Abstract
A cross‐industry survey was conducted to assess the landscape of preclinical quantitative systems pharmacology (QSP) modeling within pharmaceutical companies. This article presents the survey results, which provide insights on the current state of preclinical QSP modeling in addition to future opportunities. Our results call attention to the need for an aligned definition and consistent terminology around QSP, yet highlight the broad applicability and benefits preclinical QSP modeling is currently delivering.
MOTIVATION
The cost of developing drugs is rising rapidly (currently estimated at >$1.8 billion per newly approved drug1); a problem that is not new to the pharmaceutical industry as other reports show a decline in the number of new drug approvals per dollar spent over the last decades.2 Approximately 90% of investigational drugs fail before being approved for use in patients, mostly attributed to either lack of efficacy or to drug‐induced safety issues.3 Considering the increase in drug development costs and drug attrition rate, it was evident that new approaches were needed to expedite the drug development process and improve probability of success of new drugs in terms of clinical efficacy and safety. Interestingly, cumulative success rates as well as the overall pipeline quality have started to improve since 2012.4 Nevertheless, of all the factors that impact the overall cost of drug development, the most important one is still the poor success rate in the phase II proof‐of‐concept study.4
The Critical Path Initiative led by the US Food and Drug Administration5 proposed the utilization of model‐based approaches to improve drug development knowledge management and decision making. The concept of quantitative model‐based drug development had been introduced already some time ago through the “learn‐confirm paradigm” proposed by Sheiner6 in 1997 and its application in drug development evolved over the last decades.7, 8 A survey conducted in 2014 by the preclinical Pharmacokinetics/Pharmacodynamics (PK/PD) Discussion Group assigned by the Drug Metabolism Leadership Group (DMLG) within the International Consortium (IQ) for Innovation and Quality in Pharmaceutical Development revealed that preclinical PK/PD modeling is widely used in the pharmaceutical industry, enabling the selection and optimization of human doses and/or dose regimens, including prediction of human efficacious doses.9 Although PK/PD modeling approaches have proven invaluable, their utility in translating biological effects between species and their ability to rigorously assess the mechanism of action of novel drugs is limited.10 Because most current drug‐discovery efforts are targeted toward complex diseases, understanding the pathophysiology at a systems level has been recognized as an important aid in target validation, biomarker selection, (pre)clinical study design, and patient stratification aimed at achieving higher success rates in clinical trials.11 The growing interest in a more quantitative systems approach to assess drug disposition and drug action has been emphasized through: (1) various consortiums, white papers, working groups, conferences, or webinars focusing on quantitative and systems modeling (e.g., the Certara's Immunogenicity consortium; the Quantitative Systems Pharmacology (QSP) Workshops organized by the National Institutes of Health (NIH) in 2008 and 2010; the Rosa's Worldwide Webinar Series; the QSP Webinar organized by the IQ CPLG; the International Society of Pharmacometrics (ISoP) QSP Special Interest Group; the QSP sessions at the American Conference on Pharmacometrics, Discovery on Target, and QSP topics organized by the American College of Clinical Pharmacology (ACCP), the American Association of Pharmaceutical Scientists (AAPS) Quantitative Pharmacology Task Force, the American Society for Clinical Pharmacology and Therapeutics (ASCPT), and many more12); (2) systems and mathematical‐based training programs13; (3) industry examples of QSP based modeling14, 15, 16; and (4) acceptance of QSP models by regulators.17, 18, 19
Although there is increasing interest in QSP within the biopharmaceutical industry with a growing number of efforts since 2005, its role in research and development (R&D) has not yet reached its full potential,20 including its use within the preclinical phase. From the preclinical PK/PD survey,9 it can be concluded that the consistent use of more complex models, including systems pharmacology models, was less common compared to traditional PK/PD models. To better understand the current landscape in preclinical QSP modeling and to stimulate the exchange of knowledge in QSP practices across the biopharmaceutical industry, a preclinical QSP working group within the IQ DMLG was formed in 2016, consisting of representatives from 17 pharmaceutical companies, ranging from small biotechnical to large pharmaceutical. The objectives of the preclinical QSP working group were to understand current challenges, barriers, and opportunities for preclinical QSP modeling within R&D, as well as evaluate the organizational structures of preclinical QSP modelers within the industry and their interface with other functional experts across R&D and regulatory agencies. Therefore, a survey was conducted across 50 pharmaceutical companies in the first half of 2017. During preparation of the survey, it became apparent within the QSP working group that there was no clear definition for QSP, probably due to the fact that QSP is a newly emerging discipline and, therefore, its terminology is currently used in a broader sense. Given that a variety of QSP definitions were expected to coexist in the field, a “Terminology” section was included in the current survey to collect opinions from industrial scientists. However, to align specific survey questions around a single definition, the working group reached consensus that QSP is a quantitative or computational framework to support translational drug discovery and development by integrating knowledge on biochemical, biological, physiological, pharmacological, and clinical systems.
This article presents the survey results, which provides insights on the current state of preclinical QSP modeling within the pharmaceutical industry, including future opportunities as well as barriers that may impede its broad use in an impactful manner.
SURVEY APPROACH
The results presented in this communication were obtained by conducting an industry‐wide survey of pharmaceutical companies, with the majority being members of the IQ Consortium. Survey questions were provided and edited to their final form by members of the DMLG QSP Working Group of the IQ Consortium. The IQ Consortium is an organization of pharmaceutical and biotechnology companies with the mission of advancing science‐based and scientifically driven standards and regulations for pharmaceutical and biotechnology products worldwide. The types of questions included single and multiple choice (select all that apply or select your top five answers). The final survey consisted of 42 questions aimed to gather information in 6 different areas: demographics, terminology, organizational structure, operating logistics, applications, and impact/perspectives. In some instances, questions had a certain degree of overlapping information but were collated in the area that provided the best context to the question. “Demographics” questions provided information about the general characteristics of the pharmaceutical companies and the composition of the groups responsible for preclinical QSP modeling. “Terminology” questions provided information of definition and usage of the term “QSP.” The “Organizational structure” questions focused on gathering information regarding how preclinical QSP groups fit within the organizational structure of various pharmaceutical companies. “Operating logistics” questions provided information on practical operational aspects of preclinical QSP modeling groups, such as timing, information transfer/collaboration, data used in QSP modeling, and model development. “Application” questions gathered information on how preclinical QSP modeling is being utilized in each company and in which therapeutic areas. Finally, “Impact/perspectives” questions assessed how preclinical QSP modeling is perceived within each company, its place in the company's decision‐making processes, its impact on project progression, and the challenges for implementation of preclinical QSP modeling throughout the drug discovery and development process. Mindful that QSP modeling could be defined in a variety of different ways, the survey provided participants with the following general definition of QSP modeling: “As a general guideline, please consider QSP as a quantitative or computational framework to support translational drug discovery and development by integrating knowledge on biochemical, biological, physiological, pharmacological, and clinical systems.”
The survey was conducted using an online questionnaire and survey software (SurveyMonkey; www.surveymonkey.com) and sent to representatives of 50 pharmaceutical companies. Each representative was responsible for providing responses that were reflective of the company as a whole, because only one response was collected from each company. The collection of responses occurred over ∼3‐week period (April 21, 2017, to May 12, 2017). All responses to the questionnaire were kept anonymous. Data analysis was performed using Matlab (The Mathworks), Spotfire (TIBCO Software), and Microsoft Excel (Microsoft, Redmond, WA), whereas graphs were generated using Microsoft Excel. Correlation analysis was performed across questions to find any trends (e.g., data analyses were performed to see if the company size impacted the survey responses). Data analyses that considered company size compared pharmaceutical companies with the original designations (<500; 500–2,000; 2,001–10,000, or >10,000 employees) or simplified the designation to small (<2,000 employees), medium (2,000–10,000 employees), and large (>10,000 employees). If the respondent skipped a question, the company that the respondent represented was excluded from the total respondent count in the data analysis of the question and subsequent analyses. Finally, the authors reviewed survey responses as a group and provided potential reasons as to why certain trends were observed. These thoughts have been included in this article and may result in a certain level of bias in the discussion of survey results. However, the authoring group is a reasonable representation of individuals involved in preclinical QSP modeling across the pharmaceutical industry so these opinions should provide a realistic context to the survey results.
All survey questions and responses are presented in Supplementary Table S1.
Demographics
There were a total of 33 responses to the survey with representation from a wide range of pharmaceutical companies. Of the 33 respondents, 12 (∼36%) were from large pharmaceutical companies (with >10,000 employees), 11 (∼33%) from medium‐sized companies (2001–10,000 employees), and 9 (27%) from small pharmaceutical companies (<2,000 employees); 1 respondent did not select a company size (Supplementary Figure S1). Half of the companies (∼53%) develop both small molecule and biologics therapies, whereas 41% focus primarily on small molecules and a small fraction (6%) primarily on biologics. Within these companies, oncology, neuroscience, and autoimmune disorders are the major therapeutic areas of focus with 58%, 67%, and 55% of respondents, respectively. Other therapeutic areas of focus included infectious diseases, cardiovascular diseases, metabolic disorders, as well as rare diseases.
Terminology
There is a wide variation in the responses around the use of QSP modeling as well as the understanding of its definition. A significant number of companies (∼27%) responded that they do not use the term QSP to describe any of their modeling, suggesting that, currently, QSP is not established in one third of pharmaceutical companies (Figure 1 a). Interestingly, this observation was found to be inversely correlated to the size of the companies, varying from ∼17% in large pharma to nearly 50% of all small companies indicating that they do not use QSP modeling (Figure 1 b). These results suggest that larger companies have more resources and/or motivation to implement QSP approaches, which is reflected in Figure 1 c. Among the companies that use QSP modeling, only 25% (6 of 24, green areas in Figure 1 a,b), responded that it is clear which models are considered QSP and which are not. Interestingly, those respondents with a clear understanding of QSP modeling selected a variety of modeling approaches (Figure 2) similarly to companies in which QSP modeling is perceived as loosely defined. The majority of companies (20 of 24) that use QSP modeling consider comprehensive models of biological/therapeutic mechanisms and mechanism‐based pathway or signal transduction models as QSP models (Figure 2). As highlighted above, a lack of consistent definition of QSP is apparent because many companies also consider mechanistic PK/PD (∼79% of respondents) and physiologically based pharmacokinetic (PBPK; ∼50%) as QSP models. This uncertainty is consistent across pharmaceutical companies of different sizes (equal contribution from all companies in choosing PBPK and PK/PD as QSP models) and highlights the need for better education and definition of QSP in the modeling community and beyond.
Figure 1.
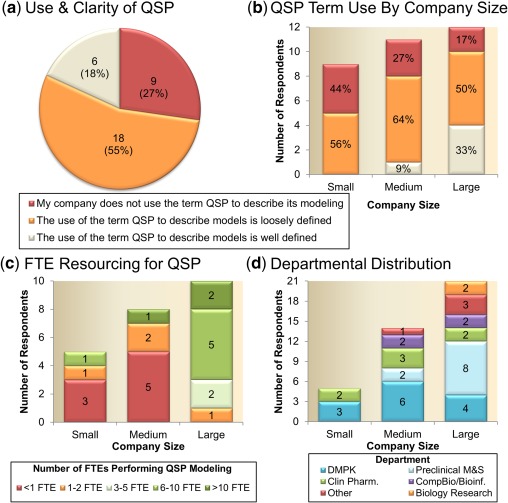
Quantitative systems pharmacology (QSP) modeling definition and resourcing. Use of QSP modeling across all surveyed pharmaceutical companies (a) show a significant number of companies (27%) do not use the term QSP to describe any of their modeling activities. When stratified based on company size (b) mid to large pharmaceutical companies were more likely to describe some of their modeling activities as QSP; however, the majority of companies, independent of size, had a loose definition of QSP. (c) A positive correlation was demonstrated between company size and QSP modeler full time equivalent (FTE) employee resource. (d) Departmental distribution of preclinical QSP modelers. Company size is defined as: small = <2,000 employees; medium = 2,000–10,000; and large = >10,000 employees. DMPK, drug metabolism and pharmacokinetics; M&S, modeling and simulation.
Figure 2.
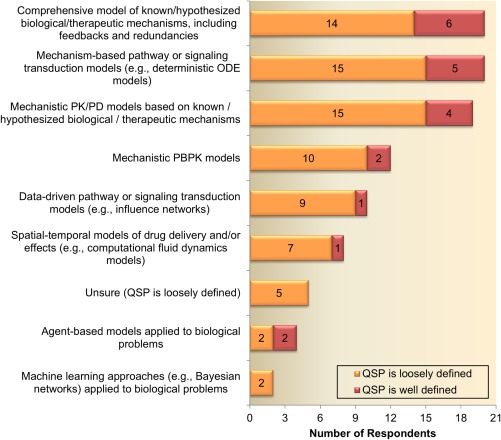
Modeling approaches considered as quantitative systems pharmacology (QSP) Models. Response patterns were comparable between Pharmaceutical companies with a clear and loose definition of QSP, highlighting the need for better education and definition of QSP in the modeling community. ODE, ordinary differential equation; PBPK, physiologically based pharmacokinetic; PK/PD, pharmacokinetic/pharmacodynamic.
ORGANIZATIONAL STRUCTURE
For the majority of companies, preclinical QSP modelers report into drug metabolism and pharmacokinetics (DMPK; 58%) and/or preclinical PK/PD modeling groups (42%), whereas a minority responded that preclinical QSP modelers also reside in the clinical pharmacology (29%), computational biology/bioinformatics department (17%), or discovery/biology department (8%) (Figure 1 d). Eighty percent of the companies in which QSP modelers report into preclinical PK/PD modeling groups is large pharmaceutical companies. This observation is reasonable as large companies either have separate preclinical modeling groups or preclinical modeling groups that report into DMPK (Schuck et al.,9 2015). In addition, the survey results show that modeling roles are not separated but rather combined with other functional roles. In addition to full‐time QSP modelers, 83% of companies reported to have part‐time QSP modelers with additional responsibilities, such as DMPK, preclinical PK/PD, clinical pharmacology, and pharmacometrics.
Additionally, most organizations had QSP modelers centralized (42%) and only a small fraction (17%) reported that QSP modelers were either divided into therapeutic areas or different geographic regions. In line with the organization structure, the academic background of QSP modelers is also diverse (Supplementary Figure S2). A significant proportion of modelers have a classical pharmacokinetic/pharmaceutical background followed by engineering, computational biology/bioinformatics pharmacology, and mathematics expertise.
As expected, the resourcing of QSP (as measured by full time equivalent (FTE) employees) was well correlated with the size of the company (Figure 1 c). Seventy percent of large pharmaceutical companies that utilized QSP modeling responded to have either 6–10 or >10 FTEs committed for QSP modeling. By contrast, seven of eight medium‐sized and four of five small companies reported a resource commitment of less than or equal to two FTEs for QSP modeling. In terms of work load, the majority of companies (46%) responded that two QSP projects are supported per FTE. Interestingly, no correlation was observed between companies’ definition of QSP modeling vs. FTE spending per project. Almost all companies reported that less than four QSP projects are supported per FTE, which is reasonable because QSP models do need significant time for development.
When asked if QSP modelers interact with projects in a direct or indirect manner, only 29% reported that QSP modelers were delegated exclusively to a service provider role. The remaining 71% of companies responded that the QSP modeler was, at least in some instances, an integral member of the project team. From this fraction, only 21% of the companies report that QSP modelers are always an integral project team member, whereas for others it depends on the project or the development stage.
OPERATING LOGISTICS
Timing
The survey sought to identify the stage at which companies initiated preclinical and/or clinical QSP modeling efforts. Based on the survey results it is clear that companies initiate QSP modeling efforts at various stages throughout the drug discovery and development process with nearly half (48%) of respondents selecting three or more stages for initiation. With regard to the specific stage, over half of the respondents (65%) indicated that QSP model development is initiated prior to clinical candidate selection (CCS), with 39% of respondents initiating QSP modeling as early as target validation, and 52% initiating at lead identification and/or lead optimization (Figure 3 a). Only three companies indicated that QSP efforts only start at the target validation stage (Figure 3 b). The majority of respondents (20 of 23; 87%) indicated that QSP modeling is sometimes initiated at the time of CCS or later, but most of these companies (n = 12) also initiate QSP efforts earlier (Figure 3 b). Eight respondents (35%) indicated that QSP activities are not initiated prior to CCS (Figure 3 b). The latter eight companies consider mechanistic PBPK or PK/PD as a type of QSP model and seven use QSP modeling to support first‐in‐human (FIH) studies (Figure 3 c) . The latter may reflect the use of PBPK and mechanistic PK/PD at later stages of preclinical drug discovery. Overall, it can be concluded that most companies start QSP modeling before the selection of clinical candidates (15 of 23), emphasizing the emerging direction of the preclinical QSP field. Only one company indicated that they only have clinical QSP modeling activities.
Figure 3.
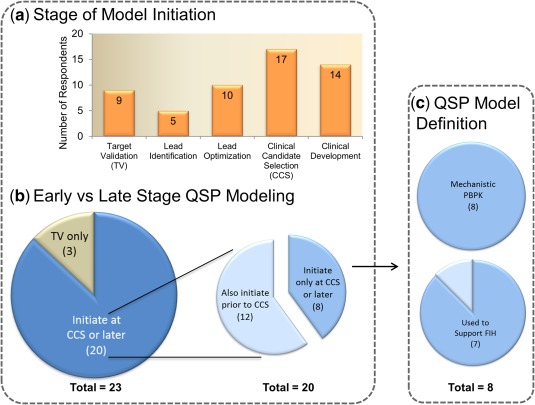
Quantitative systems pharmacology (QSP) model initiation by stage of drug discovery/development. (a) The QSP models are being implemented across all stages of drug discovery/development. (b) Three companies are implementing QSP modeling at the target validation stage only, whereas 20 initiate at clinical candidate selection (CCS) or later. Of those 20, 60% also have activities prior to CCS. Those who only initiated QSP at CCS or later were examined further to understand the role of PBPK and FIH support. (c) In these cases, all considered mechanistic physiologically based pharmacokinetic (PBPK) as a type of QSP model, suggesting that QSP may be used in clinical dose prediction or related pharmacokinetic questions. This hypothesis may be strengthened by the fact that seven of the eight responders also sited that QSP modeling was used to support first‐in‐human (FIH) studies.
Information transfer/collaboration
The survey explored how and when preclinical QSP models are shared internally and/or externally. The QSP models are most often shared as commercial code (e.g., Matlab scripts), followed by mathematical descriptions and open source code and less through markup languages, such as SBML or CellML (Supplementary Figure S3a). This may reflect (1) internal efficiencies achieved by directly sharing models; (2) the historical preference for publications to use mathematical descriptions when sharing information externally, and (3) the preference to accommodate broad use that is independent of the modeling platform. Interestingly, for companies that had a gradual handoff of the QSP model between preclinical and clinical modelers, there was a greater use of mathematical descriptions. No companies indicated that they have shared databases or official handoffs of QSP modeling activities that end the involvement of the preclinical modelers. Instead, companies either do not have separate preclinical and clinical QSP modelers, preclinical and clinical QSP modelers work together collaboratively, or they do not have a formal process in place for knowledge transfer, which implies in the latter case that a more fluid and fit‐for‐purpose (FFP) approach is taken. For those companies with a formal handoff process, this occurs prior to investigational new drug (IND)/First in Human. With regard to the software tools used for QSP modeling, there was no clear standout, with similar responses for specialized systems modeling tools, general engineering/computational/statistical languages, and PK/PD software (79%, 71%, and 63% of respondents, respectively). Approximately half the respondents indicated they use PBPK and population PK/PD tools (Supplementary Figure S3b). This is reflective of the wide variety of models defined as QSP models (Figure 2) and the diverse backgrounds of QSP modelers (Supplementary Figure S2).
Data
With regard to input data, the survey specifically examined the utilization of omics data in preclinical QSP models, whereby “omics” could reflect genomics, transcriptomics, proteomics, metabolomics, or physiomics. Despite the broad inclusion of diverse omics technologies, the results suggest that this type of data are rarely used in QSP modeling, which may be reflective of the challenges associated with proper integration of omics data into a QSP modeling framework and highlights a future development opportunity for QSP models. A recent Frontiers Research Topic was initiated in this area21 and provides examples of ongoing efforts.22 Similar to omics, gene level data have limited usage in QSP models. However, preclinical QSP models are being developed at a variety of different biological scales, including pathways, organelle, and cellular levels to tissue/organ as well as whole body and patient population, which again highlights the diversity of QSP models.
In addition to exploring the use of omics data, the survey identified another potential missed opportunity in that experimental data solely for the development of QSP models are not routinely gathered. Experimental data can be critical for confirmation of mechanistic understanding and parameter identification. Although 50% of respondents suggested that this is sometimes performed, only 8% suggested that it is done often and >30% of respondents rarely or never undertake experiments solely for the QSP modeling activities. Larger companies may have more flexibility and infrastructure to execute additional experimental studies, as indicated by 7 of 10 large companies selecting “sometimes” to this question.
MODEL DEVELOPMENT
The survey suggests that model complexity is not a major contributor to the classification of QSP models because respondents indicated that a typical preclinical QSP model has variables below 20 to beyond 100 and anywhere in between. This is consistent with the type of QSP models companies are developing, whereby a third of respondents are creating FFP models and the other two‐thirds are developing both FFP and platform models (Figure 4 a). No companies are investing in platform models only. This may be driven in part by the need to impact project decisions in a timely manner, which would be best achieved with an FFP model. In the long term, the learnings from FFP models originally developed for individual projects can be integrated into a platform framework. Figure 4 b illustrates that FFP models more often include fewer variables than those developed by companies doing both FFP and platform models. In addition, although model development time ranges from short to long for FFP models, in companies that develop both types of models, we see an increasing number of responses reflecting a longer development time, which may be attributed to the platform efforts undertaken by these companies (Figure 4 c). There was no obvious trend in the time taken to develop a QSP model and company size. In terms of how a company develops a QSP model, there was a fairly even distribution of responses for a variety of in‐house and external processes, including collaborations with academics and contract research organization (CRO) involvement. No clearly preferred method was selected to assess QSP model quality or reliability. Most often it seems to be performed using validation simulations, sensitivity analyses, and biological plausibility by nonmodelers, followed by diagnostic plots and statistical tests, uncertainty quantification methods, and assessment by other modelers (Supplementary Figure S4).
Figure 4.

Quantitative systems pharmacology (QSP) model scope, size, and development time. (a) No companies are solely using platform models. (b) Model size/complexity was assessed based on the number of model variables. Fit‐for‐purpose models (orange bars) tend to be smaller in size compared with models being developed by companies implementing fit‐for‐purpose and platform models (red bars). (c) Model development time is evenly distributed for fit‐for‐purpose models, whereas responders who are working on both types of models have a greater emphasis on longer development timelines.
APPLICATIONS
In terms of the purpose of preclinical QSP modeling, the most common responses refer to hypothesis generation, prediction of clinical efficacy, optimization of clinical proof‐of‐concept doses or regimens, biomarker identification/translation, and compound selection (Supplementary Figure S5). This suggests that, in addition to traditional PK/PD approaches, companies are striving for more biologically informed predictions utilizing QSP approaches, by exploring mechanism of action and biomarkers related to compound pharmacology and disease. In addition to the use of QSP modeling for clinical efficacy predictions, only 25% of the companies apply QSP for the assessment of safety, which highlights a future opportunity. Another missed opportunity is the use of QSP modeling in decision making, as only 33% highlighted that an internal go/no‐go decision includes QSP support. There is also room for a more significant impact of QSP in target identification/validation and to address questions regarding unexpected PK/PD relationships or special population, as it is applied for these purposes in only 25% of the companies. An illustrative example in which QSP modeling could address an unmet need and influence decisions is rational drug combinations. For example, combination therapy in oncology is becoming common practice, yet the field struggles with sequencing and regimen questions as well as prioritization of the most promising combination studies.23, 24 Given its mechanistic nature, preclinical and clinical QSP modeling has the potential to meaningfully impact rational drug combination strategies, which cannot be fully evaluated in humans using empirical approaches. It is encouraging to see that several QSP models have recently been developed to address questions in this space.25, 26
With regard to QSP modeling support across therapeutic areas (TAs), half of the respondents indicated that QSP modeling is broadly applied to various disease areas, whereas the other half indicated a limited application to only one or two TAs. Oncology and autoimmune disorders are the top two TAs in which QSP modeling currently provides the most support (12 of 24) and are expected to garner even more support in the near future (15 of 24), which is consistent with the finding that these two TAs are significant areas of focus across the respondents’ organizations (Figure 5). Although neuroscience is indicated as the top focus area for the majority of responding companies (22 of 33), it ranked very low in terms of current QSP modeling support with only four respondents (17%) currently applying QSP modeling in this area. However, it is encouraging that future QSP investments in neuroscience should increase with twice as many respondents (33%) selecting it as one of the TAs with the most potential for QSP modeling impact in the next 5 years.
Figure 5.
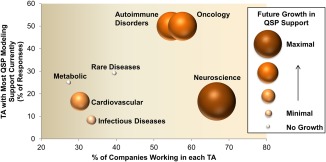
Current and future impact of quantitative systems pharmacology (QSP) across therapeutic areas (TAs). As indicated by the horizontal positioning of the circles, neuroscience, oncology, and autoimmune disorders are the three TAs with the most investment across responding companies. Oncology and autoimmune disorders have a greatest current support by QSP activities (vertical position), whereas neuroscience is similar to other TAs. However, neuroscience is expected to have the most growth in QSP modeling support in the next 5 years (size and color of circle), followed by continued investments in oncology and autoimmune disorders.
Infectious disease is indicated as the TA with the least QSP modeling support (only 2 of 24) and low future potential (only 3 of 24), despite the fact that it is the focus area for 30% of the responding companies. Potentially, this can be related to the fact that conventional PK/PD approaches and measurements (such as the time of free concentrations exceeding minimum inhibitory concentration) are widely applied and successful to guide study designs and the decision making process for infectious disease therapies.
The answers around future potential are largely consistent with those related to current QSP activities across therapeutic areas, implying that the companies are likely to continuously expand their efforts in certain TAs in which they have already seen the benefits.
Impact/perspectives
Half of the responding companies indicated that preclinical QSP was first explored between 2011 and 2015, whereas 25% indicated to have initiated efforts between 2005 and 2010, and 2 of 24 responding companies indicated having started already prior to 2005 (Supplementary Figure S6). Not surprisingly, the two companies with the longest experience with QSP were both large companies. Only 4 of the 24 companies have <2 years of experience with QSP. Among the companies that indicated a late start (2016–2017), two were mid‐sized and one was a large company.
Based on the responses in this survey, QSP modeling does not seem to be included routinely in regulatory submissions, as most respondents indicated “Rarely” (29%) and “Never” (46%), whereas only 17% and 8% answered “Frequently” or “Sometimes,” respectively (Supplementary Table S1). For the latter companies, the main purpose for the inclusion of QSP modeling in regulatory documents seems to be related to the human efficacious dose prediction (67%), and to a lesser extent to the assessment of safety (29%). One company indicated that the QSP model was included as sole supporting evidence in the submission. There are no clear correlations between company size and QSP model inclusion in regulatory submissions, except that most companies selecting “Frequently” are small to medium‐sized (500–10,000 employees) and not large. Correlation analysis between the QSP definitions and frequency of QSP model submission revealed no significant trends. For example, for those selecting “Frequently” or “Sometimes” answers, their definitions of QSP models are not biased in favor of PBPK modeling but approximately evenly distributed over all model types.
The QSP modeling is thought to be impactful by the majority of the respondents (21 of 24; Figure 6 a), although nearly 60% selected it as being only “somewhat important/impactful,” leaving room for future improvements. For 16 of the 24 respondents, QSP modeling has had a “positive impact” on team communication/alignment. As expected, impact seems to correlate somewhat with the length of time in which the company has been involved in preclinical QSP. The majority of the companies indicating a positive impact have been involved in QSP for at least 6 years (prior to 2011), although 3 companies with positive impact only had experience with QSP modeling for 1 year (Figure 6 b). The number of projects for which QSP has had an impact seems to correlate, to a certain degree, with the initiation of QSP support, as most of the respondents that indicated at least five projects are impacted per year started QSP modeling before 2011 (Figure 6 c). Companies stating that zero projects were impacted by QSP only started QSP modeling in the last 6 years, suggesting that a time frame of at least a couple of years is necessary to show significant impact. In addition, companies with larger numbers of FTEs dedicated to QSP modeling reported higher number of projects being impacted per year (Figure 6 d). No clear correlations are visible between the number of impacted QSP projects and the therapeutic area.
Figure 6.
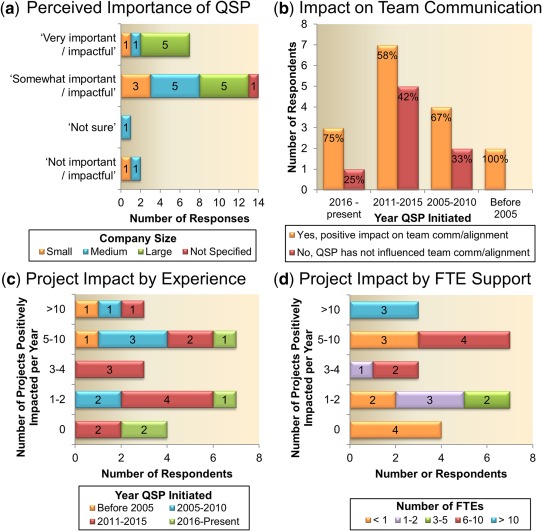
Preclinical quantitative systems pharmacology (QSP) impact. (a) The majority of surveyed companies see that preclinical QSP is important/impactful, although nearly 60% selected it as being only somewhat impactful. Small = <2,000 employees, medium = 2,000–10,000; and large = >10,000 employees. (b) Two‐thirds of respondents indicated that QSP modeling had a positive impact on team communication/alignment. The bottom panels show the number of projects positively impacted per year segmented by (c) the year QSP was initiated and (d) number of full time equivalent (FTE) employees assigned to QSP within the company.
To better understand how companies describe a successful outcome, a portion of the survey questions were related to reasons for perceived successes (Figure 7 a) or failures (Figure 7 b) of QSP modeling activities across pharmaceutical companies. One of the leading reasons for successful or failed QSP applications is whether timely delivery of results or timely project impact can be achieved. Although this response is not unexpected, previous survey responses indicate that it can take a long time to develop QSP models, which stresses the importance of proper planning and establishing proper expectations with the project teams regarding deliverables and timelines. In addition, 16 of 24 respondents indicated that clearly defined problems with an intended scope is a top reason for success, whereas lack of interest from management and delayed or insufficient modeling support are the main reasons cited for failures. The latter suggests the need for more QSP resources, funding, and/or expertise in companies to ensure timely and high quality project support to increase the impact and value of QSP modeling in the drug development process.
Figure 7.
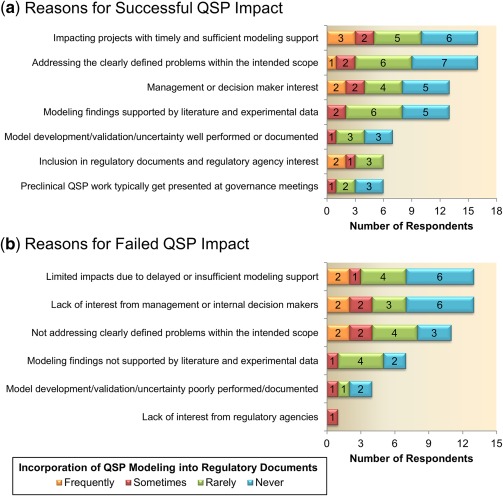
Reasons for successful (a) or failed (b) quantitative systems pharmacology (QSP) impact. Responses are listed vertically in descending order from most cited to least cited and are further segmented by the frequency with which QSP work makes it into regulatory submission documents.
To get a flavor for the expected trends in future QSP modeling support within the pharmaceutical industry, the survey also included questions around anticipated hiring and training efforts. Overall, the companies that currently conduct QSP modeling are expected to maintain or increase their QSP modeling team size as the majority of the respondents foresee an increase (14 of 24) or no change (9 of 24) in the hiring, whereas only one respondent expects a decrease in the QSP headcount. The distribution of choices is consistent across companies with different sizes. In terms of future training opportunities, the top areas selected by all companies are: “systems biology/physiology,” “PBPK/PD,” “population PK/PD,” “relevant biological/physiological/pathological/pharmacological systems,” “mathematics, statistics, engineering, or physics concepts,” and interestingly “soft skills” (Figure 8). The selection of these top areas for training opportunities is largely consistent with the observation that most companies consider QSP models as comprehensive, mechanistic models of biological systems, pathways, and therapeutic mechanisms. Interestingly, the smallest companies (<500 employers) do not see a need to train QSP modelers on soft skills, like communication, project management, or leadership.
Figure 8.
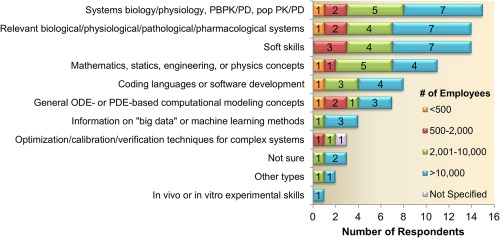
Cross‐functional training opportunities for modelers. Responses are segmented by company size. Most companies indicate a need for technical training related to comprehensive and mathematical model building, as well as the need to develop soft skills (like communication, leadership, and project management). ODE, ordinary differential equation; PBPK, physiologically based pharmacokinetic; PD, pharmacodynamic; PDE, partial differential equation; popPK/PD, population pharmacokinetic/pharmacodynamic; QSP, quantitative systems pharmacology.
CONCLUSION
The survey was sent out to 50 pharmaceutical companies and was well received, as indicated by a response rate of 33 companies, with a balanced contribution in terms of industry size and therapeutic focus. Most of the companies that indicated they do not practice QSP modeling were small companies, suggesting that larger companies enable more opportunities to explore QSP tools and invest more resources to implement QSP approaches. Indeed, the survey results show that larger companies have a significant increase in QSP FTEs (>6) as compared to smaller companies (<3). Among the companies that use QSP modeling, QSP models are used in various ways (e.g., mechanistic PBPK and PK/PD) and for the majority of respondents a clear definition of QSP is lacking, indicating the need for a general consensus and alignment in terminology as well as education around QSP. The wide variety of responses around what constitutes a QSP model could be due to the fact that existing modeling approaches are increasingly using mechanistic descriptions of biological processes to describe drug disposition (PK/PBPK), biological responses (PD), and disease progression (signaling pathways) and, thus, to some extent, rely on QSP modeling principles. In line with the wide variety of models defined as QSP, several software tools and languages are used, such as systems modeling tools, PBPK, PK/PD, and population PK/PD software as well as general engineering, computational, or statistical languages. This broad usage of the term QSP is also apparent from literature. The study by van der Graaf & Benson10 describe QSP as the “quantitative analysis of the dynamic interactions between drug(s) and a biological system that aims to understand the behavior of the system as a whole, as opposed to the behavior of its individual constituents.” Others describe QSP as mechanistic models that represent complex biological pathways in healthy and disease physiology, and downstream PD effects.27, 28 Interestingly, systems biology‐related approaches, like PBPK models, that focus on compound PK and distribution have also been described as QSP,29 as well as pharmacometric and statistical system models used to describe population data on biological pathways or disease processes.30
Larger companies seem to be the trend setters in terms of preclinical QSP modeling support (starting around 2005), and QSP efforts really emerged after 2011 across companies. Not surprisingly, the success of QSP modeling is related to the number of years a company has been exposed to QSP and the amount of FTEs assigned to QSP modeling support. In addition, a clearly defined problem with intended scope and the timely delivery of the QSP model outcomes were identified as essential factors for success, whereas delayed or insufficient modeling support, as well as a lack of interest from management, was highlighted as the main reason for failure. Overall, QSP modeling is recognized as being impactful and the majority of companies expect an increase in hiring and training of QSP modelers in the near future. In terms of the organizational structure, the results show that, in most companies, preclinical QSP modelers report into DMPK and/or preclinical PK/PD groups, in a centralized way to support across therapeutic areas. In most cases, QSP modelers (either preclinical or clinical) have other job responsibilities related to DMPK, clinical pharmacology, pharmacometrics, or PK/PD modeling, which is in line with the diversity in academic background across QSP modelers.
Most companies start QSP modeling before the selection of clinical candidates, mainly to interpret preclinical datasets, inform biomarker translation, and support clinical development of drug candidates, emphasizing the emerging direction of the preclinical QSP field. Given the mechanistic nature of QSP modeling, it is somewhat surprising that it is not routinely applied in the target identification/validation stage or for the assessment of safety issues. Even more surprising is that 35% of the companies indicated that QSP activities are not initiated prior to the selection of their clinical candidate, most likely referring to mechanistic PBPK or PK/PD modeling of clinical candidates in support of FIH trials. To become more impactful and successful, it is recommended to start earlier with preclinical QSP modeling support in drug discovery to provide greater impact on the overall decision‐making process throughout drug discovery and development and potentially support more frequently in regulatory questions and submissions. Early implementation of QSP models may necessitate experimental studies designed specifically to inform components of the model. This lack of dedicated experimental support is currently a gap within most companies and may hinder the successful implementation of QSP models, especially when such models are exploring novel biological mechanisms and in cases in which existing data and literature do not reflect the true dynamics of the biological process or provide information within the proper biological context. Access to experimental resources will require collaboration and, in many cases, a clear statement of the benefit that the model and experiments would provide.
In line with reported areas of therapeutic focus, most companies currently apply QSP models in oncology and immunology. Future QSP potential was largely consistent with areas in which current QSP activities were already ongoing, suggesting that QSP in these therapeutic areas is considered to be successful or impactful. The single outlier to this trend was in neuroscience, in which a significant number of companies focus on neuroscience, yet QSP modeling is currently not widely applied in this therapeutic area. Encouragingly and perhaps not surprisingly, a decent portion of companies do see future potential for QSP support in this therapeutic area.
In summary, preclinical QSP modeling is used across the majority of pharmaceutical companies (mainly larger‐sized) and seems to be an emerging field with expected growth in the near future. The survey outcomes indicate a clear need for a better definition and terminology around QSP. Thus, a future objective of the preclinical QSP working group is to organize an educational forum in 2018 to build consensus on the terminology and definition around QSP and to publish a follow‐on article to review the current applications of preclinical QSP modeling in the pharmaceutical industry, building on the conclusions from the current survey article. One of the main focus points will be to define a general definition of QSP as a recommendation for future use and communication. In addition, case studies illustrating the impact of preclinical QSP modeling in drug development will be included, along with a discussion of best practices and future opportunities (e.g., target identification, safety assessment, and regulatory submissions).
Source of Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
Supporting information
Supplementary Table S1. Survey Questions and Responses
Supplementary Figure S1. Size Distribution of Companies Participating in the Survey
Supplementary Figure S2. Disciplines Contributing to QSP Modeling
Supplementary Figure S3. QSP Model Sharing and Software Tools
Supplementary Figure S4. Methods to Assess QSP Model Quality or Reliability
Supplementary Figure S5. Preclinical QSP Modeling Applications
Supplementary Figure S6. Year QSP Initiated
Acknowledgments
This survey was developed with the support of the International Consortium for Innovation and Quality in Pharmaceutical Development (IQ). The IQ is a not‐for‐profit organization of pharmaceutical and biotechnology companies with a mission of advancing science‐based and scientifically driven standards and regulations for pharmaceutical and biotechnology products worldwide. Please visit www.iqconsortium.org for more information. The authors thank the IQ Drug Metabolism Leadership Group (DMLG) for their support of this work and Mira Hinman for additional support in the Spotfire analysis.
Contributor Information
Marjoleen J.M.A. Nijsen, Email: marjoleen.nijsen@abbvie.com
Mary E. Spilker, Email: mary.spilker@pfizer.com
References
- 1. DiMasi, J.A. , Grabowski, H.G. & Hansen, R.W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47, 20–33 (2016). [DOI] [PubMed] [Google Scholar]
- 2. Scannell, J.W. , Blanckley, A. , Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 11, 191–200 (2012). [DOI] [PubMed] [Google Scholar]
- 3. Arrowsmith, J. & Miller, P. Trial watch: phase II and phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 12, 569 (2013). [DOI] [PubMed] [Google Scholar]
- 4. Smietana, K. , Siatkowski, M. & Møller, M. Trends in clinical success rates. Nat. Rev. Drug Discov. 15, 379–380 (2016). [DOI] [PubMed] [Google Scholar]
- 5. U.S. Department of Health and Human Services . Food and Drug Administration. Innovation or Stagnation: Challenge and opportunity on the critical path to new medical products. Washington, DC. <https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/UCM113411.pdf> (2004).
- 6. Sheiner, L.B. Learning versus confirming in clinical drug development. Clin. Pharmacol. Ther. 61, 275–291 (1997). [DOI] [PubMed] [Google Scholar]
- 7. Woodcock, J. & Woosley, R. The FDA critical path initiative and its influence on new drug development. Annu. Rev. Med. 59, 1–12 (2008). [DOI] [PubMed] [Google Scholar]
- 8. EFPIA MID3 Workgroup et al Good practices in model‐informed drug discovery and development: practice, application, and documentation. CPT Pharmacometrics Syst. Pharmacol. 5, 93–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schuck, E. et al Preclinical pharmacokinetic/pharmacodynamic modeling and simulation in the pharmaceutical industry: an IQ consortium survey examining the current landscape. AAPS J. 17, 462–473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Graaf, P.H. & Benson, N. Systems pharmacology: bridging systems biology and pharmacokinetics‐pharmacodynamics (PKPD) in drug discovery and development. Pharm . Res. 28, 1460–1464 (2011). [DOI] [PubMed] [Google Scholar]
- 11. Leil, T.A. & Bertz, R. Quantitative systems pharmacology can reduce attrition and improve productivity in pharmaceutical research and development. Front. Pharmacol. 5, 247 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sorger, P.K. et al Quantitative and systems pharmacology in the post‐genomic era: new approaches to discovering drugs and understanding therapeutic mechanisms. An NIH White Paper by the QSP Workshop Group – October, 2011. <https://www.nigms.nih.gov/training/documents/systemspharmawpsorger2011.pdf> (2011).
- 13. Sobie, E.A. , Jenkins, S.L. , Iyengar, R. & Krulwich, T.A. Training in systems pharmacology: predoctoral program in pharmacology and systems biology at Mount Sinai School of Medicine. Clin. Pharmacol. Ther. 88, 19–22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milligan, P.A. et al Model‐based drug development: a rational approach to efficiently accelerate drug development. Clin. Pharmacol. Ther. 93, 502–514 (2013). [DOI] [PubMed] [Google Scholar]
- 15. Visser, S.A. , de Alwis, D.P. , Kerbusch, T. , Stone, J.A. & Allerheiligen, S.R. Implementation of quantitative and systems pharmacology in large pharma. CPT Pharmacometrics Syst. Pharmacol. 3, e142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benson, N. Network‐based discovery through mechanistic systems biology. Implications for applications–SMEs and drug discovery: where the action is. Drug Discov. Today Technol. 15, 41–48 (2015). [DOI] [PubMed] [Google Scholar]
- 17. Manolis, E. , Rohou, S. , Hemmings, R. , Salmonson, T. , Karlsson, M. & Milligan, P.A. The role of modeling and simulation in development and registration of medicinal products: output from the EFPIA/EMA Modeling and Simulation Workshop. CPT Pharmacometrics Syst. Pharmacol. 2, e31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romero, K. et al Modeling and simulation for medical product development and evaluation: highlights from the FDA‐C‐Path‐ISOP 2013 workshop. J. Pharmacokinet. Pharmacodyn. 41, 545–552 (2014). [DOI] [PubMed] [Google Scholar]
- 19. Peterson, M.C. & Riggs, M.M. FDA advisory meeting clinical pharmacology review utilizes a quantitative systems pharmacology (QSP) model: a watershed moment? CPT Pharmacometrics Syst. Pharmacol. 4, e00020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vicini, P. & van der Graaf, P.H. Systems pharmacology for drug discovery and development: paradigm shift or flash in the pan? Clin. Pharmacol. Ther. 93, 379–381 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Frontiers research topic: bridging the divide between omics data and mechanistic simulations: frameworks for interpreting cancer progression and immune response for drug discovery and development. <http://journal.frontiersin.org/researchtopic/4511/bridging-the-divide-between-omics-data-and-mechanistic-simulations-frameworks-for-interpreting-cancer>.
- 22. Kamisoglu, K. et al Understanding physiology in the continuum: integration of information from multiple ‐omics levels. Front. Pharmacol. 8, 91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morrissey, K.M. , Yuraszeck, T.M. , Li, C.C. , Zhang, Y. & Kasichayanula, S. Immunotherapy and novel combinations in oncology: current landscape, challenges, and opportunities. Clin. Transl. Sci. 9, 89–104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LoRusso, P.M. et al Accelerating cancer therapy development: the importance of combination strategies and collaboration. Summary of an Institute of Medicine workshop. Clin. Cancer Res. 18, 6101–6109 (2012). [DOI] [PubMed] [Google Scholar]
- 25. Schmidt, B.J. et al. Development of a quantitative systems pharmacology platform to support translational research and clinical development in immuno‐oncology. American Society for Clinical Pharmacology and Therapeutics Annual Meeting, New Orleans, LA, 3–7 March 2015.
- 26. Apgar, J. et al Quantitative systems pharmacology modeling and analysis provides biological insights into anti‐PD‐1 dosing and predicts optimal PD‐1 x TIM‐3 therapeutic properties for bispecifics and fixed dose combinations in immuno‐oncology. [abstract]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 16–20 April 2016; New Orleans, LA. Cancer Res. 76(14 suppl), Abstract 5001 (2016). [Google Scholar]
- 27. Peterson, M.C. & Riggs, M.M. A physiologically based mathematical model of integrated calcium homeostasis and bone remodeling. Bone 46, 49–63 (2010). [DOI] [PubMed] [Google Scholar]
- 28. Demin, O. et al Systems pharmacology models can be used to understand complex pharmacokinetic‐pharmacodynamic behavior: an example using 5‐lipoxygenase inhibitors. CPT Pharmacometrics Syst. Pharmacol. 2, e74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rostami‐Hodjegan, A. Physiologically based pharmacokinetics joined with in vitro‐in vivo extrapolation of ADME: a marriage under the arch of systems pharmacology. Clin. Pharmacol. Ther. 92, 50–61 (2012). [DOI] [PubMed] [Google Scholar]
- 30. Iyengar, R. , Zhao, S. , Chung, S.W. , Mager, D.E. & Gallo, J.M. Merging systems biology with pharmacodynamics. Sci. Transl. Med. 4, 126ps7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Survey Questions and Responses
Supplementary Figure S1. Size Distribution of Companies Participating in the Survey
Supplementary Figure S2. Disciplines Contributing to QSP Modeling
Supplementary Figure S3. QSP Model Sharing and Software Tools
Supplementary Figure S4. Methods to Assess QSP Model Quality or Reliability
Supplementary Figure S5. Preclinical QSP Modeling Applications
Supplementary Figure S6. Year QSP Initiated


