nidulans. Gene. 1988;68:335–344. doi: 10.1016/0378-1119(88)90036-4. [DOI] [PubMed] [Google Scholar]
- 68.Maloney A.P., VanEtten H.D. A gene from the fungal plant pathogen Nectria haematococca that encodes the phytoalexin-detoxifying enzyme pisatin demethylase defines a new cytochrome P450 family. Mol. Gen. Genet. 1994;243:506–514. doi: 10.1007/BF00284198. [DOI] [PubMed] [Google Scholar]
- 69.Funnell D.L., VanEtten H.D. Pisatin demethylase genes are on dispensable chromosomes while genes for pathogenicity on carrot and ripe tomato are on other chromosomes in Nectria haematococca. Mol. Plant Microbe Interact. 2002;15:840–846. doi: 10.1094/MPMI.2002.15.8.840. [DOI] [PubMed] [Google Scholar]
- 70.Hohn T.M., Desjardins A.E., McCormick S.P. The Tri4 gene of Fusarium sporotrichioides encodes a cytochrome P450 monooxygenase involved in trichothecene biosynthesis. Mol. Gen. Genet. 1995;248:95–102. doi: 10.1007/BF02456618. [DOI] [PubMed] [Google Scholar]
- 71.Kimura M., Tokai T., Takahashi-Ando N., Ohsato S., Fujimura M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007;71:2105–2123. doi: 10.1271/bbb.70183. [DOI] [PubMed] [Google Scholar]
- 72.Ehrlich K.C., Chang P.-K., Yu J., Cotty P.J. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 2004;70:6518–6524. doi: 10.1128/AEM.70.11.6518-6524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhatnagar D., Ehrlich K., Cleveland T. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2003;61:83–93. doi: 10.1007/s00253-002-1199-x. [DOI] [PubMed] [Google Scholar]
- 74.Wen Y., Hatabayashi H., Arai H., Kitamoto H.K., Yabe K. Function of the cypX and moxY genes in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 2005;71:3192–3198. doi: 10.1128/AEM.71.6.3192-3198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keller N.P., Kantz N.J., Adams T.H. Aspergillus nidulans verA is required for production of the mycotoxin sterigmatocystin. Appl. Environ. Microbiol. 1994;60:1444–1450. doi: 10.1128/aem.60.5.1444-1450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown D., Yu J., Kelkar H., Fernandes M., Nesbitt T., Keller N., Adams T., Leonard T. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA. 1996;93:1418–1422. doi: 10.1073/pnas.93.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu J., Chang P.-K., Cary J.W., Wright M., Bhatnagar D., Cleveland T.E., Payne G.A., Linz J.E. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus. Appl. Environ. Microbiol. 1995;61:2365–2371. doi: 10.1128/aem.61.6.2365-2371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamb D.C., Maspahy S., Kelly D.E., Manning N.J., Geber A., Bennett J.E., Kelly S.L. Purification, reconstitution, and inhibition of cytochrome P-450 sterol Δ22-desaturase from the pathogenic fungus Candida glabrata. Antimicrob. Agents Chemother. 1999;43:1725–1728. doi: 10.1128/aac.43.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doddapaneni H., Subramanian V., Yadav J.S. Physiological regulation, xenobiotic induction, and heterologous expression of P450 monooxygenase Gene pc-3 (CYP63A3), a new member of the CYP63 gene cluster in the white-rot fungus Phanerochaete chrysosporium. Curr. Microbiol. 2005;50:292–298. doi: 10.1007/s00284-005-4480-2. [DOI] [PubMed] [Google Scholar]
- 80.Prieto R., Woloshuk C. ord1, an oxidoreductase gene responsible for conversion of O-methylsterigmatocystin to aflatoxin in Aspergillus flavus. Appl. Environ. Microbiol. 1997;63:1661–1666. doi: 10.1128/aem.63.5.1661-1666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Groot P.W., Schaap P.J., Van Griensven L.J., Visser J. Isolation of developmentally regulated genes from the edible mushroom Agaricus bisporus. Microbiology. 1997;143:1993–2001. doi: 10.1099/00221287-143-6-1993. [DOI] [PubMed] [Google Scholar]
- 82.Rojas M.C., Hedden P., Gaskin P., Tudzynski B. The P450–1 gene of Gibberella fujikuroi encodes a multifunctional enzyme in gibberellin biosynthesis. Proc. Natl. Acad. Sci. USA. 2001;98:5838–5843. doi: 10.1073/pnas.091096298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tudzynski B., Rojas M.A.C., Gaskin P., Hedden P. The gibberellin 20-oxidase of Gibberella fujikuroi is a multifunctional monooxygenase. J. Biol. Chem. 2002;277:21246–21253. doi: 10.1074/jbc.M201651200. [DOI] [PubMed] [Google Scholar]
- 84.Mingot J.M., Peñalva M.A., Fernández-Cañón J.M. Disruption of phacA, an Aspergillus nidulans gene encoding a novel cytochrome P450 monooxygenase catalyzing phenylacetate 2-hydroxylation, results in penicillin overproduction. J. Biol. Chem. 1999;274:14545–14550. doi: 10.1074/jbc.274.21.14545. [DOI] [PubMed] [Google Scholar]
- 85.Ferrer-Sevillano F., Fernández-Cañón J.M. Novel phacB-encoded cytochrome P450 monooxygenase from Aspergillus nidulans with 3-hydroxyphenylacetate 6-hydroxylase and 3,4-dihydroxyphenylacetate 6-hydroxylase activities. Eukaryot. Cell. 2007;6:514–520. doi: 10.1128/EC.00226-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakayama N., Takemae A., Shoun H. Cytochrome P450foxy, a catalytically self-sufficient fatty acid hydroxylase of the fungus Fusarium oxysporum. J. Biochem. 1996;119:435–440. doi: 10.1093/oxfordjournals.jbchem.a021260. [DOI] [PubMed] [Google Scholar]
- 87.Kitazume T., Tanaka A., Takaya N., Nakamura A., Matsuyama S., Suzuki T., Shoun H. Kinetic analysis of hydroxylation of saturated fatty acids by recombinant P450foxy produced by an Escherichia coli expression system. Eur. J. Biochem. 2002;269:2075–2082. doi: 10.1046/j.1432-1033.2002.02855.x. [DOI] [PubMed] [Google Scholar]
- 88.Butchko R.A., Plattner R.D., Proctor R.H. Deletion analysis of FUM genes involved in tricarballylic ester formation during fumonisin biosynthesis. J. Agric. Food Chem. 2006;54:9398–9404. doi: 10.1021/jf0617869. [DOI] [PubMed] [Google Scholar]
- 89.Bojja R.S., Cerny R.L., Proctor R.H., Du L. Determining the biosynthetic sequence in the early steps of the fumonisin pathway by use of three gene-disruption mutants of Fusarium verticillioides. J. Agric. Food Chem. 2004;52:2855–2860. doi: 10.1021/jf035429z. [DOI] [PubMed] [Google Scholar]
- 90.The UniProt Consortium UniProt: The universal protein knowledgebase. Nucl. Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao D., Du L., Yang J., Wu W.-M., Liang H. A critical review of the application of white rot fungus to environmental pollution control. Crit. Rev. Biotechnol. 2010;30:70–77. doi: 10.3109/07388550903427272. [DOI] [PubMed] [Google Scholar]
- 92.Fu Y., Viraraghavan T. Fungal decolorization of dye wastewaters: A review. Bioresour. Technol. 2001;79:251–262. doi: 10.1016/S0960-8524(01)00028-1. [DOI] [PubMed] [Google Scholar]
- 93.Evelin H., Kapoor R., Giri B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009;104:1263–1280. doi: 10.1093/aob/mcp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Casadevall A. Determinants of virulence in the pathogenic fungi. Fungal Biol. Rev. 2007;21:130–132. doi: 10.1016/j.fbr.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desjardins A.E., Proctor R.H. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 2007;119:47–50. doi: 10.1016/j.ijfoodmicro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 96.Moss M.O. Mycotoxin review—1. Aspergillus and Penicillium. Mycologist. 2002;16:116–119. [Google Scholar]
- 97.Maragos C., Busman M. Rapid and advanced tools for mycotoxin analysis: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010;27:688–700. doi: 10.1080/19440040903515934. [DOI] [PubMed] [Google Scholar]
- 98.Hannemann F., Bichet A., Ewen K.M., Bernhardt R. Cytochrome P450 systems-biological variations of electron transport chains. Biochim. Biophys. Acta. 2007;1770:330–344. doi: 10.1016/j.bbagen.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 99.Yu J., Chang P.-K., Ehrlich K.C., Cary J.W., Bhatnagar D., Cleveland T.E., Payne G.A., Linz J.E., Woloshuk C.P., Bennett J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004;70:1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takaoka S., Kurata M., Harimoto Y., Hatta R., Yamamoto M., Akimitsu K., Tsuge T. Complex regulation of secondary metabolism controlling pathogenicity in the phytopathogenic fungus Alternaria alternata. New Phytol. 2014;202:1297–1309. doi: 10.1111/nph.12754. [DOI] [PubMed] [Google Scholar]
- 101.Ruswandi S., Kitani K., Akimitsu K., Tsuge T., Shiraishi T., Yamamoto M. Structural analysis of cosmid clone pcAFT-2 carrying AFT10-1 encoding an acyl-CoA dehydrogenase involved in AF-toxin production in the strawberry pathotype of Alternaria alternata. J. Gen. Plant Pathol. 2005;71:107–116. doi: 10.1007/s10327-004-0170-3. [DOI] [Google Scholar]
- 102.Deighton N., Muckenschnabel I., Colmenares A.J., Collado I.G., Williamson B. Botrydial is produced in plant tissues infected by Botrytis cinerea. Phytochemistry. 2001;57:689–692. doi: 10.1016/S0031-9422(01)00088-7. [DOI] [PubMed] [Google Scholar]
- 103.Siewers V., Viaud M., Jimenez-Teja D., Collado I.G., Gronover C.S., Pradier J.-M., Tudzynsk B., Tudzynski P. Functional analysis of the cytochrome P450 monooxygenase gene bcbot1 of Botrytis cinerea indicates that botrydial is a strain-specific virulence factor. Mol. Plant Microbe Interact. 2005;18:602–612. doi: 10.1094/MPMI-18-0602. [DOI] [PubMed] [Google Scholar]
- 104.Collado I.G., Sánchez A.J.M., Hanson J.R. Fungal terpene metabolites: Biosynthetic relationships and the control of the phytopathogenic fungus Botrytis cinerea. Nat. Prod. Rep. 2007;24:674–686. doi: 10.1039/b603085h. [DOI] [PubMed] [Google Scholar]
- 105.Wight W.D., Kim K.-H., Lawrence C.B., Walton J.D. Biosynthesis and role in virulence of the histone deacetylase inhibitor depudecin from Alternaria brassicicola. Mol. Plant Microbe Interact. 2009;22:1258–1267. doi: 10.1094/MPMI-22-10-1258. [DOI] [PubMed] [Google Scholar]
- 106.Chettri P., Ehrlich K.C., Cary J.W., Collemare J., Cox M.P., Griffiths S.A., Olson M.A., de Wit P.J., Bradshaw R.E. Dothistromin genes at multiple separate loci are regulated by AflR. Fungal Genet. Biol. 2013;51:12–20. doi: 10.1016/j.fgb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 107.Wallwey C., Li S.-M. Ergot alkaloids: Structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes. Nat. Prod. Rep. 2011;28:496–510. doi: 10.1039/C0NP00060D. [DOI] [PubMed] [Google Scholar]
- 108.Coyle C.M., Panaccione D.G. An ergot alkaloid biosynthesis gene and clustered hypothetical genes from Aspergillus fumigatus. Appl. Environ. Microbiol. 2005;71:3112–3118. doi: 10.1128/AEM.71.6.3112-3118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seo J.-A., Proctor R.H., Plattner R.D. Characterization of four clustered and coregulated genes associated with fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet. Biol. 2001;34:155–165. doi: 10.1006/fgbi.2001.1299. [DOI] [PubMed] [Google Scholar]
- 110.Cheng Y.-Q., Ahn J.-H., Walton J.D. A putative branched-chain-amino-acid transaminase gene required for HC-toxin biosynthesis and pathogenicity in Cochliobolus carbonum. Microbiology. 1999;145:3539–3546. doi: 10.1099/00221287-145-12-3539. [DOI] [PubMed] [Google Scholar]
- 111.Walton J.D. HC-toxin. Phytochemistry. 2006;67:1406–1413. doi: 10.1016/j.phytochem.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 112.Paterson R.R.M., Lima N. Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi. CRC Press; Boca Raton, FL, USA: 2015. [Google Scholar]
- 113.McMillan L., Carr R., Young C., Astin J., Lowe R., Parker E., Jameson G., Finch S., Miles C., McManus O. Molecular analysis of two cytochrome P450 monooxygenase genes required for paxilline biosynthesis in Penicillium paxilli, and effects of paxilline intermediates on mammalian maxi-K ion channels. Mol. Genet. Genom. 2003;270:9–23. doi: 10.1007/s00438-003-0887-2. [DOI] [PubMed] [Google Scholar]
- 114.Saikia S., Parker E.J., Koulman A., Scott B. Defining paxilline biosynthesis in Penicillium paxilli. J. Biol. Chem. 2007;282:16829–16837. doi: 10.1074/jbc.M701626200. [DOI] [PubMed] [Google Scholar]
- 115.Hidalgo P.I., Ullán R.V., Albillos S.M., Montero O., Fernández-Bodega M.Á., García-Estrada C., Fernández-Aguado M., Martín J.-F. Molecular characterization of the PR-toxin gene cluster in Penicillium roqueforti and Penicillium chrysogenum: Cross talk of secondary metabolite pathways. Fungal Genet. Biol. 2014;62:11–24. doi: 10.1016/j.fgb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Alexander N.J., Hohn T.M., McCormick S.P. The TRI11 gene of Fusarium sporotrichioides encodes a cytochrome P-450 monooxygenase required for C-15 hydroxylation in trichothecene biosynthesis. Appl. Environ. Microbiol. 1998;64:221–225. doi: 10.1128/aem.64.1.221-225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim S., Thiessen P.A., Bolton E.E., Chen J., Fu G., Gindulyte A., Han L., He J., He S., Shoemaker B.A. PubChem substance and compound databases. Nucleic Acids Res. 2015;44:D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown M., Brown-Jenco C., Payne G. Genetic and molecular analysis of aflatoxin biosynthesis. Fungal Genet. Biol. 1999;26:81–98. doi: 10.1006/fgbi.1998.1114. [DOI] [PubMed] [Google Scholar]
- 119.Fratamico P.M., Bhunia A.K., Smith J.L. Foodborne Pathogens: Microbiology and Molecular Biology. Horizon Scientific Press; Poole, UK: 2005. [Google Scholar]
- 120.Bennett J. Mycotoxins, mycotoxicoses, mycotoxicology and mycopathologia. Mycopathologia. 1987;100:3–5. doi: 10.1007/BF00769561. [DOI] [PubMed] [Google Scholar]
- 121.Yu J., Chang P.-K., Cary J.W., Bhatnagar D., Cleveland T.E. avnA, a gene encoding a cytochrome P-450 monooxygenase, is involved in the conversion of averantin to averufin in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 1997;63:1349–1356. doi: 10.1128/aem.63.4.1349-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yabe K., Ando Y., Hamasaki T. Biosynthetic relationship among aflatoxins B1, B2, G1, and G2. Appl. Environ. Microbiol. 1988;54:2101–2106. doi: 10.1128/aem.54.8.2101-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu J., Chang P.-K., Ehrlich K.C., Cary J.W., Montalbano B., Dyer J.M., Bhatnagar D., Cleveland T.E. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P-450 monooxygenase encoded by ordA that is involved in the biosynthesis of aflatoxins B1, G1, B2, and G2. Appl. Environ. Microbiol. 1998;64:4834–4841. doi: 10.1128/aem.64.12.4834-4841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Proctor R.H., Desjardins A.E., Plattner R.D., Hohn T.M. A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi mating population A. Fungal Genet. Biol. 1999;27:100–112. doi: 10.1006/fgbi.1999.1141. [DOI] [PubMed] [Google Scholar]
- 125.Howard P.C., Eppley R.M., Stack M.E., Warbritton A., Voss K.A., Lorentzen R.J., Kovach R., Bucci T.J. Carcinogenicity of fumonisin B1 in a two-year bioassay with Fischer 344 rats and B6C3F1 mice. JSM Mycotoxins. 2000;1999:45–54. doi: 10.2520/myco1975.1999.Suppl2_45. [DOI] [Google Scholar]
- 126.Caldas E.D., Sadilkova K., Ward B.L., Jones A.D., Winter C.K., Gilchrist D.G. Biosynthetic studies of fumonisin B1 and AAL toxins. J. Agric. Food Chem. 1998;46:4734–4743. doi: 10.1021/jf980210j. [DOI] [Google Scholar]
- 127.Walton J.D. Host-selective toxins: Agents of compatibility. Plant Cell. 1996;8:1723–1733. doi: 10.1105/tpc.8.10.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Friesen T.L., Faris J.D., Solomon P.S., Oliver R.P. Host-specific toxins: Effectors of necrotrophic pathogenicity. Cell. Microbiol. 2008;10:1421–1428. doi: 10.1111/j.1462-5822.2008.01153.x. [DOI] [PubMed] [Google Scholar]
- 129.Akimitsu K., Tsuge T., Kodama M., Yamamoto M., Otani H. Alternaria host-selective toxins: Determinant factors of plant disease. J. Gen. Plant Pathol. 2014;80:109–122. doi: 10.1007/s10327-013-0498-7. [DOI] [Google Scholar]
- 130.Brosch G., Ransom R., Lechner T., Walton J.D., Loidl P. Inhibition of maize histone deacetylases by HC toxin, the host-selective toxin of Cochliobolus carbonum. Plant Cell. 1995;7:1941–1950. doi: 10.1105/tpc.7.11.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Youngman R.J., Elstner E. Photodynamic and reductive mechanisms of oxygen activation by the fungal phytotoxins, cercosporin and dothistromin. In: Bors W., Saran M., Tait D., editors. Oxygen Radicals in Chemistry and Biology, Proceedings/Third International Conference, Neuherberg, Germany, 10–15 July 1983. W. de Gruyter; Berlin, Germany: 1984. [Google Scholar]
- 132.Bradshaw R. Dothistroma (red-band) needle blight of pines and the dothistromin toxin: A review. For. Pathol. 2004;34:163–185. doi: 10.1111/j.1439-0329.2004.00356.x. [DOI] [Google Scholar]
- 133.Kabir M., Ganley R., Bradshaw R. Dothistromin toxin is a virulence factor in dothistroma needle blight of pines. Plant Pathol. 2015;64:225–234. doi: 10.1111/ppa.12229. [DOI] [Google Scholar]
- 134.Govrin E.M., Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000;10:751–757. doi: 10.1016/S0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 135.Collado I., Aleu J., Hernández-Galán R., Durán-Patrón R. Botrytis species an intriguing source of metabolites with a wide range of biological activities. Structure, chemistry and bioactivity of metabolites isolated from Botrytis species. Curr. Org. Chem. 2000;4:1261–1286. doi: 10.2174/1385272003375815. [DOI] [Google Scholar]
- 136.Varga J., Rigó K., Téren J., Mesterházy Á. Recent advances in ochratoxin research I. Production, detection and occurrence of ochratoxins. Cereal Res. Commun. 2001;29:85–92. [Google Scholar]
- 137.International Agency for Research on Cancer (IARC) IARC Monographs on the evaluation of carcinogenic risks to humans: Some naturally. IARC Monogr. Eval. Carcinog. Risks Hum. 1993;56:489–521. [Google Scholar]
- 138.Gil-Serna J., García-Díaz M., González-Jaén M.T., Vázquez C., Patiño B. Description of an orthologous cluster of ochratoxin A biosynthetic genes in Aspergillus and Penicillium species: A comparative analysis. Int. J. Food Microbiol. 2018;268:35–43. doi: 10.1016/j.ijfoodmicro.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 139.Liu X., Yu F., Schnabel G., Wu J., Wang Z., Ma Z. Paralogous cyp51 genes in Fusarium graminearum mediate differential sensitivity to sterol demethylation inhibitors. Fungal Genet. Biol. 2011;48:113–123. doi: 10.1016/j.fgb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 140.Anzenbacher P., Anzenbacherova E. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci. 2001;58:737–747. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Furge L.L., Guengerich F.P. Cytochrome P450 enzymes in drug metabolism and chemical toxicology: An introduction. Biochem. Mol. Biol. Educ. 2006;34:66–74. doi: 10.1002/bmb.2006.49403402066. [DOI] [PubMed] [Google Scholar]
- 142.Danielson P.B., MacIntyre R.J., Fogleman J.C. Molecular cloning of a family of xenobiotic-inducible drosophilid cytochrome P450s: Evidence for involvement in host-plant allelochemical resistance. Proc. Natl. Acad. Sci. USA. 1997;94:10797–10802. doi: 10.1073/pnas.94.20.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ding M., Gao Q., Mo J., Cheng J.A. Construction and validation of an insecticide resistance-associated DNA microarray. J. Pestic. Sci. 2007;32:32–41. doi: 10.1584/jpestics.G06-08. [DOI] [Google Scholar]
- 144.Syed K., Yadav J.S. P450 monooxygenases (P450ome) of the model white rot fungus Phanerochaete chrysosporium. Crit. Rev. Microbiol. 2012;38:339–363. doi: 10.3109/1040841X.2012.682050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ichinose H. Cytochrome P450 of wood-rotting basidiomycetes and biotechnological applications. Biotechnol. Appl. Biochem. 2013;60:71–81. doi: 10.1002/bab.1061. [DOI] [PubMed] [Google Scholar]
- 146.Matthews D.E., Van Etten H.D. Detoxification of the phytoalexin pisatin by a fungal cytochrome P-450. Arch. Biochem. Biophys. 1983;224:494–505. doi: 10.1016/0003-9861(83)90237-0. [DOI] [PubMed] [Google Scholar]
- 147.Coleman J.J., Wasmann C.C., Usami T., White G.J., Temporini E.D., McCluskey K., VanEtten H.D. Characterization of the gene encoding pisatin demethylase (FoPDA1) in Fusarium oxysporum. Mol. Plant Microbe Interact. 2011;24:1482–1491. doi: 10.1094/MPMI-05-11-0119. [DOI] [PubMed] [Google Scholar]
- 148.Parks L.W., Smith S.J., Crowley J.H. Biochemical and physiological effects of sterol alterations in yeast—A review. Lipids. 1995;30:227–230. doi: 10.1007/BF02537825. [DOI] [PubMed] [Google Scholar]
- 149.Lepesheva G.I., Waterman M.R. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta. 2007;1770:467–477. doi: 10.1016/j.bbagen.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mellado E., Garcia-Effron G., Buitrago M., Alcazar-Fuoli L., Cuenca-Estrella M., Rodriguez-Tudela J. Targeted gene disruption of the 14-α sterol demethylase (cyp51A) in Aspergillus fumigatus and its role in azole drug susceptibility. Antimicrob. Agents Chemother. 2005;49:2536–2538. doi: 10.1128/AAC.49.6.2536-2538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sanglard D., Ischer F., Koymans L., Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sheng C., Miao Z., Ji H., Yao J., Wang W., Che X., Dong G., Lü J., Guo W., Zhang W. Three-dimensional model of lanosterol 14α-demethylase from Cryptococcus neoformans: Active-site characterization and insights into azole binding. Antimicrob. Agents Chemother. 2009;53:3487–3495. doi: 10.1128/AAC.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Van Bogaert I.N.A., Groeneboer S., Saerens K., Soetaert W. The role of cytochrome P450 monooxygenases in microbial fatty acid metabolism. FEBS J. 2011;278:206–221. doi: 10.1111/j.1742-4658.2010.07949.x. [DOI] [PubMed] [Google Scholar]
- 154.Shoun H., Fushinobu S., Jiang L., Kim S.-W., Wakagi T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos. Trans. R. Soc. B. 2012;367:1186–1194. doi: 10.1098/rstb.2011.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang X.-W., Jia L.-J., Zhang Y., Jiang G., Li X., Zhang D., Tang W.-H. In planta stage-specific fungal gene profiling elucidates the molecular strategies of Fusarium graminearum growing inside wheat coleoptiles. Plant Cell. 2012;24:5159–5176. doi: 10.1105/tpc.112.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gao S., Li Y., Gao J., Suo Y., Fu K., Li Y., Chen J. Genome sequence and virulence variation-related transcriptome profiles of Curvularia lunata, an important maize pathogenic fungus. BMC Genom. 2014;15:627. doi: 10.1186/1471-2164-15-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karlsson M., Elfstrand M., Stenlid J., Olson Å. A fungal cytochrome P450 is expressed during the interaction between the fungal pathogen Heterobasidion annosum sensu lato and conifer trees. DNA Seq. 2008;19:115–120. doi: 10.1080/10425170701447473. [DOI] [PubMed] [Google Scholar]
- 158.Zhang D.-D., Wang X.-Y., Chen J.-Y., Kong Z.-Q., Gui Y.-J., Li N.-Y., Bao Y.-M., Dai X.-F. Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci. Rep. 2016;6:27979. doi: 10.1038/srep27979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Črešnar B., Petrič Š. Cytochrome P450 enzymes in the fungal kingdom. Biochim. Biophys. Acta. 2011;1814:29–35. doi: 10.1016/j.bbapap.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 160.Leslie J.F., Summerell B.A. The Fusarium Laboratory Manual. 1st ed. Blackwell Pub.; Ames, Iowa: 2006. [Google Scholar]
- 161.Son H., Seo Y.-S., Min K., Park A.R., Lee J., Jin J.-M., Lin Y., Cao P., Hong S.-Y., Kim E.-K., et al. A phenome-based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum. PLoS Pathog. 2011;7:e1002310. doi: 10.1371/journal.ppat.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brown D.W., McCormick S.P., Alexander N.J., Proctor R.H., Desjardins A.E. A genetic and biochemical approach to study trichothecene diversity in Fusarium sporotrichioides and Fusarium graminearum. Fungal Genet. Biol. 2001;32:121–133. doi: 10.1006/fgbi.2001.1256. [DOI] [PubMed] [Google Scholar]
- 163.Harris L.J., Alexander N.J., Saparno A., Blackwell B., McCormick S.P., Desjardins A.E., Robert L.S., Tinker N., Hattori J., Piché C., et al. A novel gene cluster in Fusarium graminearum contains a gene that contributes to butenolide synthesis. Fungal Genet. Biol. 2007;44:293–306. doi: 10.1016/j.fgb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 164.Bahadoor A., Schneiderman D., Gemmill L., Bosnich W., Blackwell B., Melanson J.E., McRae G., Harris L.J. Hydroxylation of longiborneol by a Clm2-encoded CYP450 monooxygenase to produce culmorin in Fusarium graminearum. J. Nat. Prod. 2016;79:81–88. doi: 10.1021/acs.jnatprod.5b00676. [DOI] [PubMed] [Google Scholar]
- 165.Grovey J. Progress in the Chemistry of Organic Natural Products. Springer; New York, NY, USA: 2007. The trichothecenes and their biosynthesis; pp. 63–130. [PubMed] [Google Scholar]
- 166.Ueno Y. Toxicological features of T-2 toxin and related trichothecenes. Fundam. Appl. Toxicol. 1984;4:S124–S132. doi: 10.1016/0272-0590(84)90144-1. [DOI] [PubMed] [Google Scholar]
- 167.Pestka J.J., Smolinski A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 168.Minervini F., Fornelli F., Flynn K. Toxicity and apoptosis induced by the mycotoxins nivalenol, deoxynivalenol and fumonisin B1 in a human erythroleukemia cell line. Toxicol. In Vitro. 2004;18:21–28. doi: 10.1016/S0887-2333(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 169.Brown D.W., McCormick S.P., Alexander N.J., Proctor R.H., Desjardins A.E. Inactivation of a cytochrome P-450 is a determinant of trichothecene diversity in Fusarium species. Fungal Genet. Biol. 2002;36:224–233. doi: 10.1016/S1087-1845(02)00021-X. [DOI] [PubMed] [Google Scholar]
- 170.Wuchiyama J., Kimura M., Yamaguchi I. A trichothecene efflux pump encoded by Tril02 in the biosynthetic gene cluster of Fusarium graminearum. J. Antibiot. 2000;53:196–200. doi: 10.7164/antibiotics.53.196. [DOI] [PubMed] [Google Scholar]
- 171.Tokai T., Koshino H., Kawasaki T., Igawa T., Suzuki Y., Sato M., Fujimura M., Eizuka T., Watanabe H., Kitahara T. Screening of putative oxygenase genes in the Fusarium graminearum genome sequence database for their role in trichothecene biosynthesis. FEMS Microbiol. Lett. 2005;251:193–201. doi: 10.1016/j.femsle.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 172.Wang C., Zhang S., Hou R., Zhao Z., Zheng Q., Xu Q., Zheng D., Wang G., Liu H., Gao X., et al. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathog. 2011;7:e1002460. doi: 10.1371/journal.ppat.1002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fischer G.J., Keller N.P. Production of cross-kingdom oxylipins by pathogenic fungi: An update on their role in development and pathogenicity. J. Microbiol. 2016;54:254–264. doi: 10.1007/s12275-016-5620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Paxton J. A new working definition of the term “phytoalexin”. Plant Dis. 1980;64:734. [Google Scholar]
- 176.Harris L.J., Balcerzak M., Johnston A., Schneiderman D., Ouellet T. Host-preferential Fusarium graminearum gene expression during infection of wheat, barley, and maize. Fungal Biol. 2016;120:111–123. doi: 10.1016/j.funbio.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 177.Maertens J. History of the development of azole derivatives. Clinic. Microbiol. Infect. 2004;10:1–10. doi: 10.1111/j.1470-9465.2004.00841.x. [DOI] [PubMed] [Google Scholar]