Abstract
Effective decision making in the healthcare setting is highly dependent on access to reliable and robust data and information. A minimum data set is a standard assessment instrument that is used during the data collection process to ensure that decision makers have access to a consistent set of information. The objective of the current study was to develop a minimum data set for infertility patients that can be employed as the basis for an infertility registry in Iran. A systematic review resulted in the identification of 2,501 articles and 17 patient forms from infertility centers that were relevant to the study objectives. Of these, 10 articles met all the inclusion and exclusion criteria, and 232 data elements were subsequently extracted from these papers. The data elements were classified by three experts and validated via two rounds of a Delphi technique. The accessibility of the data elements was then evaluated during a focus group discussion. Finally, 146 data elements were selected as the minimum data set. The proposed minimum data set could provide the basis for standardization of infertility treatments. Synchronizing the various data sets that are currently in use will be necessary to allow sharing of data across infertility registries.
Keywords: common data elements; registries, infertility; assisted reproductive techniques
Introduction and Background
Infertility is a considerable health challenge in developing countries1 and is associated with poor mental and social outcomes.2 Depression, fear of divorce, remarriage, high treatment costs, and fear of uncertainty about the treatment outcomes are just some of the challenges that people who are suffering from infertility experience.3, 4, 5, 6 According to the World Health Organization (WHO), one in four married couples in developing countries encounter infertility problems.7 In Iran, fertility problems are experienced by 20.2 percent of couples (19.9 percent in urban areas and 22 percent in rural areas).8
A range of therapeutic methods of treating infertility are available, such as intrauterine insemination (IUI), in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI), and their application varies according to the cause of infertility. The interventions that are currently in use are costly and have negative side effects. Therefore, they should be utilized only if the chance of successful treatment is significant. Various models have been created to predict the likelihood of a successful pregnancy following medical intervention.9 When creating a predictive model, it is important to ensure that the data are accurate, complete, and aligned with the clinical goals.10 Decision makers who are responsible for the implementation of clinical and managerial healthcare policy rely on the availability of data and key information.11
One of the main objectives of data collection is to access information that can be employed to conduct an assessment of the available therapeutic interventions. In the case of infertility interventions, the final analysis of the likelihood of successful treatment is affected by the extent to which the available data are valid and comprehensive.12 For example, the growing effectiveness of assisted reproductive technology (ART) in relation to human reproduction is demonstrated by data on the efficacy and safety of such methods. Data on the safety of therapeutic methods and their outcomes are of significance to all stakeholders, including patients, healthcare planners, investigators, and ART centers.13
A minimum data set is recommended as a standard tool that can guide data collection.14 A minimum data set is a structure of information that is collated from different sources and is developed using definitions and procedures. This information facilitates the creation of a comprehensive database on a particular subject. A minimum data set can be used to standardize healthcare services in hospitals, nursing houses, and healthcare institutions. It can also be used to guide the data collection process that underpins a specific research study. Data based on a minimum data set can be used to assimilate broad views on healthcare policies.15 The recording of patient data elements improves the quality of healthcare and decreases costs.16 Patient registries are databases that often use a minimum data set to facilitate precise analysis.17, 18
To the best of our knowledge, a minimum data set has not yet been developed for infertility in Iran. The objective of the current study was to develop a minimum data set for infertility as a means of establishing an infertility registry in Iran that could expedite the collection of reliable and detailed data from patients who have been referred to infertility centers.
Methods
This descriptive, cross-sectional study was conducted in 2016. The infertility minimum data set was developed via a four-stage process:
Systematic review
Classification of the data elements
Validation of the data elements using the Delphi technique
Determination of the accessibility of data elements using focus group discussion
Systematic Review
A systematic review was conducted using sources from the PubMed, ScienceDirect, Scopus, Embase, Web of Science, IEEE Xplore, and Google Scholar databases. A keyword search of these databases was performed using words related to the concepts of minimum data set or infertility registry (dataset, dataset as topic, common data element, registries, minimum dataset) and keywords relating to infertility (in vitro fertilization, artificial insemination, intrauterine insemination, intracytoplasmic sperm injections, assisted reproductive technique, infertility). Keyword MeSH terms are shown in bold. The websites of infertility institutions were also searched for patient forms. Both searches were performed in the second week of June 2016. Databases were screened for English articles only without any limitation on time and type of study. The keywords and references of the articles identified during the initial search were also considered as a means of identifying additional keywords and other relevant articles (see Table 1).
Table 1.
Detailed Search Strategy
| Database | Reference Type | Search Fields | No. of Returned Articles |
|---|---|---|---|
| PubMed | All References | Title/abstract | 443 |
| Embase | All References | Title/Abstract/Key words | 114 |
| Web of Science | All References | Topic | 385 |
| ScienceDirect | All References | TITLE-ABSTR-KEY | 115 |
| Scopus | All References | TITLE-ABS-KEY | 1047 |
| IEEE Xplore | All References | MetaData and FullText | 397 |
The electronic database search was performed by one reviewer. The titles and abstracts of all articles were screened by two reviewers to identify articles that were relevant to the research objectives. One of these reviewers was the same person who conducted the initial database search. The full text of the articles was then assessed to ensure that the inclusion and exclusion criteria for the study were met. Data extraction was facilitated with the use of a checklist that contained the study objectives, setting, type of study, data sources, data collection methods (computer- or paper-based), main classification, and data elements. Patient forms were downloaded from the websites of the infertility institutions. The data elements were extracted from the forms and related articles, and duplicate items were deleted.
The inclusion criterion were all articles published in English that focused on the establishment of infertility registries and the development of an infertility minimum dataset, and patient forms from infertility institutions. Studies that reported registry data analysis without identifying the data elements were excluded. Seminar abstracts, letters to the editor, theses, dissertations, and position papers were also excluded.
Classification of the Data Elements
The articles identified during the first stage of the research employed various classifications of the data elements. Therefore, the classification applied to the extracted data elements was determined via separate two-hour meetings with three infertility experts. With all three experts' opinions taken into account, any classifications that the experts believed were not practical were omitted.
Validation of the Data Elements Using the Delphi Technique
The data elements were validated using two rounds of the Delphi technique. A two-column checklist was developed for the first round. The first column recorded whether each data element would be deleted or retained from the data set, while the second ranked the item according to the degree of importance based on a five-point Likert scale, ranging from low importance (1) to high importance (5). At the end of each classification, a row was provided for the data elements suggested by the experts (see Appendix 1). The concept of a minimum data set was explained to the participants, and they were asked to score the checklist elements based on the following question: “Do you think this data element is essential for an evaluation of an infertility patient's therapeutic status and to make a decision as to the appropriate treatment intervention?”
The level of agreement was considered to be a criterion for the acceptance of the data elements. Elements that were scored 4 or 5 by at least 50 percent of the experts were considered for inclusion in the minimum data set. Elements that received a score of 1 or 2 from at least 50 percent of the experts were excluded. The remaining elements were entered into the second round of the Delphi technique.
The same checklist that was used in the first round of the Delphi technique was used in the second round with one minor change: the data element suggestion row was removed. The results of the first-round analysis were given to the experts, and they were asked to determine the score for each data element listed in the checklist. Similar to the procedure followed in round 1, elements that received a score of 4 or 5 by at least 50 percent of the experts were considered for inclusion in the minimum data set. The remaining elements were disregarded.
Each round of the Delphi technique lasted four weeks. Both checklists were presented to the experts in person. The experts were blind to the scores given by the other experts. Similar scores were given to the response by the experts.
Accessibility of Data Elements Using Focus Group Discussion
To evaluate the accessibility of the proposed minimum data set, a focus group discussion was held with five experts as a means of obtaining their opinions on the recommended minimum data set. The focus group provided the experts with an opportunity to discuss and compare experiences.19 This session lasted two hours.
Results
Systematic Review
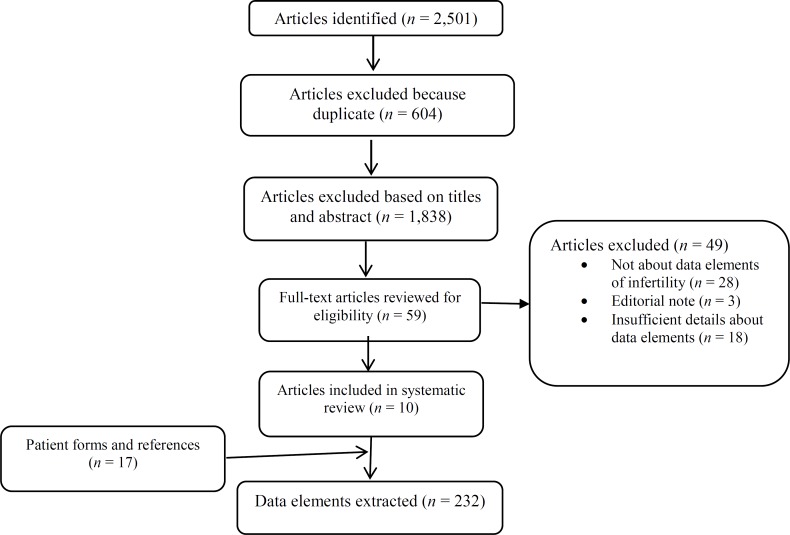
A total of 2,501 articles were obtained from different databases. After we excluded duplicate articles and reviewed the titles and abstracts of those initially identified, 66 articles were selected for the final survey. After the application of the study inclusion and exclusion criteria, 10 of these articles were considered for extraction of data elements. A further article was identified during an evaluation of the references contained in the shortlisted articles;20 however, it did not meet the inclusion criteria. A total of 17 patient forms were extracted from the International Committee Monitoring Assisted Reproductive Technologies (ICMART),21 the Infertility Family Research Registry (IFFR),22 the Society for Assisted Reproductive Technology (SART),23 and the IVF/ICSI forms of the National Health Service (NHS) of England and infertility centers.24 The patient form search continued until no new data elements were identified. A total of 232 data elements were identified on the forms and within the shortlisted articles. The details of these are provided in Figure 1.
Figure 1.
Systematic Review Flowchart
Of the 10 shortlisted articles, the classifications and data elements were completely described in four articles,25, 26, 27, 28 and these articles explained the method of determining the data elements that should be included on a registry.29, 30, 31 Only one of the related articles described the development of a minimum data set for infertility.32 Four articles focused on IVF registry,33, 34, 35, 36 four on infertility and ART registry,37, 38, 39, 40 and one on the aspects of reproduction.41 The characteristics of the 10 included articles are summarized in Table 2.
Table 2.
Characteristics of the Selected Articles
| First Author (Year) | Study Design | Setting | Source of Data | Method of Data Collection |
|---|---|---|---|---|
| Mansour et al. (2014) | Retrospective; cross-sectional survey | ART clinics | 18 centers (This report covers about 80% of the Egyptian ART activities in 2005, which means that about 20% of the data are missing.) | The International Committee Monitoring Assisted Reproductive Technology (ICMART) developed the data collection forms. The forms were sent to each ART clinic practicing in Egypt by the Egyptian IVF registry. Data came directly to the Egyptian registry anonymously. Participation was voluntary. |
| Gissler and Tiitinen (2001) | Retrospective; cross-sectional survey | Public and private IVF clinics | 19 clinics (7 public clinics and 12 private clinics) | Each year, all clinics providing IVF, intracytoplasmic sperm injection, and/or Frozen Embryo Transfer (FET) treatments receive 10-page data collection forms. All clinics returned completed questionnaires. The responsible data collector(s) checked the data collection forms and the final statistics. The clinics rechecked the forms for missing data and inconsistent information. The data collection was voluntary. |
| Guzick et al. (1990) | Development | IVF/GIFT clinic | Diverse origins; available to all staff members during a treatment cycle | Data are entered into the system on a series of nine input screens during the cycle. Data entry start with a “header” screen for background data and ends with a “notes” screen. On the network: data are entered at the site where they are created. On a single computer system: all of the data can be entered at the time of the completion of the cycle. |
| Blenstrup and Knudsen (2011) | Cross-sectional survey | Public and private fertility clinics | Public and private fertility clinics | 1994–2005: paper-based form. 2005: electronic reporting in Medical Birth Register, Danish National Patient Register |
| Germond et al. (2008) | Cross-sectional survey | ART clinics | ART clinics | An international, four-level reporting system |
| Dyer and Kruger (2011) | Retrospective; cross-sectional survey | ART clinics | 12 ART clinics | National data collection was started in a two-step process: In the first step, data collection was done using a Microsoft Excel spreadsheet (2009). In the second step, a software program was developed in collaboration with the Registro Latinoamericano de Reproduccion Asistida with the aim of online reporting of more data. Participation of centers was voluntary. |
| Rosenfeld et al. (1978) | Development | Hospital of the University of Pennsylvania | Hospital of the University of Pennsylvania | The physician completes the data abstract form. Information is recorded at the time of each visit or contact of patient. |
| Coetsee et al. (2014) | Development | Fertility clinics in South Africa | Infertility clinics | Web-based program |
| Westergaard et al. (1999) | Comparative, cross-sectional | Public and private fertility clinics | Nine private and six public clinics | Data from the IVF registry and cross-linking data to other registries |
| Westergaard et al. (2000) | Comparative, cross-sectional | Public and private fertility clinics | Nine private and six public clinics | Data from the IVF registry and cross-linking data to other registries |
Abbreviations: ART, assisted reproductive technology; IVF, in vitro fertilization.
Sources:
Mansour, M., Y. El-Faissal, and O. Kamal. “The Egyptian IVF Registry Report: Assisted Reproductive Technology in Egypt 2005.” Middle East Fertility Society Journal 19, no. 1 (2014): 16–21.
Gissler, M., and A. Tiitinen. “IVF Treatments and Their Outcomes in Finland in the 1990s.” Acta Obstetricia et Gynecologica Scandinavica 80, no. 10 (2001): 937–44.
Guzick, D. S., J. Boles, and R. Schadle. “Data Base Management System for Assisted Reproduction.” Journal of In Vitro Fertilization and Embryo Transfer 7, no. 5 (1990): 236–40.
Blenstrup, L. T., and L. B. Knudsen. “Danish Registers on Aspects of Reproduction.” Scandinavian Journal of Public Health 39, no. 7, suppl. (2011): 79–82.
Germond, M., F. Urner, A. Chanson, M. P. Primi, D. Wirthner, and A. Senn. “What Is the Most Relevant Standard of Success in Assisted Reproduction? The Cumulated Singleton/Twin Delivery Rates per Oocyte Pick-Up: The CUSIDERA and CUTWIDERA.” Human Reproduction 19, no. 11 (2004): 2442–44.
Dyer, S. J., and T. F. Kruger. “Assisted Reproductive Technology in South Africa: First Results Generated from the South African Register of Assisted Reproductive Techniques.” South African Medical Journal 102, no. 3 (2012): 167–70.
Rosenfeld, D. L., C. R. Garcia, W. Bullock, et al. “An Infertility Data Registry.” Fertility and Sterility 29, no. 1 (1978): 112–14.
Coetsee, J. L., T. F. Kruger, and D. Vine, “An Electronic Health Record for Infertility Clinics.” South African Journal of Obstetrics and Gynaecology 20, no. 1 (2014).
Westergaard, H. B., A. M. Tranberg Johansen, K. Erb, and A. Nyboe Andersen. “Danish National In-Vitro Fertilization Registry 1994 and 1995: A Controlled Study of Births, Malformations and Cytogenetic Findings.” Human Reproduction 14, no. 7 (1999): 1896–1902.
Westergaard, H. B., A. M. Tranberg Johansen, K. Erb, and A. Nyboe Andersen. “Danish National IVF Registry 1994 and 1995. Treatment, Pregnancy Outcome and Complications During Pregnancy.” Acta Obstetricia et Gynecologica Scandinavica 79, no. 5 (2000): 384–89.
Classification of the Data Elements
The demographic data of the study participants is presented in Table 3. The potential participants consisted of 19 gynecologists and infertility experts from two private infertility centers and one academic infertility center. However, six gynecologists and infertility experts did not participate in the study. Thus, 13 experts contributed. Of these, all 13 (68 percent) participated in the first round of the Delphi survey, and nine (47 percent) participated in the second round.
Table 3.
Demographic Characteristics of Participants
| Characteristics | Number of Participants |
|---|---|
| Specialty | |
| Gynecologist | 6 |
| Infertility fellowship | 7 |
| Gender | |
| Female | 13 |
| Male | 0 |
| Age (years) | |
| 30–40 | 2 |
| 40–50 | 5 |
| 50–60 | 4 |
| >60 | 2 |
| Work experience (years) | |
| <10 | 2 |
| 10–20 | 3 |
| 20–30 | 7 |
| >30 | 1 |
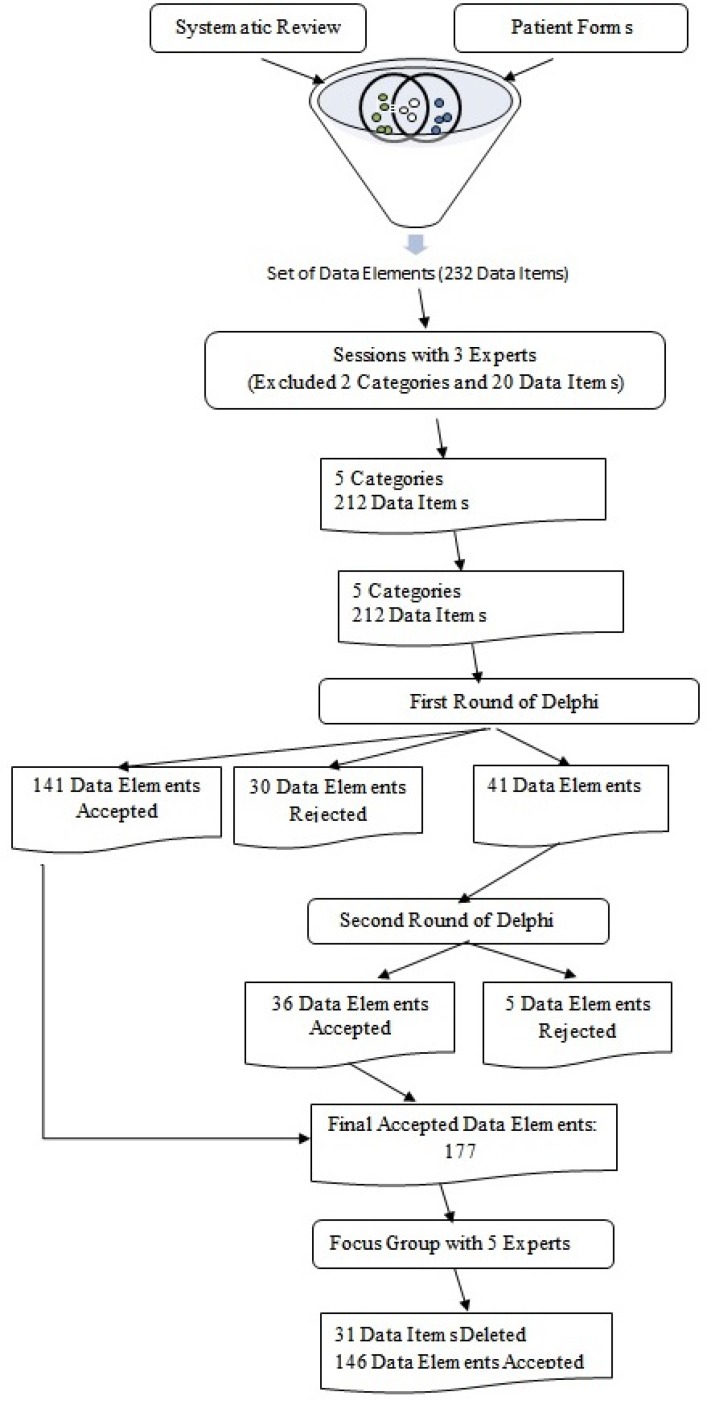
During the sessions with three experts, five classifications were identified: General Information, Patient History, Paraclinical Reports, Treatment Plan (IVF/ICSI, IUI, IO), and Treatment Outcome. The Lifestyle and Psychological classifications, in addition to their data elements (20 of the 232 data elements), were removed on the basis of the experts' opinions.
Validation of the Data Elements Using the Delphi Technique
A total of 212 final data elements were included in the Delphi survey. Of these, 141 data elements were approved in the first round, and 30 were rejected. A total of 41 data elements progressed to the second round of the Delphi survey. Of these, 36 were approved in round 2. Thus, on completion of the survey, 177 data elements were approved. Figure 2 contains a flowchart showing the process by which the elements to be included in the data set were determined.
Figure 2.
Data Validation Flowchart
Determination of the Accessibility of Data Elements Using Focus Group Discussion
In the focus group discussion, 31 data elements were removed by the experts to ensure accessibility of the data set. The final minimum data set included 146 data elements. The classification of these data elements is presented in Table 4.
Table 4.
Minimum Data Set and Classifications
| Data Elements in the General Information Class | |
| Record number | |
| National identifier | |
| Age | |
| Education | |
| Occupation | |
| Phone number | |
| Husband age | |
| Husband occupation | |
| Husband phone number | |
| Data Elements in the Patient History Class | |
| Female | Male |
| General | Addiction (smoking, addictive drugs, alcohol) |
| Height | Any medical problem |
| Weight | Name of problem |
| Duration of marriage | Previous operations Name of operations |
| Previous marriage | Previous marriage |
| Number of children in previous marriage | Number of children in previous marriage |
| Addiction (smoking, addictive drugs, alcohol) | Diseases in family |
| Any medical problem | Infertility problems |
| Name of problem | Recurrent miscarriage |
| Previous operations | Difficulties with ejaculation |
| Name of operation | Difficulties with erection |
| Medication allergies | Exposure of genitals to excessive heat |
| Name of medications | Injury to genitals |
| Diseases in family | Infection of prostate glands, penis, or testicles |
| Cancer | |
| Premature menopause | |
| Birth defects | |
| Hormonal disorders | |
| Infertility problems | |
| Recurrent miscarriage | |
| Blood clots | |
| Family relationship | |
| Menstrual | |
| Duration of bleeding | |
| Usual menstrual interval | |
| Spotting between menses | |
| Premenstrual syndrome (PMS) | |
| Dyspareunia | |
| Galactorrhea | |
| Hirsutism | |
| Dysmenorrhea | |
| Number of intercourses per week | |
| Normal | |
| Lower than normal | |
| No intercourse | |
| Pregnancy | |
| Duration of infertility | |
| Form of contraception used | |
| Period of time of contraception used | |
| Number of previous pregnancies | |
| Number of children | |
| Previous treatment of infertility | |
| In vitro fertilization (IVF)/ intracytoplasmic sperm injection (ICSI) | |
| Intrauterine insemination (IUI) | |
| Induction of ovulation (IO) | |
| Drugs for IUI/IO | |
| Number of treatment cycles received | |
| Outcome of previous treatment | |
| Complications during or after pregnancy | |
| Number of preterm births | |
| Number of miscarriages | |
| Number of ectopic pregnancies | |
| Data Elements in the Paraclinical Reports Class | |
| Female | Male |
| Laboratory tests | Sperm motility |
| Thyroid-stimulating hormone (TSH) | Sperm count |
| Follicle-stimulating hormone (FSH) | Morphology |
| Prolactin | Venereal disease research laboratory (VDRL) |
| Luteinizing hormone (LH) | Human immunodeficiency virus (HIV) 1, 2 |
| Anti-Mullerian hormone (AMH) | Hepatitis B surface antigen (HBsAg) |
| Fasting blood sugar (FBS) | Hepatitis C virus (HCV) |
| Complete blood count (CBC) | Human T-lymphotropic virus (HTLV) 1, 2 |
| Pap smear | |
| Venereal disease research laboratory (VDRL) | |
| Human immunodeficiency virus (HIV) 1, 2 | |
| Hepatitis B surface antigen (HBsAg) | |
| Hepatitis C virus (HCV) | |
| Human T-lymphotropic virus (HTLV) 1, 2 | |
| Ultrasound | |
| Antral follicle count (AFC) | |
| Number of mature follicles | |
| Uterine | |
| Right ovary (RO) | |
| Left ovary (LO) | |
| Endometrial thickness | |
| Laparoscopy, Hysteroscopy | |
| Uterine cavity | |
| Right tube (RT) | |
| Left tube (LT) | |
| Right ovary (RO) | |
| Left ovary (LO) | |
| Data Elements of Treatment Plan (IVF/ICSI, IUI, IO) Class | |
| Cause of infertility | |
| Male factor | |
| Endometriosis | |
| Ovarian factors | |
| Hormonal problems | |
| Male severe (ICSI) | |
| Tubal pathology ovary | |
| Unexplained | |
| Mix | |
| Type of infertility (primary or secondary) | |
| Number of IUI/IO cycle | |
| Number of IVF/ICSI cycle | |
| Drugs for stimulation | |
| Gonadotropin dose | |
| Sperm catch | |
| Type of cycle | |
| Antagonist protocol | |
| Agonist protocol | |
| Long | |
| Poor | |
| Shanghai protocol | |
| Donor (embryo, oocytes, surrogate's uterus) | |
| Heterologous oocytes | |
| Autologous oocytes | |
| Number of oocytes | |
| Quality of oocytes | |
| Number of transferred embryos | |
| Quality of transferred embryos | |
| Stage of embryos (cells) | |
| Embryo transfer (easy, difficult) | |
| Number of frozen embryos | |
| Thawed embryos for frozen embryo transfer | |
| Data Elements of Treatment Outcome Class | |
| Lost to follow-up of pregnancy | |
| Clinical pregnancy | |
| Intrauterine live pregnancy at week 7 or later | |
| Gestational sacs | |
| Fetal reductions | |
| Miscarriage | |
| Ectopic pregnancy | |
Discussion
According to the results of the study, 146 data elements were identified and subsequently categorized into the General Information, Patient History, Paraclinical Reports, Treatment Plan (IVF/ICSI, IUI, IO), and Treatment Outcome classifications as a minimum data set for the purpose of establishing an infertility registry in Iran. At present, there is no consistency in terms of the availability of resources and facilities used to treat infertility between developed and developing countries. Therefore, a minimum data set that was created in a developed country would not apply to a developing one. The minimum data set developed in the current study provides a mechanism by which information can be standardized and exchanged between infertility registries.
To ensure the inclusion of all relevant data elements, a systematic review was conducted before the experts were consulted to gauge their opinion. Hence, the new data elements were not suggested by the experts during round 1 of the Delphi. In total, 68 percent of the experts from three private and academic infertility institutions participated in the first round of the Delphi technique. It was assumed that the experts agreed to participate because they recognize the need for data recording systems, the standardization of patient care forms, better treatment follow-up, and access to reliable data for research purposes. Essentially, measuring change over time represents the golden key to health monitoring.42
The minimum data set developed in the current study included the demographic characteristics of the patients, medical history, laboratory test results, diagnosis, and treatment. No complete data on pregnancy outcomes and no data on treatment complications were included in the final minimum data set, with the exception of those items that relate to patients' referral back to the infertility center for treatment, which is recorded in the medical history. Data on labor, such as delivery week, infant's birth weight, type of delivery, and treatment complications, were not included in the minimum data set for infertility. This decision was based on the consensus of the respondents who attended the focus group discussion and was according to the accessibility criteria. Usually, patients continue to attend the infertility center until the point at which fetal heart activity is registered. Thereafter, they are referred to private or academic centers to receive prenatal care. If a birth registration system were in place, it could potentially be linked with the data in the minimum data set via the patient's national identifying code. Although a cumulative delivery rate has been referred to as the gold standard for successful infertility treatment,43 exact information of this type is not currently available. Therefore, despite the significance of live and stillbirth data elements, these data elements were excluded from the minimum data set.
An international data element of “clinical pregnancy” can be employed to assess the effectiveness of infertility treatment;44 therefore, this data element was included in the infertility minimum data set. Treatment complications were considered in 3 of the 10 included articles.45, 46, 47 Also, the South Africa ART registry reported that patients ceased being referred to infertility centers after the registration of pregnancy (fetal heartbeat).48
The Lifestyle and Psychological classifications, in addition to their data elements, were removed after the sessions with the three experts. These two classifications and their associated data elements were not included in any of the 10 related articles. The extent to which the data elements complied with the accessibility criteria were evaluated during the focus group discussions. One of the characteristics of data quality was the accessibility of data. This means that data elements should be easily acquirable and can be legally collected.49, 50 According to the World Health Organization, accessibility plays a significant role in the development of healthcare services.51 Data collection is costly and time consuming.52 Therefore, the accessibility of data elements was assessed because accessibility is important for minimizing missing data and accelerating data collection. Accessibility criteria were not evaluated in any of the 10 selected articles.
In the current study, three different methods were used to develop the minimum data set: individual sessions with experts, a Delphi technique, and a focus group discussion. Experts from three different infertility centers participated in the study, and coordination between them was difficult. Hence, it was not possible to hold several focus group discussions. The Delphi technique facilitated the process by which information was shared among specialists from different geographical areas. The Delphi technique is a structured, iterative method through which the approval and consensus of experts in related fields is sought.53 Therefore, we used this technique to determine which elements would be included in the minimum data set. We then needed to assess the extent to which the minimum data set was accessible. To decide which data elements should be collected by all infertility centers, experts from all three infertility centers discussed and finalized the data set during a focus group discussion. During this process, a distinct emphasis was placed on interaction among group members.54
The current study has some limitations. First, the opinions and evaluations that were employed to finalize the data set were derived from experts from only one city. This city is the second most populated city in Iran. Nevertheless, the minimum data set developed in the current study could be updated by specialists in other cities to develop infertility registries therein. Second, the infertility registries employ different terms to describe aspects of infertility. Therefore, after we initially searched for and reviewed related keywords, the search strategy was modified, and new keywords were added. This process led to the inclusion of registries and a minimum data set for the different infertility treatments in the second search. An additional minimum data set is necessary for prenatal care and pediatric care to capture data on the outcomes and effectiveness of infertility treatments. Therapeutic protocols and effective parameters for diagnosis and treatment may be changed. Thus, the minimum data set developed for infertility in the current study should be updated in the future.
Conclusion
The minimum data set developed for infertility in the current study could potentially pave the way for the development of a standardized approach to treating patients with infertility. At a minimum, it offers a means by which the different data sets that are currently used in different fertility registers can be combined into a single data set. The ability to assess infertility treatment and associated outcomes with respect to mothers and infants is facilitated by the current minimum data set. Developing an infertility registry using this minimum data set could help to generate higher-quality data that would lead to better clinical decisions.
Acknowledgments
This study was part of the first author's PhD dissertation, which was supported by a grant from Mashhad University of Medical Sciences. We would like to thank all experts from the three infertility centers who participated in the study and played a role in the validation of the data elements. We would also like to thank Mahdi Mohammadi, who contributed as a second reviewer.
Appendix 1
Delphi Technique Checklist Round 1
| 1- General Information | Removal=0 Retention=1 | Rating (1–5) | |
|---|---|---|---|
| 1-1 | Record number | ||
| 1-2 | National code | ||
| 1-3 | Age | ||
| 1-4 | Occupation | ||
| 1-5 | Education | ||
| 1-6 | Phone number | ||
| 1-7 | Husband national code | ||
| 1-8 | Husband age | ||
| 1-9 | Husband occupation | ||
| 1-10 | Husband education | ||
| 1-11 | Husband phone number | ||
| Comments: | |||
| Suggested Data Elements: | |||
| 2- History | Removal=0 Retention=1 | Rating (1–5) | |
|---|---|---|---|
| General (women) | |||
| 2-1 | Height | ||
| 2-2 | Weight | ||
| 2-3 | BMI | ||
| 2-4 | Race | ||
| 2-5 | Previous marriage | ||
| 2-5-1 | Number of pregnancies | ||
| 2-6 | Duration of marriage | ||
| 2-7 | Medication allergies | ||
| 2-7-1 | Name of medications | ||
| 2-7-2 | Type of reaction | ||
| 2-8 | Any allergy | ||
| 2-9 | Smoking | ||
| 2-9-1 | Number of cigarettes in a day | ||
| 2-9-2 | Number of years of consumption | ||
| 2-10 | Addiction and/or alcohol consumption | ||
| 2-10-1 | Number of glasses per week | ||
| 2-10-2 | Name of drug | ||
| 2-11 | Any medical problem | ||
| 2-11-1 | Name of problem | ||
| 2-12 | Previous operations | ||
| 2-12-1 | Name of operation | ||
| 2-14-2 | Treatment outcome | ||
| 2-13 | Diseases in family | ||
| 2-13-1 | Family relationship | ||
| 2-13-2 | Infertility | ||
| 2-13-3 | Premature menopause | ||
| 2-13-4 | Hormonal disorders | ||
| 2-13-5 | Recurrent miscarriage | ||
| 2-13-6 | Colon cancer | ||
| 2-13-7 | Uterine cancer | ||
| 2-13-8 | Ovarian cancer | ||
| 2-13-9 | Breast cancer | ||
| 2-13-10 | Blood clot (emboli) or DVT | ||
| 2-13-11 | Birth defects | ||
| Comments: | |||
| Suggested Data Elements: | |||
| General (men) | |||
| 2-14 | Previous marriage | ||
| 2-14-1 | Number of children from previous marriage | ||
| 2-15 | Smoking | ||
| 2-15-1 | Number of cigarettes per day | ||
| 2-15-2 | Number of years of consumption | ||
| 2-16 | Drugs and alcohol consumption | ||
| 2-16-1 | Number of glasses per week | ||
| 2-16-2 | Name of drug | ||
| 2-17 | Any medical problem | ||
| 2-17-1 | Name of problem | ||
| 2-18 | Previous operations | ||
| 2-18-1 | Name of operation | ||
| 2-18-2 | Treatment outcome | ||
| 2-19 | Diseases in family | ||
| 2-19-1 | Family relationship | ||
| 2-19-2 | Infertility problems | ||
| 2-19-3 | Recurrent miscarriage | ||
| 2-20 | Difficulties with ejaculation | ||
| 2-21 | Difficulties with erection | ||
| 2-22 | Exposure of genitals to excessive heat | ||
| 2-23 | Injury to genitals | ||
| 2-24 | Infection of prostate glands, penis, or testicles | ||
| Comments: | |||
| Suggested Data Elements: | |||
| Menstrual | |||
| 2-25 | Number of bleeding days | ||
| 2-25-1 | Maximum number of days | ||
| 2-25-2 | Minimum number of days | ||
| 2-26 | Spotting between menses | ||
| 2-27 | Usual menstrual interval | ||
| 2-27-1 | Maximum number of days | ||
| 2-27-2 | Minimum number of days | ||
| 2-28 | Premenstrual syndrome (PMS) | ||
| 2-28-1 | Low | ||
| 2-28-2 | Moderate | ||
| 2-28-3 | Severe | ||
| 2-29 | Number of intercourses per week | ||
| 2-29-1 | Normal | ||
| 2-29-2 | Lower than normal | ||
| 2-29-3 | No intercourse | ||
| 2-30 | Dyspareunia | ||
| 2-31 | Hirsutism | ||
| 2-32 | Galactorrhea | ||
| 2-33 | Dysmenorrhea | ||
| Comments: | |||
| Suggested Data Elements: | |||
| Pregnancy | |||
| 2-34 | Number of previous pregnancies | ||
| 2-34-1 | Number of children | ||
| 2-34-2 | Was pregnancy natural or by ART? | ||
| 2-35 | Duration of infertility | ||
| 2-36 | Any complication during or after pregnancy | ||
| 2-36-1 | Number of preterm births (before 37 weeks) | ||
| 2-36-2 | Number of miscarriages | ||
| 2-36-3 | Number of ectopic pregnancies | ||
| 2-37 | Form of contraception used | ||
| 2-38 | Previous treatment of infertility | ||
| 2-38-1 | In vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) | ||
| 2-38-2 | Intrauterine insemination (IUI) | ||
| 2-38-3 | Induction of ovulation (IO) | ||
| 2-38-3-1 | Drugs for IUI/IO | ||
| 2-38-4 | Number of treatment cycles received | ||
| 2-38-5 | Outcome of previous treatment | ||
| Comments: | |||
| Suggested Data Elements: | |||
| 3- Paraclinical Reports | Removal=0 Retention=1 | Rating (1–5) | |||
|---|---|---|---|---|---|
| Women | |||||
| 3-1 | Fasting blood sugar (FBS) | ||||
| 3-2 | Red blood cell count (RBC) | ||||
| 3-3 | White blood cell count (WBC) | ||||
| 3-4 | Hemoglobin | ||||
| 3-5 | Hematocrit | ||||
| 3-6 | Mean Corpuscular Hemoglobin(MCH) | ||||
| 3-7 | Mean Corpuscular Hemoglobin Concentration(MCHC) | ||||
| 3-8 | Mean Corpuscular Volume (MCV) | ||||
| 3-9 | Complete blood count (CBC) | ||||
| 3-10 | Blood group, Rh | ||||
| 3-11 | Cholesterol | ||||
| 3-12 | Triglycerides | ||||
| 3-13 | Venereal disease research laboratory (VDRL) | ||||
| 3-14 | Hepatitis C virus (HCV) | ||||
| 3-15 | Human immunodeficiency virus (HIV) 1, 2 | ||||
| 3-16 | Hepatitis B surface antigen (HBsAg) | ||||
| 3-17 | Human T-lymphotropic virus (HTLV) 1, 2 | ||||
| 3-18 | Rubella | ||||
| 3-19 | Follicle-stimulating hormone (FSH) | ||||
| 3-20 | Luteinizing hormone (LH) | ||||
| 3-21 | Estradiol (E2) | ||||
| 3-22 | Anti-Mullerian hormone (AMH) | ||||
| 3-23 | Progesterone | ||||
| 3-24 | Thyroid-stimulating hormone (TSH) | ||||
| 3-25 | Prolactin | ||||
| 3-26 | Testosterone | ||||
| 3-27 | Dehydroepiandrosterone | ||||
| 3-28 | Thrombophilia | ||||
| 3-29 | Pap smear | ||||
| 3-30 | Genetic testing | ||||
| 3-31 | Torch syndrome | ||||
| Ultrasound | |||||
| 3-32 | AFC | ||||
| 3-33 | Uterine | ||||
| 3-34 | Left ovary | ||||
| 3-35 | Right ovary | ||||
| 3-36 | Endometrial thickness | ||||
| Laparoscopy and hysteroscopy | |||||
| 3-37 | Uterine cavity (normal, abnormal) | ||||
| 3-38 | Right tube (open, blocked) | ||||
| 3-39 | Left tube (open, blocked) | ||||
| 3-40 | Right ovary | ||||
| 3-41 | Left ovary | ||||
| Comments: | |||||
| Suggested Data Elements: | |||||
| Men | |||||
| 3-42 | Semen analysis | ||||
| 3-42-1 | Sperm count | ||||
| 3-42-2 | Morphology | ||||
| 3-42-3 | Motility | ||||
| 3-42-4 | Degree of motility | ||||
| 3-42-5 | Ratio of progressive motile sperm | ||||
| 3-43 | Venereal disease research laboratory (VDRL) | ||||
| 3-44 | Hepatitis C virus (HCV) | ||||
| 3-45 | Human immunodeficiency virus (HIV) 1, 2 | ||||
| 3-46 | Hepatitis B surface antigen (HBsAg) | ||||
| 3-47 | Human T-lymphotropic virus (HTLV) 1, 2 | ||||
| Comments: | |||||
| Suggested Data Elements: | |||||
| 4- Treatment Plan | Removal=0 Retention=1 | Rating (1–5) | |
|---|---|---|---|
| 4-1 | Type of infertility (primary or secondary) | ||
| 4-2 | Cause of infertility | ||
| 4-2-1 | Male factor | ||
| 4-2-2 | Endometriosis | ||
| 4-2-3 | Ovarian factors | ||
| 4-2-4 | Hormonal problems | ||
| 4-2-5 | Male severe (ICSI) | ||
| 4-2-6 | Tubal pathology ovary | ||
| 4-2-7 | Unexplained | ||
| 4-2-8 | Mix | ||
| 4-3 | Number of IUI/IO cycles | ||
| 4-4 | Number of IVF/ICSI cycles | ||
| 4-5 | Type of cycle | ||
| 4-5-1 | Agonist (long, poor) | ||
| 4-5-2 | Antagonist (flexible, fixed) | ||
| 4-5-3 | Shanghai protocol | ||
| 4-5-4 | Donor (embryo, oocytes, surrogate's uterus) | ||
| 4-6 | Drugs for stimulation | ||
| 4-7 | Gonadotrophins | ||
| 4-7-1 | Type of gonadotrophins (follicle-stimulating hormone [FSH], luteinizing hormone [LH], Human Molecular Genetics(HMG)) | ||
| 4-7-2 | Number of ampoules | ||
| 4-7-3 | Gonadotropin dose | ||
| 4-8 | Number of mature follicles (day of Human Chorionic Gonadotropin (HCG)) | ||
| 4-9 | Number of oocytes | ||
| 4-10 | Heterologous oocyte | ||
| 4-11 | Antilog oocyte | ||
| 4-12 | Fertilization method | ||
| 4-13 | Sperm origin (husband, donor) | ||
| 4-14 | Sperm catch (ejaculation, Testicular Sperm Extraction, Percutaneous Epididymal Sperm Aspiration, cryopreservation) | ||
| 4-15 | Number of embryos | ||
| 4-16 | Number of embryos cryopreserved | ||
| 4-17 | Quality of embryo (A, B, C, D) | ||
| 4-18 | Day of transfer (2, 3, 4, 5, 6) | ||
| 4-19 | Stage of embryos (cells) | ||
| 4-20 | Number of fresh embryos transferred | ||
| 4-21 | Number of thawed embryos transferred | ||
| 4-22 | Preimplantation genetic diagnosis (PGD) cycle | ||
| 4-23 | Genetic disorder (monogenic, chromosomal) | ||
| 4-24 | Preimplantation genetic screening (PGS) cycle | ||
| 4-25 | Embryo transfer (easy/difficult) | ||
| Comments: | |||
| Suggested Data Elements: | |||
| 5- Treatment Outcome | Removal=0 Retention=1 | Rating (1–5) | |
|---|---|---|---|
| 5-26 | Lost to follow-up (pregnancy) | ||
| 5-26-1 | Clinical pregnancy | ||
| 5-26-2 | Intrauterine live pregnancy at week 7 or later | ||
| 5-26-3 | Gestational sacs | ||
| 5-26-4 | Fetal reductions | ||
| 5-26-5 | Miscarriage | ||
| 5-26-6 | Ectopic pregnancy | ||
| 5-27 | Lost to follow-up of delivery | ||
| 5-28 | Delivery (live birth, stillbirth) | ||
| 5-28-1 | Mode of delivery | ||
| 5-28-2 | Gestational weeks at delivery | ||
| 5-28-3 | Birth weight | ||
| 5-28-4 | Number of infants at delivery | ||
| 5-28-5 | Sex of newborn | ||
| 5-29 | Presence of complication | ||
| 5-29-1 | Ovarian hyperstimulation syndrome (OHSS) requiring admission | ||
| 5-29-2 | Thrombosis | ||
| 5-29-3 | Pelvic infection, requiring admission | ||
| 5-29-4 | Maternal death | ||
| Comments: | |||
| Suggested Data Elements: | |||
Contributor Information
Masoumeh Abbasi, Department of Medical Informatics in the School of Medicine at Mashhad University of Medical Sciences in Mashhad, Iran.
Leila Ahmadian, Research Center of the Institute for Future Studies in Health at Kerman University of Medical Sciences in Kerman, Iran.
Malihe Amirian, School of Medicine at Mashhad University of Medical Sciences in Mashhad, Iran.
Hamed Tabesh, Department of Medical Informatics in the School of Medicine and manager of the Health Information Technology Center at Mashhad University of Medical Sciences in Mashhad, Iran.
Saeid Eslami, Department of Medical Informatics in the School of Medicine and the Pharmaceutical Research Center at Mashhad University of Medical Sciences in Mashhad, Iran and a researcher in the Department of Medical Informatics in the Academic Medical Center at University of Amsterdam in Amsterdam, the Netherlands.
Notes
- 1.Zhang H., Wang S., Zhang S., Wang T., Deng X. “Increasing Trend of Prevalence of Infertility in Beijing.”. Chinese Medical Journal. 2014;127(4):691–95. [PubMed] [Google Scholar]
- 2.Iliyasu Z., Galadanci H.S., Abubakar I.S., Bashir F.M., Salihu H.M., Aliyu M.H. “Perception of Infertility and Acceptability of Assisted Reproduction Technology in Northern Nigeria.”. Nigerian Journal of Medicine. 2013;22(4):341–47. [PubMed] [Google Scholar]
- 3.Wiersema N. J., Drukker A.J., Mai B.T., Giang H.N., Nguyen T.N., Lambalk C.B. “Consequences of Infertility in Developing Countries: Results of a Questionnaire and Interview Survey in the South of Vietnam.”. Journal of Translational Medicine. 2006;4:54. doi: 10.1186/1479-5876-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyer S. J., Patel M. “The Economic Impact of Infertility on Women in Developing Countries—a Systematic Review.”. Facts, Views & Vision in ObGyn. 2012;4(2):102–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Harvard Mental Health Letter. “The Psychological Impact of Infertility and Its Treatment.” May 2009. Harvard Health Publication. Available at https://www.health.harvard.edu/newsletter_article/The-psychological-impact-of-infertility-and-its-treatment.
- 6.Van Balen F., Bos H.M.W. “The Social and Cultural Consequences of Being Childless in Poor-Resource Areas.”. Facts, Views & Vision in ObGyn. 2009;1(2):106–21. [PMC free article] [PubMed] [Google Scholar]
- 7.“Sexual and Reproductive Health.” World Health Organization. Available at http://www.who.int/reproductivehealth/topics/infertility/burden/en/
- 8.Akhondi M. M. (2015). Fertility status in Iran. International Congress on Obstetrics and Gynecology, Tehran, Iran. “Speech of Deputy of Research and Technology, Ministry of Health and Medical Education at the Opening of Obstetrics and Gynecology Congress.” 2015.
- 9.McLernon D. J., et al. “Clinical prediction models to inform individualized decision-making in subfertile couples: a stratified medicine approach.”. Hum Reprod. 2014;29(9):1851–1858. doi: 10.1093/humrep/deu173. [DOI] [PubMed] [Google Scholar]
- 10.Royston P., Moons K.G., Altman D.G., Vergouwe Y. “Prognosis and Prognostic Research: Developing a Prognostic Model.”. British Medical Journal. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 11.Wager K. A., Lee F.W., Glaser J.P. In Health Care Information Systems: A Practical Approach for Health Care Management. San Francisco, CA: Jossey-Bass; 2009. “Health Care Data Quality.”; pp. 41–60. [Google Scholar]
- 12.Germond M., Wirthner D., Senn A. “Core Data for Assisted Reproductive Technology Registers: Results of a Consensus Meeting.”. Reproductive Biomedicine Online. 2008;17(6):834–40. doi: 10.1016/s1472-6483(10)60412-9. [DOI] [PubMed] [Google Scholar]
- 13.Dyer S. J., Kruger T.F. “Assisted Reproductive Technology in South Africa: First Results Generated from the South African Register of Assisted Reproductive Techniques.”. South African Medical Journal. 2012;102(3):167–70. doi: 10.7196/samj.5311. [DOI] [PubMed] [Google Scholar]
- 14.Abdelhak M., Grostick S., Hanken M. A. Health Information: Management of a Strategic Resource. 3rd ed. St. Louis, MO: Elsevier; 2007. [Google Scholar]
- 15.Gould D. J. “Minimum Data Set.”. In: Loue S. J. D., Sajatovic M., editors. Encyclopedia of Aging and Public Health. Boston, MA: Springer US; 2008. [Google Scholar]
- 16.Ahmadi M., Mohammadi A., Chraghbaigi R., Fathi T., Shojaee Baghini M. “Developing a Minimum Data Set of the Information Management System for Orthopedic Injuries in Iran.”. Iranian Red Crescent Medical Journal. 2014;16(7) doi: 10.5812/ircmj.17020. e17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gliklich R. E., Dreyer N. A., editors. Registries for Evaluating Patient Outcomes: A User's Guide. 3rd ed. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 18.de Souza M. P., Miller V. R. “Significance of Patient Registries for Dermatological Disorders.”. Journal of Investigative Dermatology. 2012;132:1749–52. doi: 10.1038/jid.2012.168. [DOI] [PubMed] [Google Scholar]
- 19.McCann L. J., Kirkham J.J., Wedderburn L.R., Pilkington C., Huber A.M., Ravelli A., et al. “Development of an Internationally Agreed Minimal Dataset for Juvenile Dermatomyositis (JDM) for Clinical and Research Use.”. Trials. 2015;16:268. doi: 10.1186/s13063-015-0784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tadir Y., Kaplan-Kraicer R., Kelly R., Tepper R., Amit S., Toker R., et al. “Practical and Informative Charts for in Vitro Fertilization and Embryo Transfer.”. Journal of In Vitro Fertilization and Embryo Transfer. 1987;4(6):348–52. doi: 10.1007/BF01555385. [DOI] [PubMed] [Google Scholar]
- 21.Nygren K. G., Zegers-Hochschild F., Adamson D., de Mouzon J., Mansour R., Ishihara O O., et al. The ICMART Tool Box for ART Data Collection. Palo Alto, CA: International Committee Monitoring Assisted Reproductive Technologies (ICMART); 2011. [Google Scholar]
- 22.Infertility Family Research Registry. Available at https://www.ifrr-registry.org/.
- 23.Society for Assisted Reproductive Technology “What Are My Chances with ART?” Available at https://www.sartcorsonline.com/Predictor/Patient
- 24.National Health Service (England) Application for Prior Approval for Funding N-SC037 IVF/ICSI. 2016.
- 25.Germond M., Wirthner D., Senn A. “Core Data for Assisted Reproductive Technology Registers: Results of a Consensus Meeting.”. [DOI] [PubMed]
- 26.Rosenfeld D. L., Garcia C.R., Bullock W., et al. “An Infertility Data Registry.”. Fertility and Sterility. 1978;29(1):112–14. [PubMed] [Google Scholar]
- 27.Guzick D. S., Boles J., Schadle R. “Data Base Management System for Assisted Reproduction.”. Journal of In Vitro Fertilization and Embryo Transfer. 1990;7(5):236–40. doi: 10.1007/BF01129525. [DOI] [PubMed] [Google Scholar]
- 28.Coetsee J. L., Kruger T. F., Vine D. “An Electronic Health Record for Infertility Clinics.”. South African Journal of Obstetrics and Gynaecology. 2014;20(1) [Google Scholar]
- 29.Ibid.
- 30.Gissler M., Tiitinen A. “IVF Treatments and Their Outcomes in Finland in the 1990s.”. Acta Obstetricia et Gynecologica Scandinavica. 2001;80(10):937–44. doi: 10.1034/j.1600-0412.2001.801011.x. [DOI] [PubMed] [Google Scholar]
- 31.Mansour M., El-Faissal Y., Kamal O. “The Egyptian IVF Registry Report: Assisted Reproductive Technology in Egypt 2005.”. Middle East Fertility Society Journal. 2014;19(1):16–21. [Google Scholar]
- 32.Germond M., Wirthner D., Senn A. “Core Data for Assisted Reproductive Technology Registers: Results of a Consensus Meeting.”. doi: 10.1016/s1472-6483(10)60412-9. [DOI] [PubMed] [Google Scholar]
- 33.Gissler M., Tiitinen A. “IVF Treatments and Their Outcomes in Finland in the 1990s.”. doi: 10.1034/j.1600-0412.2001.801011.x. [DOI] [PubMed] [Google Scholar]
- 34.Mansour M., El-Faissal Y., Kamal O. “The Egyptian IVF Registry Report: Assisted Reproductive Technology in Egypt 2005.” [Google Scholar]
- 35.Westergaard H. B., Tranberg Johansen A.M., Erb K., Nyboe Andersen A. “Danish National In-Vitro Fertilization Registry 1994 and 1995: A Controlled Study of Births, Malformations and Cytogenetic Findings.”. Human Reproduction. 1999;14(7):1896–1902. doi: 10.1093/humrep/14.7.1896. [DOI] [PubMed] [Google Scholar]
- 36.Westergaard H. B., Tranberg Johansen A.M., Erb K., Nyboe Andersen A. “Danish National IVF Registry 1994 and 1995. Treatment, Pregnancy Outcome and Complications During Pregnancy.”. Acta Obstetricia et Gynecologica Scandinavica. 2000;79(5):384–89. [PubMed] [Google Scholar]
- 37.Dyer S. J., Kruger T.F. “Assisted Reproductive Technology in South Africa: First Results Generated from the South African Register of Assisted Reproductive Techniques.”. doi: 10.7196/samj.5311. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld D. L., Garcia C.R., Bullock W., et al. “An Infertility Data Registry.”. [PubMed] [Google Scholar]
- 39.Guzick D. S., Boles J., Schadle R. “Data Base Management System for Assisted Reproduction.”. doi: 10.1007/BF01129525. [DOI] [PubMed] [Google Scholar]
- 40.Coetsee J. L., Kruger T. F., Vine D. “An Electronic Health Record for Infertility Clinics.” [Google Scholar]
- 41.Blenstrup L. T., Knudsen L.B. “Danish Registers on Aspects of Reproduction.”. Scandinavian Journal of Public Health. 2011;39(no. 7, suppl.):79–82. doi: 10.1177/1403494811399957. [DOI] [PubMed] [Google Scholar]
- 42.Temmerman M., Foster L.B., Hannaford P., Cattaneo A., Olsen J., Bloemenkamp K.W., et al. “Reproductive Health Indicators in the European Union: The REPROSTAT Project.”. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2006;126(1):3–10. doi: 10.1016/j.ejogrb.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 43.Germond M., Urner F., Chanson A., Primi M.P., Wirthner D., Senn A. “What Is the Most Relevant Standard of Success in Assisted Reproduction? The Cumulated Singleton/Twin Delivery Rates per Oocyte Pick-Up: The CUSIDERA and CUTWIDERA.”. Human Reproduction. 2004;19(11):2442–44. doi: 10.1093/humrep/deh501. [DOI] [PubMed] [Google Scholar]
- 44.Germond M., Wirthner D., Senn A. “Core Data for Assisted Reproductive Technology Registers: Results of a Consensus Meeting.”. doi: 10.1016/s1472-6483(10)60412-9. [DOI] [PubMed] [Google Scholar]
- 45.Ibid.
- 46.Coetsee J. L., Kruger T. F., Vine D. “An Electronic Health Record for Infertility Clinics.” [Google Scholar]
- 47.Mansour M., El-Faissal Y., Kamal O. “The Egyptian IVF Registry Report: Assisted Reproductive Technology in Egypt 2005.” [Google Scholar]
- 48.Dyer S. J., Kruger T.F. “Assisted Reproductive Technology in South Africa: First Results Generated from the South African Register of Assisted Reproductive Techniques.”. doi: 10.7196/samj.5311. [DOI] [PubMed] [Google Scholar]
- 49.Wager K. A., Lee F.W., Glaser J.P. “Health Care Data Quality.” [Google Scholar]
- 50.American Health Information Management Association (AHIMA) “Statement on Quality Healthcare Data and Information.” December 2007. Available at http://bok.ahima.org/doc?oid=101304.
- 51.Ghaneie M., Rezaie A., Ghorbani N.R., Heidari R., Arjomandi M., Zare M. “Designing a Minimum Data Set for Breast Cancer: A Starting Point for Breast Cancer Registration in Iran.”. Iranian Journal of Public Health. 2013;42(1):66–73. [PMC free article] [PubMed] [Google Scholar]
- 52.Germond M., Wirthner D., Senn A. “Core Data for Assisted Reproductive Technology Registers: Results of a Consensus Meeting.”. doi: 10.1016/s1472-6483(10)60412-9. [DOI] [PubMed] [Google Scholar]
- 53.Booth A., Clarke M., Ghersi D., Moher D., Petticrew M., Stewart L. “Establishing a Minimum Dataset for Prospective Registration of Systematic Reviews: An International Consultation.”. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027319. e27319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ping W.L. “Focus Group Discussion: A Tool for Health and Medical Research.”. Singapore Medical Journal. 2008;49(3):256–61. [PubMed] [Google Scholar]