Abstract
Objectives
There is growing evidence for the role of systemic inflammation in Alzheimer’s disease (AD) and other neurodegenerative diseases; however the systemic inflammatory profile in dementia with Lewy bodies (DLB) has never before been investigated. This study aimed to characterise systemic inflammatory mediators in established DLB and AD, as well as in their prodromal, mild cognitive impairment (MCI) phases.
Methods
We obtained plasma samples from patients with DLB (n=37), AD (n=20), MCI with DLB profile (n=38), MCI with AD profile (n=20) and healthy control subjects (n=20). The following inflammatory biomarkers were measured using Roche cobas c702 and Meso Scale Discovery V-Plex Plus: high-sensitivity C-reactive protein, interferon-gamma, interleukin (IL)-10, IL-12p70, IL-13, IL-1beta, IL-2, IL-4, IL-6, IL-8 and tumour necrosis factor-alpha.
Results
We found significantly higher levels of IL-10, IL-1beta, IL-4 and IL-2 in both MCI groups (P<0.001), while there was no significant difference in inflammatory markers between dementia groups and controls. Furthermore, increased disease severity was associated with lower levels of IL-1beta, IL-2 and IL-4 (P<0.05).
Interpretation
We have shown for the first time that in both DLB and AD, increased peripheral inflammation occurs early at the MCI disease stages. These data support a role for inflammation early in the disease process, and have important implications for the stage of disease where trials of anti-inflammatory medication should be focused.
Introduction
Dementia affects around 50 million people worldwide, and this is expected to rise to 131.5 million by 2050. One of the major problems in dementia care is the lack of effective treatments, with current pharmacological therapies having limited benefit.
Theories about the role of neuroinflammation in the progression of degenerative dementia have been gaining interest due to their therapeutic potential. It has been suggested that neuronal damage in chronic neurodegeneration leads to a damaging proinflammatory microglial response.1 In Alzheimer’s disease (AD) there has been considerable evidence to show that peripheral and central inflammations play a key role in the pathogenesis of the disease. While serum tumour necrosis factor (TNF)-alpha in particular appears to be highly associated with cognitive decline,2 associations have been made with several other systemic inflammatory markers.3 Longitudinal studies have found that inflammation occurs years before AD onset,4 5 and cross-sectional studies have found a large increase in inflammatory markers in the mild stages of disease.6 Furthermore, genome-wide association studies (GWAS) have identified several risk AD candidate genes for inflammatory pathways, which are strongly supportive of inflammation playing a critical role in early AD aetiology.7
Dementia with Lewy bodies (DLB) is the second most common cause of degenerative dementia, and shares many clinical characteristics with AD. A recent meta-analysis showed that there is increased systemic inflammation in patients with Parkinson’s disease, which is closely related to DLB.8 It is therefore likely that the neuroinflammatory processes occurring in AD are also involved in driving neurodegeneration in DLB, with cytokines such as interleukin (IL)-1 being implicated in the neuropathological changes characteristic of both conditions.9
Only two studies have assessed inflammation in patients with DLB, both using cerebrospinal fluid (CSF), with one finding lower levels of IL-6 and the other finding no significant difference.10 11 No studies have examined inflammatory markers in the blood compared with controls, although a small exploratory study found that increased peripheral levels of IL-6 were associated with cognitive impairment in patients with DLB.12 The accessibility and practicality of using peripheral blood to monitor neuroinflammation in patients make this an attractive option. Furthermore, the increasing evidence that peripheral inflammation and neuroinflammation are closely related suggests that altered systemic inflammatory markers reflect neurodegenerative disease.13 Therefore, there is a need to investigate systemic inflammatory markers in patients with DLB as markers of brain inflammation.
The aim of this study was to characterise the plasma cytokine profile and C-reactive protein (CRP) levels of patients with DLB and compare this with patients with AD and healthy control subjects. Furthermore, we aimed to compare this with patients with mild cognitive impairment (MCI) thought to be due to AD and DLB to assess inflammation at this earlier pre-dementia stage. We hypothesised that the inflammatory profile in patients with DLB would be similar to that seen in patients with AD, and would be raised compared with healthy control subjects. Furthermore, we hypothesised that patients with MCI would have raised inflammatory markers compared with healthy control subjects and patients with dementia.
Methods
Subjects
Patients with dementia and MCI were recruited from the same memory clinics and dementia services including neurology and geriatrics in the North-East of England. All patients were aged over 60 and had provided written informed consent, or in cases where capacity was lacking their participation in the study was discussed with a consultee in accordance with UK legislation.
Patients with dementia had a Mini Mental State Examination (MMSE) score of at least 12. Diagnosis of DLB was made based on the International Consensus criteria,14 and patients with AD were diagnosed based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria.15 16 Patients with MCI met the National Institute on Aging and Alzheimer’s Association MCI criteria.16 Probable Lewy Body MCI (MCI-LB) was diagnosed using the Diagnostic and Statistical Manual of Mental Disorders 5 criteria,17 with the inclusion of dopaminergic imaging so that probable MCI-LB was diagnosed when at least two core or suggestive features of DLB were present, but in the absence of dementia. If none of these symptoms were present, the patient was classified as MCI-AD. Dementia and MCI diagnoses were made by a consensus panel of three experienced clinicians. Healthy control subjects were recruited from patients’ friends or family, or people who had already indicated a willingness to participate in research.
We further classified participants as having amnestic subtype if their Rey delay score was less than 3 and as non-amnestic if their Rey delay score was 3 or more in order to ascertain whether there was any difference in inflammation.
Exclusion criteria included severe physical, neurological or psychiatric illness, history of alcohol excess, and use of psychotropic drugs. We also excluded people who had a history or evidence of a stroke and those with possible or probable vascular dementia. We did not exclude people with inflammatory or autoimmune diseases; however, information regarding this was collected and taken into account during analysis.
Assessments
All patients were assessed by the equivalent of a board-certified medical practitioner, including a physical and neurological examination, and assessment of parkinsonism used the Movement Disorder Society Unified Parkinson’s Disease Rating Scale Part III (UPDRS). They were free from acute inflammatory illness, and their illness burden was assessed using the Cumulative Illness Rating Scale-Geriatrics (CIRS-G). Cognition was assessed using the Addenbrooke’s Cognitive Examination Revised (ACE-R) and MMSE.
Psychiatric symptoms were assessed using the Neuropsychiatric Inventory and the Geriatric Depression Scale. Activities of daily living were assessed using the Instrumental Activities of Daily Living Scale and Bristol Activities of Daily Living Scale. Doses of levodopa and antidementia medications were documented. Based on these assessments, healthy control subjects did not show any evidence of dementia. Positron emission tomography (PET) imaging was carried out on all dementia and healthy comparison subjects to investigate the amyloid burden in the brain.
Measurement of cytokines and high-sensitivity CRP
Venous blood samples were taken from all subjects using EDTA tubes, which were then centrifuged and the plasma removed. Samples were stored at −80° until assays were performed. Cytokine assays were performed using the Meso Scale Discovery V-Plex Plus Proinflammatory Panel 1, which included interferon (IFN)-gamma, IL-1beta, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13 and TNF-alpha. Assays were analysed at the National Institute for Health and Research-Newcastle University Biomarkers Facility according to the manufacturer’s protocol, and samples were processed in triplicates. Samples that were under the limit of detection for a particular cytokine (<0.05 for IFN-gamma, <0.03 for IL-13, <0.02 for IL-12p70 and <0.01 for all other cytokines) had cytokine levels that were low enough to be undistinguishable from background noise, and therefore these samples were treated as having ‘zero’ levels of that cytokine. High-sensitivity CRP (hsCRP) was analysed using Roche cobas c702, and was carried out at the Royal Victoria Infirmary in Newcastle upon Tyne.
Analysis
Statistical analysis was completed using IBM SPSS Statistics V.23 software (http://www-03.ibm.com/software/products/en/spss-statistics). Data distribution and normality were assessed using the Shapiro-Wilk test, and log transformations were performed to attempt to normalise any skewed data. Comparisons between diagnostic groups were carried out using analysis of variance or Kruskal-Wallis tests depending on normality of the data following log transformations. Where gender differences between groups were present, gender was added as a covariant in the analysis. Bonferroni correction was used to correct for multiple comparisons, so that P<0.001 was required for significance. Correlations were sought between inflammatory markers and patient characteristics using Spearman’s rank correlation, due to some of the data not being normally distributed following log transformation. Finally, significant differences in cytokine levels were sought between participants with amyloid-positive and amyloid-negative PET scans, and between participants with amnestic and non-amnestic subtype.
Results
One hundred and thirty-six subjects took part in this study. Of these, 20 were healthy control subjects, 20 with AD, 37 with DLB, 38 with MCI-LB and 21 with MCI-AD (see table 1 for full details of subject characteristics). The mean age of the whole cohort was 76.2 (±6.9) years, and this was not significantly different between any of the groups. In all groups except the MCI-AD group, there were more men than women. Disease duration was around 2 years in both the DLB and AD patients. As expected, UPDRS was higher in the DLB groups compared with all other groups, the ACE-R and MMSE scores were lower in the dementia and MCI groups compared with healthy control subjects, and a higher proportion of patients with dementia were taking anti-dementia medications than patients with MCI. The proportion of patients taking anti-inflammatory medications ranged between 25% and 66%, but in the majority of cases this was low-dose aspirin (75 mg), prescribed for its vascular effects. Eight participants were taking a non-aspirin non-steroidal anti-inflammatory drug, and seven were taking a steroid; these were spread evenly between all groups.
Table 1.
Differential diagnosis for decreased conscious level
| Controls (n=20) | DLB (n=37) | AD (n=20) | MCI-LB (n=38) | MCI-AD (n=21) | |
| Age (years±SD) | 75.9±7.3 | 76.1±6.6 | 75.9±6.7 | 75.6±7.5 | 78.5±6.4 |
| Sex (M:F) | 16:4 | 30:7§ | 15:5 | 25:13 | 7:14* |
| Disease duration (months±SD) | 25.2±20.4 | 24.2±20.9 | |||
| MMSE (score±SD) | 29.1±0.9*†‡§ | 21.2±4.6‡§¶ | 20.3±4.7‡§¶ | 26.4±2.3*†¶ | 26.5±2.1*†¶ |
| ACE (score±SD) | 94.8±3.0*†‡§ | 62.1±15.4‡§¶ | 60.5±16.5‡§¶ | 78.2±9.6*†¶ | 79.4±11.1*†¶ |
| UPDRS (score±SD) | 5.6±3.5*†‡§ | 43.0±18.1†‡§¶ | 13.6±7.2*‡¶ | 27.2±16.2*†§¶ | 15.7±6.5*‡¶ |
| NPI (score±SD) | 20.4±19.9§ | 14.0±13.3§ | 14.3±10.5§ | 5.1±6.2*†‡ | |
| Levodopa use (no of patients, %) | 0 (0%) | 13 (35%) | 0 (0%) | 8 (21%) | 0 (0%) |
| Antidementia medication (no of patients, %) | – | 35 (96%)‡§ | 20 (100%)‡§ | 18 (47%)*† | 5 (24%)*† |
| Anti-inflammatory medication (no of patients, %) | 13 (65%) | 14 (38%) | 5 (25%) | 23 (66%) | 9 (43%) |
| CIRS-G (score±SD) | 6.7±4.3* | 11.5±4.1¶ | 8.6±3.6 | 9.5±4.3 | 9.5±3.9 |
| CIRS-G without neuro/psych (score±SD) | 6.5±4.1 | 8.1±3.8 | 6.2±3.4 | 8.4±4.5 | 8.2±4.4 |
Results are presented as mean and SD.
Antidementia medication includes donepezil, rivastigmine, galantamine and memantine. Anti-inflammatory medication includes all non-steroidal anti-inflammatory drugs and steroids.
*Significantly different from DLB group (P<0.05).
†Significantly different from AD group (P<0.05)
‡Significantly different from MCI-LB group (P<0.05)
§Significantly different from MCI-AD group (P<0.05)
¶Significantly different from controls (P<0.05).
ACE, Addenbrooke’s Cognitive Examination; AD, Alzheimer’s Disease; CIRS-G, Cumulative Illness Rating Scale-Geriatric; CIRS-G without neuro/psych, CIRS-G score without the neurology or psychiatric components included; DLB, dementia with Lewy bodies; MCI, mild cognitive impairment; MCI-AD, MCI with probable Alzheimer’s disease; MCI-LB, MCI with probable Lewy body dementia; MMSE, Mini Mental State Examination; NPI, Neuropsychiatric Inventory; UPDRS, Unified Parkinson’s Disease Rating Scale.
The CIRS-G identified that the DLB group had a significantly higher general medical illness score than other groups. However, this was due to the DLB group scoring more highly in the neurological and psychiatric aspects of the assessment, as would be expected by the increased incidence of parkinsonism and hallucinations in these groups of patients. Once these neurological and psychological aspects had been removed from analysis, there were no significant differences between any of the groups (see table 1).
We also looked specifically at the prevalence of autoimmune comorbidities in each group due to the impact it can have on inflammation. Overall one control participant had rheumatoid arthritis, one or two participants in each group had hypothyroidism or hyperthyroidism, one control participant had giant cell arteritis, two patients with DLB had polymyalgia, one patient with DLB and one patient with MCI-LB had gout, and one control participant had ‘immune system disease’ not otherwise specified. Therefore the prevalence of autoimmune inflammatory disease was spread evenly across groups. Overall all other comorbidities including vascular comorbidities were spread evenly between groups.
Inflammatory marker levels
Table 2 shows all cytokine and hsCRP results. Overall, patients in both MCI groups had significantly higher levels of IL-10, IL-1beta, IL-4 and IL-2 than control, AD or DLB groups. Both MCI groups had lower levels of TNF-alpha than the control or dementia groups. IL-13 was removed because more than 50% of the samples had non-detectable cytokine levels. Furthermore, IL-12p70 was removed due to the high interassay variability. There were no significant differences between either of the dementia groups and control subjects, and there were no significant differences between the two dementia groups or between the two MCI groups (table 2). Cohen’s effect sizes were calculated and were large (d>0.8) for all significant differences, apart from that between the AD group and MCI groups for IL-10, which were moderate (d=0.55 and d=0.56 for AD vs MCI-LB and AD vs MCI-AD respectively). The median values for intra-assay and interassay coefficient of variance across all cytokines were 9.87% and 16.04%, respectively. Correction calculations were carried out based on the values obtained for internal controls to account for this variation. There were no significant differences in hsCRP level between any of the groups (table 2).
Table 2.
Level of inflammatory markers in patients and controls
| Control | DLB | AD | MCI-LB | MCI-AD | |
| CRP, mg/mL | 3.35±5.72 | 1.73±2.12 | 6.29±14.56 | 4.12±6.44 | 3.41±3.34 |
| IFN-gamma, pg/L | 7.56±6.13 | 5.13±4.13 | 9.01±12.06 | 6.52±4.30 | 8.75±12.08 |
| IL-10, pg/L | 0.46±0.62 | 0.43±0.52 | 0.60±0.75 | 0.96±0.54*†‡ | 0.93±0.36*†‡ |
| IL-1beta, pg/L | 0.51±2.16 | 0.07±0.33 | 0.03±0.08 | 1.92±1.24*†‡ | 2.72±1.51*†‡ |
| IL-2, pg/L | 0.38±0.42 | 0.34±0.37 | 0.39±0.68 | 3.68±0.85*†‡ | 3.56±0.92*†‡ |
| IL-4, pg/L | 0.03±0.05 | 0.02±0.03 | 0.01±0.03 | 0.80±0.16*†‡ | 0.85±0.21*†‡ |
| IL-6, pg/l | 1.66±0.99 | 2.02±2.00 | 2.78±2.21 | 2.90±10.39 | 3.32±4.34 |
| IL-8, pg/L | 4.85±1.49 | 5.10±2.03 | 5.11±2.25 | 6.75±17.50 | 4.34±2.88 |
| TNF-alpha, pg/L | 4.26±1.32 | 4.02±1.61 | 4.28±2.38 | 2.23±2.01*†‡ | 3.08±3.53*† |
Results are presented as mean±SD.
*Significantly different from controls (P≤0.001).
†Significantly different from DLB group (P≤0.001).
‡Significantly different from AD group (P≤0.001).
AD, Alzheimer’s disease; CRP, C reactive protein; DLB, dementia with Lewy bodies; IFN-gamma, interferon-gamma; IL, interleukin; MCI, mild cognitive impairment; MCI-AD, MCI with probable Alzheimer’s disease; MCI-LB, MCI with probable Lewy body dementia; TNF-alpha, tumour necrosis factor-alpha.
Correlations
Spearman’s rank correlation was used to explore any correlations between inflammatory markers and patient characteristics. Healthy control subjects were excluded from the correlation analysis. Increasing age was correlated with a higher level of IFN-gamma. Greater severity of cognitive impairment measured using the ACE-R and the MMSE was associated with having a significantly lower level of IL-1beta, IL-2 and IL-4, and a higher level of IL-6 and TNF-alpha (figure 1).
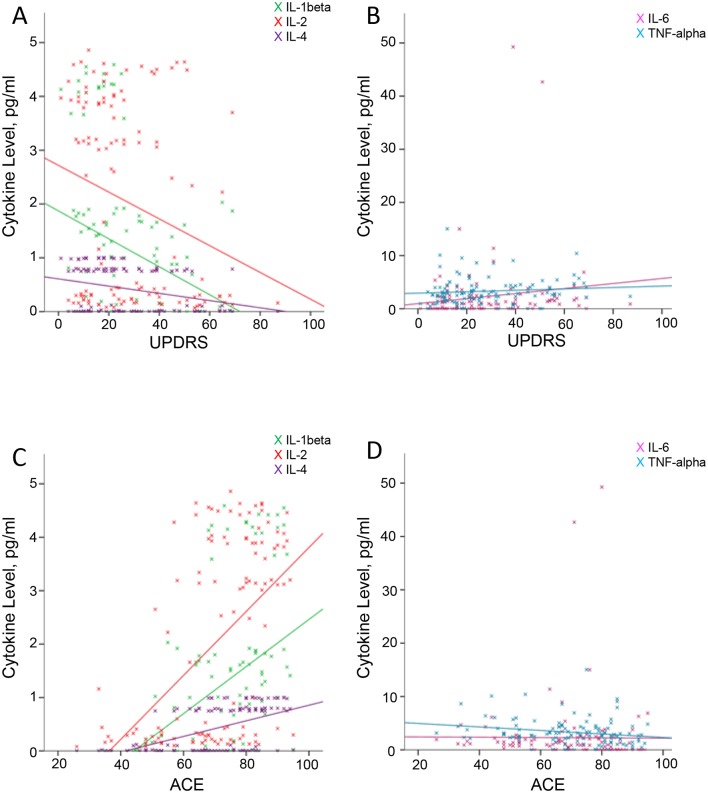
Figure 1.
Correlations between Unified Parkinson’s Disease Rating Scale (UPDRS) and Addenbrooke’s Cognitive Examination (ACE) scores with cytokine levels. (A,B) Correlations between UPDRS and interleukin (IL)-1beta (r=−0.285, P=0.002), IL-2 (r=−0.220, P=0.018) and IL-4 (r=−0.217, P=0.019), and between UPDRS and IL-6 (r=0.208, P=0.025) and tumour necrosis factor (TNF)-alpha (r=0.168, P=0.071). (C,D) Correlations between the ACE score and IL-1beta (r=0.494, P=0.000), IL-2 (r=0.485, P=0.000) and IL-4 (r=0.540, P=0.000), and between the ACE and IL-6 (r=−0.343, P=0.000) and TNF-alpha (r=−0.253, P=0.006).
Correlations were also sought between the level of parkinsonism and inflammatory markers using the UPDRS. AD groups were also removed from this analysis, as parkinsonism is not a feature in this group and scores were consequently low. Overall, a significantly higher score on the UPDRS was associated with having lower levels of IL-1beta, IL-2 and IL-4, and a higher level of IL-6 and TNF-alpha.
When looking at the MCI and dementia groups separately, a greater level of parkinsonism was associated with a lower level of IL-1beta and IL-4 in the MCI-LB group only. In the DLB group, the only significant correlation was between having more severe parkinsonian symptoms and a higher level of IL-10.
Inflammatory markers and amyloid
There were no significant differences in any of the cytokine levels between participants with amyloid-positive and amyloid-negative PET scans.
Inflammatory marker in amnesic versus non-amnesic patients
There were no significant differences in any of the cytokine levels between participants with amnestic and non-amnestic MCI subtype.
Discussion
We investigated for the first time the peripheral cytokine profile in DLB and AD both at the MCI stage and at the dementia stage, and compared with healthy control subjects. We found that while there was no difference in the level of inflammatory markers between control and dementia subjects, there was a significant increase in inflammatory markers at the MCI stage. Specifically, we found significantly higher levels of IL-10, IL-1beta, IL-4 and IL-2 in both MCI-LB and MCI-AD compared with dementia and control subjects. Furthermore, greater disease severity, whether measured for cognition or parkinsonism, was associated with lower levels of IL-1beta, IL-2 and IL-4, further supporting our finding that inflammatory markers decrease with disease progression. To our knowledge this is the first study to investigate baseline inflammatory markers in DLB with control subjects, and we found that peripheral inflammation in DLB shows a similar pattern to that seen in AD.
The results from this study are consistent with previous studies of AD. Previous studies have demonstrated lower levels of IL-1beta, Il-6, IL-12, IL-16, IL-18 and TGF-β1 in severe AD compared with mild or moderate disease.6 18 CSF chemokine levels have also been found to be increased in MCI compared with patients with AD.19 Peripheral blood mononuclear cell stimulation studies have found similar patterns of immune dysfunction; one study found higher levels of cytokine production in patients with MCI than AD or controls,20 and another found significantly lower levels of cytokine release in severe AD.18 21 A recent meta-analysis found that increased peripheral levels of inflammatory markers are associated with an increased risk of later dementia, indicating that peripheral inflammation may occur before clinical symptoms are present.22
Few studies have investigated inflammatory markers in DLB. One study found that IL-6 in CSF was negatively correlated with MMSE,11 and a correlation study found that increasing IL-6 is associated with cognitive decline.12 In our study IL-6 was one of the only cytokines that were negatively correlated with cognitive function, and it was also correlated with worsening parkinsonian symptoms. However, studies investigating Parkinson’s disease suggest that high IL-6 levels increase the risk of later Parkinson’s disease, implying that high IL-6 may be associated with triggering disease onset.23 These findings suggest that IL-6 may show a more complex relationship with disease progression, perhaps increasing both early and late in disease.
The only cytokine that we found to be decreased in patients with MCI was TNF-alpha, which was also associated with worsening cognition and parkinsonism. Previous studies investigating TNF-alpha in AD have found mixed results. While some studies have found increased levels of TNF-alpha in patients with AD,24 25 some have found decreased levels26 27 and others have found no difference.28 Studies investigating TNF-alpha levels at different stages of disease have in general found higher levels of TNF-alpha in patients with severe AD compared with mild AD and MCI,29 30 and therefore it may be that the increase in TNF-alpha is seen at a later stage in disease than other cytokines.
The underlying cause for this inflammation seen early in disease is still not fully understood, although it has been suggested that in the early stages of disease, aberrant protein deposition induces a microglial inflammatory response in the brain, leading to peripheral inflammation.31 It has been suggested that it is this initial microglial activation that produces toxic products, producing further neuronal death and thereby perpetuating the inflammatory reaction.32 Previously, studies have also found increased inflammation peripherally in Parkinson’s disease,8 33 suggesting that inflammation may indeed be associated with aberrant protein aggregation and not specifically beta amyloid (Aβ). However studies investigating inflammation in Parkinson’s disease dementia have found mixed results,34 35 which could perhaps be a reflection of the severity of disease of this cohort of participants, with such patients being less likely to be in the early stages of disease.
Although it is not clear how systemic inflammation relates to disease processes occurring in the brain, studies suggest that peripheral inflammation and central inflammation are closely related.2 Microglia expressing IL-1 appear to be more highly associated with early-stage than late-stage Aβ plaques in AD brains, and IL-1 may be important in driving plaque progression.36 Furthermore, administration of Aβ centrally in mice induces a dose-dependent increase in peripheral IL-6,37 suggesting that there is some relationship between peripheral inflammatory markers and central disease progression. It has been suggested that Aβ deposition may drive cognitive decline in people with AD, DLB and PD, perhaps by inducing inflammation; however, evidence is limited and results are inconsistent.38 The present study found no relationship between Aβ and inflammatory marker levels, suggesting that inflammation may not be a direct consequence of Aβ deposition. A previous study showed that inflammation appears to be a better marker for synapse loss seen in AD than Aβ deposition, suggesting that there is a role for inflammation driving neurodegeneration.39 This study suggests that while there is a clear increase in inflammation at the MCI stage, there appears to be an imbalance between proinflammatory and anti-inflammatory cytokines, something that is thought to be highly important in the progression from acute inflammation to chronic disease.40
This study did not investigate patients with severe dementia; however, the correlation analysis suggests that as disease progresses, the level of inflammation decreases. Previous studies investigating this have indeed shown that there appears to be a gradual decrease in the level of inflammatory markers with disease progression, reaching levels below healthy in severe disease.6 18 However, although chronic inflammation appears to decrease with disease severity, acute inflammation still likely has an impact on the rate of decline, as it has been shown that acute increases in TNF-alpha in patients with dementia predict more rapid disease progression.2
This decrease in chronic inflammation seen later in disease may relate to the chronic neurodegenerative processes involved in dementia progression. It has been shown in transgenic mice that chronic Aβ exposure in the brain is associated with a decreased immune response.41 It may be that chronic exposure to Aβ in AD leads to increased tolerance for Aβ deposition. This may result in immune hyporesponsiveness, which may contribute towards further Aβ deposition. It seems likely that a similar inflammatory process is occurring in DLB; however, more evidence is needed to ascertain whether this is also related to beta deposition, or whether this is as a result of alpha-synuclein. Therefore, it could be hypothesised that while aberrant protein deposition in the brain in AD and DLB may initially trigger an immune response leading to an increase in inflammatory markers and therefore neuronal toxicity, chronic exposure may lead to tolerance and a hyporesponsive immune system.
These findings have important implications for the management of dementia. Previous studies have investigated the use of anti-inflammatory medication in the treatment of AD and found no clinical benefit.42 However, because the patients in these cohorts had already progressed from MCI to AD, it is likely that the stage of dementia was too advanced for the anti-inflammatory medication to have had any clinical benefit. Observational studies have found that long-term use of anti-inflammatory medications is associated with a reduced risk of dementia, indicating that the beneficial effects of anti-inflammatory medications may only apply in very early stages of disease.43 Further studies are needed to ascertain the optimum time, dose and type of anti-inflammatory medication to be used in the management of patients with MCI. More recent studies have suggested that there may be a role for more specific anti-inflammatory agents; a pilot study found improvements in cognition in patients with AD taking specific TNF-alpha inhibitors.44 Randomised controlled trials are needed to ascertain the benefit of anti-inflammatory antibody therapies in AD and DLB.
Strengths of this study include the large patient group used and the thorough clinical assessment of each patient by an experienced clinician using detailed rating scales. Robust exclusion criteria were used, including routine blood tests to assess for systemic disease. Limitations include the cross-sectional design of the study, the high number of samples falling below the detection level for IL-13 leading to exclusion of IL-13, and the severity of the dementia groups, having excluded patients with an MMSE score of less than 12.
In conclusion, we have shown for the first time that in both AD and DLB, increased peripheral inflammation occurs early at the MCI stage and decreases with severity of disease. It seems likely therefore that there is an early microglial response to aberrant protein deposition, which progresses to an immune tolerance in chronic disease leading to immune hyporesponsiveness. Early treatment of AD and DLB at the MCI stage with anti-inflammatory medication may therefore be of benefit, and our results indicate that further trials should focus on early-stage disease.
Acknowledgments
We would like to thank Claire Kolenda and Craig Parker for the processing of the plasma samples. We would also like to thank Melanie Maitland and friends for their donations to our research in Lewy body dementia.
Footnotes
Contributors: EK contributed towards planning the study, analysing data and interpreting results, and writing the manuscript. JTOB contributed towards designing the study and drafting the manuscript. PD contributed towards acquiring data and drafting the manuscript. CM contributed towards designing the study and drafting the manuscript. NB contributed towards acquiring the data and drafting the manuscript. KO contributed towards acquiring the data and drafting the manuscript. CM-R contributed towards analysing the data and drafting the manuscript. JPT contributed towards acquiring data and drafting the manuscript. AT contributed towards design of the study, acquiring data, interpreting results and drafting the manuscript.
Funding: We would like to acknowledge our funders: the National Institute for Health Research (NIHR) Newcastle Biomedical Research Unit in Lewy Body Dementia based at Newcastle upon Tyne NHS Foundation Trust and Newcastle University. Thanks to the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN). EK is also grateful to the Royal College of Psychiatrists Pathfinder Fellowship for the grant that was provided for this project. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests: JTOB reports grants from Avid/Lilly during the conduct of the study. He also reports personal fees from GE Healthcare, personal fees from TauRx, grants and personal fees from Avid/Lilly, and personal fees from Axon, outside the submitted work. AT reports grants from NIHR BRU in Lewy Body Dementia and grants from Alzheimer’s Research UK during the conduct of the study. He also reports grants from GE Healthcare, outside the submitted work. All other authors have no further competing interests to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol 2013;35:601–12. 10.1007/s00281-013-0382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009;73:768–74. 10.1212/WNL.0b013e3181b6bb95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swardfager W, Lanctôt K, Rothenburg L, et al. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 2010;68:930–41. 10.1016/j.biopsych.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 4. Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 2004;61:668-72 10.1001/archneur.61.5.668 [DOI] [PubMed] [Google Scholar]
- 5. Kuo HK, Yen CJ, Chang CH, et al. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol 2005;4:371–80. 10.1016/S1474-4422(05)70099-5 [DOI] [PubMed] [Google Scholar]
- 6. Motta M, Imbesi R, Di Rosa M, et al. Altered plasma cytokine levels in Alzheimer’s disease: correlation with the disease progression. Immunol Lett 2007;114:46–51. 10.1016/j.imlet.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 7. Zhang Z-G, Li Y, Ng CT, et al. Inflammation in Alzheimer’s disease and molecular genetics: recent update. Arch Immunol Ther Exp 2015;63:333–44. 10.1007/s00005-015-0351-0 [DOI] [PubMed] [Google Scholar]
- 8. Qin XY, Zhang SP, Cao C, et al. Aberrations in peripheral inflammatory Cytokine levels in parkinson disease: a systematic review and meta-analysis. JAMA Neurol 2016;73:1316–24. 10.1001/jamaneurol.2016.2742 [DOI] [PubMed] [Google Scholar]
- 9. Griffin WS, Liu L, Li Y, et al. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation 2006;3:5 10.1186/1742-2094-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gómez-Tortosa E, Gonzalo I, Fanjul S, et al. Cerebrospinal fluid markers in dementia with lewy bodies compared with Alzheimer disease. Arch Neurol 2003;60:1218–22. 10.1001/archneur.60.9.1218 [DOI] [PubMed] [Google Scholar]
- 11. Wennström M, Hall S, Nägga K, et al. Cerebrospinal fluid levels of IL-6 are decreased and correlate with cognitive status in DLB patients. Alzheimers Res Ther 2015;7:63 10.1186/s13195-015-0145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clough Z, Jeyapaul P, Zotova E, et al. Proinflammatory cytokines and the clinical features of dementia with lewy bodies. Alzheimer Dis Assoc Disord 2015;29:97–9. 10.1097/WAD.0b013e3182969905 [DOI] [PubMed] [Google Scholar]
- 13. Holmes C. Review: systemic inflammation and Alzheimer’s disease. Neuropathol Appl Neurobiol 2013;39:51–68. 10.1111/j.1365-2990.2012.01307.x [DOI] [PubMed] [Google Scholar]
- 14. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–72. 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 15. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:263–9. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:270–9. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Psychiatric Association. Major or mild neurocognitive disorder with Lewy bodies Diagnostic and statistical manual of mental disorders. 5 ed: American Psychiatric Publishing, 2013. [Google Scholar]
- 18. Sala G, Galimberti G, Canevari C, et al. Peripheral cytokine release in Alzheimer patients: correlation with disease severity. Neurobiol Aging 2003;24:909–14. 10.1016/S0197-4580(03)00010-1 [DOI] [PubMed] [Google Scholar]
- 19. Galimberti D, Schoonenboom N, Scheltens P, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol 2006;63:538–43. 10.1001/archneur.63.4.538 [DOI] [PubMed] [Google Scholar]
- 20. Magaki S, Mueller C, Dickson C, et al. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol 2007;42:233–40. 10.1016/j.exger.2006.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richartz E, Batra A, Simon P, et al. Diminished production of proinflammatory cytokines in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 2005;19:184–8. 10.1159/000083497 [DOI] [PubMed] [Google Scholar]
- 22. Koyama A, O’Brien J, Weuve J, et al. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: a meta-analysis. J Gerontol A Biol Sci Med Sci 2013;68:433–40. 10.1093/gerona/gls187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen H, O’Reilly EJ, Schwarzschild MA, et al. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol 2008;167:90–5. 10.1093/aje/kwm260 [DOI] [PubMed] [Google Scholar]
- 24. Fillit H, Ding WH, Buee L, et al. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci Lett 1991;129:318–20. 10.1016/0304-3940(91)90490-K [DOI] [PubMed] [Google Scholar]
- 25. Alvarez A, Cacabelos R, Sanpedro C, et al. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging 2007;28:533–6. 10.1016/j.neurobiolaging.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 26. Cacabelos R, Alvarez XA, Franco-Maside A, et al. Serum tumor necrosis factor (TNF) in Alzheimer’s disease and multi-infarct dementia. Methods Find Exp Clin Pharmacol 1994;16:29–35. [PubMed] [Google Scholar]
- 27. Alvarez XA, Franco A, Fernández-Novoa L, et al. Blood levels of histamine, IL-1 beta, and TNF-alpha in patients with mild to moderate Alzheimer disease. Mol Chem Neuropathol 1996;29:237–52. 10.1007/BF02815005 [DOI] [PubMed] [Google Scholar]
- 28. Yasutake C, Kuroda K, Yanagawa T, et al. Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: comparison between Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci 2006;256:402–6. 10.1007/s00406-006-0652-8 [DOI] [PubMed] [Google Scholar]
- 29. Paganelli R, Di Iorio A, Patricelli L, et al. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer’s disease patients. Exp Gerontol 2002;37:257–63. 10.1016/S0531-5565(01)00191-7 [DOI] [PubMed] [Google Scholar]
- 30. Bonotis K, Krikki E, Holeva V, et al. Systemic immune aberrations in Alzheimer’s disease patients. J Neuroimmunol 2008;193:183–7. 10.1016/j.jneuroim.2007.10.020 [DOI] [PubMed] [Google Scholar]
- 31. Minter MR, Taylor JM, Crack PJ. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J Neurochem 2016;136:457–74. 10.1111/jnc.13411 [DOI] [PubMed] [Google Scholar]
- 32. McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol 1998;33:371–8. 10.1016/S0531-5565(98)00013-8 [DOI] [PubMed] [Google Scholar]
- 33. Reale M, Iarlori C, Thomas A, et al. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun 2009;23:55–63. 10.1016/j.bbi.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 34. Rota E, Bellone G, Rocca P, et al. Increased intrathecal TGF-beta1, but not IL-12, IFN-gamma and IL-10 levels in Alzheimer’s disease patients. Neurol Sci 2006;27:33–9. 10.1007/s10072-006-0562-6 [DOI] [PubMed] [Google Scholar]
- 35. Lindqvist D, Hall S, Surova Y, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease--associations with depression, fatigue, and cognitive impairment. Brain Behav Immun 2013;33:183–9. 10.1016/j.bbi.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 36. Griffin WS, Sheng JG, Roberts GW, et al. Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J Neuropathol Exp Neurol 1995;54:276–81. [DOI] [PubMed] [Google Scholar]
- 37. Song DK, Im YB, Jung JS, et al. Central beta-amyloid peptide-induced peripheral interleukin-6 responses in mice. J Neurochem 2001;76:1326–35. 10.1046/j.1471-4159.2001.00121.x [DOI] [PubMed] [Google Scholar]
- 38. Donaghy P, Thomas AJ, O’Brien JT. Amyloid PET imaging in Lewy body disorders. Am J Geriatr Psychiatry 2015;23:23–37. 10.1016/j.jagp.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 39. Lue LF, Brachova L, Civin WH, et al. Inflammation, a beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J Neuropathol Exp Neurol 1996;55:1083–8. 10.1097/00005072-199655100-00008 [DOI] [PubMed] [Google Scholar]
- 40. Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal 2012;2012:1–15. 10.1100/2012/756357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Monsonego A, Maron R, Zota V, et al. Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci U S A 2001;98:10273–8. 10.1073/pnas.191118298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miguel-Álvarez M, Santos-Lozano A, Sanchis-Gomar F, et al. Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: a systematic review and meta-analysis of treatment effect. Drugs Aging 2015;32:139–47. 10.1007/s40266-015-0239-z [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Tan L, Wang HF, et al. Anti-inflammatory drugs and risk of Alzheimer’s disease: an updated systematic review and meta-analysis. J Alzheimers Dis 2015;44:385–96. 10.3233/JAD-141506 [DOI] [PubMed] [Google Scholar]
- 44. Tobinick E. Tumour necrosis factor modulation for treatment of Alzheimer’s disease: rationale and current evidence. CNS Drugs 2009;23:713–25. 10.2165/11310810-000000000-00000 [DOI] [PubMed] [Google Scholar]