Abstract
Purpose
To assess the 5-year progression from unilateral to bilateral age-related macular degeneration (AMD) and associated risk factors.
Design
Pooled data analyses of three prospective population-based cohorts, the Blue Mountains Eye Study, Beaver Dam Eye Study and Rotterdam Study.
Methods
Retinal photography and interview with comprehensive questionnaires were conducted at each visit of three studies. AMD was assessed following the modified Wisconsin AMD grading protocol. Progression to bilateral any (early and late) or late AMD was assessed among participants with unilateral involvement only. Factors associated with the progression were assessed using logistic regression models while simultaneously adjusting for other significant risk factors.
Results
In any 5-year duration, 19–28% of unilateral any AMD cases became bilateral and 27–68% of unilateral late AMD became bilateral. Factors associated with the progression to bilateral involvement of any AMD were age (per year increase, adjusted OR 1.07), carrying risk alleles of the complement factor H and age-related maculopathy susceptibility 2 genes (compared with none, OR 1.76 for 1 risk allele and OR 3.34 for 2+ risk alleles), smoking (compared with non-smokers, OR 1.64 for past and OR 1.67 for current smokers), and the presence of large drusen area or retinal pigmentary abnormalities in the first eye.
Conclusion
One in four to one in five unilateral any AMD cases, and up to one in two unilateral late AMD cases, progressed to bilateral in 5 years. Known AMD risk factors, including smoking, are significantly associated with the progression to bilateral involvement.
INTRODUCTION
Age-related macular degeneration (AMD) is a leading cause of blindness in western populations.1 While the presence of AMD in one eye can be debilitating, vision loss and blindness in both eyes due to bilateral AMD will have severe consequences for the affected individuals.2,3
Previous studies report the development of late AMD in the second eye to be 20–50% over 5–10 years.4–9 However, the progression from unilateral early AMD to bilateral early or any (early and late) AMD,10 and its associated risk factors has been less well described.
We aimed to report the 5-year progression of unilateral AMD cases to bilateral involvement in three population-based cohorts, the Three Continent AMD Consortium (3CC). We also aimed to investigate this progression in relation to risk factors and early AMD lesion characteristics.
METHODS
Among the 3CC, we included non-Hispanic white, population-based cohort studies conducted in Australia, the USA and the Netherlands.11,12 Written informed consent was obtained from each participant at each visit, in all three studies. All studies adhered to the tenets of the Declaration of Helsinki.
Blue Mountains Eye Study
The Blue Mountains Eye Study (BMES) recruited 3654 participants (82.4% of those eligible) aged ≥49 years living in two postcode regions west of Sydney between 1992 and 1994.13 Of these participants, 2334, 1952 and 1149 were re-examined after 5, 10 and 15 years, respectively. Examinations were approved by the University of Sydney and the Sydney West Area Health Service Human Research Ethics Committees.
Beaver Dam Eye Study
The Beaver Dam Eye Study (BDES), conducted in Beaver Dam, Wisconsin, examined 4926 participants (83.2% of those eligible) aged 43–86 years from 1988 to 1990.14 Of these participants, 3721, 2962, 2375 and 1913 were re-examined after 5, 10, 15 and 20 years, respectively. The University of Wisconsin-Madison approved all study visits in conformity with federal and state laws and compliance with the Health Insurance Portability and Accountability Act.
Rotterdam study (RS)
At baseline (1990–1993), the RS examined 7983 participants (77.7% participation rate) aged 55+ years, of whom 6419 had ophthalmic examinations and retinal photography performed.15 Of 6419 participants, 4977, 3637 and 2674 were re-examined at the second (1993–1995), third (1997–1999) and fourth (2002–2004) visits, respectively. The mean follow-up period was 10 years. Only data from the first, third and fourth visits were used. Examinations were approved by the Medical Ethics Committee of the Erasmus Medical Centre and the Ministry of Health, Welfare and Sport of the Netherlands, implementing the Wet Bevolkingsonderzoek: ERGO (Population Studies Act: Rotterdam Study).
Retinal photography
Mydriatic stereoscopic colour fundus photographs were taken at each study visit. Zeiss fundus cameras (Carl Zeiss, Oberkochen, Germany) were used in the first three visits of BMES (FF3) and all visits of BDES (FF4), and 30° stereoscopic colour fundus photographs of the macula and optic disc, and non-stereoscopic photographs of the other retinal fields of both eyes were taken in both studies. Topcon TRV-50VT fundus camera (Topcon Optical Co, Tokyo, Japan) was used in the RS during the first visits, and 35° stereoscopic colour fundus photographs of the macular were taken. In the fourth visit, the BMES used a Canon CF-60 DSi with DS Mark II body (Canon, Tokyo, Japan) to take 40° digital photographs; and the RS used a Topcon TRC 50EX fundus camera with Sony DXC-950P digital camera (Topcon Optical Co) to take 35° digital photographs.
Photographic grading and definitions of AMD
Retinal photographs of both eyes were initially graded by trained graders of each study following the Wisconsin Age-related Maculopathy Grading System (WARMGS).16 All late AMD incident cases detected from each study were adjudicated and confirmed by the retinal specialists of each study team initially, then cross-checked among chief investigators of the three cohorts.17
A 5-step severity scale for AMD, developed after phenotype harmonisation11 was used to define AMD severity stage. Levels 10, 20, 30, 40 and 50 corresponded to normal, mild early, moderate early, severe early and late AMD (see online supplementary appendix). We grouped levels 20–40 as early AMD. Unilateral any AMD was defined if either early or late AMD were present in one eye only. Unilateral late AMD was defined as late AMD presence in one eye with no late AMD in the fellow eye (regardless of presence of early AMD). Bilateral any and late AMD were defined as presence of any and late AMD in both eyes, respectively. Progression was defined as the transition from unilateral any or late AMD to bilateral.
Total drusen area, measured as a proportion of the WARMGS grid, and the presence of retinal pigment epithelium (RPE) abnormalities were assessed at first detection of unilateral AMD, as prognostic factors for bilateral involvement. Methods used to calculate total drusen area differed across studies thus we derived quintiles of drusen area within each study to obtain comparable measures. Drusen area was categorised as small, intermediate or large, representing participants who had the lowest 20%, the middle 60% and the highest 20% of drusen area in each population accordingly.
Assessment of risk factors
Smoking status was assessed using interviewer-administered questionnaires. In the BMES, participants were classified as ‘non-smokers’ if they answered ‘no’ to smoking regularly, ‘past smokers’ if they quit smoking >1 year prior to the examination and ‘current smokers’ if they currently smoked or stopped smoking <1 year before the examination. In the BDES, participants were classified as ‘non-smokers’ if they had smoked fewer than 100 cigarettes in their lifetime, ‘past smokers’ if they smoked ≥100 cigarettes but had stopped smoking before the examination or ‘current smokers’ if they had not stopped smoking.18 In the RS, smoking status was defined as never, past or current according to participants responses ‘no’, ‘yes, stopped smoking’ and ‘yes, still smoking’, respectively.19
Mean systolic and diastolic blood pressures were taken from an average of two readings, except for BMES baseline visit, when one measure was taken. Serum total cholesterol levels, high density lipoprotein (HDL) levels and white blood cell count were measured at baseline from non-fasting blood samples in the BDES and RS and fasting blood samples in the BMES.20
Genotyping methods
We used two major AMD-associated genes to represent AMD genetic susceptibility, the complement factor H (CFH; OMIM 134370) and age-related maculopathy susceptibility 2 (ARMS2; OMIM 611313). Genotyping methods are described in the online supplementary appendix.17,21,22
Statistical analyses
All statistical analyses were performed using SAS V.9.3 (SAS Institute, Cary, North Carolina, USA).
Progression to bilateral AMD was assessed using discrete time survival analysis, focusing solely on the first 5-year interval since initial detection of unilateral cases. Participants were included at first detection of unilateral AMD and assessed for progression to bilateral involvement at the subsequent 5-year visit.
Progression rates were compared across categories of age, genetic risk levels (carrying 0, 1 or 2–4 risk alleles of the CFH and ARMS2 combined) and smoking status, by individual and pooled study samples, using Mantel-Haenzel χ2 tests for linear trend.
Associations between progression to bilateral involvement and age, sex, smoking status, genetic risk, blood pressure, white blood cell count, total cholesterol and HDL cholesterol levels were assessed in age-adjusted and multivariable-adjusted logistic regression models. Age, drusen area and RPE abnormalities were time-dependent variables corresponding to the visit when unilateral AMD was first detected. All other co-variables were defined at baseline. Final models included age, sex, smoking status, total drusen area, presence of RPE abnormalities that remained statistically significant in the model. Indicators of study site were included in models using pooled-data. Association estimates are presented as adjusted ORs and 95% CIs.
We obtained a receiver operating characteristic (ROC) curve and area under the ROC curve (AUC) to assess how useful the final model might be in predicting progression to bilateral any AMD in 5 years. The AUC is a measure of discrimination and, here indicates the probability that a person with progression will have a higher predicted value in the model than a person without progression.
Due to limited numbers of cases, associations with progression to bilateral late AMD were examined using pooled-data only, and associations between early AMD lesion characteristics and progression to bilateral late AMD could not be assessed.
RESULTS
Participants with unilateral any or late AMD from the BMES, BDES and RS were included (ages 51+, 44+ and 55+ years, respectively). Among 1490 participants (BMES n=335, BDES n=625 and RS n=530) with unilateral any AMD detected at any visit, 94 (28%), 119 (19%) and 126 (24%) progressed to bilateral in the corresponding cohorts, respectively. Of 96 participants (BMES n=25, BDES n=51 and RS n=20) with unilateral late AMD, 17 (68%), 14 (27%) and 11 (55%) progressed to bilateral in the three cohorts respectively.
Progression to bilateral any AMD
Table 1 compares baseline characteristics between those who did and did not progress in the separate and pooled cohorts. Compared with participants who remained unilateral, those who progressed to bilateral were older, and more likely to have 2+ risk alleles from combined CFH and ARMS2 genes.
Table 1.
Comparison of baseline characteristics of participants who did and those who did not progress from unilateral to bilateral any AMD, or from unilateral to bilateral late AMD, in the BMES, BDES, RS individually and combined three cohorts
| 5-year progression
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMES
|
BDES
|
RS
|
Combined
|
|||||||||
| No progression | Progression | p Value* | No progression | Progression | p Value* | No progression | Progression | p Value* | No progression | Progression | p Value* | |
| Unilateral any AMD to bilateral any AMD | ||||||||||||
| Participants, n (%) | 241 | 94 (28.1) | 506 | 119 (19.0) | 404 | 126 (23.8) | 1151 | 339 (22.8) | ||||
| Mean age (SD), years | 66.1 (7.5) | 72.4 (7.12) | <0.0001 | 63.6 (9.4) | 69.8 (9.0) | <0.0001 | 68.1 (7.3) | 70.9 (7.8) | 0.0002 | 65.7 (8.5) | 70.9 (8.1) | <0.0001 |
| Sex (male), % | 41.5 | 46.8 | 0.4 | 49.2 | 42.9 | 0.2 | 44.1 | 40.5 | 0.5 | 45.8 | 43.1 | 0.4 |
| Smoking status, % | ||||||||||||
| Never | 57.5 | 49.5 | 0.3 | 43.1 | 40.3 | 0.9 | 34.3 | 29.6 | 0.6 | 42.9 | 38.9 | 0.3 |
| Past | 28.8 | 37.6 | 37.2 | 39.5 | 45.0 | 48.8 | 38.2 | 42.4 | ||||
| Current | 13.7 | 12.9 | 19.8 | 20.2 | 20.8 | 21.6 | 18.9 | 18.7 | ||||
| CFH (rs1061170), % | ||||||||||||
| TT | 44.2 | 32.1 | 0.04 | 40.3 | 21.9 | 0.0003 | 45.9 | 34.5 | 0.02 | 43.0 | 29.2 | <0.0001 |
| CT | 40.9 | 41.7 | 47.8 | 57.1 | 42.0 | 44.5 | 44.5 | 48.5 | ||||
| CC | 14.9 | 26.2 | 11.9 | 21.0 | 12.2 | 21.0 | 12.6 | 22.4 | ||||
| ARMS2 (rs10490924), % | ||||||||||||
| GG | 66.7 | 46.8 | 0.003 | 62.5 | 47.1 | 0.003 | 65.7 | 53.8 | 0.004 | 64.4 | 49.5 | <0.0001 |
| GT | 31.8 | 46.8 | 33.0 | 42.9 | 32.2 | 38.7 | 32.5 | 42.3 | ||||
| TT | 1.5 | 6.3 | 4.6 | 10.1 | 2.1 | 7.6 | 3.1 | 8.2 | ||||
| Combined genetic risk category†, % | ||||||||||||
| 0 risk alleles | 27.8 | 11.4 | 0.007 | 23.5 | 10.9 | <0.0001 | 29.6 | 18.5 | 0.003 | 26.5 | 13.9 | <0.001 |
| 1 risk allele | 43.3 | 45.6 | 46.3 | 35.3 | 43.1 | 38.7 | 44.6 | 39.1 | ||||
| 2–4 risk alleles | 28.9 | 43.0 | 30.2 | 53.8 | 27.3 | 42.9 | 28.9 | 47.0 | ||||
| Mean systolic BP (SD), mm Hg | 142.9 (19.4) | 147.6 (21.5) | 0.05 | 130.4 (19.9) | 131.9 (18.7) | 0.4 | 140.9 (21.4) | 140.5 (20.6) | 0.9 | 136.7 (21.1) | 139.4 (21.1) | 0.03 |
| Mean diastolic BP (SD), mm Hg | 84.6 (10.2) | 83.2 (9.9) | 0.3 | 78.4 (10.1) | 76.3 (10.0) | 0.04 | 75.6 (11.7) | 71.3 (10.0) | 0.0002 | 78.7 (11.1) | 76.4 (11.0) | 0.0006 |
| Mean WBCC (SD), ×109 cells/L | 6.6 (1.8) | 6.4 (1.6) | 0.4 | 7.2 (1.9) | 7.2 (1.9) | 0.8 | 6.4 (1.8) | 6.3 (1.6) | 0.5 | 6.8 (1.9) | 6.7 (1.8) | 0.2 |
| Mean total cholesterol (SD), mmol/L | 6.1 (0.9) | 6.3 (1.0) | 0.09 | 6.0 (1.1) | 6.1 (1.0) | 0.7 | 6.4 (1.1) | 6.2 (1.1) | 0.09 | 6.2 (1.1) | 6.2 (1.0) | 0.9 |
| Mean HDL cholesterol (SD), mmol/L | 1.4 (0.4) | 1.5 (0.5) | 0.2 | 1.4 (0.5) | 1.3 (0.4) | 0.4 | 1.4 (0.4) | 1.4 (0.4) | 0.6 | 1.4 (0.4) | 1.4 (0.4) | 0.6 |
| Unilateral late AMD to bilateral late AMD | ||||||||||||
| Participants (n) | 8 | 17 (68.0) | 37 | 14 (27.5) | 9 | 11 (55.0) | 54 | 42 (43.8) | ||||
| Mean age (SD) | 77.3 (6.2) | 76.5 (6.2) | 0.8 | 73.2 (8.0) | 79.4 (6.8) | 0.01 | 73.4 (6.8) | 75.9 (5.3) | 0.4 | 73.8 (7.6) | 77.3 (6.2) | 0.02 |
| Sex (male) % | 25.0 | 23.5 | 0.9 | 46.0 | 35.7 | 0.5 | 66.7 | 63.6 | 0.9 | 46.3 | 38.1 | 0.4 |
| Smoking status, % | ||||||||||||
| Never | 37.5 | 47.1 | 0.6 | 56.8 | 50.0 | 0.3 | 11.1 | 9.1 | 0.6 | 46.3 | 38.1 | 0.5 |
| Past | 50.0 | 29.4 | 32.4 | 50.0 | 66.7 | 45.5 | 40.7 | 40.5 | ||||
| Current | 12.5 | 23.5 | 10.8 | 0.0 | 22.2 | 45.5 | 13.0 | 21.4 | ||||
| CFH (rs1061170), % | ||||||||||||
| TT | 12.5 | 31.3 | 0.6 | 16.2 | 14.3 | 0.9 | 33.3 | 9.1 | 0.3 | 18.5 | 19.5 | 0.6 |
| CT | 50.0 | 43.8 | 67.6 | 64.3 | 55.6 | 54.6 | 63.0 | 53.7 | ||||
| CC | 37.5 | 25.0 | 16.2 | 21.4 | 11.1 | 36.4 | 18.5 | 26.8 | ||||
| ARMS2 (rs10490924), % | ||||||||||||
| GG | 62.5 | 31.3 | 0.2 | 29.7 | 21.4 | 0.03 | 33.3 | 27.3 | 0.6 | 35.2 | 26.8 | 0.2 |
| GT | 37.5 | 50.0 | 40.5 | 78.6 | 66.7 | 63.6 | 44.4 | 63.4 | ||||
| TT | 0.0 | 18.8 | 29.7 | 0.0 | 0.0 | 9.1 | 20.4 | 9.8 | ||||
| Combined genetic risk category†% | ||||||||||||
| 0 risk alleles | 12.5 | 6.3 | 0.8 | 13.5 | 0.0 | 0.3 | 22.2 | 9.1 | 0.4 | 14.8 | 4.9 | 0.3 |
| 1 risk allele | 37.5 | 31.3 | 16.2 | 21.4 | 22.2 | 9.1 | 20.4 | 22.0 | ||||
| 2–4 risk alleles | 50.0 | 62.5 | 70.3 | 78.6 | 55.6 | 81.8 | 64.8 | 73.2 | ||||
| Mean systolic BP (SD), mm Hg | 146.4 (20.3) | 147.5 (16.2) | 0.9 | 136.4 (19.8) | 136.6 (18.0) | 0.97 | 137.6 (12.2) | 144.3 (17.7) | 0.4 | 138.1 (18.9) | 143.0 (6.2) | 0.2 |
| Mean diastolic BP (SD), mm Hg | 82.9 (8.5) | 83.5 (8.2) | 0.9 | 75.7 (9.3) | 71.7 (11.2) | 0.2 | 71.6 (10.2) | 78.4 (11.2) | 0.2 | 76.1 (9.7) | 78.2 (11.0) | 0.3 |
| Mean WBCC (SD), ×109 cells/L | 6.9 (2.3) | 6.8 (1.1) | 0.8 | 7.2 (2.3) | 7.1 (2.1) | 0.9 | 6.6 (1.2) | 6.9 (1.6) | 0.6 | 7.0 (2.2) | 6.9 (1.6) | 0.8 |
| Mean total cholesterol (SD), mmol/L | 6.6 (1.3) | 6.3 (0.9) | 0.4 | 6.1 (1.2) | 5.7 (0.8) | 0.3 | 5.9 (1.0) | 5.8 (0.9) | 0.7 | 6.1 (1.2) | 5.9 (0.9) | 0.4 |
| Mean HDL cholesterol (SD), mmol/L | 1.5 (0.3) | 1.4 (0.5) | 0.6 | 1.4 (0.5) | 1.6 (0.4) | 0.4 | 1.4 (0.3) | 1.6 (0.8) | 0.4 | 1.4 (0.4) | 1.5 (0.5) | 0.4 |
p Value for association between baseline characteristics and progression of AMD from unilateral to bilateral (categorical factors) and p value for difference in mean baseline level (continuous factors).
Total number of risk alleles from CFH and ARMS2 are combined.
AMD, age-related macular degeneration; ARMS2, age-related maculopathy susceptibility gene 2 (risk allele T); BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; BP, blood pressure; CFH, complement factor H (risk allele C); HDL, high density lipoprotein; RS, Rotterdam Study; WBCC, white blood cell count.
Table 2 presents proportions of progression to bilateral any AMD by age, genetic risk and smoking status in separate and pooled populations. Progression was associated with increasing age and increased risk alleles of the CFH and ARMS2 genes. There was no significant crude association between smoking status and progression to bilateral any AMD.
Table 2.
Five-year progression from unilateral to bilateral any and late age-related macular degeneration, by age, genotype and smoking status in the Blue Mountains Eye Study, Beaver Dam Eye Study, Rotterdam Study individually and combined three cohort
| Factors | 5-year progression from unilateral to bilateral AMD
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blue mountains eye study
|
Beaver dam eye study
|
Rotterdam study
|
Combined
|
|||||||||||||
| Any AMD*
|
Late AMD†
|
Any AMD*
|
Late AMD†
|
Any AMD*
|
Late AMD†
|
Any AMD*
|
Late AMD†
|
|||||||||
| No. of cases/No. at risk | Per cent | No. of cases/No. at risk | Per cent | No. of cases/No. at risk | Per cent | No. of cases/No. at risk | Per cent | No. of cases/No. at risk | Per cent | No. of cases/No. at risk | Per cent | No. of cases/No. at risk | Per cent | No. of cases/No. at risk | Per cent | |
| Age (years) | ||||||||||||||||
| 40–49 | – | – | – | – | 3/44 | 6.8 | 0/0 | 0.0 | – | – | – | – | 3/44 | 6.8 | 0/0 | 0.0 |
| 50–59 | 4/47 | 8.5 | 0/0 | 0.0 | 11/137 | 8.0 | 0/1 | 0.0 | 11/58 | 19.0 | 0/0 | 0.0 | 26/242 | 10.7 | 0/1 | 0.0 |
| 60–69 | 24/136 | 17.7 | 2/3 | 66.7 | 42/241 | 17.4 | 1/11 | 9.1 | 40/233 | 17.2 | 1/3 | 33.3 | 106/610 | 17.4 | 4/17 | 23.5 |
| 70–79 | 51/127 | 40.2 | 10/14 | 71.4 | 46/163 | 28.2 | 8/27 | 29.6 | 59/197 | 30.0 | 7/12 | 58.3 | 156/487 | 32.0 | 25/53 | 47.2 |
| 80+ | 15/25 | 60.0 | 5/8 | 62.5 | 17/40 | 42.5 | 5/12 | 41.7 | 16/42 | 38.1 | 3/5 | 60.0 | 48/107 | 44.9 | 13/25 | 52.0 |
| Total | 94/335 | 28.1 | 17/25 | 68.0 | 119/625 | 19.0 | 14/51 | 27.5 | 126/530 | 23.8 | 11/20 | 55.0 | 339/1490 | 22.8 | 42/96 | 43.8 |
| p Trend‡ | <0.0001 | 0.8 | <0.0001 | 0.07 | 0.0005 | 0.5 | <0.0001 | 0.06 | ||||||||
| Smoking status | ||||||||||||||||
| Never | 46/180 | 25.6 | 8/11 | 72.7 | 48/266 | 18.1 | 7/28 | 25.0 | 37/174 | 21.3 | 1/2 | 50.0 | 131/620 | 21.1 | 16/41 | 39.0 |
| Past | 35/102 | 34.3 | 5/9 | 55.6 | 47/235 | 20.0 | 7/19 | 36.8 | 61/241 | 25.3 | 5/11 | 45.5 | 143/578 | 24.7 | 17/39 | 43.6 |
| Current | 12/44 | 27.3 | 4/5 | 80.0 | 24/124 | 19.4 | 0/4 | 0.0 | 27/110 | 24.6 | 5/7 | 71.4 | 63/278 | 22.7 | 9/16 | 56.3 |
| p Trend‡ | 0.1 | 0.4 | 0.6 | 0.8 | 0.4 | 0.3 | 0.4 | 0.2 | ||||||||
| CFH (rs1061170) | ||||||||||||||||
| TT | 27/119 | 22.7 | 5/6 | 83.3 | 26/230 | 11.3 | 2/8 | 25.0 | 41/218 | 18.8 | 1/4 | 25.0 | 94/567 | 16.6 | 8/18 | 44.4 |
| CT | 35/120 | 29.2 | 7/11 | 63.6 | 68/310 | 21.9 | 9/34 | 26.5 | 53/215 | 24.7 | 6/11 | 54.6 | 156/645 | 24.2 | 22/56 | 39.3 |
| CC | 22/53 | 41.5 | 4/7 | 57.1 | 25/85 | 29.4 | 3/9 | 33.3 | 25/72 | 34.7 | 4/5 | 80.0 | 72/210 | 34.3 | 11/21 | 52.4 |
| p Trend‡ | 0.01 | 0.3 | <0.0001 | 0.6 | 0.006 | 0.1 | <0.0001 | 0.5 | ||||||||
| ARMS2 (rs10490924) | ||||||||||||||||
| GG | 37/169 | 21.9 | 5/10 | 50.0 | 56/372 | 15.1 | 3/14 | 21.4 | 64/317 | 20.2 | 3/6 | 50.0 | 157/858 | 18.3 | 11/30 | 36.7 |
| GT | 37/100 | 37.0 | 8/11 | 72.7 | 51/218 | 23.4 | 11/26 | 42.3 | 46/124 | 27.1 | 7/13 | 53.9 | 134/488 | 27.5 | 26/50 | 52.0 |
| TT | 5/8 | 62.5 | 3/3 | 100.0 | 12/35 | 34.3 | 0/11 | 0.0 | 9/17 | 52.9 | 1/1 | 100.0 | 26/60 | 43.3 | 4/15 | 26.7 |
| p Trend‡ | 0.0008 | 0.09 | 0.0006 | 0.3 | 0.003 | 0.5 | <0.0001 | 0.8 | ||||||||
| Combined genetic risk category§ | ||||||||||||||||
| 0 risk alleles | 9/63 | 14.3 | 1/2 | 50.0 | 13/132 | 9.9 | 0/5 | 0.0 | 22/136 | 16.2 | 1/3 | 33.3 | 44/331 | 13.3 | 2/10 | 20.0 |
| 1 risk allele | 36/120 | 30.0 | 5/8 | 62.5 | 42/276 | 15.2 | 3/9 | 33.3 | 46/212 | 21.7 | 1/3 | 33.3 | 124/608 | 20.4 | 9/20 | 45.0 |
| 2–4 risk alleles | 34/90 | 37.8 | 10/14 | 71.4 | 64/217 | 29.5 | 11/37 | 29.7 | 51/156 | 32.7 | 9/14 | 64.3 | 149/463 | 32.2 | 30/65 | 46.2 |
| p Trend‡ | 0.002 | 0.5 | <0.0001 | 0.3 | 0.0008 | 0.2 | <0.0001 | 0.2 | ||||||||
Unilateral any AMD progression to bilateral any AMD.
Unilateral late AMD progression to bilateral late AMD.
p Trend calculated using Mantel-Haenszel χ2 test for linear association.
Combined risk dichotomised as 0 or 1 risk allele of CFH or ARMS2 or 2 to 4 risk alleles of CFH and/or ARMS2.
AMD, age-related macular degeneration; ARMS2, age-related maculopathy susceptibility 2 (risk allele T); CFH, complement factor H (risk allele C).
Table 3 presents factors associated with progression to bilateral any AMD by individual cohorts. After adjustment, age and the presence of ≥2 risk alleles from the two genes combined were associated with increased risks of progression across three cohorts, while smoking was non-significantly associated with this progression. Large total drusen area (highest compared with lowest quintile) contributed significantly to the risk of progression in each cohort. The presence of RPE abnormalities was significantly associated with increased risk of progression in the BMES and BDES but not the RS.
Table 3.
Associations of AMD risk factors with 5-year progression from unilateral to bilateral any AMD in the BMES, BDES and RS populations
| Risk factors | BMES
|
BDES
|
RS
|
|||
|---|---|---|---|---|---|---|
| Age-adjusted OR (95% CI) |
Multivariable-adjusted OR* (95% CI) |
Age-adjusted OR (95% CI) |
Multivariable-adjusted OR* (95% CI) |
Age-adjusted OR (95% CI) |
Multivariable-adjusted OR* (95% CI) |
|
| Age per year | 1.12 (1.08 to 1.16) | 1.15 (1.09 to 1.21) | 1.08 (1.05 to 1.10) | 1.07 (1.04 to 1.10) | 1.05 (1.02 to 1.09) | 1.04 (1.00 to 1.07) |
| Sex (male) | 1.54 (0.91 to 2.60) | 1.02 (0.49 to 2.12) | 0.90 (0.59 to 1.36) | 0.76 (0.47 to 1.24) | 0.96 (0.63 to 1.46) | 0.92 (0.54 to 1.56) |
| Smoking status | ||||||
| Never | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Past | 1.74 (0.98 to 3.08) | 1.43 (0.65 to 3.12) | 1.33 (0.83 to 2.12) | 1.57 (0.91 to 2.70) | 1.51 (0.93 to 2.45) | 1.79 (0.98 to 3.29) |
| Current | 1.61 (0.70 to 3.67) | 2.10 (0.73 to 5.98) | 1.48 (0.83 to 2.63) | 1.47 (0.78 to 2.77) | 1.71 (0.93 to 3.13) | 1.69 (0.83 to 3.43) |
| Combined genetic risk category† | ||||||
| 0 risk alleles | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 risk allele | 2.93 (1.22 to 7.05) | 3.46 (1.33 to 9.02) | 1.71 (0.87 to 3.37) | 1.57 (0.77 to 3.20) | 1.42 (0.80 to 2.49) | 1.52 (0.83 to 2.79) |
| 2–4 risk alleles | 5.36 (2.16 to 13.30) | 5.19 (1.93 to 13.93) | 4.75 (2.43 to 9.27) | 4.25 (2.11 to 8.54) | 2.49 (1.40 to 4.40) | 2.51 (1.35 to 4.67) |
| Blood pressure (per 10 mm Hg) | ||||||
| Systolic | 1.03 (0.91 to 1.17) | – | 0.99 (0.90 to 1.11) | – | 0.95 (0.86 to 1.05) | – |
| Diastolic | 0.90 (0.70 to 1.16) | 0.96 (0.68 to 1.34) | 0.97 (0.78 to 1.21) | 0.93 (0.73 to 1.18) | 0.72 (0.59 to 0.86) | 0.71 (0.57 to 0.88) |
| WBCC (per SD increase) | 0.93 (0.69 to 1.23) | – | 1.12 (0.88 to 1.43) | – | 0.96 (0.77 to 1.19) | – |
| Total Cholesterol (per SD increase) | 1.25 (0.92 to 1.69) | – | 0.95 (0.76 to 1.19) | – | 0.88 (0.71 to 1.08) | – |
| HDL cholesterol (per SD increase) | 1.15 (0.86 to 1.54) | – | 0.93 (0.75 to 1.15) | – | 1.04 (0.85 to 1.27) | – |
| Drusen area‡ | ||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Intermediate | 1.32 (0.58 to 2.99) | 1.83 (0.75 to 4.49) | 1.79 (0.88 to 3.66) | 2.75 (1.26 to 5.99) | 2.99 (1.52 to 5.90) | 2.65 (1.32 to 5.31) |
| High | 9.62 (3.77 to 24.55) | 15.26 (5.08 to 45.83) | 7.93 (3.69 to 17.04) | 12.32 (5.10 to 29.79) | 9.11 (4.27 to 19.43) | 9.62 (4.29 to 21.57) |
| Presence of RPE abnormality | 1.18 (0.65 to 2.15) | 2.61 (1.21 to 5.66) | 0.93 (0.61 to 1.42) | 1.73 (1.03 to 2.90) | 1.02 (0.67 to 1.56) | 1.49 (0.91 to 2.43) |
Bold values indicate significant ORs.
Adjusted for age, sex, smoking, combined genetic risk score, diastolic blood pressure, drusen area and RPE abnormalities.
Total number of risk alleles from the complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) genes combined (reference: 0 risk alleles).
Total drusen area categorised as low, intermediate and high representing the lowest 20% of drusen area, the central 60% and highest 20%, respectively.
AMD, age-related macular degeneration; BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; HDL, high density lipoprotein; RPE, retinal pigment epithelium; RS, Rotterdam Study; WBCC, white blood cell count.
Table 4 presents factors associated with progression to bilateral any AMD in pooled data. Progression was more commonly observed in the BMES compared with the BDES. Older age, smoking and carrying ≥1 risk allele from the CFH and ARMS2 were significantly associated with an increased risk of progression. Large total drusen area and RPE abnormalities also contributed significantly to an increased risk of progression.
Table 4.
Associations of age-related macular degeneration (AMD) risk factors with 5-year progression from unilateral to bilateral any AMD and late AMD in pooled data of the Blue Mountains Eye Study, Beaver Dam Eye Study and Rotterdam Study
| Risk factors | Bilateral any AMD
|
Bilateral late AMD
|
||
|---|---|---|---|---|
| Age-adjusted OR (95% CI) | Multivariable-adjusted OR* (95% CI) | Age-adjusted OR (95% CI) | Multivariable-adjusted OR* (95% CI) | |
| Study population (ref: BDES) | ||||
| BMES | 1.42 (1.03 to 1.97) | 1.71 (1.16 to 2.54) | 5.45 (1.86 to 15.90) | 7.30 (2.05 to 25.96) |
| RS | 1.06 (0.79 to 1.42) | 1.10 (0.79 to 1.53) | 3.54 (1.16 to 10.80) | 3.43 (0.82 to 14.31) |
| Age (per year) | 1.08 (1.06 to 1.09) | 1.07 (1.05 to 1.09) | 1.08 (1.01 to 1.15) | 1.13 (1.05 to 1.23) |
| Sex (male) | 1.06 (0.82 to 1.37) | 0.89 (0.65 to 1.22) | 0.86 (0.33 to 2.23) | 0.81 (0.28 to 2.34) |
| Smoking status | ||||
| Never | 1.00 | 1.00 | 1.00 | 1.00 |
| Past | 1.51 (1.13 to 2.01) | 1.64 (1.16 to 2.33) | 1.32 (0.46 to 3.77) | 1.97 (0.59 to 6.55) |
| Current | 1.65 (1.14 to 2.38) | 1.67 (1.10 to 2.55) | 2.14 (0.47 to 9.76) | 2.01 (0.38 to 10.57) |
| Combined genetic risk category‡ | ||||
| 0 risk alleles | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 risk allele | 1.72 (1.17 to 2.54) | 1.76 (1.17 to 2.64) | 3.61 (0.52 to 25.34) | 4.91 (0.60 to 40.03) |
| 2–4 risk alleles | 3.56 (2.42 to 5.25) | 3.34 (2.21 to 5.04) | 6.39 (1.04 to 39.09) | 12.46 (1.52 to 101.97) |
| Blood pressure (per 10 mm Hg) | ||||
| Systolic | 0.99 (0.93 to 1.05) | – | 1.05 (0.81 to 1.36) | – |
| Diastolic | 0.84 (0.74 to 0.95) | 0.82 (0.71 to 0.95) | 1.07 (0.67 to 1.70) | – |
| WBCC (per SD increase) | 1.01 (0.87 to 1.17) | – | 1.04 (0.65 to 1.64) | – |
| Total cholesterol (per SD increase) | 0.98 (0.86 to 1.12) | – | 0.62 (0.36 to 1.04) | 0.47 (0.26 to 0.84) |
| HDL cholesterol (per SD increase) | 1.02 (0.88 to 1.17) | – | 1.19 (0.77 to 1.83) | – |
| Drusen area§ | ||||
| Low | 1.00 | 1.00 | – | – |
| Intermediate | 2.04 (1.34 to 3.10) | 2.32 (1.50 to 3.59) | – | – |
| High | 8.57 (5.42 to 13.56) | 10.67 (6.45 to 17.67) | – | – |
| RPE abnormality presence | 0.99 (0.76 to 1.29) | 1.68 (1.23 to 2.29) | – | – |
Bold values indicate significant ORs.
Adjusted for study population, age, sex, smoking, combined genetic risk, diastolic blood pressure, drusen area and RPE abnormalities.
Adjusted for study population, age, sex, smoking, combined genetic risk and total cholesterol.
Total number of risk alleles from complement factor H and age-related maculopathy susceptibility 2 genes combined (reference: 0 risk alleles).
Total drusen area categorised as low, intermediate and high representing the lowest 20% of drusen area, the central 60% and highest 20%, respectively.
BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; HDL, high density lipoprotein; RPE, retinal pigment epithelium; RS, Rotterdam Study; WBCC, white blood cell count.
Figure 1 presents the ROC curve for bilateral any AMD using the multivariable-adjusted model shown in table 4. The AUC of this model was 0.79, improved by 0.02 from a model without genetic risk categories (0.77) and by 0.11 from an age-sex-adjusted model (0.68).
Figure 1.
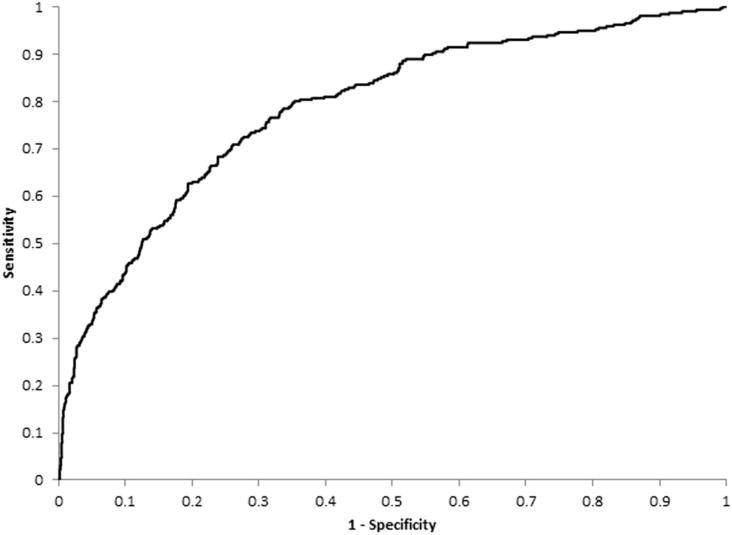
ROC curve indicating the prognostic performance of the model in predicting probabilities of 5-year progression from unilateral to bilateral involvement by any age-related macular degeneration. A ROC curve that follows the left-hand and top axes of the graph (AUC=1) indicates that the model provides perfect discrimination, whereas a diagonal line from the bottom left-hand corner of the graph to the top right-hand corner (AUC=0.5) indicates a model with no discriminative value. Sensitivity (the true positive rate) indicates the proportion of participants with progression who were correctly identified by the model, whereas 1−specificity (the false positive rate) indicates the proportion of participants without progression who were misidentified by the model. AUC, area under the ROC curve; ROC, receiver operating characteristic.
Supplementary analyses using comprehensive gene–environment risk scores23 found no additional improvement to the fully adjusted model in predicting progression to bilateral any AMD (data not shown). There was no meaningful difference in the association between these risk factors and progression to bilateral any AMD after inclusion of secular trend terms in the model, accounting for different detection timing of the unilateral cases (data not shown).
Progression to bilateral late AMD
Compared with participants who remained unilateral late AMD over 5 years, those who progressed to bilateral late AMD were older (table 1). However, there was no significant trend for age, smoking or genetic crude associations with this progression in separate or pooled cohorts (table 2).
After adjustment, progression to bilateral late AMD was more commonly observed in the BMES compared with the BDES (table 4). The presence of ≥2 risk alleles from CFH and ARMS2 genes combined was associated with a high risk of progression. Increased serum total cholesterol was associated with a decreased risk.
DISCUSSION
We found that over a 5-year period, about 19–28% of unilateral any AMD cases progressed to bilateral, and 27–68% of unilateral late AMD cases progressed to bilateral in our three population-based cohorts. In addition to age and AMD genetic risk, smoking and early AMD lesion characteristics were associated with increased risk of progression from unilateral to bilateral involvement in 5 years.
The BDES population includes a younger age spectrum (age 44+ years) compared with the BMES (51+ years) and the RS (55+ years), which may explain why higher proportions of progression were observed in the BMES and RS than the BDES.
The 5-year incidence of late AMD in the fellow eye of unilateral late patients with AMD enrolled in a randomised clinical trial was 26%9 which is similar to that found in the BMES and BDES (29% and 22%, respectively).4,6 In the RS 5-year cumulative incidence of late AMD in the fellow eye was 39%.8 The rates we report currently are greater as the mean age of participants are older than the mean age of the aforementioned samples at baseline, by including additional unilateral AMD cases detected at follow-up visits.
In addition to older age and AMD genetic risk, we documented that past or current smoking was significantly associated with increased risk of progression to bilateral any AMD in pooled analyses. Despite the heterogeneity in definitions, the contribution of smoking to the risk of developing any AMD in the second eye was evident when the sample size increased. The relatively small numbers of participants with unilateral late AMD in each individual population and in pooled data, or the narrow difference in smoking rates between participants with unilateral and bilateral late AMD are likely reasons for the lack of association of smoking with bilateral late AMD found in this report.
The inverse association between increased diastolic blood pressure and reduced risk of progression to bilateral any AMD is not readily explained. The inverse association between increased cholesterol level and reduced risk of progression to bilateral late AMD is not yet understood. Although elevated total cholesterol levels were found to be associated with a reduced incidence of neovascular AMD in a previous study,20 the relationship between total cholesterol levels and AMD risk has been largely inconsistent.12,24,25
Increasing severity levels of early AMD lesions in one eye were previously reported to be associated with increased incidence of AMD in the fellow eye.10 We found drusen area to be the strongest predictor for progression to bilateral any AMD within 5 years.
An AUC of 0.79 and 0.77 for bilateral any AMD suggests that both full model and the model without genetic risk categories are satisfactory in distinguishing persons who will progress to bilateral involvement from those who will not. Previous studies found minimal improvement in AUC after including genetic information in prediction models.23,26 Genetic testing in clinical practice is not supported by ours or other previous findings.27
We assembled a large number of unilateral any AMD cases from the 3CC. Care has been taken to harmonise AMD grading, confirm late AMD cases across three cohorts,17 and use uniformly the severity scale developed11 in the 3CC. Limitations include small numbers of unilateral late AMD cases even after pooling, resulting in low power to assess modifiable risk factors. The cohort samples are mostly Caucasians of Northern and Western European descent, and the findings may not be applicable to other ethnic populations.
In summary, 20–25% of unilateral any AMD cases, and up to 50% of unilateral late AMD cases on average, progressed to bilateral in 5 years. Of risk factors associated with the progression to bilateral involvement, only smoking is modifiable. The protective association between cholesterol level and bilateral late AMD warrant further investigation.
Supplementary Material
Acknowledgments
The BMES investigators would like to acknowledge the Blue Mountain Eye Study GWAS team and the Welcome Trust Case Control Consortium 2 (WTCCC2). Lists of members of the Blue Mountains Eye Study GWAS team and the WTCCC2 are available at http://aaojournal.org.
Funding This work was supported by the National Health and Medical Research Council (Canberra, Australia) (Project Grant IDs 512423 and 590204 to JJW). The Blue Mountains Eye Study (BMES) was supported by the National Health and Medical Research Council (NHMRC), Canberra, Australia (NHMRC project grant IDs 974159, 211069, 457349, 302068, and Centre for Clinical Research Excellence in Translational Clinical Research in Eye Diseases, CCRE in TCR-Eye, grant ID 529923). The BMES GWAS and genotyping costs was supported by the NHMRC, Canberra, Australia (NHMRC project grant IDs 512423, 475604 and 529912), and the Wellcome Trust, UK as part of Wellcome Trust Case Control Consortium 2 (A Viswanathan, P McGuffin, P Mitchell, F Topouzis, P Foster, grant IDs 085475/B/08/Z and 085475/08/Z). The Beaver Dam Eye Study was supported by National Institutes of Health (NIH) grant EY006594 (BEK Klein and R Klein), an NIH Research Vision Core grant EY016665, and, in part, by an unrestricted grant from Research to Prevent Blindness, New York, NY. The Rotterdam Study (RS) was supported by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. In addition, the RS was supported by Rotterdamse Stichting Blindenbelangen, Rotterdam; Macula Fonds, Utrecht; Henkes Stichting, Rotterdam; Stichting Oogfonds Nederland, Utrecht; and Landelijke Stichting voor Blinden en Slechtzienden, Utrecht. JJW is funded by a NHMRC (Canberra, Australia) Senior Research Fellowship (Grant ID 358702, 2005–2009 and ID 632909, 2010–2015). NHMRC Australia, the NIH and other funding bodies mentioned above had no role in the design or conduct of this research.
Footnotes
PM, CCWK, RK, JJW are co-senior authors.
Contributors All the authors meet the ICMJE recommendations for authorship credit (substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data; drafting the work or revising it critically for important intellectual content; final approval of the version published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved).
Competing interests There are no financial disclosures for all authors except for PM and CCWK, who have provided consultancy to Bayer (PM, CCWK), Novartis (PM, CCWK) and Allergan (PM) and received payment and travel/accommodations from these companies for lectures.
Ethics approval The University of Sydney and the Sydney West Area Health Service Human Research Ethics Committees; University of Wisconsin-Madison; Erasmus Medical Centre and Ministry of Health, Welfare and Sport of the Netherlands.
Provenance and peer review Not commissioned; externally peer reviewed.
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/bjophthalmol-2016-309729).
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Sengupta S, Nguyen AM, van Landingham SW, et al. Evaluation of real-world mobility in age-related macular degeneration. BMC Ophthalmol. 2015;15:9. doi: 10.1186/1471-2415-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill MT, Banks AD, Stinnett SS, et al. Vision-related quality of life in patients with bilateral severe age-related macular degeneration. Ophthalmology. 2005;112:152–8. doi: 10.1016/j.ophtha.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P, Wang JJ, Foran S, et al. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–7. doi: 10.1016/s0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 5.Wang JJ, Rochtchina E, Lee AJ, et al. Ten-year incidence and progression of age-related maculopathy: the blue Mountains Eye Study. Ophthalmology. 2007;114:92–8. doi: 10.1016/j.ophtha.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Jensen SC, et al. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Klein BE, Tomany SC, et al. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam eye study. Ophthalmology. 2002;109:1767–79. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen R, Klaver CC, Vingerling JR, et al. The risk and natural course of age-related maculopathy: follow-up at 6 ½ years in the Rotterdam study. Arch Ophthalmol. 2003;121:519–26. doi: 10.1001/archopht.121.4.519. [DOI] [PubMed] [Google Scholar]
- 9.Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Macular Photocoagulation Study Group. Arch Ophthalmol. 1993;111:1189–99. doi: 10.1001/archopht.1993.01090090041018. [DOI] [PubMed] [Google Scholar]
- 10.Gangnon RE, Lee KE, Klein BE, et al. Severity of age-related macular degeneration in 1 eye and the incidence and progression of age-related macular degeneration in the fellow eye: the beaver dam eye study. JAMA Ophthalmol. 2015;133:125–32. doi: 10.1001/jamaophthalmol.2014.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Meuer SM, Myers CE, et al. Harmonizing the classification of age-related macular degeneration in the three-continent AMD consortium. Ophthalmic Epidemiol. 2014;21:14–23. doi: 10.3109/09286586.2013.867512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein R, Myers CE, Buitendijk GH, et al. Lipids, lipid genes, and incident age-related macular degeneration: the three continent age-related macular degeneration consortium. Am J Ophthalmol. 2014;158:513–24. doi: 10.1016/j.ajo.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell P, Smith W, Attebo K, et al. Prevalence of age-related maculopathy in Australia: The Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 15.Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001;42:2237–41. [PubMed] [Google Scholar]
- 16.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang JJ, Buitendijk GH, Rochtchina E, et al. Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations. Ophthalmology. 2014;121:667–75. doi: 10.1016/j.ophtha.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein BE, Tomany SC, et al. Ten-year incidence of age-related maculopathy and smoking and drinking: the Beaver Dam Eye Study. Am J Epidemiol. 2002;156:589–98. doi: 10.1093/aje/kwf092. [DOI] [PubMed] [Google Scholar]
- 19.Vingerling JR, Hofman A, Grobbee DE, et al. Age-related macular degeneration and smoking. The Rotterdam Study. Arch Ophthalmol. 1996;114:1193–6. doi: 10.1001/archopht.1996.01100140393005. [DOI] [PubMed] [Google Scholar]
- 20.Tomany SC, Wang JJ, van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–7. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Wang JJ, Rochtchina E, Smith W, et al. Combined effects of complement factor H genotypes, fish consumption, and inflammatory markers on long-term risk for age-related macular degeneration in a cohort. Am J Epidemiol. 2009;169:633–41. doi: 10.1093/aje/kwn358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131:383–92. doi: 10.1001/jamaophthalmol.2013.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buitendijk GH, Rochtchina E, Myers C, et al. Prediction of age-related macular degeneration in the general population: the Three Continent AMD Consortium. Ophthalmology. 2013;120:2644–55. doi: 10.1016/j.ophtha.2013.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds R, Rosner B, Seddon JM. Serum lipid biomarkers and hepatic lipase gene associations with age-related macular degeneration. Ophthalmology. 2010;117:1989–95. doi: 10.1016/j.ophtha.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Leeuwen R, Klaver CC, Vingerling JR, et al. Cholesterol and age-related macular degeneration: is there a link? Am J Ophthalmol. 2004;137:750–2. doi: 10.1016/j.ajo.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Seddon JM, Reynolds R, Yu Y, et al. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology. 2011;118:2203–11. doi: 10.1016/j.ophtha.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chew EY, Klein ML, Clemons TE, et al. Genetic testing in persons with age-related macular degeneration and the use of the AREDS supplements: to test or not to test? Ophthalmology. 2015;122:212–5. doi: 10.1016/j.ophtha.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


