Abstract
Aspergillus carbonarius, belonging to the group Nigri, is the main species responsible for contamination by ochratoxin A (OTA) in grapes and derivative products. OTA can accumulate in the mycelium and in black conidia of the fungus and released into the matrix. Here, we have deleted in A. carbonarius the alb1 orthologue gene of A. fumigatus, involved in melanin biosynthesis. Three A. carbonarius Δalb1 mutants were characterized for morphologic traits and OTA production on different media and temperatures. Δalb1 mutants showed a fawn color of conidia associated with a significant reduction of the conidiogenesis and a statistically significant increase (p ≤ 0.01) of total OTA production as compared to the wild type (WT) strain. The alb1 gene somehow affected OTA partitioning since in Δalb1 mutants OTA amount was lower in conidia and was more abundantly secreted into the medium as compared to the WT. On grape berries the Δalb1 mutants and the WT caused lesions with similar sizes but OTA amount in berry tissues was higher for the mutants. These results demonstrate that A. carbonarius conidia pigmentation is largely dependent on polyketide biosynthesis. The gene is not directly involved in virulence and its deletion affects morphological features and OTA production in the fungus.
Keywords: melanin, alb1, OTA partitioning, gene disruption, conidiation, sclerotia
1. Introduction
Aspergillus carbonarius, belonging to the section Nigri (black Aspergilli), is the main species responsible for the contamination by ochratoxin A (OTA) in grapes and derivatives [1,2,3]. OTA is a mycotoxin with potent nephrotoxic and immunosuppressive effects and it has been classified as a possible human carcinogen (group 2B) [4].
A common feature of all Aspergillus species belonging to section Nigri, including A. carbonarius, is the dark pigmentation of conidia, probably due to the synthesis of aspergillin resulting from the combination of the green pigment hexahydroxyl pentacyclic quinoid and the brown pigment melanin [5]. Several studies focused on melanin in Aspergillus, but most information on this issue comes from A. fumigatus and several aspects, such as its precise location, amount, type, and physico-chemical nature remain to be clarified [6]. For A. carbonarius, in particular, Babitskaya et al. [7] proved that the pigments synthesized by the fungus are melanins of the dihydronaphthalene type.
The genetic basis of conidia pigmentation has been fully elucidated in A. fumigatus, in which it is due to the synthesis of 1,8-dihydroxynaphtalene (1,8-DHN) melanin [8]. A cluster of six genes was identified to be involved in the biosynthetic pathway to produce 1,8-DHN melanin and inactivation of single genes in the cluster caused different pigmentation of conidia [9]. The cluster contains the genes alb1 (synonymous of pksP), ayg1, arp2, arp1, abr1, abr2 encoding, in the order, a polyketide synthase (PKS) involved in the production of the heptaketide naphtopyrone YWA1 [10], a putative hydrolase converting YWA1 into 1,3,6,8-tetrahydroxynaphtalene (1,3,6,8-THN) [11], an hydroxynaphthalene (HN) reductase yielding both scytalone and vermelone [8], a dehydratase converting scytalone in 1,3,8-THN [8], a putative multicopper oxidase converting vermelone to 1,8-DHN [12] and, finally, a laccase that polymerize 1,8-DHN in 1,8-DHN melanin [13]. In contrast, in A. niger, the genes fwnA, olvA and brnA (orthologues of alb1/pksP, ayg1 and abr1, respectively) are involved in the production of the characteristic black pigment of conidia but are not organized in a cluster [14].
PKS are multi-domain enzymes playing a key role in fungal secondary metabolism [15]. Recently, a RNA-Seq study using four A. carbonarius strains showed that different pks were up-regulated under OTA-inducing conditions. In addition to the pks gene involved in OTA production (ID: 173482; DOE Joint Genome Institute; http://www.jgi.doe.gov), the most up-regulated pks gene (ID: 172075) under the tested conditions corresponded to the orthologue of alb1/fwnA genes of A. fumigatus/A. niger, not included in a gene cluster [16].
In the present study, Agrobacterium tumefaciens-mediated transformation (ATMT) was used for gene disruption of the pks gene (ID: 172075) in A. carbonarius. Three Δalb1 strains were characterized for phenotypic features, including morphological traits, OTA production in vitro and in vivo and its partitioning in conidia, mycelium and medium in comparison to the wild-type strain.
2. Results
2.1. A. carbonarius alb1 Gene Description and Phylogenetic Analysis
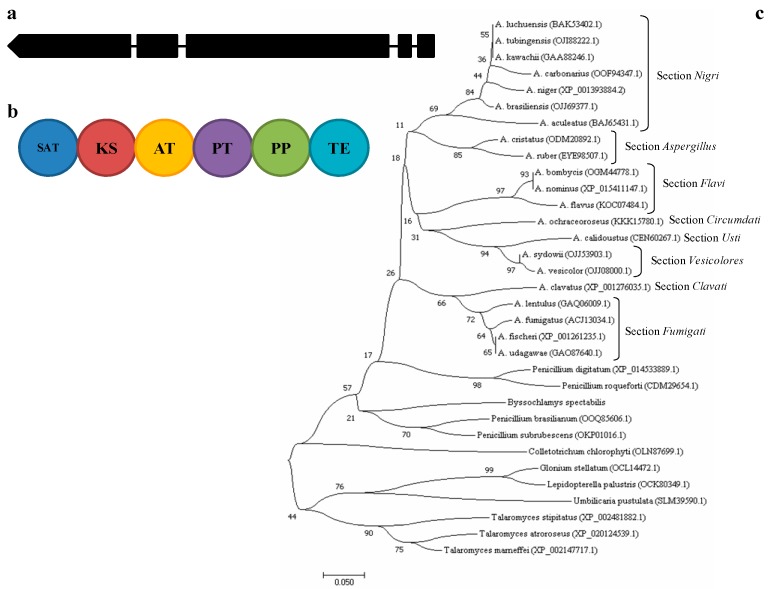
The pks ID: 172075 of A. carbonarius is the orthologue gene of the deeply studied alb1 gene of A. fumigatus (identity 72%) and the fwnA gene of A. niger (identity 89%) involved in the first step of DHN-melanin biosynthesis. It consists of five exons separated by short (48–62 bp) introns and encodes for a type I PKS, composed by the domains ACP-transcyclase (SAT), β-ketoacyl synthase (KS), acyl transferase (AT), product template (PT), phosphopantetheine attachment site (PP) and thioesterase (TE) (Figure 1a,b).
Figure 1.
Gene structure (a) and domains structure (b) of alb1(ALB1) in A. carbonarius, and phylogenetic analysis (c) of ALB1 AT domain in different fungal species. SAT: ACP-transcyclase; KS: β-ketoacyl synthase; AT: acyl transferase; PT: product template; PP: phosphopantetheine attachment site; TE: thioesterase. The tree was constructed by the neighbor-joining method using Poisson correction. Bootstrap values were calculated from 1000 trees. The sequence accession number for each species is reported in parenthesis.
The gene seems to be conserved in the ascomycetes (Table S1) and according to phylogenetic analysis of the AT domain, the sequence is generally clade based on sections and species within the genus Aspergillus, in fact the highest bootstrap values include species having similar conidia pigmentation (Figure 1c).
2.2. Generation of A. carbonarius Δalb1 Mutants
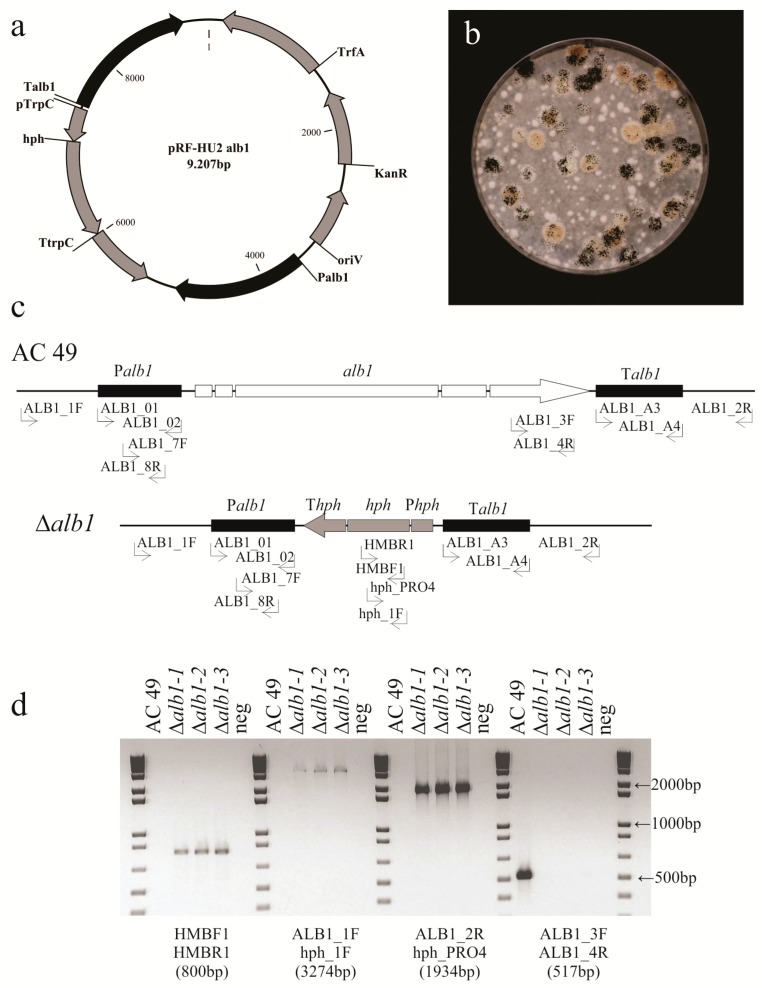
To investigate whether the alb1 gene is involved in the biosynthesis of melanin and in the production of OTA, the gene was deleted in the A. carbonarius AC49 strain by replacing it with a hygromycin resistance cassette. The gene replacement binary plasmid pRF-HU2alb1 was constructed with the 1.4 kb upstream and downstream fragments of the alb1 gene, which were cloned in the vector pRF-HU2 [17] flanking the hygromycin resistance marker. The resulting plasmid (Figure 2a) was used to obtain the A. carbonarius alb1 knockout mutants (Δalb1) via A. tumefaciens mediated transformation (ATMT). One hundred and six hygromycin resistant colonies were obtained in co-cultivation of 1.5 × 104 conidia/mL with A. tumefaciens, corresponding to a transformation frequency of 0.7%. About 70% of the colonies yielded fawn pigmented conidia while the remaining colonies displayed wild-type morphologic traits (Figure 2b). Thirty fawn-colored colonies were selected for subsequent analysis. To confirm the presence of the hygromycin gene we used the primers HMBF1 and HMBR1 (Table 1 and Figure 2c). The expected 800-bp PCR fragment was detected in all transformants and was absent in the untransformed AC49 strain (Figure 2d). Confirmation of the deletion of the target gene was obtained using primers that flanked either the 5′ or 3′ end of the construct in combination with primers within the hygromycin resistant marker (ALB1_1F and hph_1F for the 5′ part, and ALB1_2R and hph_PRO4 for the 3′ part, Figure 2c), as only homologous recombinants (deletants) would amplify with these two primer pairs (Figure 2d). Monosporic isolates were obtained from these transformants and were further validated by PCR using primers located within the coding regions (ALB1-3F and ALB1-4R, Figure 2c), which is not present in the T-DNA. As expected, these primers amplified in the wild-type, but failed to amplify the knockout Δalb1 mutants (Figure 2d).
Figure 2.
Analysis of Aspergillus carbonarius alb1 transformants. (a) Map of plasmid pRF-HU2_alb1. (b) Transformation plate containing colonies displaying fawn pigmented conidia (putative Δalb1) or wild-type-like morphology regarding conidia pigmentation. (c) Diagram of the wild-type locus and the alb1 replacement with the hygromycin resistant selectable marker from pRF-HU2_alb1 by homologous recombination to generate the Δalb1 mutants. Primers used in the construction of plasmid pRF-HU2_alb1 and those used for the analysis of the transformants are shown. (d) Polymerase chain reaction (PCR) analysis of the wild-type AC49 strain and three knockout transformants (Δalb1-1, Δalb1-2, Δalb1-3).
Table 1.
Primers used in the present work.
| Primer Name | Primer Sequence (5′-3′) | Target DNA (Organism) |
|---|---|---|
| Amplification of promoter and terminator | ||
| ALB1_O1 | GGTCTTAAUGGAATACCTGGACGCTGTTG | Promoter (A. carbonarius WT) |
| ALB1_O2 | GGCATTAAUCGGATCGATTGGGTTGCATT | |
| ALB1_A3 | GGACTTAAUCCAGTGAATGACCGAATGCA | Terminator (A. carbonarius WT) |
| ALB1_A4 | GGGTTTAAUAGACTTCGTACGCCACAGAA | |
| Screening of transformants | ||
| RF-5 | GTTTGCAGGGCCATAGAC | Promoter (E. coli DH5α) |
| RF-2 | TCTCCTTGCATGCACCATTCCTTG | |
| RF-1 | AAATTTTGTGCTCACCGCCTGGAC | Terminator (E. coli DH5α) |
| RF-6 | ACGCCAGGGTTTTCCCAGTC | |
| ALB1_1F | GAACTCACGGCCCTCAAAGA | Promoter (A. carbonarius Δalb1) |
| hph_1F | ACGAGGTCGCCAACATCTTCTTCT | |
| ALB1_2R | ATTCACCCCGGTTTCCTCAC | Terminator (A. carbonarius Δalb1) |
| hph_PRO4 | GCACCAAGCAGCAGATGATA | |
| HMBF1 | CTGTCGAGAAGTTTCTGATCG | Hygromicin (A. carbonarius Δalb1) |
| HMBR1 | CTGATAGAGTTGGTCAAGACC | |
| ALB1_3F | CTTGGTAGGATCCGCGAGAC |
Alb1 (A. carbonarius Δalb1) |
| ALB1_4R | CGGCATCGAAAGCGCAAATA | |
| Determination of T-DNA copy number | ||
| ALB1_7F | ATTTCCGAACGGGGTAACTC |
Alb1 (A. carbonarius Δalb1) |
| ALB1_8R | CAAGGTCTCTTGCAATGCTG | |
| nrps_1F | GAGCAGCTACCGGAGCTATT |
nrps (A. carbonarius Δalb1) |
| nrps_2R | GCATCGCATGAGTGAGTTGT | |
Underlined: part of the primer useful for the treatment with the USER enzyme mix in the generation of 3′ single stranded overhangs.
Six knockout transformants were selected for determination of the number of T-DNA copies integrated in the genome by quantitative PCR using the wild-type AC49 strain as a control and the nrps gene as a reference (Table 1). The six Δalb1 mutants analyzed contained a single T-DNA integration, so we selected three of them (Δalb1-1, Δalb1-2, and Δalb1-3) for further functional characterization of the alb1 gene (Table S1).
2.3. Phenotypic Characterization
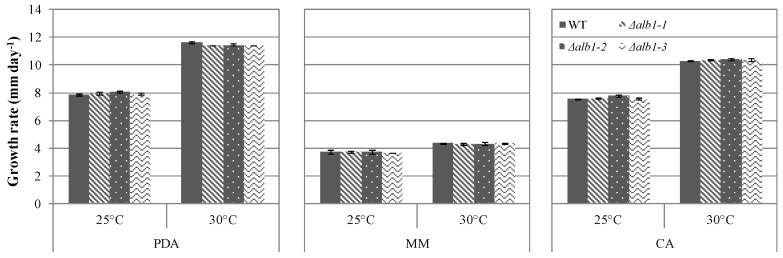
No statistical differences in the daily colony growth were observed among Δalb1 and WT strains (Figure 3). The colony growth was medium-dependent and on all the tested medium the strains grew faster at 30 °C than at 25 °C. On MM the daily growth rate was reduced of 50–62% as compared to CA and PDA (Figure 3).
Figure 3.
Growth rate at 25 °C and 30 °C of the Δalb1 mutants and the WT strain on different media. Figures are the mean values of three technical replicates. No statistical differences were observed among strains through three-way ANOVA analysis.
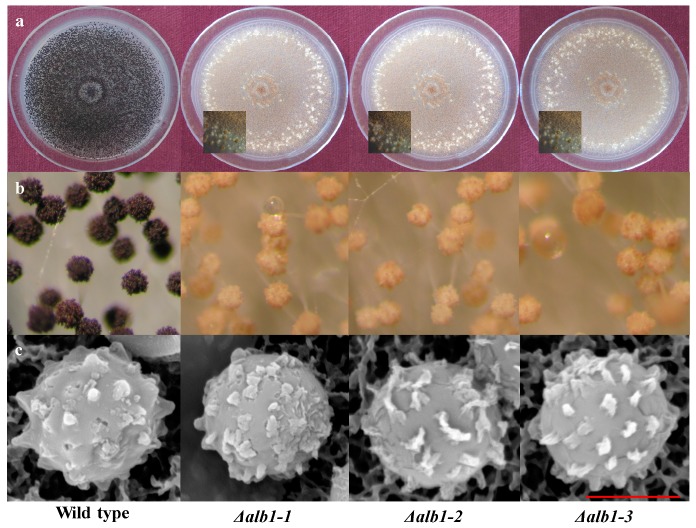
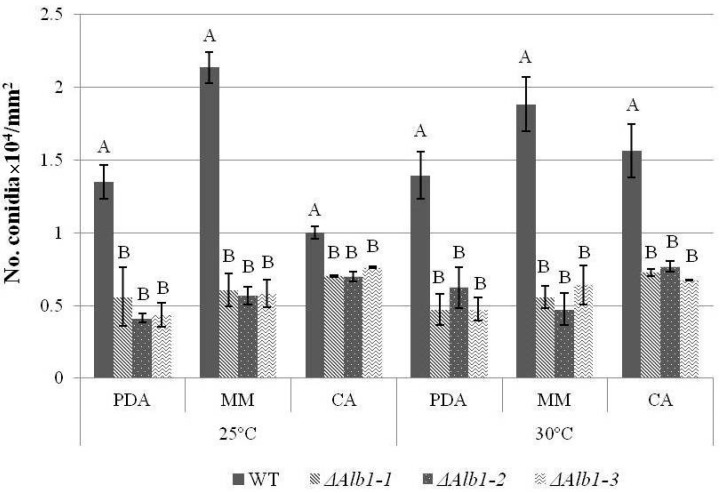
Substantial changes occurred in asexual sporulation. Conidia produced by Δalb1 mutants showed a fawn pigmentation as opposed to the typical black pigmentation of WT conidia (Figure 4a). Stereomicroscope observations showed that the conidiophore vesicles of Δalb1 mutants carried a lower number of conidial chains as compared to the WT strain (Figure 4b). The numbers of conidia produced at 7 DAI by Δalb1 mutants were significantly (p ≤ 0.01) lower than those produced by the WT strain in all the tested conditions (Figure 5), whereas temperature and media did not significantly influence the conidiogenesis (Table S2). In detail, the WT strain produced from 1.0 × 104 (CA, 25 °C) to 2.1 × 104 conidia/mm2 (MM, 25 °C), and the Δalb1 mutants produced from 0.4 × 104 (Δalb1-2, PDA, 25 °C) to 0.8 × 104 conidia/mm2 (Δalb1-3, CA, 25 °C). The morphology of the conidial surface was investigated through SEM observations and similar echinulate surfaces were observed in WT and Δalb1 strains (Figure 4c).
Figure 4.
Morphology of 7-day-old colonies grown on PDA of the A. carbonarius Δalb1 mutants and the WT strain. (a) Colony with detail of sclerotia produced by the Δalb1 mutants; (b) Conidiophores observed by stereomicroscopy; (c) SEM study of conidial surface (Scale bar: 5 μm).
Figure 5.
Production of conidia in the Δalb1 mutants and the WT strain. Figures are the mean values of three technical replicates. Values followed by the same letter within the same medium for each temperature are not statistically different (Tukey’s test at probability level p ≤ 0.01).
In Δalb1 mutants the production of sclerotia was promoted as compared to WT on all the media (PDA, MM and CA) and at both temperatures (25 °C and 30 °C) (Table 2). After 7 DAI the sclerotia at 25 °C ranged from 4 to 11 on MM, from 9 to 23 on PDA and from 44 to 56 on CA. The number of sclerotia remarkably increased at 30 °C on MM (25 to 26) and PDA (81 to 108) and much less on CA (49 to 66). No differences in sclerotial size were recorded and sclerotia were 800–1300 μm in diameter, in agreement with previously reported findings [18].
Table 2.
Number of sclerotia observed in the Δalb1 mutants as compared to the WT strain.
| Strain | Sclerotia (Number ± Standard Error) | |||||
|---|---|---|---|---|---|---|
| PDA | MM | CA | ||||
| 25 °C | 30 °C | 25 °C | 30 °C | 25 °C | 30 °C | |
| WT | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Δalb1-1 | 9 ± 1 | 81 ± 1 | 4 ± 0 | 26 ± 2 | 53 ± 2 | 61 ± 4 |
| Δalb1-2 | 23 ± 3 | 82 ± 1 | 11 ± 1 | 25 ± 1 | 56 ± 5 | 66 ± 10 |
| Δalb1-3 | 20 ± 2 | 108 ± 1 | 10 ± 1 | 26 ± 2 | 44 ± 10 | 49 ± 4 |
2.4. OTA Production and Partitioning
Evaluation on total OTA showed that Δalb1 mutants produced more toxin than the WT strain (Table 3) and that it was significantly (p ≤ 0.01) influenced by temperature and media (Table S3). Indeed, a higher amount of OTA was produced at 25 °C than at 30 °C, and it was more abundant on CA followed by PDA and MM. However, the biggest differences in OTA production between Δalb1 mutants and the WT strain was recorded on MM (227.2 to 755.9%), followed by CA (66.3 to 168.9%) and PDA (32.5 to 70.8%) (Table 3).
Table 3.
Total OTA produced by the Δalb1 mutants and the WT strain of A. carbonarius in 4-mm-diam agar plugs with mycelium and conidia after 7 days of incubation.
| Strain | OTA (ng/mm2 ± Standard Error) | ||
|---|---|---|---|
| MM * | PDA | CA | |
| 25 °C | |||
| WT | 11.59 ± 0.67 B | 87.89 ± 2.39 B | 296.00 ± 22.41 B |
| Δalb1-1 | 90.15 ± 12.73 A (677.8) ** | 124.78 ± 1.19 A (41.9) | 492.26 ± 34.18 A (66.3) |
| Δalb1-2 | 85.81 ± 7.00 A (640.4) | 116.46 ± 3.39 A (32.5) | 503.45 ± 13.59 A (70.1) |
| Δalb1-3 | 99.21 ± 6.71 A (755.9) | 119.69 ± 5.75 A (36.2) | 550.03 ± 41.31 A (85.5) |
| 30 °C | |||
| WT | 12.03 ± 0.55 B | 28.80 ± 1.14 B | 47.11 ± 2.92 B |
| Δalb1-1 | 44.41 ± 5.28 A (269.2) | 50.34 ± 1.33 A (70.8) | 126.68 ± 5.40 A (168.9) |
| Δalb1-2 | 43.97 ± 0.98 A (265.5) | 48.35 ± 1.82 A (67.9) | 117.97 ± 4.90 A (150.4) |
| Δalb1-3 | 39.37 ± 1.19 A (227.2) | 43.08 ± 2.64 A (49.6) | 112.08 ± 12.43 A (137.9) |
Letters refer to comparisons within the same medium for each temperature (Tukey’s test at probability level p ≤ 0.01). * MM: Minimal Medium; PDA: Potatoes Dextrose Agar; CA: Coconut Agar. ** In parenthesis: percentage of OTA increase as compared to the wild type calculated with the formula [(OTAΔalb1 − OTAWT)/OTAWT] × 100.
The OTA partitioning in conidia, mycelium and medium was assessed at different DAI in the Δalb1 mutants and the WT strain grown on CA medium. Generally, the higher amount of OTA in the mycelium was quantified at 4 DAI with no differences between Δalb1 and WT (Table 4). In the WT strain the OTA rapidly decreased from 3.3 ng mg−1 (4 DAI) to 0.4 ng mg−1 (6 DAI), and then lightly increased to 1.0 ng mg−1 (8–10 DAI). The same behavior was observed for the Δalb1 mutants, even if a greater amount of OTA was quantified at 6 (0.9–1.6 ng mg−1), 8 (1.3–2.9 ng mg−1) and 10 (1.9–3.3 ng mg−1) DAI as compared to WT. OTA concentrations were always higher in WT conidia (3.0–23.5 pg 10−2 conidia) than in conidia of the Δalb1 mutants (1.2–12.1 pg 10−2 conidia) and statistically significant differences (p ≤ 0.05) were observed at 6 and 10 DAI. In contrast to this, Δalb1 mutants secreted more OTA than WT in the medium (6.6 to 13.9 and 3.8 to 9.7 ng mg−1, respectively) and differences were statistically significant (p ≤ 0.05) only at 4 and 6 DAI (Table 4).
Table 4.
OTA partitioning in A. carbonarius Δalb1 mutants and the WT strain grown on CA medium.
| Strain | Mycelium (ng mg−1) | Conidia (pg 10−2 Conidia) | Medium (ng mg−1) |
|---|---|---|---|
| 4 DAI | |||
| WT | 3.3 ± 0.8 (a) | 21.1 ± 1.8 (a A) | 3.8 ± 0.6 (cC) |
| Δalb1-1 | 3.0 ± 0.5 (a) | 8.9 ± 1.7 (bc B) | 9.5 ± 2.1 (ab AB) |
| Δalb1-2 | 3.1 ± 0.8 (a) | 9.1 ± 1.2 (b AB) | 12.8 ± 2.1 (a A) |
| Δalb1-3 | 2.6 ± 0.2 (a) | 6.1 ± 1.0 (c B) | 6.6 ± 0.8 (bc BC) |
| 6 DAI | |||
| WT | 0.4 ± 0.1 (b A) | 23.5 ± 3.9 (a A) | 5.1 ± 1.1 (bB) |
| Δalb1-1 | 0.9 ± 0.3 (ab A) | 12.1 ± 2.5 (ab A) | 12.0 ± 1.5 (aAB) |
| Δalb1-2 | 1.6 ± 0.4 (a A) | 11.3 ± 2.8 (ab A) | 13.9 ± 2.2 (a A) |
| Δalb1-3 | 1.1 ± 0.5 (ab A) | 7.1 ± 2.0 (b A) | 12.2 ± 1.7 (a AB) |
| 8 DAI | |||
| WT | 1.0 ± 0.1 (b A) | 3.0 ± 0.5 (a) | 9.7 ± 2.4 (a) |
| Δalb1-1 | 1.3 ± 0.2 (b A) | 1.2 ± 0.2 (a) | 13.5 ± 1.5 (a) |
| Δalb1-2 | 2.9 ± 0.8 (a A) | 1.5 ± 0.2 (a) | 12.5 ± 1.6 (a) |
| Δalb1-3 | 2.2 ± 0.4 (ab A) | 1.3 ± 0.3 (a) | 14.5 ± 3.7 (a) |
| 10 DAI | |||
| WT | 1.0 ± 0.1 (b A) | 5.6 ± 0.5 (a A) | 8.0 ± 1.0 (b A) |
| Δalb1-1 | 2.6 ± 0.7 (ab A) | 2.7 ± 0.5 (b B) | 13.7 ± 2.5 (a A) |
| Δalb1-2 | 1.9 ± 0.3 (ab A) | 2.5 ± 0.4 (b B) | 12.2 ± 0.7 (ab A) |
| Δalb1-3 | 3.3 ± 0.9 (a A) | 2.0 ± 0.3 (b B) | 11.4 ± 1.0 (ab A) |
For each DAI, letters refer to comparisons within the analyzed matrix among strains according to the Tukey’s test at probability levels p ≤ 0.05 (lowercase letters) and p ≤ 0.01 (uppercase letters).
In Δalb1 mutants, OTA was accumulated also in the sclerotia, and when they were differentiated concentrations ranged from 29.8 ng mg−1 to 1378.1 ng mg−1.
2.5. Artificial Inoculation on Grape Berries
The behavior of Δalb1 mutants on artificially-inoculated grape berries was evaluated at 7 DAI (Figure S1). Generally, the lesions caused by the Δalb1 mutants were bigger in size than those caused by the WT strain, although with no statistical significance. The Δalb1 mutants, but not the WT strain differentiated sclerotia also on berries and produced higher amount of OTA than the WT strain at both 25 and 30 °C (Table 5).
Table 5.
Lesion diameter, OTA production and number of sclerotia for the ΔAlb1 mutants and the WT strain on artificially-inoculated grape berries incubated at two temperatures at 7 DAI.
| Strain | 25 °C | 30 °C |
|---|---|---|
| Lesion diameter (mm) | ||
| WT | 33.2 ± 2.8 a | 47.0 ± 1.0 a |
| ΔAlb1-1 | 35.2 ± 3.0 a | 51.3 ± 3.8 a |
| ΔAlb1-2 | 33.3 ± 0.9 a | 52.7 ± 1.4 a |
| ΔAlb1-3 | 33.3 ± 1.6 a | 54.2 ± 5.8 a |
| OTA (ng g−1) | ||
| WT | 315.1 ± 14.8 a | 272.2 ± 26.3 bB |
| ΔAlb1-1 | 450.4 ± 67.5 a | 1380.7 ± 204.8 aAB |
| ΔAlb1-2 | 515.3 ± 77.3 a | 1548.9 ± 187.8 a A |
| ΔAlb1-3 | 482.8 ± 33.6 a | 1929.7 ± 388.3 a A |
| Sclerotia (No.) | ||
| WT | 0 ± 0 bC | 0 ± 0 bB |
| ΔAlb1-1 | 2 ± 0 abAB | 19 ± 2 aA |
| ΔAlb1-2 | 1 ± 0 bAB | 14 ± 2 aAB |
| ΔAlb1-3 | 3 ± 0 aA | 24 ± 1 aA |
Values are the mean values of five replicates. For lesion diameter, OTA, and number of sclerotia, letters refer to comparisons within of the analyzed temperature among strains according to the Tukey’s test at probability levels p ≤ 0.05 (lowercase letters) and p ≤ 0.01 (uppercase letters).
3. Discussion
A. carbonarius is a fungus well known as an important source of OTA contamination in food and feed [19]. The fungus belongs to the section Nigri of the genus, the so called “black Aspergilli”, which characteristically present dark-brown to black conidia, and hyaline or lightly pigmented hyphae near the apex [20]. DHN melanin is responsible for the dark-brown pigmentation of conidia but it was also proved to be involved in the resistance of conidia to environmental stress and as virulence factor [8,21].
In A. fumigatus, a cluster of six genes includes the alb1 gene encoding a PKS involved in dihydroxynaphtalene (DHN)-melanin biosynthesis [22], which is expressed during conidiation [12]. In addition, deletion of the alb1 gene determines the albino phenotype of conidia, demonstrating that DHN-melanin is the only pigment responsible for conidia pigmentation in A. fumigatus [21]. Although three orthologs of the A. fumigatus DHN-melanin biosynthetic genes (alb1, ayg1 and abr1) were found in A. niger, the deletion of the alb1 orthologue gene leads to conidia with fawn pigmentation. Hence the residual pigmentation suggests that in addition to DHN melanin, one or more pigments are involved in the blackness of conidia [14].
The gene orthologue to A. fumigatus alb1 has been detected in A. carbonarius and it encodes a type I PKS [16]. The gene is conserved in the ascomycetes and in the Aspergillus genus clades largely reflect the sections. The function of the gene was investigated in A. carbonarius by comparing three Δalb1 mutants with the WT strain. The three Δalb1 mutants showed the same behavior and our findings on A. carbonarius fit better with genetic data on A. niger rather than those on A. fumigatus. In fact, in both A. niger and A. carbonarius the genes close to the pks orthologue of A. fumigatus alb1 gene were not co-expressed and the genes orthologue respectively to A. fumigatus ayg1 and abr1 were in a different genetic position, so there is no the evidence for a DHN-melanin biosynthetic gene cluster [14,15,16]. In both species conidia still show a fawn pigmentation following the deletion of the alb1 gene; hence, in A. carbonarius and A. niger one or more additional pigment(s) are supposed to be responsible for the black color of conidia and this corroborates the hypothesis that aspergillin, a compound with molecular size of about 20,000 Da, is formed starting from two precursors identified as a hexahydroxyl pentacyclic quinoid (HPQ) and a melanin pigment [5]. A secondary metabolites profile of conidial pigmentation was developed for fungi belonging to A. niger group, demonstrating that their conidial pigmentation depends on polyketide-derivative compounds, especially the chemical family of naptho-γ-pyrones, that include aspergillin [23].
The functional characterization of the alb1 gene was deeply studied in A. fumigatus and Δalb1 strains produced nearly smooth conidia differently from WT conidia that exhibited echinulate surfaces [8]. Observations through SEM on A. carbonarius showed that the surface of conidia of the Δalb1 mutants exhibited the same echinulate surface of those of the WT strain (Figure 3), demonstrating that, differently from A. fumigatus, the analogue of DHN-melanin is not responsible for conidia ornamentation. In A. nidulans, the wA gene (orthologue of alb1) is regulated by wetA, a regulator of spore-specific gene expression [24]. wetA together with brlA and abaA are transcription factors proposed as Central Regulator Pathway (CRP) of conidiogenesis, conserved in the Aspergillus genus [25,26]. In a RNA-Seq study on A. flavus, brlA, abaA and wetA were shown to be down-regulated in sclerotia [27] and comparable results were obtained by Jin et al. [28] on A. oryzae, demonstrating that the down regulation of CRP occurred when sclR, a gene involved in the regulation of sclerotia production, was over expressed. Our results showed that the WT strain did not differentiate sclerotia under the adopted growth conditions, whereas all the three Δalb1mutants were able to produce them on all media. The sclerotia pigmentation of Δalb1 mutants was not affected, as observed in Botrytis cinerea by deleting the same orthologue gene [29]. The relationship between regulatory factors controlling the formation of sclerotia and asexual sporulation is not well understood. However, data described herein showed that in the Δalb1 mutants of A. carbonarius the conidiogenesis was also significantly reduced as compared to the WT parental strain (Figure 4). These concurrent developmental changes occurring in Δalb1mutants suggest that, probably, the deletion of alb1 induces the activation of a specific regulation pathway promoting differentially sclerotia production and asexual sporulation processes.
Fungal secondary metabolism and morphological development have been shown to be associated at the genetic level [30,31,32]; for example, in A. nidulans and in Aspergillus spp. producing aflatoxins, it was demonstrated that a FadA-dependent signal transduction pathway regulate both conidiation and sterigmatocystin-aflatoxins biosynthesis [33]. Another interesting consequence of alb1 loss in A. carbonarius was the statistically significant increase of total OTA production under all tested conditions. In A. carbonarius, OTA is differentially partitioned among mycelium, conidia, sclerotia and the medium [34,35]. The amount of OTA accumulated in the WT conidia, ranging from 3.0 to 23.5 pg per 102 conidia, agrees with data by Atoui et al. [35] on the A. carbonarius 2Mu134 strain grown on different media. OTA was accumulated in conidia up to three-fold more in the WT strain than in the Δalb1 mutants. Conversely, Δalb1 mutants were much more active in secreting OTA into the medium as compared to the WT strain and accumulated high amount of OTA in sclerotia. On grape berries artificially inoculated on wounds, Δalb1 mutants caused lesions slightly bigger that those caused by the WT strain, demonstrating that the gene is not directly involved in rotting development. They also produced more OTA that the WT strain either at 25 °C or 30 °C confirming the results obtained in vitro on different media.
The biosynthesis of both OTA and DHN-melanin analogues is depending on the PKSs activity, which can be regulated by different environmental factors (e.g., light, pH, nitrogen, and carbon sources) via the activation of specific transcription factors [15]. The existence of common genetic networks between fungal development and secondary metabolism [36] and the different pigmentation of conidia and OTA production and partitioning in different fungal organs showed by the Δalb1 mutants and the WT strain suggest that the biosynthesis of both polyketides, OTA, and DHN-melanin, may share common genetic regulation.
In conclusion, this study clarified that the function of the PKS encoding gene alb1 on conidial pigmentation in A. carbonarius is similar to that of the orthologous gene in A. niger [14] and different from the genetic mechanism in A. fumigatus [8]. The changes observed in Δalb1 mutants concerning conidia pigmentation as well as fungal development (conidiogenesis and sclerotia production), OTA production (in vitro and on grape berries) and its partitioning in different fungal organs represent new insights that are worthwhile to be investigated in more detail in A. carbonarius.
4. Materials and Methods
4.1. Strains and Growth Conditions
The A. carbonarius wild type (WT) AC49 strain, stored in the culture collection of the Department of Soil, Plant and Food Sciences of the University of Bari (Italy), and three selected alb1-deleted mutants (Δalb1-1, Δalb1-2 and Δalb1-3), obtained in the present study, were used. Fungal strains were stored at −80 °C until use and routinely grown on Potato Dextrose Agar (PDA; infusion from 200 g peeled and sliced potatoes kept at 60 °C for 1 h, 20 g dextrose, adjusted at pH 6.5, 20 g agar Oxoid no. 3, per liter) in the dark at 28 ± 1 °C. When required, the medium was supplemented with 100 μg mL−1 of hygromycin B (HygB; InvivoGen, San Diego, CA, USA).
Escherichia coli DH5α and A. tumefaciens AGL-1 strains stored in the culture collection of the Department of Food Biotechnology, Instituto de Agroquimica y Tecnología de Alimentos (IATA-CSIC, Valencia, Spain), were respectively used for the generation of chemically and electrocompetent cells.
4.2. Analysis of the A. carbonarius alb1 Gene
The alb1 gene was identified through blastx analysis on A. carbonarius filtered proteins database (http://www.jgi.doe.gov) using the A. fumigatus alb1 gene (NCBI Accession: AF025541.1) as input sequence. The A. carbonarius pks gene (ID: 172075), orthologue of alb1, was then used as input for blastx analysis on non-redundant protein sequences of NCBI database (http://www.ncbi.nlm.nih.gov/BLAST/) to identify orthologue genes in other fungi.
The amino acid sequences of the ALB1 acetyl-transferase (AT) domain of 21 Aspergillus species and other 15 genera of Ascomycetes were obtained by using the Simple Modular Architecture Research Tool (SMART; http://smart.embl-heidelberg.de/). The domain sequences were then aligned through MEGA6 software [37] with the MUSCLE algorithm, and the phylogenetic tree was computed using the neighbor-Joining method [38].
4.3. Generation of A. carbonarius Δalb1 Mutants
All primer pairs were designed with the Primer3 software [39]. The amplification of the promoter and the terminator regions (~1.5 kb) from A. carbonarius AC49 genomic DNA (10 ng) was performed using Top-Taq DNA polymerase (Bioron GmbH, Ludwigshafen, Germany), according to manufacturer’s instructions, and the primer pairs ALB1_O1/ALB1_O2 for the promoter and ALB1_A3/ALB1_A4 for the terminator (Table 1). Cycling conditions were: 94 °C for 3 min, 35 cycles of 94 °C for 15 s, 58 °C for 20 s and 72 °C for 2 min and 72 °C for 10 min.
The plasmid pRFHU2-alb1 (Figure 1a) was obtained using the Uracil-Specific Excision Reagent (USER) enzyme (New England Biolabs, Ipswich, MA, USA) by mixing the amplified promoter and terminator fragments with the digested vector pRFHU2 [17] (ratio 30:30:120 ng). Aliquots (1 µL) of the mixture were used for transformation of E. coli DH5α chemically-competent cells [17]. After 18 h of incubation at 37 °C on Luria-Bertani (LB) agar medium (bacto tryptone 10 g, yeast extract 5 g, NaCl 5 g, agar 14 g, per liter) supplied with 25 µg mL−1 of kanamycin (Invitrogen, Carlsbad, CA, USA), resistant transformants were screened by PCR using the primer pairs RF-5/RF-2 and RF-1/RF-6 (Table 1) and the fusion was confirmed by DNA sequencing.
The plasmid pRFHU2-alb1 was then introduced in electrocompetent A. tumefaciens AGL-1 cells using a Gene Pulser apparatus (Bio-Rad, Richmond, CA, USA), generating pulses of up to 2500 V from a 25 μF capacitor. A. tumefaciens AGL-1 carrying the plasmid pRFHU2-alb1 was grown at 28 °C for 2 days on LB agar supplemented with kanamycin (50 µg mL−1, Invitrogen), rifampicin (20 µg mL−1, Sigma-Aldrich, St. Louis, MO, USA) and carbenicillin (75 µg mL−1, Sigma-Aldrich, St. Louis, MO, USA). A single colony was used to inoculate LB medium (10 mL) containing the same antibiotics and the culture was incubated for 24 h. AGL-1 cells were centrifuged, washed with the induction medium (IM) [40] and newly suspended (OD600 = 0.15) in the same medium amended with 200 µM acetosyringone (AS; Sigma-Aldrich, St. Louis, MO, USA) (IMAS). Cells were grown at 28 ± 1 °C and 200 rpm until an OD600 of 0.5–0.75 was reached. A 150 µL aliquot of the culture was mixed with an equal volume of a conidial suspension (105 conidia mL−1) of A. carbonarius, and aliquots (100 µL) of the mixture were spread onto paper filter layered on agar plates containing IMAS. After co-cultivation at 24 ± 1 °C for 40 h, the paper filters were transferred on PDA containing hygromycin B (HygB, 100 µg mL−1, InvivoGen), as selective agent for fungal transformants, and cefotaxime (200 µg mL−1, Sigma-Aldrich, St. Louis, MO, USA), inhibiting the growth of A. tumefaciens cells. A. carbonarius AC49 HygB-resistant colonies appeared after 3–4 days of incubation at 28 ± 1 °C and monosporic cultures were obtained. The genomic DNA was extracted as described by Crespo-Sempere et al. [41]. Transformants were screened for the sequences enclosed between the promoter and the terminator of alb1 and the HygB genes (primer pairs: ALB_1F and hph_1F, ALB_2R and hph_PRO4, respectively), as well as for the deletion of the alb1 gene (primers ALB1_3F and ALB1_4R) and the insertion of the selection marker HygB (primers HMBF1 and HMBR1) (Table 1).
Finally, qPCR was carried out on a sample of 5 selected transformants for assessing the number of T-DNA copies integrated in the genome. The primers ALB1_7F and ALB1_8R (Table 1) were designed in the promoter region of the alb1 gene close to the selection marker. As reference, the non-ribosomal peptide synthetase (nrps) gene (ID: 132610) was used with the primers nrps_1F and nrps_2R (Table 1). qPCR reactions were performed in a final volume of 10 μL, containing 1 × of LightCycler® 480 SYBR Green I Master (Bio-Rad, Hercules, CA, USA), 250 nM of each primer and 10 ng of template DNA. Thermal cycler conditions were: 95 °C for 5 min, followed by 35 cycles at 95 °C for 10 s, 58 °C for 45 s and 72 °C for 10 s. Two technical replicates were performed. All amplifications were carried out in a LightCycler 480 Instrument (Roche Diagnostics, Mannheim, Germany) equipped with LightCycler SW 1.5 software. For each sample, raw data were used to calculate the qPCR efficiency (E) and the quantification cycle (Cq) through LinRegPCR software [42]. The number of T-DNA copies integrated in the genome of each transformant was calculated according to Pfaffl [43].
4.4. Morphological Studies and OTA Production
Three A. carbonarius Δalb1 mutants, Δalb1-1, Δalb1-2 and Δalb1-3, were compared with the WT strain for colony growth, conidiogenesis and OTA production. Three different media [PDA; minimal medium (MM; 10 mL solution A (10 g KH2PO4, 100 mL−1 water), 10 mL solution B (20 g NaNO3, 5 g KCl, 5 g MgSO4·7H2O, 0.1 g FeSO4, 100 mL−1 water), 1 mL of micronutritive solution [44], 20 g glucose, per liter); and coconut-agar medium (CA; 200 g of blended coconut, 18 g agar Oxoid no. 3, per liter)], dispensed in aliquots of 20 mL in Petri dishes of 100 mm diameter, were used in the assays. Mycelial plugs of 4 mm diameter from the edges of actively growing colonies on PDA were used to inoculate three replicated Petri dishes per medium. The orthogonal diameters of developing colonies were measured after 2, 5 and 7 days of incubation at 25 and 30 ± 1 °C in the darkness, which are the optimal conditions for OTA production and vegetative growth in A. carbonarius, respectively [45]. Additionally, the production of conidia and OTA was determined in three agar plugs (4 mm diameter) with mycelium and conidia collected from the inner, middle, and outer part of 7-day-old growing colonies [41]. Mature sclerotia were removed before OTA extraction.
4.5. OTA Partitioning in WT and Δalb1 Strains
OTA partitioning among conidia, mycelium and the medium was evaluated according to the procedure described by Atoui et al. [31], slightly modified. A sterile cellophane disc was overlayered on CA medium, poured in aliquots (30 mL) in each Petri dish (100 mm diameter), and five replicated dishes were inoculated with mycelial plugs (4 mm diameter) from colonies actively growing on PDA. Colonies were grown up to 10 days at 25 ± 1 °C and at 4, 6, 8 and 10 Days. After Inoculation (DAI) the orthogonal diameters of the developing colonies were measured. Plates were destructively sampled. The entire colony was scraped from the cellophane layer, suspended in 10 mL of sterile water containing 0.01% Tween 20 and shaken vigorously for 5 min. The suspension was filtered through a layer of sterile miracloth, and the filtrate was centrifuged for pelletizing conidia. Mycelium, collected from the miracloth was deprived of sclerotia, when present, and washed three-times with 30 mL of sterile distilled water until complete clearing of conidia. Three 4-mm agar plugs in correspondence of the inner, middle, and outer regions of the growing culture were collected from the same plates for OTA analysis.
4.6. Artificial Inoculation on Grape Berries
For each strain, five ripe table-grape berries cv Italia (~35 mm in diameter) collected from two bunches were surface-sterilized with 2% sodium hypochlorite for 1 min, rinsed three times with sterile distilled water and air dried. Conidia, collected by scraping the surface of 7-day-old colonies grown on PDA were suspended in water and adjusted to 106 conidia mL−1. Aliquots (10 µL) of the conidial suspension were singly placed on berry skin which was wounded with a needle (3-mm-deep) under the drop. Berries were kept at 100% relative humidity. After 7 DAI at 25 or 30 ± 1 °C in darkness the orthogonal diameters of developing lesion were measured. Five replicated berries inoculated with sterile water were used as control. OTA concentration in berries was assessed as described below.
4.7. OTA Extraction and Quantification
OTA was extracted from conidia, mycelium and medium samples adding 5 mL of methanol, vortexing for 2 min, incubating at room temperature for 1 h and filtering through 0.22 µm-pore-size Nuclepore filter (Nucleopore Corp., Pleasanton, CA, USA). All the samples were stored at −20 °C before HPLC analysis. OTA was extracted also from sclerotia when present. All the sclerotia differentiated per plate were separated from the mycelium, weighed, and transferred in a microtube before being crushed with a glass micro-pestle in 5 mL of methanol.
The extraction of OTA from berries was performed according to Visconti et al. [46], slightly modified. Briefly, each berry was suspended in 20 mL of dilution buffer [1% PEG 8000 (Sigma-Aldrich, St. Louis, MO, USA), 0.5% NaHCO3 (Carlo Erba Reagents, Milan, Italy)] and homogenized by using a T-25 high-performance dispersing (Ultra-Turrax, Staufen, Germany). One mL of diluted samples (1:50) was loaded on a Ochratest™ immunoaffinity columns (Vicam, Milford, MA, USA), washed with 5 mL of washing buffer (2% NaCl (Sigma Aldrich, St. Louis, MO, USA), 0.5% NaHCO3 (Carlo Erba Reagents, Milan, Italy)) and 5 mL of distilled water, then eluted with 2 mL of methanol.
OTA was quantified through High Performance Liquid Chromatography (HPLC). Briefly, 20 μL of methanol extracts were injected into the chromatographic apparatus made up of an isocratic pump (HP 1100, Agilent Technologies, Santa Clara, CA, USA) equipped with an injection valve (mod. 7125, Rheodyne, Cotati, CA, USA), a fluorometric detector (HP 1100, λex = 333 nm, λem = 460 nm) and a Chemstation Rev A.08.03 data system (Agilent Technologies, Santa Clara, CA, USA). The analytical column was a reversed-phase Discovery C18 (15 cm × 4.6 mm, 5 mm particles) (Supelco, Bellefonte, PA, USA) preceded by a Security Guard (Phenomenex, Torrance, CA, USA). The identification of OTA in extracts was carried out comparing the retention time with that of the OTA standard (Supelco Sigma-Aldrich, St. Louis, MO, USA).
OTA concentration was expressed per mm2 of surface of the three sampled agar plugs in the experiment on different media, and the percentage of OTA increase in Δalb1 mutants compared to WT was calculated according to the formula: [(OTAΔalb1 − OTAWT)/OTAWT] × 100. In the OTA partitioning experiment, OTA concentration was expressed per g (fresh weight) of mycelium, sclerotia, medium, berry, or number of conidia in the other experiments.
4.8. Scanning Electron Microscopy (SEM) Studies
Conidia from 7-day-old colonies on PDA were collected on a 0.22-µm-pore-size Nuclepore filter. The filters were dehydrated, mounted on aluminum stubs, and coated with gold palladium alloy. Conidia were observed under a low-pressure scanning electron microscope Hitachi TM3000 (Hitachi, Tokyo, Japan).
4.9. Statistical Analysis
All data were analyzed by ANOVA followed by the Tukey’s honestly significant different test (HSD), using CoStat-software (CoHort Software, Monterey, CA, USA), at the significance levels p ≤ 0.05 and p ≤ 0.01.
Acknowledgments
This research was partially carried out in the framework of the Projects: “Laboratory network for the selection, characterization and conservation of germplasm and for preventing the spread of economically-relevant and quarantine pests (SELGE) No. 14”, funded by the Apulia Region, PO FESR 2007–2013—Axis I, Line of intervention 1.2., Action 1.2.1; and “Promotion of ECO-friendly processes for the enhancement of quality of apulian food productions” granted by the “Distretto Agroalimentare Regionale—D.A.Re. s.c.ar.l. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge the cooperation of Ana Izquierdo and José M. Coll-Marqués, both from the IATA-CSIC, for the technical assistance in obtaining the deletion mutants and the help with the microscopy, respectively, Donato Perrelli and Patrizia Natale of Centro di Ricerca, Sperimentazione e Formazione in Agricoltura “Basile Caramia”, Locorotondo (Bari) for technical assistance in chemical analysis of OTA and Pasquale Trotti Department of Soil, Plant and Food Sciences—University of Bari (Bari) for the technical assistance in SEM observations.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6651/10/3/120/s1. Figure S1: Grape berries at 7 days after inoculation with Δalb1 mutants and WT, Table S1: Determination of the number of T-DNA copies integrated in the genome of A. carbonarius transformants, Table S2: Three-way ANOVA on data of conidia and OTA production of A. carbonarius Δalb1 and WT strains.
Author Contributions
D.G., L.G.-C., S.P., R.M.D.M.A. and F.F. conceived and designed the experiments; D.G., A.-R.B., S.P. performed the experiments; D.G., A.-R.B. and S.P. analyzed the data; D.G. wrote the paper, D.G., L.G.-C., A.-R.B., S.P., R.M.D.M.A., F.F. supervised the writing, F.F. coordinated the collaboration of the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Deletion of alb1 gene reduces asexual sporulation and promotes the production of sclerotia in Aspergillus carbonarius. In Δalb1 strains the OTA production is increased and its partitioning is different respect to the wild type.
References
- 1.Bellì N., Marın S., Sanchis V., Ramos A.J. Influence of water activity and temperature on growth of isolates of Aspergillus section Nigri obtained from grapes. Int. J. Food Microbiol. 2004;96:19–27. doi: 10.1016/j.ijfoodmicro.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Cabañes F.J., Accensi F., Bragulat M.R., Abarca M.L., Castellá G., Minguez Pons A. What is the source of Ochratoxin A in wine? Int. J. Food Microbiol. 2002;79:213–215. doi: 10.1016/S0168-1605(02)00087-9. [DOI] [PubMed] [Google Scholar]
- 3.Pollastro S., Dongiovanni C., Abbatecola A., Tauro G., Natale P., Pascale M., Visconti A., Faretra F. Wine contamination by Ochratoxin A in South Italy: Causes and preventive actions. J. Plant Pathol. 2003;85:281. [Google Scholar]
- 4.International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 1st ed. Volume 56. IARC Press; Lyon, France: 1993. Ochratoxin A; pp. 489–521. [PMC free article] [PubMed] [Google Scholar]
- 5.Ray A.C., Eakin R.E. Studies on the biosynthesis of Aspergillin by Aspergillus niger. Appl. Microbiol. 1975;30:909–915. doi: 10.1128/am.30.6.909-915.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal A.K., Gajjar D.U., Vasavada A.R. DOPA and DHN pathways orchestrate melanin synthesis in Aspergillus species. Med. Mycol. 2014;52:10–18. doi: 10.3109/13693786.2013.826879. [DOI] [PubMed] [Google Scholar]
- 7.Babitskaya V.G., Shcherba V., Filimonova T.V., Grigorchuk E.A. Melanin Pigments from the Fungi Paecilomycesvariotii and Aspergillus carbonarius. Appl. Biochem. Microbiol. 2000;36:128–133. doi: 10.1007/BF02737906. [DOI] [PubMed] [Google Scholar]
- 8.Tsai H.F., Wheeler M.H., Chang Y.C., Kwon-Chung K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1998;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai H.F., Fujii I., Watanabe A., Wheeler M.H., Chang Y.C., Yasuoka Y., Ebizuka Y., Kwon-Chung K.J. Pentaketide melanin biosynthesis in Aspergillus fumigatus requires chain-length shortening of a heptaketide precursor. J. Biol. Chem. 2001;276:29292–29298. doi: 10.1074/jbc.M101998200. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe A., Fujii I., Tsai H.F., Chang Y.C., Kwon-Chung K.J., Ebizuka Y. Aspergillus fumigatus alb1 encodes Naphthopyrone synthase when expressed in Aspergillus oryzae. FEMS Microbiol. Lett. 2000;192:39–44. doi: 10.1111/j.1574-6968.2000.tb09356.x. [DOI] [PubMed] [Google Scholar]
- 11.Fujii I., Yasuoka Y., Tsai H.F., Chang Y.C., Kwon-Chung K.J., Ebizuka Y. Hydrolytic polyketide shortening by ayg1p, a novel enzyme involved in fungal melanin biosynthesis. J. Biol. Chem. 2004;279:44613–44620. doi: 10.1074/jbc.M406758200. [DOI] [PubMed] [Google Scholar]
- 12.Pihet M., Vandeputte P., Tronchin G., Renier G., Saulnier P., Georgeault S., Mallet R., Chabasse D., Symoens F., Bouchara J.P. Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol. 2009;9:1. doi: 10.1186/1471-2180-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugareva V., Härtl A., Brock M., Hübner K., Rohde M., Heinekamp T., Brakhage A.A. Characterisation of the laccase-encoding gene abr2 of the dihydroxynaphthalene-like melanin gene cluster of Aspergillus fumigatus. Arch. Microbiol. 2006;186:345–355. doi: 10.1007/s00203-006-0144-2. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen T.R., Park J., Arentshorst M., van Welzen A.M., Lamers G., vanKuyk P.A., Damveld R.A., van den Hondel C.A.M., Nielsen K.F., Frisvard J.C., et al. The molecular and genetic basis of conidial pigmentation in Aspergillus niger. Fungal Genet. Biol. 2011;48:544–553. doi: 10.1016/j.fgb.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Keller N.P., Turner G., Bennett J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 16.Gerin D., De Miccolis Angelini R.M., Pollastro S., Faretra F. RNA-Seq Reveals OTA-related gene transcriptional changes in Aspergillus carbonarius. PLoS ONE. 2016;11:e0147089. doi: 10.1371/journal.pone.0147089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frandsen R.J., Andersson J.A., Kristensen M.B., Giese H. Efficient four fragment cloning for the Construction of vectors for targeted gene replacement in filamentous fungi. BMC Mol. Biol. 2008;9:70. doi: 10.1186/1471-2199-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisvad J.C., Petersen L.M., Lyhne E.K., Larsen T.O. Formation of Sclerotia and production of Indoloterpenes by Aspergillus niger and other species in Section Nigri. PLoS ONE. 2014;9:e94857. doi: 10.1371/journal.pone.0094857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abarca M.L., Accensi F., Bragulat M.R., Castella G., Cabanes F.J. Aspergillus carbonarius as the main source of ochratoxin A contamination in dried vine fruits from the Spanish market. J. Food Prot. 2003;66:504–506. doi: 10.4315/0362-028X-66.3.504. [DOI] [PubMed] [Google Scholar]
- 20.Simões M.F., Santos C., Lima N. Structural diversity of Aspergillus (section Nigri) spores. Microsc. Microanal. 2013;19:1151–1158. doi: 10.1017/S1431927613001712. [DOI] [PubMed] [Google Scholar]
- 21.Langfelder K., Jahn B., Gehringer H., Schmidt A., Wanner G., Brakhage A.A. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med. Microbiol. Immunol. 1998;187:79–89. doi: 10.1007/s004300050077. [DOI] [PubMed] [Google Scholar]
- 22.Krijgsheld P., Bleichrodt R.V., Van Veluw G.J., Wang F., Müller W.H., Dijksterhuis J., Wösten H.A.B. Development in Aspergillus. Stud. Mycol. 2013;74:1–29. doi: 10.3114/sim0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen K.F., Mogensen J.M., Johansen M., Larsen T.O., Frisvad J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 2009;395:1225–1242. doi: 10.1007/s00216-009-3081-5. [DOI] [PubMed] [Google Scholar]
- 24.Adams T.H., Wieser J.K., Yu J.H. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 1998;62:35–54. doi: 10.1128/mmbr.62.1.35-54.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall M.A., Timberlake W.E. Aspergillus nidulanswet A activates spore-specific gene expression. Mol. Cell. Biol. 1991;11:55–62. doi: 10.1128/MCB.11.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J.H. Regulation of development in Aspergillus nidulans and Aspergillus fumigatus. Mycobiology. 2010;38:229–237. doi: 10.4489/MYCO.2010.38.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X., Zhou B., Yin C., Guo Y., Lin Y., Pan L., Wang B. Characterization of natural antisense transcript, sclerotia development and secondary metabolism by strand-specific RNA sequencing of Aspergillus flavus. PLoS ONE. 2014;9:e97814. doi: 10.1371/journal.pone.0097814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin F.J., Takahashi T., Matsushima K.I., Hara S., Shinohara Y., Maruyama J.I. SclR, a basic helix-loop-helix transcription factor, regulates hyphal morphology and promotes sclerotial formation in Aspergillus oryzae. Eukaryot. Cell. 2011;10:945–955. doi: 10.1128/EC.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumacher J. DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes. Mol. Microbiol. 2016;99:729–748. doi: 10.1111/mmi.13262. [DOI] [PubMed] [Google Scholar]
- 30.Chang P.K., Scharfenstein L.L., Li R.W., Arroyo-Manzanares N., De Saeger S., Di Mavungu J.D. Aspergillus flavusasw, a gene homolog of Aspergillus nidulansoef, regulates sclerotial development and biosynthesis of sclerotium-associated secondary metabolites. Fungal Genet. Biol. 2017;104:29–37. doi: 10.1016/j.fgb.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhi Q.Q., Li J.Y., Liu Q.Y., He Z.M. A cytosine Methyltransferase ortholog dmtA is involved in the sensitivity of Aspergillus flavus to environmental stresses. Fungal Biol. 2017;121:501–514. doi: 10.1016/j.funbio.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Calvo A.M., Cary J.W. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 2015;6:62. doi: 10.3389/fmicb.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hicks J.K., Yu J.H., Keller N.P., Adams T.H. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA Gα protein-dependent signaling pathway. EMBO J. 1997;16:4916–4923. doi: 10.1093/emboj/16.16.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wicklow D.T., Dowd P.F., Alfatafta A.A., Gloer J.B. Ochratoxin A: An antiinsectan metabolite from the sclerotia of Aspergillus carbonarius NRRL 369. Can. J. Microbiol. 1996;42:1100–1103. doi: 10.1139/m96-141. [DOI] [PubMed] [Google Scholar]
- 35.Atoui A., Mitchell D., Mathieu F., Magan N., Lebrihi A. Partitioning of ochratoxin A in mycelium and conidia of Aspergillus carbonarius and the impact on toxin contamination of grapes and wine. J. Appl. Microbiol. 2007;103:961–968. doi: 10.1111/j.1365-2672.2007.03320.x. [DOI] [PubMed] [Google Scholar]
- 36.Calvo A.M., Wilson R.A., Bok J.W., Keller N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002;66:447–459. doi: 10.1128/MMBR.66.3.447-459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 39.Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3—New capabilities and interfaces. Nucl. Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michielse C.B., Hooykaas P.J., van den Hondel C.A., Ram A.F. Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nat. Protoc. 2008;3:1671–1678. doi: 10.1038/nprot.2008.154. [DOI] [PubMed] [Google Scholar]
- 41.Crespo-Sempere A., Marin S., Sanchis V., Ramos A.J. VeA and LaeA transcriptional factors regulate ochratoxin A biosynthesis in Aspergillus carbonarius. Int. J. Food Microbiol. 2013;166:479–486. doi: 10.1016/j.ijfoodmicro.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Ramakers C., Ruijter J.M., Deprez R.H.L., Moorman A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 43.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanderson K.E., Srb A.M. Heterokaryosis and parasexuality in the fungus Ascochyta imperfecta. Am. J. Bot. 1965;52:72–81. doi: 10.1002/j.1537-2197.1965.tb06759.x. [DOI] [PubMed] [Google Scholar]
- 45.Pollastro S., De Miccolis Angelini R.M., Natale P., Pastore C., Pascale M., Faretra F. Observations on ochratoxin A production in Aspergillus carbonarius and Aspergillus niger; Proceedings of the International Workshop: Ochratoxin A in Grapes and Wine: Prevention and Control; Marsala, Italy. 20–21 October 2005. [Google Scholar]
- 46.Visconti A., Pascale M., Centonze G. Determination of ochratoxin A in wine by means of immunoaffinity column clean-up and high-performance liquid chromatography. J. Chromatogr. 1999;864:89–101. doi: 10.1016/S0021-9673(99)00996-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.