Abstract
The oral microbiome is a complex ecological niche where both commensal and pathogenic bacteria coexist. Previous reports have cited that the plant isolate honokiol is a potent inhibitor of S. mutans biofilms. Herein we report a cross-coupling method that provides access to a concise library of honokiolinspired analogs. Through this work we determined that the inhibitory activity of honokiol is highly dependent on the growth conditions. Further, we identify a series of analogs that display significant potency against oral bacteria leading to the discovery of a potent antimicrobial.
Keywords: oxidative coupling, antibiotic, oral microbiome, natural product
Graphical abstract
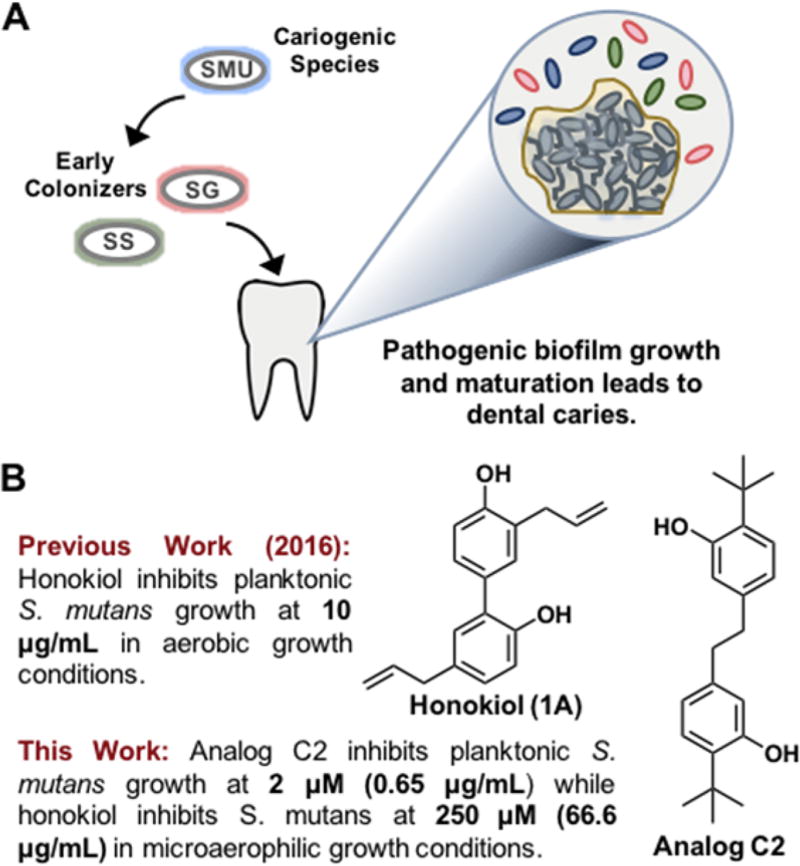
In the past decade studies have correlated that healthy human microbiomes play a role in combating “modern plagues” such as diabetes, obesity, Crohn’s, and celiac disease.1 The oral microbiome, a specific microbial niche residing in the oral cavity of humans, has gained attention due to the complexity in which the microorganisms interact both in commensal and pathogenic manners. This ecosystem is home to various species of bacteria that have adapted to live in the microaerophilic and/or anaerobic environments that exist surrounding the teeth, gingiva, and tongue.2,3 Scientists have turned to chemical biology to develop new tools to study these systems and understand them at the molecular level. Interest in S. mutans has grown since it has been highly associated with the formation of carries via environmental acidification which leads to the corrosion of tooth enamel (Figure 1A).4,5 Early colonizers such as commensal Streptococcus sanguinis and Streptococcus gordonii are responsible for early plaque formation by anchoring adhesion proteins to the pellicle of the tooth and producing glucan polymers that constitute the matrix of dental plaque. S. mutans is able to invade this matrix, form microcolonies, and eventually develop into a mature biofilm that is responsible for tooth decay via acidification.6,7 Another lesser known and more harrowing disease that has been associated with S. mutans biofilm growth is infective endocarditis, or inflammation of the inner tissues of the heart.8 S. mutans has the capability to nest itself in the heart as a mature biofilm and block the blood supply to the inner heart tissues causing inflammation. To date, few natural products have been reported to be effective inhibitors of the oral pathogen S. mutans. One such example is the natural product carolacton which has attracted the attention of our group as well as the Kirshning and Wagner-Döbler laboratories.9–11 Carolacton specifically targets S. mutans cells as they transition into a biofilm. In contrast, the phenolic natural product honokiol has received attention due to the reportedly potent inhibitory activity against S. mutans (Figure 1B).12,13 Although isolated and first reported in 1982 from the bark or seeds of a magnolia tree, honokiol has been used as a therapeutic in Chinese, Japanese, and Korean traditional herbal remedies for centuries.14,15 Previously, our group developed a concise synthesis of honokiol via oxidative phenolic coupling.16 In this report we leverage this method to develop a focused library of honokiol-inspired analogs to better understand the structure–activity relationship against oral bacteria.
Figure 1.
A) Early colonizers S. sanguinis and S. gordonii allow cariogenic S. mutans to form biofilms on the surface of the tooth by adhering to the pellicle. B) The natural product honokiol has been previously reported to inhibit S. mutans growth. Here we demonstrate that analog C2 is a more potent bactericidal agent against oral microbiome bacteria.
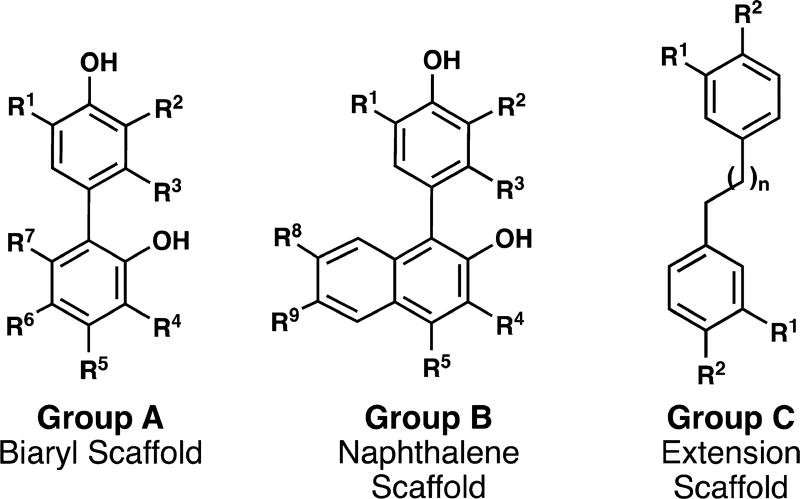
Our group has developed an expedited method to access this natural product scaffold.17 Accordingly, we sought to apply this method in a general sense for two reasons: 1) to demonstrate the scope of this method for uniting aryl moieties and 2) to provide a library of analogs to answer specific structure–activity relationship questions. The analog design was organized into three groups based on the scaffold (Figure 2). Group A mimics the biaryl architecture of the natural product honokiol, Group B focuses on the naphthalene scaffold, and Group C examines the necessity of the biaryl linkage.
Figure 2.
Analogs are classified in three groups. Group A = biaryl scaffold; Group B = napthalene scaffold; Group C = extension scaffold.
As mentioned previously, the oxidative coupling reactions developed in our lab were used to synthesize the specific congeners of the general subclasses outlined in Figure 2. A vanadium-catalyzed phenol homocoupling was used to assemble A4 and A5 (eq 1).18 Selective cross-coupling of two different phenols was accomplished with a chromium catalyst developed previously.16 Table 1 illustrates how the technique was used to rapidly assemble an array to investigate structure–activity relationships; in these cases no optimization of the yields was performed as the bioactivity was the focus. To investigate an alternate biaryl union, C2 was prepared by Friedel–Crafts alkylaton of the parent bisphenol (eq 2). The analogs described in Table 1 are all congeners of the parent structures in Figure 2.
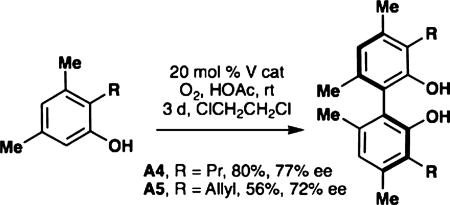 |
(1) |
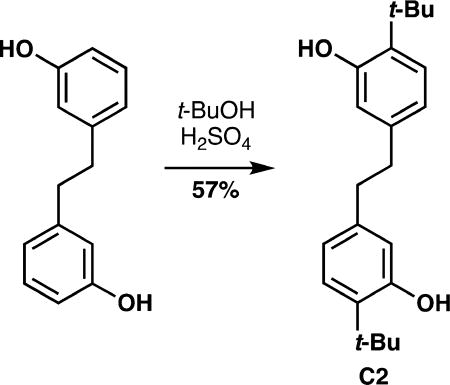 |
(2) |
Table 1.
Cr-Salen Catalyzed Cross-Couplings
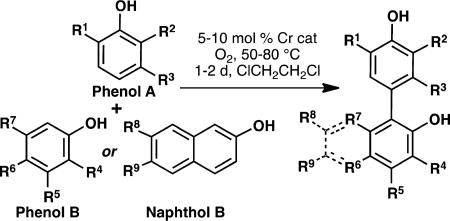
| |||
|---|---|---|---|
|
| |||
| product | phenol A R1, R2, R3 |
phenol B/naphthol B R4, R5, R6, R7/ R8, R9 |
yield (%) |
| A2 | t-Bu, t-Bu, H | Me, HO, H, Me | 38 |
| A3 | Me, Me, H | 63a,b,c | |
| A6 | allyl, t-Bu, OH | H, Me, Me, Me | 33 |
| A7 | t-Bu, Me, H | Me, H, H, i-Pr | 74a,b |
| A8 | allyl, t-Bu, OH | H, H, Me, H | 28 |
| A9 | i-Pr, i-Pr, H | H, Me, Me, Me | 18 |
| A10 | allyl, allyl, H | Me, H, H, i-Pr | 4b |
| A11 | t-Bu, t-Bu, H | Me, OH, H, Me | 29d |
| B1 | Me, Me, H | H, H | 65 |
| B2 | MeO, MeO, H | H, H | 72 |
| B3 | t-Bu, t-Bu, H | H, H | 83a |
| B4 | t-Bu, t-Bu, H | HO, H | 88 |
| B5 | t-Bu, t-Bu, H | H, Br | 24 |
| B6 | i-Pr, i-Pr, H | H, Br | 72 |
| B7 | allyl, t-Bu, H | H, MeO | 49 |
| B8 | allyl, t-Bu, H | H, Br | 85 |
| B9 | Me, t-Bu, H | H, H | 13 |
| B10 | i-Pr, i-Pr, H | MeO2C, HO | 56 |
| B11 | n-Pr, t-Bu, H | H, MeO | 18a, |
| B12 | n-Pr, t-Bu, H | H, Br | 93e |
| B13 | i-Pr, i-Pr, H | H, pyrazole | 28 |
| B14 | allyl, t-Bu, H | H, OH | 21d |
See ref 16.
para–para Coupling.
Homo coupling.
Trimer from two molecules of phenol A and one of phenol/naphthol B.
Obtained by hydrogenation of B7 or B8.
At the beginning of our investigation we were interested in comparing the inhibitory activity of honokiol (1A) to that of our newly synthesized analogs. Minimum inhibitory concentration (MIC) assays, minimum biofilm inhibitory concentration (MBIC) assays, and minimum bactericidal concentration (MBC) assays were undertaken. We initially performed the MIC assays in a 5% CO2-supplemented environment to promote growth of S. mutans in an environment that most closely mimics a healthy oral cavity. The MIC of honokiol was determined to be 250 µM (66.6 µg/mL), which was in stark contrast to the literature value of 10 µg/mL (Table 2). After revisiting the original procedures, we recognized that the original assays were completed in an aerobic environment, which precludes the growth of S. mutans.12 As expected, when aerobic conditions were employed in the assay, the potency of honokiol increased to 125 µM (33.3 µg/mL). These results demonstrate that although S. mutans growth is inhibited by honokiol, the overall efficacy of the compound will be less under physiological conditions. Furthermore, our studies show that honokiol is unable to inhibit biofilm growth (Figure S3) and was not bactericidal at concentrations of 250 µM or lower.
Table 2.
Summary of MIC (S. mutans, S. sanguinis, and S. gordonii) and MBC (S. mutans) Values for Analogsa
| Analog | MIC S. mutans |
MIC S. sanguinis |
MIC S. gordonii |
MBC S. mutans |
|
|---|---|---|---|---|---|
| A | 1 | 250 | 125 | 125 | - |
| 2 | 32 | 32 | 32 | 63 | |
| 3 | >250 | >250 | >250 | - | |
| 4 | >250 | >250 | >250 | - | |
| 5 | >250 | >250 | >250 | - | |
| 6 | >250 | >250 | >250 | - | |
| 7 | 32 | 32 | 16 | 32 | |
| 8 | >250 | 125 | 125 | - | |
| 9 | >250 | >250 | >250 | - | |
| 10 | >250 | 250 | 250 | - | |
| 11 | >250 | >250 | >250 | - | |
| B | 1 | >250 | >250 | >250 | - |
| 2 | >250 | >250 | >250 | - | |
| 3 | 63 | 63 | 63 | - | |
| 4 | 125 | 125 | 125 | - | |
| 5 | 8 | 4 | 16 | 125 | |
| 6 | >250 | >250 | >250 | - | |
| 7 | 125 | 63 | 125 | - | |
| 8 | 16 | 8 | 16 | 32 | |
| 9 | 32 | 32 | 32 | 32 | |
| 10 | 32 | 8 | 8 | 32 | |
| 11 | 16 | 8 | 16 | 63 | |
| 12 | >250 | 250 | 250 | - | |
| 13 | >250 | >250 | >250 | - | |
| 14 | 63 | 16 | 32 | - | |
| C | 1 | >250 | 125 | 125 | - |
| 2 | 2 | 2.5 | 1.25 | 4 | |
MIC and MBC values were completed in biological triplicate. MBC assays were completed for analogs with MIC values <32 µM.
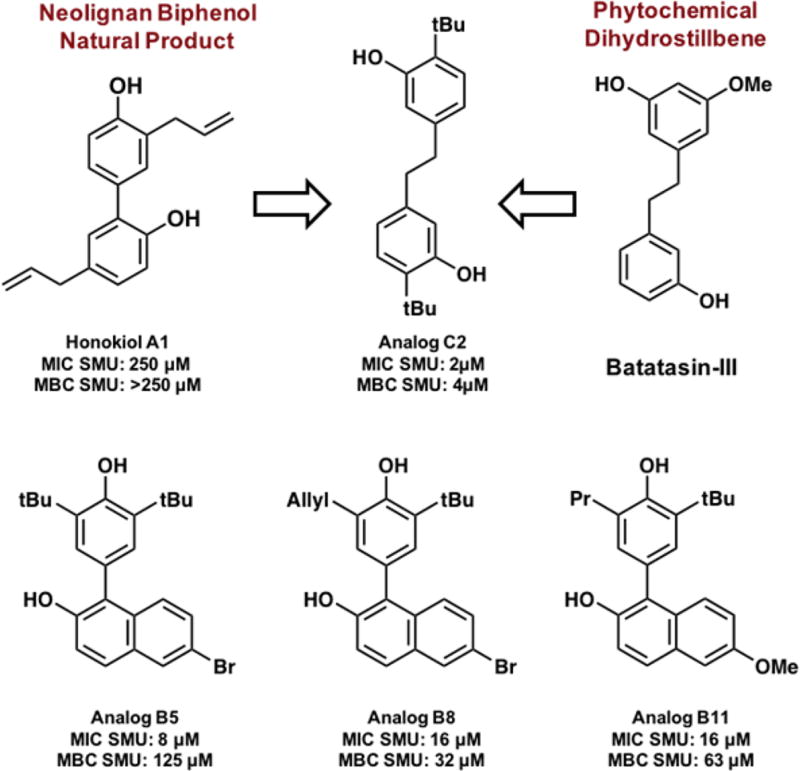
Undeterred by these findings, we sought to evaluate the bioactivity of our honokiol-inspired analogs against a panel of representative oral bacteria via MIC, MBIC, and MBC assays (Table 2 and Figure S3). Out of the 26 honokiol analogs (see Figures S1 and S2 for all structures) four compounds showed significant inhibition at low concentrations (≤16 µM). Analogs C2, B5, B8, and B11 were the most impressive with MIC values of 2 µM, 8 µM, 16 µM, and 16 µM, respectively, against planktonic S. mutans. The analogs were also tested against two commensal strains that are early colonizers: S. gordonii and S. sanguinis. Generally, the MICs for these commensal strains mirrored the values for the pathogenic S. mutans hinting at a broad-spectrum inhibitory mechanism.
Biofilms are of particular importance to the oral cavity as they protect the bacteria and allow them to outcompete other colonizers by decreasing the local pH, thereby causing enamel erosion and other pathologies resulting from the acidified environment.19 For these reasons, the analogs were tested for their ability to inhibit the formation of S. mutans biofilm (Figure S3). Initial reports identified honokiol as a biofilm inhibitor; however in our hands, the MBIC values were within one dilution of the MIC. Based on these results, it is likely that biofilm inhibition is an effect of the inherent toxicity of the compounds to the planktonic bacteria and not by a biofilmspecific mechanism. Honokiol and the analogs tested herein all potently deterred the formation of biofilms when the cells were grown in the presence of sucrose, albeit at the previously determined MIC values. This likely indicates that the compounds are targeting the bacteria in a general fashion and do not show any preferential killing to biofilms.
We next sought to determine if the compounds were working in a bacteriostatic or bactericidal manner. Toward this end, a regrow analysis was completed to determine the MBC values of the active analogs against S. mutans (Figure S4). The MBC values reported refer to the concentration at which there is a 3-Log reduction in CFU count corresponding to 99.9% bacterial cell death (Table 2). Analog C2 had the lowest MBC value at 4 µM confirming that the molecule is bactericidal.20 Analogs B8 and B11 were also shown to be bactericidal; however, the MIC and MBC of analog B5 differs by four dilutions hinting at a bacteriostatic mechanism. These findings suggest that compounds C2 and B5 may be inhibiting the growth of S. mutans by different mechanisms.
Research has focused mainly on the antitumor, antifungal, and anti-inflammatory activities of honokiol resulting in the identification of various cellular targets.21–24 In contrast, the mechanism of action of honokiol, or the derivatives reported, in these oral pathogens has been elusive. Therefore, future work will focus on determining the mechanism by which these molecules elicit their response.
Our initial interest in the natural product honokiol was 2-fold: 1) to probe the bioactivity profile of the compound and 2) as a means to showcase our newly developed synthetic method to access biaryl scaffolds. By extending the aryl connectivity with a two-carbon linker, the potency of the lead compound increased from 250 µM to 2 µM. Intriguingly, the phytochemical dihydrostillbene Batatasin-III, which possesses an analogous scaffold has been studied for its antifouling abilities and has ties to ancient Chinese herbal remedies along with honokiol (Figure 3).25 Based on these chemical similarities, it is possible that the extended scaffold has unrealized biological activity against cariogenic agents and warrants further study. Future work will include a more expansive analog library that will address these hypotheses and will be reported in due course.
Figure 3.
Summary of biologically active honokiol-inspired analogs. Comparison of the structure of C2 and the natural product batatasin- III.
In conclusion, the work presented herein highlights the importance of performing biological testing at physiologically relevant conditions. Our results have demonstrated that the bioactivity of honokiol may be overstated. However, our curiosity surrounding the natural product led to the serendipitous discovery of a highly potent, bactericidal analog, C2, with an MIC value of 2 µM (66 ng/mL). This compound serves as an exciting starting point for future translational studies which may be of particular interest to the oral care industry based on its simple structural architecture and potent bioactivity.
Supplementary Material
Acknowledgments
We are grateful to the NIH (GM112684 [M.C.K.], DE025837 [W.M.W.]) and the NSF (CHE1464778 [M.C.K.], CHE1755698 [W.M.W.]) for financial support of this research. Partial instrumentation support was provided by the NIH and NSF (1S10RR023444, 1S10RR022442, CHE 0840438, CHE-0848460, 1S10OD011980). C.O. acknowledges NIH training grant T32 GM071339. Drs. Rakesh Kohli and Charles W. Ross III are acknowledged for obtaining HRMS data.
Footnotes
ASSOCIATED CONTENT
- Synthetic characterization and biological assay procedures (PDF)
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): W.M.W. and M.C.K. have filed a patent on the technology disclosed.
References
- 1.Blaser MJ. Missing Microbes: How the Overuse of Antibiotics is Fueling our Modern Plagues. Henry Holt and Company, LLC; New York: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi N. Oral Microbiome Metabolism: From “Who Are They?” to “What Are They Doing? J. Dent. Res. 2015;94(12):1628–1837. doi: 10.1177/0022034515606045. [DOI] [PubMed] [Google Scholar]
- 3.Gomez A, Nelson KE. The Oral Microbiome of Children: Development, Disease, and Implications Beyond Oral Health. Microb. Ecol. 2017;73(2):492–503. doi: 10.1007/s00248-016-0854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemos JA, Quivey RG, Koo H, Abranches J. Streptococcus mutans: a new Gram-positive paradigm? Microbiology. 2013;159:436–445. doi: 10.1099/mic.0.066134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. Am. J. Dent. 2009;22(1):3–8. [PubMed] [Google Scholar]
- 6.Kreth J, Merritt J, Qi F. Bacterial and host interactions of oral streptococci. DNA Cell Biol. 2009;28:397–403. doi: 10.1089/dna.2009.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2:1599–1607. doi: 10.1016/s1286-4579(00)01316-2. [DOI] [PubMed] [Google Scholar]
- 8.Vose JM, Smith PW, Henry M. Recurrent streptococcus mutans endocarditis. Am. J. Med. 1987;82:630–632. doi: 10.1016/0002-9343(87)90111-2. [DOI] [PubMed] [Google Scholar]
- 9.Solinski AE, Koval AB, Brzozowski RS, Morrison KR, Fraboni AJ, Carson CE, Eshraghi AR, Zhou G, Quivey RG, Voelz VA, Buttaro BA, Wuest WM. Diverted Total Synthesis of Carolacton-Inspired Analogs Yields Three Distinct Phenotypes in Streptococcus mutans Biofilms. J. Am. Chem. Soc. 2017;139:7188–7191. doi: 10.1021/jacs.7b03879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stumpp N, Premnath P, Schmidt T, Ammermann J, Dräger G, Reck M, Jansen R, Stiesch M, Wagner-Döebler I, Kirschning A. Synthesis of new carolacton derivatives and their activity against biofilms of oral bacteria. Org. Biomol. Chem. 2015;13:5765–5774. doi: 10.1039/c5ob00460h. [DOI] [PubMed] [Google Scholar]
- 11.Donner J, Reck M, Bergmann S, Kirschning A, Müller R, Wagner- Döebler I. The biofilm inhibitor Carolacton inhibits planktonic growth of virulent pneumococci via a conserved target. Sci. Rep. 2016;6:29677. doi: 10.1038/srep29677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaue Y, Domon H, Oda M, Takenaka S, Kubo M, Fukuyama Y, Okiji T, Terao Y. Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on Streptococcus mutans. Microbiol. Immunol. 2016;60:10–16. doi: 10.1111/1348-0421.12343. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg M, Dodds M, Tian M. Naturally Occurring Phenolic Antibacterial Compounds Show Effectiveness against Oral Bacteria by a Quantitative Structure-Activity Relationship Study. J. Agric. Food Chem. 2008;56:11151–11156. doi: 10.1021/jf8020859. [DOI] [PubMed] [Google Scholar]
- 14.Fujita M, Itokawa H, Sashida Y. Honokiol, a New Phenolic Compound isolated from the Bark of Magnolia obovata. Chem. Pharm. Bull. 1972;20:212–213. [Google Scholar]
- 15.Namba T, Tsunezuk M, Hatori M. Dental Caries Prevention by Traditional Chinese Medicines. Planta Med. 1982;44:100–106. doi: 10.1055/s-2007-971412. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y-E, Cao T, Torruellas C, Kozlowski MC. Selective Oxidative Homo- and Cross-Coupling of Phenols with Aerobic Catalysts. J. Am. Chem. Soc. 2014;136:6782–6785. doi: 10.1021/ja500183z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaracz S, Kozlowski MC, Lee YE, Kim SM. WO/2017/070568 Improved Synthesis of Honokiol. 2017 Apr 27;
- 18.Kang H, Lee Y-E, Reddy PVG, Dey S, Allen SE, Niederer KA, Sung P, Hewitt K, Torruellas C, Herling MR, Kozlowski MC. Asymmetric Oxidative Coupling of Phenols and Hydroxycarbazoles. Org. Lett. 2017;19:5505–5508. doi: 10.1021/acs.orglett.7b02552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dashper SG, Reynolds EC. Lactic acid excretion by Streptococcus mutans. Microbiology. 1996;142:33–39. doi: 10.1099/13500872-142-1-33. [DOI] [PubMed] [Google Scholar]
- 20.Macia MD, Rojo-Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014;20:981–990. doi: 10.1111/1469-0691.12651. [DOI] [PubMed] [Google Scholar]
- 21.Pan J, Lee Y, Zhang Q, Xiong D, Wan TC, Wang Y, You M. Honokiol Decreases Lung Cancer Metastasis through Inhibition of the STAT3 Signaling Pathway. Cancer Prev. Res. 2017;10:133–141. doi: 10.1158/1940-6207.CAPR-16-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Shen Y. Antifungal compound honokiol triggers oxidative stress responsive signaling pathway and modulates central carbon metabolism. Mycology. 2016;7:124–133. doi: 10.1080/21501203.2016.1221862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X-D, Wang Y-L, Gao W-F. Honokiol possesses potential anti-inflammatory effects on rheumatoid arthritis and GM-CSF can be a target for its treatment. Int. J. Clin. Exp. Pathol. 2015;8:7929–7936. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen P-J, Wang Y-L, Kuo L-M, Lin C-F, Chen C-Y, Tsai Y-F, Shen J-J, Hwang T-L. Honokiol suppresses TNF-α-induced neutrophil adhesion on cerebral endothelial cells by disrupting polyubiquitination and degradation of IκBα. Sci. Rep. 2016;6:26554. doi: 10.1038/srep26554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moodie LWK, Trepos R, Cervin G, Bråthen KA, Lindgård B, Reiersen R, Cahill P, Hellio C, Svenson J. Probing the Structure–Activity Relationship of the Natural Antifouling Agent Polygodial against both Micro- and Macrofoulers by Semisynthetic Modification. J. Nat. Prod. 2017;80:2001–2011. doi: 10.1021/acs.jnatprod.6b01056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.