Abstract
Background
Being married is associated with healthier lifestyle behaviours and lower mortality and may reduce risk for dementia due to life-course factors. We conducted a systematic review and meta-analysis of studies of the association between marital status and the risk of developing dementia.
Methods
We searched medical databases and contacted experts in the field for relevant studies reporting the relationship, adjusted for age and sex, between marital status and dementia. We rated methodological quality and conducted random-effects meta-analyses to summarise relative risks of being widowed, divorced or lifelong single, compared with being married. Secondary stratified analyses with meta-regression examined the impact of clinical and social context and study methodology on findings.
Results
We included 15 studies with 812 047 participants. Compared with those who are married, lifelong single (relative risk=1.42 (95% CI 1.07 to 1.90)) and widowed (1.20 (1.02 to 1.41)) people have elevated risk of dementia. We did not find an association in divorced people.
Further analyses showed that less education partially confounds the risk in widowhood and worse physical health the elevated risk in lifelong single people. Compared with studies that used clinical registers for ascertaining dementia diagnoses, those which clinically examined all participants found higher risk for being unmarried.
Conclusions
Being married is associated with reduced risk of dementia than widowed and lifelong single people, who are also underdiagnosed in routine clinical practice. Dementia prevention in unmarried people should focus on education and physical health and should consider the possible effect of social engagement as a modifiable risk factor.
Introduction
The rising number of people living with dementia1 makes it the current global public health priority,2 and there is a pressing need to identify modifiable risk factors. Although there are more people with dementia overall, there has been a small decline in the age-specific incidence of dementia in many developed countries3 4 over the past two decades suggesting that differential lifetime exposure to risk factors in successive generations affects their dementia risk.4
Marital status has potential to affect dementia risk by increasing daily social interaction. This may improve cognitive reserve, meaning that an individual has a greater ability to cope with neuropathological damage by using compensatory cognitive approaches from a physically more resilient brain to maintain cognitive ability and daily function.5 Marriage may result in more frequent social contact, which is associated with reduced dementia risk,6 and reduced harmful lifestyle behaviours.7 8 Bereavement or divorce in people who had been married may promote dementia development through stress, which is pathogenic9 and associated with increased dementia risk.10 Being unmarried is associated with adverse health behaviours7 and a range of poorer health outcomes. A meta-analysis of observational studies found lower mortality for married than unmarried people11; health of unmarried Americans is worse than that of married people8; being married is related to improved cancer survival12; and widowhood is associated with disability in older people.13
In this study, we aim to synthesise evidence from published studies examining the effect of marital status (married/cohabiting, widowed, divorced/separated and lifelong single) on dementia incidence and the extent to which this risk is modified by sociodemographic factors, study design and methodological quality of the study. We hypothesise that married people are at lower risk of developing dementia compared with unmarried people and that previously married people are at lower risk than those who have been lifelong single.
Methods
Search strategy
We searched Embase, MEDLINE and PsycInfo databases from their inception to 5 December 2016. Our search terms (online supplementary table 1) identified papers whose titles, abstracts or keywords included terms encompassing marital status and dementia, and we used the Scottish Intercollegiate Guidelines Network filters for observational studies (http://www.sign.ac.uk/methodology/filters.html). We searched references of included studies and systematic reviews and contacted two experts in this field aiming to identify additional studies.
jnnp-2017-316274supp001.pdf (577.2KB, pdf)
Inclusion criteria
A study was included if:
it used a prospective or retrospective cohort, case–control or cross-sectional study design
it reported quantitative data measuring the relationship between dementia and marital status or partner/spouse presence
it presented results of analyses that were adjusted for age and sex; we contacted authors of studies who reported unadjusted results and included new adjusted data if provided
marital status was measured and reported separately from other aspects of social network, for example, contact with other family
the sample consisted of at least 50% of individuals aged 65 years or over at time of dementia ascertainment, or if a younger population was sampled, a study was included if it presented stratified results for an over-65 population
the sample was derived from a general community-dwelling population. For cohort studies, participants had to be screened for dementia at baseline and prevalent dementia cases excluded.
it was a published research paper or dissertation; when we found relevant conference abstracts, we contacted the author for details of any eligible published research
it was published in English.
When two studies reported different analyses of cohort studies, so to avoid duplication, we used only the analysis that had a longer follow-up duration.
Data extraction
One researcher (AS) screened the abstracts of all studies to identify those potentially meeting the inclusion criteria and reviewed full-text articles to confirm eligibility. A second researcher (JR) reviewed a random sample of 10% of the studies to assess agreement and reviewed all included studies to approve eligibility. We used a standardised form (online supplementary table 2) to extract data for evidence synthesis. Extracted information included results and information for the assessment of the risk of bias.
In the one study14 that used lifelong single people as the reference group, we inverted the ORs, and for this study and another,15 we calculated CIs based on raw published data.16 Where marital status categories had been combined (eg, divorced and single people) or results for dementia subtypes rather than all-cause dementia presented, we requested additional data from study authors. We have included new data for three papers.17–19
We registered the study protocol prospectively in the PROSPERO register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016043161).
Quality rating
We rated methodological quality of included studies using an adapted version of the Newcastle-Ottawa Criteria 20 for cohort and case–control studies and the Joanna Briggs Institute’s Checklist 21 for cross-sectional studies. Full details are in online supplementary tables 3a–c but, in summary, these tools rated the quality of selection, measurement and comparability for all studies and gave a score for cohort and case–control studies (maximum of 9) and cross-sectional studies (maximum 6). Two researchers (AS and JR) assessed the quality of all included studies and discussed discrepancies until consensus was reached.
Statistical analysis
We provide a narrative synthesis of findings from included studies and have pooled results where studies have used the same measurements, calculating random-effects estimates using STATA V.14. The random-effects model allows for HRs and ORs to be incorporated into the same meta-analysis22 and accounts for heterogeneity between studies.23 All included studies provided an estimate of relative risk and CI that we used for the analysis. We measured heterogeneity between the studies using the χ2 test and the I 2 statistic and considered, a priori, that I 2 >50% indicated substantial heterogeneity. Where studies provided estimates of relative risk from different multivariate models, we included the result from the model with the largest number of covariates.
Our main analyses compared risk of all-cause dementia in married people to those who were widowed, divorced or lifelong single for studies that ascertained dementia diagnosis status from clinical assessment. We conducted prespecified secondary analyses. We analysed the association between marital status and risk of Alzheimer’s or vascular dementia. We conducted stratified analyses and used meta-regression24 to quantify the effect of various study design factors on the association between marital status and all-cause mortality: (1) dementia case ascertainment method: clinical assessment of study participants versus clinical register data; (2) study type: cohort versus other studies; (3) study quality rating; and (4) time period of study conduct, based on mean year of birth of study participants.
We assessed the effect of confounder adjustment on the relative risk using stratified analyses of studies that adjusted only for age and sex versus studies that additionally adjusted for education or baseline cognition versus studies that additionally adjusted for physical health. We assessed for evidence of publication bias using funnel plots and Egger’s weighted regression method.25
Results
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) diagram (figure 1) shows our search results and reasons for study exclusion. Sixteen studies fulfilled our inclusion criteria, but we excluded one publication26 from our meta-analysis as it reported data from the same cohort as another study27 but with shorter follow-up. The 15 studies in our analyses included 812 047 people, of whom 29 610 had any form of dementia. Of these, 61 012 had a clinical assessment for dementia and 751 035 had dementia status ascertained from clinical records.
Figure 1.
PRISMA diagram of study identification and selection. PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis.
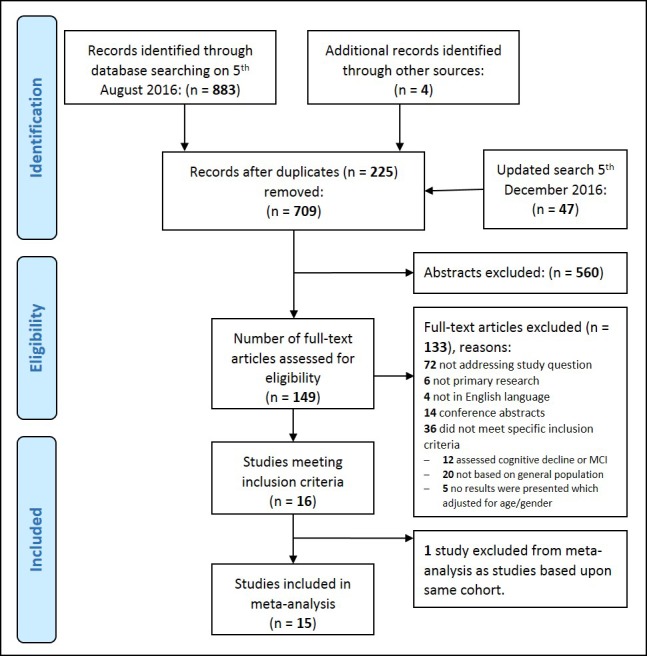
MCI, Mild cognitive impairment.
Table 1 describes key study characteristics. Nine were cohort studies,17–19 27–32 two case–control14 15 and four cross-sectional.33–36 Eight included studies were set in European countries, four in Asia, two from USA and one from Brazil. The mean year of birth of study participants ranged from 1897 to 1939. Studies typically measured marital status at study inception (mean age 72.8 (SD 7.2) years.) In the cohort studies, the duration of follow-up before dementia assessment was 3 to 20.9 (mean 8.5, SD 5.5) years.
Table 1.
Characteristics of included studies
| Study (first author and year of publication) | Study design | Country | Number of participants | Mean year of birth | Mean age at marital status evaluation (years) | Baseline marital status of participants (%) | Mean/range of follow-up (years) | Method of dementia case ascertainment | Quality rating | |||
| Married | Widowed | Divorced | Lifelong single | |||||||||
| Amieva 201027 | Cohort | France | 2089 | 1914 | 74 | 60.7 | 32.5 | 2.7 | 4.2 | 5–15 | Clinical assessment | 5 |
| Arai 200428 | Cohort | Japan | 853 | 1929 | 69 | 71 | 29 (unmarried) | 5 | Clinical assessment | 3 | ||
| Bae 201417 | Cohort | S Korea | 359 | 1936 | 72 | 70.2 | 29.8 | 0 | 0 | 3.5 | Clinical assessment | 3 |
| Bickel 199418 | Cohort | Germany | 331 | 1918 | 74 | 42.4 | 47.5 | 3.8 | 6.4 | 7–8 | Clinical assessment | 5 |
| Fratiglioni 200019 | Cohort | Sweden | 1368 | 1905 | 82 | 27.8 | 45.4 | 5.9 | 20.9 | 3 | Clinical assessment | 6 |
| Håkansson 200929 | Cohort | Sweden | 2000 | 1926 | 51 | 80.1 | 7.8 | 4.4 | 7.8 | 20.9 | Clinical assessment | 8 |
| Hatch 201330 | Cohort | USA | 5092 | 1920 | 75 | 65.9 | 29.9 | 4.1 | N/A | 12 | Clinical assessment | 8 |
| Sundström 201431 | Cohort | Sweden | 1677 | 1919 | 75 | 57.6 | 14.2 | 5.7 | 32.6 | 8.6 | Clinical assessment | 7 |
| Sundström 201632 | Cohort | Sweden | 7 50 129 | 1928 | 69 | 64.9 | 8.4 | 16.0 | 10.8 | 6 | Clinical register/death register | 9 |
| Beard 199215 | Case-control | USA | 482 | 1897 | 80 | 28.8 | 48.0 | 5.4 | 17.8 | N/A | Secondary care clinical register | 3 |
| Seidler 200314 | Case-control | Germany | 424 | 1924 | 77 | 78.5 | 11.1 | 3.8 | 6.6 | N/A | General practice clinical register | 2 |
| Correa Ribeiro 201333 | Cross-sectional | Brazil | 683 | 1931 | 78 | 41.6 | 40.8 | 7.5 | 10.1 | N/A | Clinical assessment | 3 |
| Fan 201534 | Cross-sectional | Taiwan | 10 432 | 1936 | 76 | 64.2 | 31.0 | 4.8 (Div/single) | N/A | Clinical assessment | 4 | |
| Guaita 201535 | Cross-sectional | Italy | 1321 | 1939 | 72 | 67.1 | 24.6 | 2.2 | 6.1 | N/A | Clinical assessment | 4 |
| Zhang 200636 | Cross-sectional | China | 34 807 | 1929 | 68 | 77.4 | 20.8 | 1.6 (Div/single) | N/A | Clinical assessment | 4 | |
N/A, Not applicable
Married people accounted for between 27.8% and 80.1% of the sample (widowed=7.8% to 48.0%, divorced=0% to 16%, lifelong single=0% to 32.6%). Two studies34 36 combined divorced and lifelong single people (6.1% and 10.1%). The mean methodological quality score for the cohort studies was 5.4/9, 2/9 for case–control studies and 3.8/6 for cross-sectional studies. Full details of methodological assessment are in online supplementary tables 3a–c. All included cohort studies analysed complete cases, excluding participants who had withdrawn from study.
Marital status was, in all but two of the cohort studies30 32 which used registry data, reported by the participant or a close informant. No studies provided further details about this assessment nor was there any information on duration of exposure to a particular marital status category. In one cohort study,32 marital status was ascertained from a Swedish central population register, and in another cohort,30 a marriage registry was used to confirm marital status. For the two case–control studies, those with dementia (or, if incapable of answering, an informant) were asked about their marital status at age 30 and 50 years and 10 years prior to interview14 or at time of diagnosis.15
All but three of the studies clinically examined all participants for ascertaining diagnostic status (outcome). The other studies14 15 32 ascertained diagnostic status from routine clinical registers and, for one of these studies,32 death registers. Except for the cohort study32 that exclusively used register data, none reported whether they ascertained dementia status from death registers. The clinical examination used in the majority of studies was a staged approach: a screening phase followed by a more detailed neuropsychological and functional assessment and an expert consensus panel to establish diagnostic status.
Main meta-analysis: widowed, divorced or lifelong single versus married people and risk of all-cause dementia
We pooled risk estimates from studies that evaluated the risk of all-cause dementia according to marital status category, with dementia case ascertainment based on clinical examination (figure 2). Nine studies analysed the risk of all-cause dementia in widowed versus married people and we found that in widowed, compared with married, people, the relative risk of dementia=1.20 (95% CI 1.02 to 1.41). The relative risk for divorced versus married people from seven studies=0.99 (0.71 to 1.37) and for the six studies that analysed dementia risk for lifelong single people, RR=1.42 (1.07 to 1.90).
Figure 2.
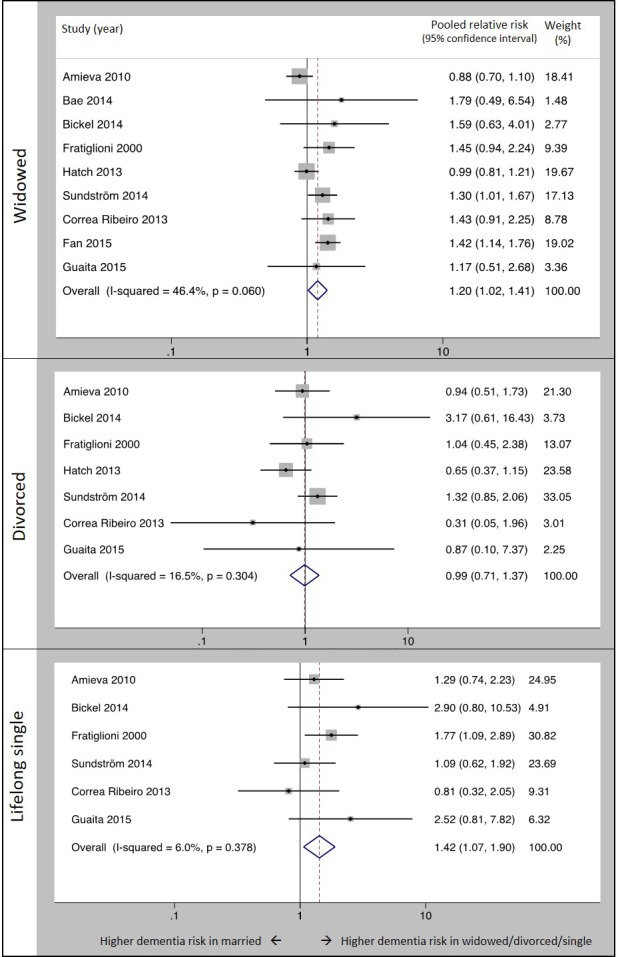
Forest plot showing pooled relative risk of dementia in widowed, divorced and lifelong single people versus married people when dementia was ascertained by clinical examination. Notes: figures are based on random-effects meta-analysis; included studies ascertained dementia diagnostic status using a clinical examination of study participants.
Secondary analyses
Widowed, divorced or lifelong single versus married people and risk of Alzheimer’s disease and vascular dementia
Fewer studies examined the risk of dementia subtypes according to marital status. Eight14 15 17 27 29 30 35 36 examined the risk of Alzheimer’s disease (1891 cases) in widowed versus married people and found a pooled relative risk of 1.24 (0.97 to 1.60). The risk of Alzheimer’s disease in five14 15 27 30 35 studies of divorced (0.89 (0.58 to 1.36)) and three15 27 35 of lifelong single (1.07 (0.75 to 1.52)) people was not different to that of married people. For vascular dementia (372 cases), no effect of marital status on dementia risk was found in pooled estimates from the three studies14 35 36 that examined the risk for widowed versus married people (pooled RR=0.90 (0.40 to 2.04)) or the two14 35 studies that examined risk in lifelong single people versus married (2.66 (0.85 to 8.28)). Only one study14 compared the risk of vascular dementia in divorced and married people and found no difference.
Widowed, divorced or lifelong single versus married people and risk of all-cause dementia stratified by sex
Two studies (online supplementary table 2) analysed the relationship between marital status and dementia separately for men and women. For one study,32 the outcome was all-cause dementia, and for the other,15 it was Alzheimer’s disease so meta-analysis was not possible. Neither study found any difference between men and women in the association of marital status and dementia.
Impact of study design on association between marital status and all-cause dementia
Widowed, divorced or lifelong single versus married people and risk of all-cause dementia stratified by case ascertainment method
There was evidence that the method of dementia case ascertainment affected the risk estimates (table 2). Studies using clinical examination for dementia ascertainment produced higher pooled estimates for the effect of being widowed (1.20 (1.02 to 1.41) versus 1.12 (1.07 to 1.18)) or lifelong single (1.42 (1.07 to 1.90) versus 1.23 (1.17 to 1.29)), and this difference nearly reached significance for the comparison of single and married people (p=0.06). The risk of dementia for divorced compared with married people was slightly lower but neither risk estimate was significant.
Table 2.
Meta-regression of the risk of all-cause dementia according to marital status, stratified by study time period, case ascertainment methodology, study type and study quality
| Widowed versus married | Divorced versus married | Lifelong single versus married | |||||
| Stratified analysis: Relative risk (95% CI) Number of studies |
Meta-regression coefficient (95% CI) p value | Stratified analysis: Relative risk (95% CI) Number of studies |
Meta-regression coefficient (95% CI) p-value | Stratified analysis: Relative risk (95% CI) Number of studies |
Meta-regression coefficient (95% CI) p value | ||
| Method of case ascertainment | Clinical assessment | 1.20 (1.02 to 1.41) n=9 |
b=−0.06 (−0.18 to 0.05) p=0.29 | 0.99 (0.71 to 1.37) n=7 |
b=0.34 (0.06 to 0.62) p=0.02 |
1.42 (1.07 to 1.90) n=6 |
b=−0.27 (−0.55 to 0.01) p=0.06 |
| Clinical registers | 1.12 (1.07 to 1.18) n=2 |
1.11 (0.52 to 2.38) n=2 |
1.23 (1.17 to 1.29) n=2 |
||||
| Study type | Cohort | 1.10 (1.05 to 1.28) n=7 |
b=0.28 (0.09 to 0.46) p=0.004 |
1.16 (0.87 to 1.55) n=6 |
b=−0.83 (−1.69 to 0.03) p=0.06 |
1.24 (1.17 to 1.30) n=5 |
b=0.08 (−0.45 to 0.62) p=0.76 |
| Case–control/cross-sectional | 1.39 (1.16 to 1.67) n=4 |
0.55 (0.23 to 1.31) n=3 |
1.21 (0.67 to 2.18) n=3 |
||||
| Global quality score | Higher quality ≥6 | 1.13 (1.02 to 1.31) n=4 |
b=0.08 (-0.06 to 0.23) p=0.27 |
1.16 (0.83 to 1.62) n=4 |
b=−0.40 (-0.88 to 0.08) p=0.10 |
1.26 (1.09 to 1.45) n=3 |
b=0.20 (-0.17 to 0.57) p=0.29 |
| Lower quality <6 | 1.22 (0.96 to 1.54) n=7 |
0.88 (0.54 to 1.44) n=5 |
1.33 (0.92 to 1.92) n=5 |
||||
| Increase in quality by one point | b=−0.04 (−0.08 to −0.002) p=0.04 n=11 |
b=0.12 (0.01 to 0.24) p=0.04 n=9 |
b=−0.05 (−0.13 to 0.03) p=0.21 n=8 |
||||
| Time period | Mean DoB before 1927 | 1.11 (0.93 to 1.31) n=6 |
b=0.15 (−0.14 to 0.43) p=0.32 |
0.98 (0.71 to 1.37) n=6 |
b=0.35 (0.08 to 0.63) p=0.01 |
1.40 (1.06 to 1.85) n=5 |
b=−0.22 (−0.50 to 0.06) p=0.13 |
| Mean DoB after 1927 | 1.23 (1.06 to 1.43) n=5 |
1.08 (0.50 to 2.35) n=3 |
1.24 (0.94 to 1.62) n=3 |
||||
| Mean year of birth 10 years later | b=0.08 (−0.08 to 0.23) p=0.34 n=11 |
b=0.24 (0.01 to 0.47) p=0.04 n=9 |
b=−0.15 (−0.33 to 0.02) p=0.09 n=8 |
||||
Figures are based on random-effects meta-analysis.
DoB, date of birth.
Widowed, divorced or lifelong single versus married people and risk of all-cause dementia stratified by study type
The pooled risk estimate (table 2) for dementia in widowed versus married people was lower (meta-regression: p=0.004) from the seven cohort studies17–19 27 30–32 (1.10 (1.05 to 1.28)) than the four cross-sectional or case–control studies14 33 34 36 (1.39 (1.16 to 1.67)) that examined this association. There were no differences between cohort and other studies in pooled estimates of dementia risk in lifelong single versus married people or divorced versus married people.
Widowed, divorced or lifelong single versus married people and risk of all-cause dementia stratified by study quality
Stratified analyses of higher versus lower quality studies and meta-regression analysis of the effect of study quality on risk estimates found no effect of study quality on relative risk for widowed or lifelong single people. The four higher quality studies19 30–32 produced a slightly increased risk for divorced people than the five lower quality studies14 18 27 33 35 but in neither strata was divorce related to dementia risk.
Widowed, divorced or lifelong single versus married people and risk of all-cause dementia by time period
Meta-regression analysis suggested that the relative risk of dementia in divorced people increased by 24% (95% CI 1% to 47%) for studies of participants born 10 years later (table 2), although risk remained non-significant when comparing the newer and older studies. There was some evidence that time period modified the effect of being lifelong single on risk of dementia: the risk of dementia in single people was 15% lower (9% CI 33% lower to 2% higher) for every 10 years later that participants were born. In the oldest studies (participants born on average before 1927), the risk of dementia in lifelong single versus married people was 1.40 (1.06 to 1.85) and for the most recent studies (of people born after 1927), the risk was 1.24 (0.94 to 1.62). No significant modifying effect of time period was found for the risk of dementia in widowed people.
Effect of covariate adjustment on risk estimates
For dementia risk in widowed versus married people, the pooled risk estimates (table 3) from the three studies17 18 31 that adjusted only for age and sex (1.33 (1.05 to 1.69)) was higher than the five studies14 19 30 33 35 that adjusted additionally for education or baseline cognitive function (1.12 (0.95 to 1.31)). No further attenuation of the effect was found in three studies27 32 34 that additionally adjusted for physical health (1.12 (0.92 to 1.37)).
Table 3.
Meta-analyses of the risk of all cause dementia according to marital status stratified by covariate adjustment.
| Widowed versus married | Divorced versus married | Lifelong single versus married | ||||
| Relative risk (95% CI) p value | Number of studies Heterogeneity statistic |
Relative risk (95% CI) p value | Number of studies Heterogeneity statistic |
Relative risk (95% CI) p value | Number of studies Heterogeneity statistic |
|
| Studies adjusted for age and sex | 1.33 (1.05 to 1.69) p=0.02 |
n=3 I2=0% |
1.41 (0.90 to 2.21) p=0.14 |
n=2 I2=1.5% |
1.49 (0.61 to 3.63) p=0.38 |
n=2 I2=46.1% |
| Studies adjusted for age, sex and education | 1.12 (0.95 to 1.31) p=0.19 |
n=5 I2=0% |
0.70 (0.47 to 1.06) p=0.10 |
n=5 I2=0% |
1.45 (0.97 to 2.19) p=0.005 |
n=4 I2=14.6% |
| Studies adjusted for age, sex, education and physical health | 1.12 (0.92 to 1.37) p=0.26 |
n=3 I2=77.8% |
1.30 (0.93 to 1.81) p=0.12 |
n=2 I2=42.5% |
1.23 (1.17 to 1.29) p=0.36 |
n=2 I2=0% |
Figures are based on random-effects meta-analysis.
For lifelong single people, the risk estimate for dementia was not affected by adjustment for education, but the relative risk of dementia in single versus married people fell from 1.45 (0.97 to 2.19) to 1.23 (1.17 to 1.29) in studies that adjusted for physical health.
Publication bias
In funnel plots (online supplementary figure 1), there was no clear evidence of asymmetry suggesting publication bias. Weighted regression (Egger) test indicated that there was unlikely to be publication bias in studies examining widowed (p=0.30) or lifelong single (p=0.35) people but that there may have been for studies of divorced people (p=0.04).
Discussion
Our study summarised all accessible published evidence and found that people who are lifelong single have a 42% higher risk and that those who are widowed have a 20% higher risk of developing dementia than those who are married in studies adjusted for age and sex. We found no evidence that dementia risk in divorced people differs from married people. The reduced risk in married people persisted in sensitivity analyses, indicating the robustness of the findings. Similar direction and magnitude of effect were found for dementia subtypes, but these estimates were non-significant as these analyses had fewer participants. Study design affects estimates of dementia risk. Higher relative risk of dementia for lifelong single and widowed people was found in studies that diagnosed dementia following clinical examination of all participants than in those that ascertained diagnostic status from routinely collected data; and lower risk was found for widowed people in cohort studies than in case–control or cross-sectional studies. There is some indication that the elevated risk in lifelong single people has decreased over time, with more recent studies finding smaller associations. We find that much of the increased risk in widowed people is attenuated after adjustment for education and that confounding by physical health explains part of the increased risk of dementia in lifelong single people.
Our findings may be explained in one or more ways. First, being married may change individuals’ exposure to other protective and risk factors throughout their subsequent lifespan; this is supported by our identification of confounding factors affecting this risk and evidence showing married people to be more likely to have a healthy lifestyle. The residual increased risk for lifelong single people in studies that adjusted for age, sex, education and physical health is likely to be due to different social engagement in married and single people,37 which may contribute to building cognitive reserve and reducing dementia risk6 over the lifespan. The magnitude of effect of marital status on dementia is higher than the risk for mortality in unmarried compared with married people (RR=1.1),11 supporting the idea that marriage’s effect on dementia risk is more than just improving physical health and that there may a direct cognitive benefit of being married.
Second, the end of marriage through bereavement could act directly to increase dementia risk, through the detrimental effect of stress on hippocampal neurons9 or cognition,10 and this theory could explain the increased dementia risk for widowed, but not divorced, people, as studies have found widowhood to be more stressful than divorce.38 39 Third, developing dementia could be related to other underlying cognitive or personality traits meaning that in societies where marriage was the social norm, people with difficulties in flexibility of thought or communication and consequent smaller lifelong cognitive reserve (therefore more likely to develop dementia) may be less likely to marry. This explanation may be supported by our finding that the risk for lifelong single people is possibly reduced in more recent times. Remaining unmarried has become more common,40 41 and it may be that single people born in the latter half of the 20thcentury have fewer unusual cognitive and personality characteristics.
Our findings, from large populations, across numerous countries and time periods are the strongest evidence yet that married people are less likely to develop dementia. We searched the literature systematically, sought additional studies where possible by contacting authors to gain additional data where published information was insufficient and followed PRISMA guidance in the conduct and reporting of this study.42 The main limitations of this review relate to the methodology of included studies. We could not investigate the effect of the duration of being widowed or divorced as the included studies did not report this, and we could only investigate the impact of potential confounders that were measured and analysed in studies, limiting our investigation of potential explanations for our findings. Our findings in relation to divorced people are less robust as there were fewer divorced people in the included studies. While our search terms were thorough, supporting our belief that we identified all studies examining this relationship, we may have missed eligible studies. This is a particular risk for observational studies examining the effect of other exposures on dementia risk, which may have reported marital status as a potential covariate, although less likely for this review as we aimed to only include studies that adjusted the relationship between marital status and dementia for age and sex.
Our finding of a 42% increased risk in lifelong single people compares closely to other known dementia risk factors incorporated in National Institute for Health and Care Excellence guidelines43 such as physical inactivity (RR=1.4) and less education, hypertension or smoking (RR for each=1.6).44 Our findings support the need for further work to develop preventative approaches in these lifestyle domains and indicate this may be particularly important for the high-risk groups of widowed and lifelong single people.
We also found that routine clinical registers underestimate the risk of dementia in these groups, which is likely to be because register data has poor sensitivity for detecting dementia45 and unmarried people are more likely to be undiagnosed in routine practice.46 Diagnosing dementia in people who attend clinic alone is more difficult, due to lack of collateral information and because individuals with dementia may not complain of memory impairment,47 so clinicians should have a high index of suspicion for dementia in these groups.
Future research should explore the mechanism of the relationship between marital status and dementia in order to develop interventions. It should, in particular, evaluate the contribution of social contact and health behaviours; use studies with sufficient follow-up to allow exploration of premarriage cognitive characteristics; and use cohort studies with sufficient detail on the duration of marriage, widowhood or divorce to allow the exploration of a dose–response effect.
Acknowledgments
We would like to thank Professor Horst Bickel, Dr Jong Bin Bae and Prof Hue-Xin Wang for providing additional data for inclusion in this meta-analysis and Dr Daniel Hatch, Dr Krister Håkansson and Professor Knut Engedal for their response to correspondence.
Footnotes
Contributors: AS, AS-M, GL and GL conceived and designed the study. AS conducted the literature search. AS and JR assessed manuscripts for inclusion in the study and rated them for quality. AS extracted data and performed analyses. All authors contributed substantially to drafting the article and revising it critically for intellectual content and gave final approval to the submitted manuscript.
Funding: AS received funding from the Wellcome Trust (200163/Z/15/Z) during the conduct of the submitted work. AS, GL and GL are supported by the UCLH NIHR Biomedical Research Centre. The authors analysed results and prepared this manuscript independently of the funding body. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Wellcome Trust or NIHR. Funders had no role in the study design and the collection, analysis and interpretation of data and the writing of the article and the decision to submit it for publication. The researchers were independent from funders and sponsors. All researchers could access all the data.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Prince M, Wimo A, Guerchet M, et al. ; World alzheimer report 2015. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International, 2015. [Google Scholar]
- 2.Prince M, Guerchet M, Prina M. The global impact of dementia 2013-2050. London: Alzheimer’s Disease International, 2013. [Google Scholar]
- 3.Matthews FE, Arthur A, Barnes LE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405–12. 10.1016/S0140-6736(13)61570-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Internal Medicine 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012;11:1006–12. 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuiper JS, Zuidersma M, Oude Voshaar RC, et al. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 2015;22:39–57. 10.1016/j.arr.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Joung IM, Stronks K, van de Mheen H, et al. Health behaviours explain part of the differences in self reported health associated with partner/marital status in The Netherlands. J Epidemiol Community Health 1995;49:482–8. 10.1136/jech.49.5.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller TD, Tech V. Relationship status, health, and health behavior: an examination of cohabiters and commuters. Sociological Perspectives 2010;53:221–45. 10.1525/sop.2010.53.2.221 [DOI] [Google Scholar]
- 9.Rothman SM, Mattson MP. Adverse stress, hippocampal networks, and Alzheimer’s disease. Neuromolecular Med 2010;12:56–70. 10.1007/s12017-009-8107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson L, Guo X, Hällström T, et al. Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer’s disease: a 38-year longitudinal population study. BMJ Open 2013;3:e003142 10.1136/bmjopen-2013-003142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzoli L, Villari P, M Pirone G, et al. Marital status and mortality in the elderly: a systematic review and meta-analysis. Soc Sci Med 2007;64:77–94. 10.1016/j.socscimed.2006.08.031 [DOI] [PubMed] [Google Scholar]
- 12.Kravdal O. The impact of marital status on cancer survival. Soc Sci Med 2001;52:357–68. [DOI] [PubMed] [Google Scholar]
- 13.Goldman N, Korenman S, Weinstein R. Marital status and health among the elderly. Soc Sci Med 1995;40:1717–30. 10.1016/0277-9536(94)00281-W [DOI] [PubMed] [Google Scholar]
- 14.Seidler A, Bernhardt T, Nienhaus A, et al. Association between the psychosocial network and dementia--a case-control study. J Psychiatr Res 2003;37:89–98. 10.1016/S0022-3956(02)00065-1 [DOI] [PubMed] [Google Scholar]
- 15.Beard CM, Kokmen E, Offord KP, et al. Lack of association between Alzheimer’s disease and education, occupation, marital status, or living arrangement. Neurology 1992;42:2063–8. 10.1212/WNL.42.11.2063 [DOI] [PubMed] [Google Scholar]
- 16.Morris J : Gardner MJ, Altman DG, Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates Statistics with Confidence–Confidence Intervals and Statistical Guidelines. London: BMJ Publishing Group, 1995. [Google Scholar]
- 17.Bae JB, Kim YJ, Han JW, et al. Incidence of and risk factors for Alzheimer’s disease and mild cognitive impairment in Korean elderly. Dement Geriatr Cogn Disord 2015;39:105–15. 10.1159/000366555 [DOI] [PubMed] [Google Scholar]
- 18.Bickel H, Cooper B. Incidence and relative risk of dementia in an urban elderly population: findings of a prospective field study. Psychol Med 1994;24:179–92. 10.1017/S0033291700026945 [DOI] [PubMed] [Google Scholar]
- 19.Fratiglioni L, Wang HX, Ericsson K, et al. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet 2000;355:1315–9. 10.1016/S0140-6736(00)02113-9 [DOI] [PubMed] [Google Scholar]
- 20.Wells G, Shea B, O’connell D, et al. ; The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2000. [Google Scholar]
- 21. Institute TJB. Joanna briggs institute reviewers' manual: 2016 edition. Australia: The Joanna Briggs Institute, 2016. [Google Scholar]
- 22.Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011;64:1187–97. 10.1016/j.jclinepi.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 24.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–73. 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 25.Sterne JA, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. British Medical Journal 2001;323:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmer C, Damon D, Letenneur L, et al. Marital status and risk of Alzheimer’s disease: a French population-based cohort study. Neurology 1999;53:1953–8. 10.1212/WNL.53.9.1953 [DOI] [PubMed] [Google Scholar]
- 27.Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med 2010;72:905–11. 10.1097/PSY.0b013e3181f5e121 [DOI] [PubMed] [Google Scholar]
- 28.Arai A, Katsumata Y, Konno K, et al. Sociodemographic factors associated with incidence of dementia among senior citizens of a small town in Japan. Care Manag J 2004;5:159–65. 10.1891/cmaj.2004.5.3.159 [DOI] [PubMed] [Google Scholar]
- 29.Håkansson K, Rovio S, Helkala EL, et al. Association between mid-life marital status and cognitive function in later life: population based cohort study. BMJ 2009;339:b2462–12. 10.1136/bmj.b2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatch DJ. The influence of widowhood and sociodemographic moderators on dementia and alzheimer’s disease risk, 2013. [Google Scholar]
- 31.Sundström A, Westerlund O, Mousavi-Nasab H, et al. The relationship between marital and parental status and the risk of dementia. Int Psychogeriatr 2014;26:749–57. 10.1017/S1041610213002652 [DOI] [PubMed] [Google Scholar]
- 32.Sundström A, Westerlund O, Kotyrlo E. Marital status and risk of dementia: a nationwide population-based prospective study from Sweden. BMJ Open 2016;6:e008565 10.1136/bmjopen-2015-008565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correa Ribeiro PC, de Souza Lopes C, Lourenço RA. Prevalence of dementia in elderly clients of a private health care plan: a study of the FIBRA-RJ, Brazil. Dement Geriatr Cogn Disord 2013;35:77–86. 10.1159/000345984 [DOI] [PubMed] [Google Scholar]
- 34.Fan LY, Sun Y, Lee HJ, et al. Marital status, lifestyle and dementia: a nationwide survey in Taiwan. PLoS One 2015;10:e0139154 10.1371/journal.pone.0139154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guaita A, Vaccaro R, Davin A, et al. Influence of socio-demographic features and apolipoprotein E epsilon 4 expression on the prevalence of dementia and cognitive impairment in a population of 70-74-year olds: the InveCe.Ab study. Arch Gerontol Geriatr 2015;60:334–43. 10.1016/j.archger.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 36.Zhang ZX, Zahner GE, Román GC, et al. Socio-demographic variation of dementia subtypes in china: Methodology and results of a prevalence study in Beijing, Chengdu, Shanghai, and Xian. Neuroepidemiology 2006;27:177–87. 10.1159/000096131 [DOI] [PubMed] [Google Scholar]
- 37.Sarkisian N, Gerstel N. Does singlehood isolate or integrate? Examining the link between marital status and ties to kin, friends, and neighbors. Journal of Social and Personal Relationships 2015:0265407515597564. [Google Scholar]
- 38.Gardner J, Oswald AJ. Do divorcing couples become happier by breaking up? J R Stat Soc Ser A Stat Soc 2006;169:319–36. 10.1111/j.1467-985X.2006.00403.x [DOI] [Google Scholar]
- 39.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res 1967;11:213–8. 10.1016/0022-3999(67)90010-4 [DOI] [PubMed] [Google Scholar]
- 40. Pew Research Centre. The decline of marriage and rise of new families. Washington DC, USA, 2010. [Google Scholar]
- 41.McLaren E. Marriages in England and Wales: 2013. London: Office for National Statistics, 2016. [Google Scholar]
- 42.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Jama 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 43. NICE. Dementia, disability and frailty in later life – mid-life approaches to delay or prevent onset: National Institute for Health and Clinical Excellence, 2015. [Google Scholar]
- 44.Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13:788–94. 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 45.Jin YP, Gatz M, Johansson B, et al. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology 2004;63:739–41. 10.1212/01.WNL.0000134604.48018.97 [DOI] [PubMed] [Google Scholar]
- 46.Savva GM, Arthur A. Who has undiagnosed dementia? a cross-sectional analysis of participants of the Aging, Demographics and Memory Study. Age Ageing 2015;44:642–7. 10.1093/ageing/afv020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livingston G, Leavey G, Manela M, et al. Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK. BMJ 2010;341:c4184 10.1136/bmj.c4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2017-316274supp001.pdf (577.2KB, pdf)


