Summary
Artemisinin and its derivatives (ARTs) are frontline antimalarial drugs. However, ART monotherapy is associated with a high frequency of recrudescent infection, resulting in treatment failure. A subset of parasites is thought to undergo ART-induced latency, but the mechanisms remain unknown. Here we report that ART treatment results in phosphorylation of the parasite eukaryotic initiation factor-2α (eIF2α), leading to repression of general translation and latency induction. Enhanced phosphorylated eIF2α correlates with high rates of recrudescence following ART, and inhibiting eIF2α dephosphorylation renders parasites less sensitive to ART treatment. ART-induced eIF2α phosphorylation is mediated by the Plasmodium eIF2α kinase, PK4. Overexpression of a PK4 dominant-negative or pharmacological inhibition of PK4 blocks parasites from entering latency and abolishes recrudescence after ART treatment of infected mice. These results show that translational control underlies ART-induced latency and that interference with this stress response may resolve the clinical problem of recrudescent infection.
Keywords: Plasmodium, artemisinin, recrudescence, eIF2α, PK4, resistance
eTOC paragraph
The antimalarial drug artemisinin is associated with a high frequency of recrudescent infection. Zhang et al. identified that artemisinin induces latency through Plasmodium eukaryotic initiation factor 2α (eIF2α) phosphorylation. Inhibiting the Plasmodium eIF2α kinase PK4 blocks parasites from entering latency and abolishes recrudescence after artemisinin therapy.
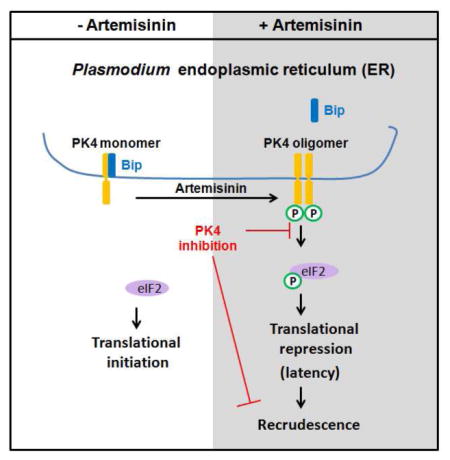
Introduction
The malaria parasite Plasmodium spp. was estimated to cause 212 million new cases and over 0.4 million deaths in 2015 (World Health Organization., 2015). Artemisinin and its derivatives (ARTs) are the most potent antimalarial drugs to date (Chen, 2016; O’Neill et al., 2010; Tu, 2011), but their use is stymied by recrudescence rates as high as 60% (Codd et al., 2011; de Vries and Dien, 1996; Meshnick et al., 1996). Malaria recrudescence, defined as the reappearance of the original strain of the parasite after antimalarial medications, is considered as treatment failure (Shaukat et al., 2012). To minimize this problem, the World Health Organization recommends against monotherapy in favor of ART-based combination therapy (ACT). Nevertheless, recrudescent P. falciparum infections have still been reported (Ndounga et al., 2015; Yeka et al., 2005).
Most clinical antimalarials are schizonticidal drugs, targeting blood stage parasites that are responsible for the clinical symptoms associated with malaria. Within blood cells, merozoites develop to form either gametocytes or schizonts. Plasmodium schizonts release daughter merozoites and the free merozoites re-invade erythrocytes and differentiate into rings, trophozoites, and schizonts. Recrudescence following antimalarial therapy has been thought to occur as a result of drug-resistant parasites (O’Brien et al., 2011). However, recrudescent parasites isolated from patients remain sensitive to ARTs (Dondorp et al., 2009; Noedl et al., 2008; Phyo et al., 2012). As the in vivo half-life of ARTs is short (Li et al., 2014), it has been proposed that the concentration of the drug in plasma was insufficient to suppress all parasites (White, 2013). Consequently, longer courses of therapy or twice-a-day dosing intervals are recommended to efficiently clear the infection (Dogovski et al., 2015). Recent studies also indicate that ARTs induce transition of a subset of parasites into latent rings that are responsible for recrudescence (Codd et al., 2011; Grobler et al., 2014; LaCrue et al., 2011; Teuscher et al., 2012; Teuscher et al., 2010). Despite its clinical importance, the molecular mechanism of ART-induced parasite latency remains unknown.
We have previously demonstrated that latent stages of apicomplexan parasites, including Toxoplasma and Plasmodium, coincide with increased phosphorylation of the α subunit of eukaryotic translation initiation factor-2 (eIF2), which delivers initiator tRNA to ribosomes. Phosphorylation of eIF2α, which occurs during cellular stress, results in a reduction of general protein synthesis, accompanied by preferential translation of mRNAs encoding products that are important for recovery from the stress (Young and Wek, 2016). We hypothesized that translation control through parasite eIF2α phosphorylation contributes to ART-induced latency. Consistent with this idea, RNA sequencing and microarray studies have shown that ribosomal protein genes are down-regulated in response to ART in the human malaria parasite, P. falciparum, and the rodent malaria parasite, P. berghei (Shaw et al., 2015). Furthermore, proteins involved in the translation process were among the most down-regulated gene products in ART-treated parasites (Shaw et al., 2015).
In Plasmodium, there are three eIF2α kinases: eIK1, eIK2, and PK4 (Ward et al., 2004). The eIK1 protein kinase responds to amino acid deprivation during the erythrocytic stage (Babbitt et al., 2012; Fennell et al., 2009), while eIK2 controls sporozoite latency in the mosquito salivary glands (Zhang et al., 2010). Neither eIK1 nor eIK2 are essential for erythrocytic stage development (Fennell et al., 2009; Zhang et al., 2010). In contrast, PK4 phosphorylates eIF2α in mature schizonts and is essential for parasite development in erythrocytes (Solyakov et al., 2011; Zhang et al., 2012a). In this study, we establish that eIF2α phosphorylation by PK4 is critical for the ART-induced latency that leads to recrudescence and treatment failure. Importantly, small molecule inhibition of PK4 blocks the ability of the parasite transition into this latent phase and as a consequence prevents malaria recrudescence following ART treatment.
Results
ART induces eIF2α phosphorylation in Plasmodium
Phosphorylation of eIF2α has been associated with slow-growing and latent stages of parasite development (Babbitt et al., 2012; Fennell et al., 2009; Zhang et al., 2013; Zhang et al., 2012a). We sought to determine the status of eIF2α phosphorylation in malaria parasites treated with ARTs as well as recrudescent parasites post-ART treatment. Two derivatives of artemisinin have been employed to study malaria recrudescence: dihydroartemisinin (DHA) is used in cultured human malaria and artesunate (AS) in rodent malaria (Chen et al., 2014; Codd et al., 2011; LaCrue et al., 2011; Peatey et al., 2015; Shaw et al., 2015; Teuscher et al., 2012; Teuscher et al., 2010). We established recrudescence post-AS treatment in P. berghei ANKA infected Swiss Webster mice (Figure S1 and Table S1). Phosphorylation of P. berghei eIF2α (PbIF2α) was significantly enhanced following AS treatment and dephosphorylated in the recrudescent parasites (Figure 1A). Similar results were observed in P. falciparum cultures treated with DHA followed by magnetic column purification to remove parasites unaffected by DHA (Figure 1B) (Teuscher et al., 2010). High levels of Plasmodium eIF2α phosphorylation in the small population (<1%, Figure S2) of latent parasites induced by ART were maintained with salubrinal (Sal) treatment, which has been established to inhibit the dephosphorylation of eIF2α in multiple species, including apicomplexan parasites (Tsaytler and Bertolotti, 2013; Zhang et al., 2010) (Figure 1C). Addition of Sal to the latent parasites delayed parasite recrudescence in a dose-dependent manner (Figures 1D, 1E and S2). These results show that ARTs induce eIF2α phosphorylation in Plasmodium, which is then removed in recrudescing parasites.
Figure 1. Phosphorylation of eIF2α in response to ART treatment in Plasmodium parasites.
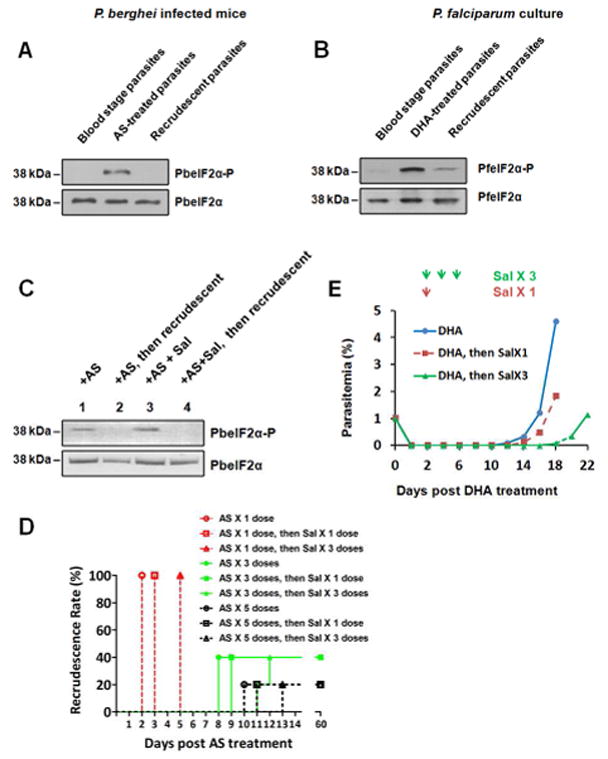
(A) P. berghei ANKA infected mice were treated with 3 doses of AS (64 mg/kg/dose, 12-hour interval), and AS-treated parasites were collected 6 hours after the final dose of AS. Parasite recrudesced 8 days post-AS treatment, and the recrudescent parasites were collected on day 8. The levels of phosphorylated PbeIF2α (PbeIF2α-P) and total PbeIF2α were measured by immunoblot in the untreated, AS-treated and recrudescent parasites. (B) Asynchronized P. falciparum 3D7 culture was treated for 6 hours with 200 nM DHA followed by magnetic column purification to remove unaffected parasites by the drug. DHA-treated parasites were collected immediately after column purification. Parasite recrudesced 12 days post-DHA treatment, and the recrudescent parasite were collected on day 12. The untreated parasites without magnetic column purification were included as a control. Levels of PfeIF2α-P and total PfeIF2α in the untreated, DHA-treated, and recrudescent parasites were analyzed by immunoblot. (C) Mice infected with P. berghei ANKA were treated orally with 64 mg/kg AS. One day later, the normal erythrocytic stage parasites were undetectable by Giemsa stain and there was increased levels of phosphorylated PbeIF2α (lane 1). The mice were then separated into 2 groups: mice in one group were i.v. injected with Sal (2mg/kg) and the other group was vehicle control. 24 hours after Sal administration, normal erythrocytic stage parasites recurred in the control group harboring dephosphorylated PbeIF2α (lane 2); recrudescence was undetectable in the Sal-treated mice and PbeIF2α was phosphorylated (lane 3). Two days post-Sal treatment the parasites recrudesce and their PbeIF2α was dephosphorylated (lane 4). (D) Sal prolongs AS-induced latency in P. berghei infected mice. One to five doses of AS were administered orally to the infected mice, then Sal was i.v. injected into mice on day 1 or on days 1–3 post-AS treatment. Giemsa stain (sensitivity of 0.01% parasitemia) was performed to determine recrudescent rate. (E) Sal prolongs DHA-induced latency in P. falciparum culture. DHA-treated P. falciparum 3D7 cultures followed by magnetic column purification were treated with 10 μM Sal on day 2 or on days 2, 4, and 6 post-DHA treatment. Data are representative of 3 independent experiments. Related to Figure S1, S2 and Table S1.
Parasites bearing phosphorylated eIF2α are less sensitive to ART
Blood stage schizogony is a replicative process in which the parasite undergoes multiple rounds of nuclear division: ring stages differentiate to trophozoites and then mature into schizonts, which rupture and release merozoites. Free merozoites invade other erythrocytes to continue the asexual blood-stages of the life cycle. Phosphorylation of Plasmodium eIF2α was not detectable in rings or trophozoites, but was observed in late schizonts (Figures 2A and B), consistent with the diminished protein synthesis seen in late schizonts (Fang et al., 2014; Zhang et al., 2012a). Schizonts bearing enhanced levels of phosphorylated eIF2α were associated with high rates of recrudescence following ART treatment (Figures 2C and D). The level of eIF2α phosphorylation was significantly enhanced in rings in response to ART treatment (Figures 2A and B). Recrudescence occurred in ring stages following ART treatment compared with trophozoites (Figures 2C and D). Thus, ARTs induce ring-stage parasites to increase the levels of phosphorylated eIF2α and ring-stage parasites are less sensitive to ARTs.
Figure 2. Parasites bearing phosphorylated eIF2α are less sensitive to ART.
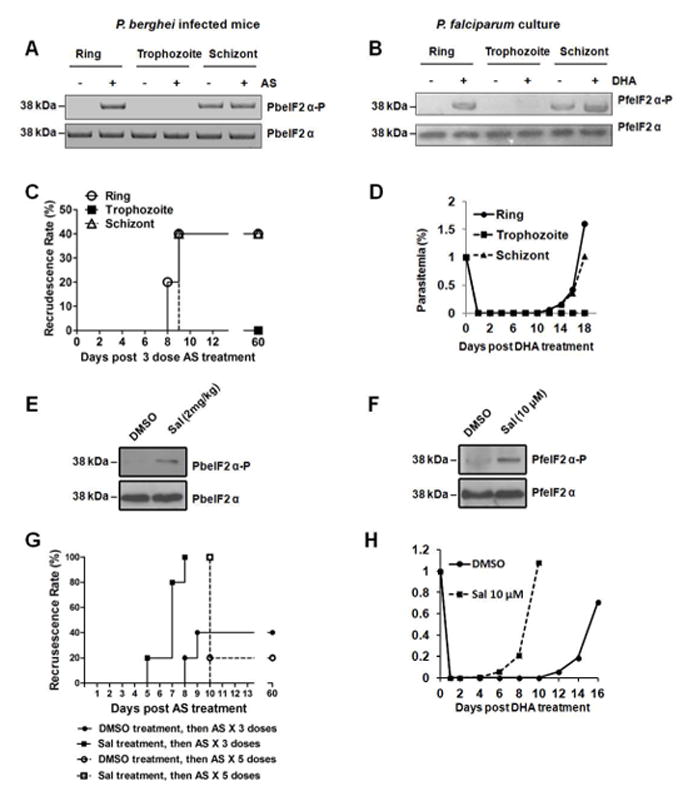
(A) Ring, trophozoite, and schizont stages of P. berghei ANKA were treated with 64 mg/kg AS for 15 min and levels of PbeIF2α-P and total PbeIF2α were determined by immunoblot. (B) Ring, trophozoite, and schizont stages of P. falciparum 3D7 were treated with 200 nM DHA for 15 min. Amounts of PfeIF2α-P and total PfeIF2α were determined by immunoblot. (C) Recrudescence of P. berghei rings, trophozoites, and schizonts following 3 doses of 64 mg/kg AS as determined by Giemsa stain. (D) Recrudescence of P. falciparum rings, trophozoites, and schizonts after a 6 hour treatment with 200 nM DHA treatment, followed by magnetic column purification to the unaffected parasites by DHA. (E) PbeIF2α-P levels after pre-treatment with Sal. Mice infected with asynchronized P. berghei were intravenously injected with 2 mg/kg Sal 24 hours before AS treatment. PbeIF2α-P levels were analyzed by immunoblots. (F) PfeIF2α-P levels after pre-treatment with Sal. Asynchronized P. falciparum were treated with 10 μM Sal for 2 hours. (G) Recrudescence rates of AS-treated P. berghei following increased levels of eIF2α phosphorylation by Sal treatment. P. berghei infected mice were intravenously injected with Sal 24 hours before the mice were orally administered 3 or 5 doses of 64 mg/kg AS. (H) Recrudescence rates of DHA-treated P. falciparum 3D7 after inflating eIF2α phosphorylation with Sal. Asynchronized P. falciparum 3D7 were treated with Sal for 2 hours. The medium was then refreshed and the parasites were treated with 200 nM DHA for 6 hours, followed by a step of magnetic column purification to the unaffected parasites by DHA. Data are representative of 3 independent experiments.
Inclusion of Sal in either P. berghei or P. falciparum cultures increases the amount of eIF2α phosphorylation (Figures 2E and F). Enhanced Plasmodium eIF2α phosphorylation coincided with the parasites being less sensitive to ART treatment (Figures 2G and H), suggesting that translational control has a protective effect against the drug. Recrudescence following AS exposure occurred in all mice pretreated with Sal, compared to a 20–40% recrudescence rate in control groups with only basal levels of PbeIF2α phosphorylation. In addition, recrudescence occurred earlier in some mice pretreated with Sal. The earlier recrudescence following ART treatment was also observed in P. falciparum cultures pretreated with Sal. These findings show that increased levels of phosphorylated eIF2α protects parasites from ART treatment.
The effect of Sal on the parasite’s recrudescence post ART therapy depends on the time of Sal delivery. In the scenario of adding Sal after ART therapy, recrudescence was postponed (Figures 1D and E). The explanation for this finding is that the parasite transits to latency after ART therapy, and adding Sal to the latent parasite prolongs the latency by maintaining the eIF2α phosphorylation level. In the scenario of adding Sal prior to ART therapy, the recrudescence occurred earlier and with higher rate (Figures 2G and H). The rationale for this observation is that pretreatment of Sal increases eIF2α phosphorylation level and the parasite transits into latency before ART therapy. The latent parasite is tolerant to ART treatment.
ART exposure activates the ER-resident eIF2α kinase, PK4
Three eIF2α kinases have been identified in Plasmodium: eIK1, eIK2, and PK4 (Tewari et al., 2010; Ward et al., 2004). We determined whether parasites lacking each of these eIF2α kinases exhibited a defect in recrudescence following ART treatment. Figure S3 shows our successful generation of PbeIK1 (−) parasites, and our generation of PbeIK2 (−) parasites was described earlier (Zhang et al., 2010). PbeIK1 (−) and PbeIK2 (−) parasites proliferated normally in erythrocytes (Figure S3C) and no defect was observed in the enhanced eIF2α phosphorylation in response to AS treatment (Figure S4A). Moreover, mice infected with either PbeIK1 (−) or PbeIK2 (−) parasites and treated with 3 or 5 doses of AS showed the same degree of recrudescence as wild-type (Figures S4B and S4C). These results show that neither PbeIK1 nor PbeIK2 contributes to AS-induced latency.
Plasmodium PK4, which has been shown to be essential for the parasite (Solyakov et al., 2011; Tewari et al., 2010; Zhang et al., 2012a), is related to the human eIF2α kinase, PERK. Like PERK, PK4 contains a predicted transmembrane domain situated upstream of its protein kinase domain (Figure 3A). Also like PERK (Harding et al., 1999), we found that PK4 localizes to the parasite endoplasmic reticulum (ER) (Figure 3B and S5) in a complex with the ER-resident chaperone BIP (Kumar et al., 1991) (Figure 3C). We previously verified that the kinase domain of PK4 is able to phosphorylate eIF2α using an in vitro assay (Zhang et al., 2012a). Notably, this reaction required oligomerization via the fused GST domain for both eIF2α phosphorylation and autophosphorylation of PK4 (Zhang et al., 2012a), which is consistent with the reported mechanism of PERK activation (Harding et al., 1999; Walter and Ron, 2011). Additionally, we show that endogenous PK4 oligomerized post DHA treatment (Figure 3D), supporting the idea that oligomerization of PK4 leads to enhanced phosphorylation of eIF2α in response to AS in 15 min (Figures 2B). Of importance, ART treatment also led to displacement of BIP from PK4, which coincides with PK4 oligomerization/autophosphorylation, and eIF2α phosphorylation, which are processes that are also important for activation of PERK. Taken together, the results indicate that Plasmodium ER-resident eIF2α kinase PK4 phosphorylates eIF2α in response to AS treatment using a mechanism of kinase activation that is analogous to PERK.
Figure 3. ART treatment and PK4 activation.
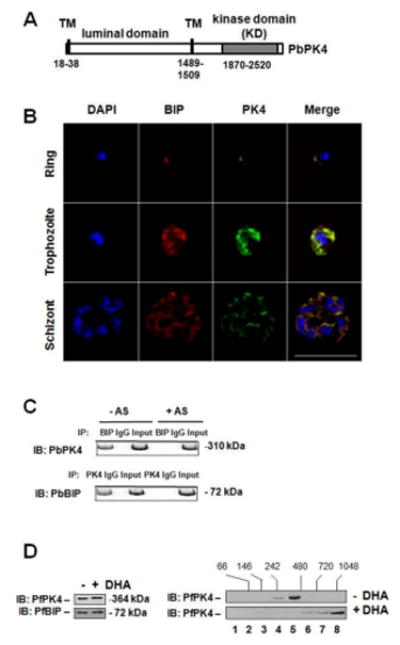
(A) A box represents the PK4 protein, with the luminal domain, transmembrane domain (TM), and eIF2α kinase domain indicated, along with corresponding residue numbers. (B) Subcellular co-localization of PK4 (green) and BIP (red) as revealed by immunofluorescence in erythrocytic stages of P. berghei. DAPI (blue) was used as a co-stain to show the parasite nuclei. Scale bar, 10 μm. (C) Whole cell extracts from P. berghei blood stage parasites untreated or treated with AS for 15 min were immediately subjected to immunoprecipitation (IP) with anti-PK4 or anti-BIP antibodies followed, by immunoblot (IB) analysis with the designated antibody. (D) PfPK4 oligomerizes in response to DHA. Left panel, contents of PK4 and BIP from lysates of untreated or 200 nM DHA-treated (15 min) P. falciparum 3D7 culture. Right panels, measurements of PK4 protein after size fractionation of lysates by sedimentation on a 20–40% glycerol gradient. The fractionation numbers are indicated and PK4 levels were measured in gradient fractions by immunoblot. Related to Figure S3, S4 and S5.
Expression of a dominant-negative form of PK4 blocks recrudescence
Since PK4 is essential (Zhang et al., 2012a), we used a dominant-negative strategy to study its role in AS-induced latency and recrudescence. A dominant-negative version of PK4 was created by mutating the autophosphorylation site in PbPK4, threonine-2436 (Zhang et al., 2010); the mutation ablated PK4 autophosphorylation and eIF2α phosphorylation (Figure 4A). An episomal plasmid was engineered to express the T2436A PK4 mutant or a control version containing a change to a synonymous threonine codon (wt). These plasmids were transfected into P. berghei schizonts, which were then inoculated into mice. It was previously shown that expression of a mutant version of PERK can oligomerize with endogenous wild-type kinase and lower eIF2α (Ma et al., 2002). Six hours later when the parasites had developed into rings, PK4 mRNA levels representing the endogenous, synonymous, and T2436A mutant were analyzed by qRT-PCR. The mRNA levels of T2436A PK4 were 10-fold higher than that of endogenous PK4 (Figure 4B). The mRNA levels of wild-type PK4 in the parasites transfected with the synonymous PK4 expression cassette were ~2-fold higher relative to the parasites transfected with empty vector. Similar results were also reported for the same mutated version of a related mammalian eIF2α kinase (Romano et al., 1998). Overexpression of eIF2α kinases inhibits translation and eukaryotic cell growth, but analogous expression of a mutant eIF2α kinase lacking activity are tolerated and therefore can be expressed at higher levels. At the protein level, total PK4 was increased in synonymous and dominant-negative PK4 overexpressing parasites (Figure 4C). As expected, eIF2α phosphorylation was increased in parasites expressing the synonymous PK4 mutation, and protein synthesis was inhibited in these parasites accordingly (Figure 4D). Conversely, levels of eIF2α phosphorylation were decreased in parasites expressing the T2436A PK4 mutant (Figure 4C), with a corresponding alleviation of translational repression (Figure 4D). Recrudescence following ART treatment occurred earlier and at a higher rate in parasites transfected with the functional PK4 containing a synonymous codon change, whereas no recrudescence was observed in parasites transfected with dominant-negative version of PK4 (Figure 4E). These findings further support the model that interference with PK4-mediated eIF2α phosphorylation blocks the ART-induced latency that leads to recrudescent infection.
Figure 4. Effect of PK4 on parasite recrudescence following ART treatment.
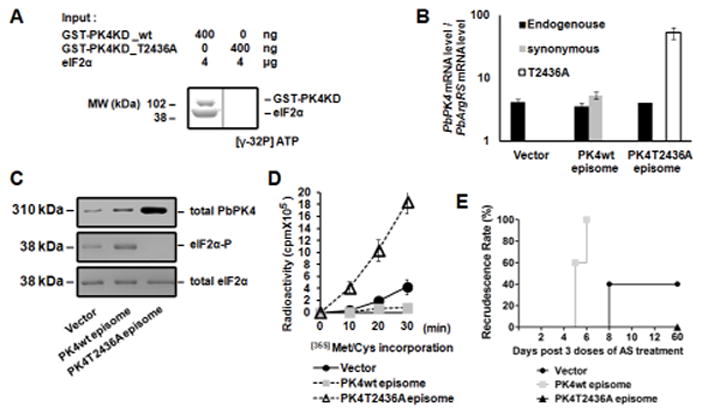
(A) A dominant-negative form of PK4 was created by mutating the autophosphorylation site (T2436A), which inhibits kinase activity in vitro. GST-tagged PbPK4KD wt or T2436A mutant proteins were incubated with PbeIF2α and [γ-32P] ATP. Samples were then resolved on SDS-PAGE for autoradiography. (B) The mRNA levels of PK4 in P. berghei parasites harboring empty vector, episomal plasmid expressing functional PK4 with a synonymous codon change or a version encoding the T2436A mutant. P. berghei ring stage parasites were analyzed for the levels of endogenous, synonymous, and T2436A mutant PK4 mRNAs. The arginyl-tRNA synthetase (PbArgRS, PB000094.03.0) was used as internal control. Each value is the mean ± standard deviation (SD) of two independent experiments. (C) Expression of dominant-negative PK4 inhibits phosphorylation of P. berghei eIF2α. Levels of total PK4 protein, phosphorylated PbeIF2α, and total PbeIF2α in the parasites harboring the different episomes were revealed by immunoblotting. (D) Expression of dominant-negative PK4 enhances protein synthesis in the parasite. Protein synthesis was analyzed by incorporation of [35S]Met/Cys. Each value is the mean ± SD of two independent experiments. (E) Expression of dominant-negative PK4 abolishes recrudescence following AS treatment. Mice infected with P. berghei parasites harboring the three designated episomes were treated with 3 doses of 64 mg/kg AS. Data are representative of 3 independent experiments.
Pharmacological inhibition of PK4 blocks parasite differentiation from trophozoites into schizonts and attenuates recrudescence
We used another approach to test whether PK4 inhibition blocks ART-induced latency. Parasites were treated with GSK2606414, an established inhibitor of PERK (Axten et al., 2012). We first confirmed that PK4 kinase activity was specifically inhibited by GSK2606414 in vitro, and that GSK2606414 had no effect on eIK1 or eIK2 (Figure S6A–C). We next measured the effect of GSK2606414 on P. berghei-infected mice and P. falciparum cultures. GSK2606414 inhibits eIF2α phosphorylation in asynchronized parasites (Figures 5A and B). In the control groups, asynchronized parasites containing all stages showed schizonts bearing phosphorylated eIF2α (Figures 2A, 2B, 5A, and 5B). GSK2606414 reversibly inhibited parasite development in erythrocytes (Figures 5C, 5D and S6D–F), consistent with previous findings that the phosphorylation of eIF2α by PK4 is essential for the development of the parasite in the erythrocytic cycle (Solyakov et al., 2011; Zhang et al., 2012a). During GSK2606414 treatment, parasites entered the trophozoite stage but did not progress to schizogony (Figure 5E and S6E), which is consistent with our previous observation that PK4 phosphorylates eIF2α in late schizonts (Zhang et al., 2012a). We conclude that the inhibition of eIF2α phosphorylation by GSK2606414 disrupts parasite differentiation from trophozoites into schizonts.
Figure 5. PK4 inhibitor GSK2606414 prevents recrudescence following ART treatment.
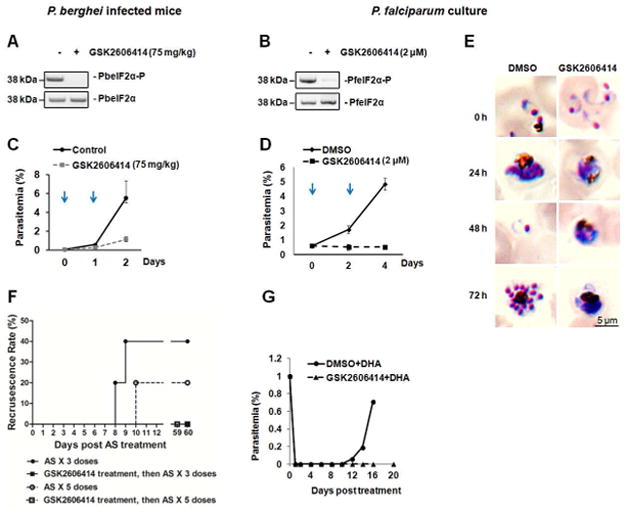
Effects of GSK2606414 on the levels of Plasmodium eIF2α phosphorylation 24 hours after 75 mg/kg GSK2606414 treatment of mice infected with asynchronized P. berghei ANKA (A) and 48 hours after 2 μM GSK2606414 treatment of asynchronized P. falciparum 3D7 parasites in culture (B). Plasmodium eIF2α phosphorylation levels were evaluated by immunoblot. Parasitemia ± SD of P. berghei ANKA infected mice (C) and P. falciparum 3D7 culture (D) treated with GSK2606414 were determined by microscopy after Giemsa stain. Arrows indicate the two days that GSK2606414 was added. (E) Giemsa stains of P. falciparum 3D7 culture treated with 2 μM GSK2606414. DMSO-treated parasites complete the erythrocytic cycle in ~48 hours, in contrast to GSK2606414-treated parasites, which halt differentiation at the trophozoite stage. (F) Recrudescence of P. berghei after combination therapy. P. berghei infected mice were intravenously injected with 75 mg/kg Sal and 1 hr later were orally administered 3 or 5 doses of 64 mg/kg AS. Giemsa stain was performed to determine recrudescence rate. (G) Recrudescence of P. falciparum 3D7 in culture after combination therapy of GSK2606414 and DHA. Asynchronized P. falciparum 3D7 parasites were treated with 2μM GSK2606414 or DMSO for 1 hr, followed by a treatment of 200 nM DHA for 6 hrs. Data are representative of 3 independent experiments. Related to Figure S6.
We next addressed whether GSK2606414 could affect ART-induced latency and recrudescent infection by inhibiting the PK4-mediated eIF2α phosphorylation that occurs in response to ART. The combination of ART and GSK2606414 abolished parasite recrudescence in P. berghei-infected mice and P. falciparum cultures (Figure 5F and G), suggesting that inhibition of PK4 restores parasite sensitivity to ART.
PfeIF2α phosphorylation in the ART-resistant parasite Dd2C580Y
A transcriptome survey of P. falciparum clinical isolates found that ART resistance is associated with increased expression of genes involved in the unfolded protein response (UPR) pathways and with arrest in rings (Mok et al., 2015). In mammalian cells, the UPR is a cellular stress response resulting in phosphorylation of eIF2α. Phosphorylation of eIF2α can promote mammalian cell survival under stress (Ron and Walter, 2007; Walter and Ron, 2011). We sought to determine the status of eIF2α phosphorylation in the ART-resistant parasite Dd2C580Y before and after DHA treatment. PfK13-propeller mutations, including C580Y, confer ART resistance in P. falciparum (Straimer et al., 2015). The ART-sensitive and chloroquine-resistant parasite line Dd2 was included as a control. PfeIF2α was phosphorylated in the young ring-stage of the ART-resistant parasite Dd2C580Y, but not in the young ring-stage of the ART-sensitive parasite Dd2 (Figure 6A). However, PfeIF2α was dephosphorylated in late rings and trophozoites. The phosphorylation of eIF2α in late schizont-stage was observed in both sensitive and resistant parasites. The ART-resistant parasite Dd2C580Y remained in a state of decelerated development at ring stage in culture. At 40 hours, the ART-resistant parasite reached the late schizont-stage just like the sensitive parasite (Figure 6A). This is consistent with the phenotype of ex-vivo culture of clinical ART-resistant isolates (Mok et al., 2015). Nevertheless, DHA did not induce higher levels of PfeIF2α phosphorylation in the ART-resistant parasite Dd2C580Y, in contrast to that observed in the sensitive Dd2 (Figure 6B). GSK2606414 reduced ART resistance in the parasite Dd2C580Y (Figure 6C). An explanation of these results is that PfeIF2α is basally phosphorylated in the young rings of the ART-resistant parasite, likely by PK4, and the resistant parasite do not response to DHA by phosphorylating eIF2α compared to the ART-sensitive parasite.
Figure 6. Phosphorylation of PfeIF2α in a PfK13 C580Y mutant parasite.
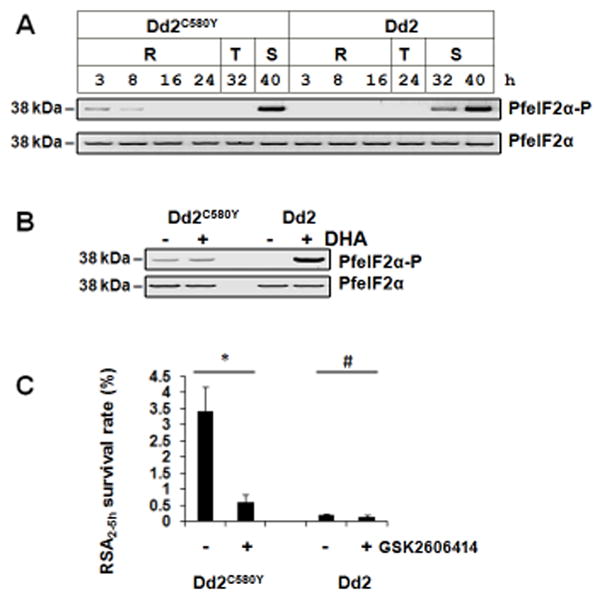
(A) ART-resistant Dd2C580Y and ART-sensitive Dd2 parasites collected at different hours post the incubation of late-stage schizonts and fresh red blood cells were subjected to immunoblot analysis of PfeIF2α-P and total PfeIF2α. R, ring; T, trophozoite; S, schizont. (B) Young rings (0–3 hours post invasion of erythrocytes) of Dd2C580Y and Dd2 were treated with 700 nM DHA for 15 min, and PfeIF2α phosphorylation levels were evaluated by immunoblot. Data are representative of 3 independent experiments. (C) GSK2606414 reduced ART resistance. Young rings (0–3 h post invasion) of the ART-resistant parasite Dd2C580Y and the ART-sensitive parasite Dd2 were pre-treated with 2 μM GSK2606414 for 2 h, and then the rings (2–5 h) were treated with a pulse of 700 nM DHA for 6 h. RSA survival rate ± SD of the parasite was measured. DMSO-treated parasites were used as controls. *P<0.05; #P>0.05. Data are representative of 5 independent experiments.
Discussion
ARTs are a formidable weapon against malaria, but a small proportion of parasites become latent during treatment and later reemerge in a phenomenon known as recrudescence (Cheng et al., 2012; Codd et al., 2011; LaCrue et al., 2011; Teuscher et al., 2012; Teuscher et al., 2010). These recrudescent parasite isolates are not drug-resistant, suggesting that disruption of latency could remedy the treatment failure (Dondorp et al., 2009; Noedl et al., 2008; Phyo et al., 2012). Our findings indicate that the Plasmodium eIF2α kinase PK4 is localized to the parasite ER and is activated by oligomerization and autophosphorylation in response to ARTs. Activated PK4 phosphorylates eIF2α and inhibits global protein synthesis, promoting latency and providing for an adaptive state that allows for the parasite to better manage the damage accrued upon exposure to ARTs. Attenuation of PK4 activity through expression of a dominant-negative PK4 or application of a PERK inhibitor was successful in curbing recrudescent infection.
ART contains an endoperoxide group that causes oxidative stress, which leads to parasite clearance (O’Neill et al., 2010). Upon entering parasites, ARTs have been reported to traffic to a wide variety of subcellular organelles, including the ER, food vacuole, mitochondrion and other membrane-bound organelles (Eckstein-Ludwig et al., 2003; Hartwig et al., 2009; Stocks et al., 2007; Wang et al., 2010) and result in protein damage (Chen et al., 2017). ARTs induce PK4 activation in the ER of ring stages, resulting in the phosphorylation of eIF2α that promotes latency associated with recrudescent infection (Cheng et al., 2012; Codd et al., 2011; LaCrue et al., 2011; Teuscher et al., 2012; Teuscher et al., 2010). Ring stage parasites respond to ARTs by phosphorylating eIF2α (Figure 2), which is concordant with previous reports describing the induction of latent rings by ARTs (Grobler et al., 2014; LaCrue et al., 2011; O’Brien et al., 2011; Teuscher et al., 2012; Teuscher et al., 2010; Tucker et al., 2012; Witkowski et al., 2010).
In response to diverse stress stimuli, eukaryotic cells activate an adaptive pathway termed the integrated stress response (ISR) to restore cellular homeostasis. The ISR centers on the ability of cells to modulate translation of mRNAs through the phosphorylation of eIF2α. Severe or prolonged stress will overwhelm the capacity of the adaptive response and initiate cell death (Pakos-Zebrucka et al., 2016). Plasmodium activates the parasitic ISR by phosphorylating eIF2α in response to diverse cell perturbations, including stress induced by drugs such as ART or chloroquine (Surolia and Padmanaban, 1991), or amino acid deprivation (Babbitt et al., 2012; Fennell et al., 2009). The percentage of parasites that retreat into a latent state during ART treatment depends on the initial parasitemia and drug dosage (Klonis et al., 2013; Teuscher et al., 2012). Longer courses of therapy or shorter intervals (e.g., twice daily regimen) are recommended to efficiently clear parasite infections (Dogovski et al., 2015). The recrudescence rate of a twice-daily regimen was lower than a once-daily regimen (Figure S1).
Recrudescence has also been reported to occur during monotherapy with other first-line antimalarial drugs (Bennett et al., 2013; Happi et al., 2004; Kugasia et al., 2014). Some clinical drugs have less inhibitory effect on schizonts compared with trophozoites (Table 1). Of interest, Plasmodium eIF2α is basally phosphorylated in schizonts (Figures 2A and B), which likely explains their decreased sensitivity to drugs, including ART therapy (Klonis et al., 2013; LaCrue et al., 2011; Sullivan, 2013). The median lethal dose (LD50) of DHA in the parasite rings and late schizonts is higher than that of trophozoites (Klonis et al., 2013; Sullivan, 2013). Latency induced by eIF2α phosphorylation during the schizont stage may be triggered by the depletion of hemoglobin from host erythrocytes, leading to nutritional deprivation. The decreased drug sensitivity in the schizonts indicates that latency triggered by an underlying stress is accompanied by activation of an eIF2α kinase which provides the parasite protection against a subsequent stresses, including those caused by drug exposure. This pre-conditioning mechanism affords the parasite an adaptive strategy that also helps to explain previous findings that Plasmodium preferentially progress to arrest as schizonts in response to antimalarials (Wilson et al., 2013). Consistent with this idea, augmentation of eIF2α phosphorylation by virtue of Sal increased recrudescence rates to 100% (Figure 2G). Additional mechanisms may also contribute to schizonts being less sensitive to some drugs. Clearly, the role of Plasmodium eIF2α phosphorylation in response to other clinical antimalarial drugs requires further investigation.
Table 1.
Inhibition of P. falciparum 3D7 growth after 4 hour treatment with antimalarial drugs*.
| Fluorescence (485/530 nm) | Concentration | Rings | Trophozoites | Schizonts |
|---|---|---|---|---|
| Amodiaquin | 600 nM | 5,801 | 5,065 | 93,628 |
| Atovaquone | 5 μM | 5,231 | 5,051 | 6,177 |
| Pyrimethamine | 4 uM | 5,579 | 5,477 | 84,981 |
| Primaquine | 1uM | 102,496 | 6,900 | 67,726 |
| DHA | 200 nM | 100,835 | 6,025 | 87,180 |
| DMSO | 155,341 | 154,326 | 155,253 |
P. falciparum 3D7 0–3 h rings, 21–24 h trophozoites, and 32–35 h schizonts were simultaneously treated with a pulse of antimalarial drugs for 4 hours. DMSO-treated parasites maintained their ring, trophozoite, or schizont stages during the 4 hour treatment period. Parasites were washed and medium was refreshed after the 4-hour drug treatment. Ninety-six hours later, parasite load was evaluated by SYBR Green I nucleic acid staining dye (Plouffe et al., 2008). Data are representative of 3 independent experiments. Fluorescence emissions with >10% difference relative to trophozoites are marked in bold.
As proof of concept, we tested the PERK inhibitor, GSK2606414, on Plasmodium PK4 and subsequently malaria infection models. GSK2606414 inhibited PK4 alone, with no effect on the other two Plasmodium eIF2α kinases that respond to alternative stress signals (Figure S6). Importantly, GSK2606414 inhibited Plasmodium eIF2α phosphorylation and abolished recrudescence post-ART therapy (Figure 5). Since the ER, and hence PERK, are absent in mature erythrocytes, GSK2606414 is most likely exerting its activity through the parasite. In agreement with the PERK inhibitor study, over-expression of a mutated PK4 lacking eIF2α kinase activity also inhibited recrudescence (Figure 4). Together, these results show that interference with the PK4-mediated stress response pathway offers a means to resolve the clinical problem of recrudescent infection.
In addition to recrudescence that has been reported decades ago (Li et al., 1984), the emergence and spread of ART resistance in recent years threatens malaria control and global public health (Dondorp et al., 2009). A transcriptome survey of P. falciparum clinical isolates found that ART resistance is associated with increased expression of genes in the unfolded protein response (UPR) (Mok et al., 2015). In mammalian cells, the UPR is a cellular stress response that is regulated by phosphorylation of eIF2α (Ron and Walter, 2007; Walter and Ron, 2011). Lowered global protein synthesis controlled through PfeIF2α phosphorylation could explain slow growth in the young rings of ART-resistant parasites (Mok et al., 2015) (Figure 6A). The young rings of ART-resistant parasite Dd2C580Y are latent, and the phosphorylation levels of PfeIF2α were not changed in response to 700 nM DHA treatment for 15 min by (Figure 6B). PfeIF2α phospohorylation level in different erythrocytic stages of clinical isolates post longer time ART treatment requires further investigation. In higher eukaryotic cells, basal phosphorylation of eIF2α is responsible for resistance to stress induced cell death (Zeng et al., 2011). Phosphorylation of PfeIF2α may be responsible for resistance to ARTs. The eIF2α kinase responsible for PfeIF2α phosphorylation in ART-resistant parasites has not yet been identified. As PERK appears to be the only UPR responder conserved in Apicomplexa, it makes for a highly attractive drug target. PK4 is homologous to the human eIF2α kinase PERK, and essential for the parasite erythrocytic cycle (Solyakov et al., 2011; Zhang et al., 2012a). Nevertheless, GSK2606414 reversibly inhibits the parasite differentiation into schizont and does not kill the parasite (Figure 5D,E and S6D–F). This result is consistent with the previous finding that the barcode of the PK4 mutant was still detected in the mouse 4–8 days post transfection of PK4 knockout plasmid (Gomes et al., 2015). The PK4 inhibitor GSK2606414 reduced ART resistance in Dd2C580Y (Figure 6C), and PK4 may be responsible for phosphorylating PfeIF2α in the ART-resistant parasite. Further investigation into the translational control, including genetically validating the PfeIF2α kinase, in ART-resistant parasites may reveal additional ways to interfere with this critical pathway.
It has been reported that P. falciparum phosphatidylinositol-3-kinase (PfPI3K) and its lipid product phosphatidylinositol-3-phosphate (PI3P) are increased in ART-resistant parasites, and PI3P is the key mediator of ART resistance (Mbengue et al., 2015). It is of interest to investigate the relationship between the PfPI3K/PI3P and PfPK4/PfeIF2α pathways using the PfPK4 inhibitor GSK2606414.
The discovery that translational control is critical for parasite recrudescence has far-reaching implications for other parasites with latent stages relevant to pathogenesis, such as Toxoplasma gondii. Toxoplasma is currently incurable because treatment of the replicative stage is believed to drive a subset of parasites back into the latent tissue cyst stage. It would be worthwhile to determine whether disruption of eIF2α phosphorylation during drug treatment of toxoplasmosis prevents the formation of additional tissue cysts.
STAR METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Plasmodium eIF2α-P | William J. Sullivan, Jr | (Narasimhan et al., 2008b) |
| Anti-total Plasmodium eIF2α | William J. Sullivan, Jr | (Sullivan et al., 2004) |
| Anti-Plasmodium PK4 | This paper | N/A |
| Anti-Plasmodium BIP | Nirbhay Kumar | (Kumar et al., 1991) |
| Parasites | ||
| P. berghei ANKA | NYU Insectary Core Facility and Parasite Culture | https://med.nyu.edu/microbiology-parasitology/research/parasitology/insectary-core-facility-and-parasite-culture |
| P. falciparum 3D7 | NYU Insectary Core Facility and Parasite Culture | https://med.nyu.edu/microbiology-parasitology/research/parasitology/insectary-core-facility-and-parasite-culture |
| P. falciparum Dd2 | David Fidock | (Straimer et al., 2015) |
| P. falciparum Dd2C580Y | David Fidock | (Straimer et al., 2015) |
| PbeIK1 (−) | This paper | Figure S3 |
| PbeIK2 (−) | Victor Nussenzweig | (Zhang et al., 2010) |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Artesunate | Tokyo Chemical Industries Co. Ltd. | Cat#A2191 |
| Dihydroartemisinin | AK Scientific, Inc. | Cat#K453 |
| Salubrinal | Tocris Bioscience | Cat#2347 |
| GSK2606414 | MedChemexpress | Cat#HY-18072 |
| Amodiaquin | Sigma-Aldrich | Cat#1031004 |
| Atovaquone | Sigma-Aldrich | Cat#A7986 |
| Pyrimethamine | Sigma-Aldrich | Cat#46706 |
| Primaquine | Sigma-Aldrich | Cat# 1561507 |
| Recombinant Protein GST-PbPK4KD T2436A mutant | This paper | N/A |
| Recombinant Protein GST-PfPK4KD | This paper | N/A |
| Recombinant Protein GST-PfeIK1KD | This paper | N/A |
| Recombinant Protein GST-PfeIK2KD | This paper | N/A |
| Recombinant Protein PfeIF2α-His | This paper | N/A |
| Experimental Models: Cell Lines | ||
| Human red blood cells | Interstate Blood Bank | http://www.interstatebloodbank.com/products.asp |
| Experimental Models: Organisms/Strains | ||
| Mouse Swiss Webster | Taconic Biosciences | Tac:SW |
| Oligonucleotides | ||
| wt PbPK4
qPCR caaatgattggaacccctggatataca |
This paper | N/A |
| synonymous PbPK4
qPCR cagatgataggtacgccaggttacact |
This paper | N/A |
| PbPK4 T2436A mutant
qPCR cagatgataggtgcaccaggttacact |
This paper | N/A |
| PbArgRS qPCR | Eurofins | (Zhang et al., 2010) |
| Recombinant DNA | ||
| plasmid pBC_PbeIK1KO | This paper | N/A |
| plasmid pGST-PfeIK1KD | This paper | N/A |
| plasmid pGST-PfeIK2KD | This paper | N/A |
| plasmid pGST-PfePK4KD | This paper | N/A |
| plasmid pPfeIF2α-His | This paper | N/A |
| plasmid pPK4wt episome | This paper | N/A |
| plasmid pPK4T2436A episome | This paper | N/A |
| plasmid pVector episome | This paper | N/A |
| Software and Algorithms | ||
| DNASTAR | DNASTAR, Inc. | https://www.dnastar.com/ |
| Applied Biosystems 7500 Fast Real-Time PCR System | Applied Biosystems | http://www.appliedbiosystems.com/absite/us/en/home/support/software/real-time-pcr/ab-7500.html |
| Prism | GraphPad Software, Inc. | https://www.graphpad.com/ |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Min Zhang (zhanmin@iu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal model
All animal work has been conducted according to Institutional Animal Care and Use Committee (IACUC) Laboratory Animal Protocol: 140102. Six-eight week old female Swiss Webster mice from Taconic Biosciences (genetics: recessive mutation Pde6brd1) were used in this study. All animals were housed at New York University Medical Center animal facilities under the care of the Division of Laboratory Animal Resources veterinary staff.
Human cells
Human red blood cells from O+ Caucasian donors were purchased from Interstate Blood Bank with authentification. All work on P. falciparum has been conducted according to Institutional Biosafety Committee protocols 16-000072 and IN-777. P. falciparum infected red blood cells were cultured in RPMI-based medium containing 5 mM glucose, 25 mM HEPES, 2 g/L NaHCO3, 100 μM hypoxanthine, 50 μg/L gentamicin and 5% Albumax II, pH 7.2 at 37 °C in a mixture of nitrogen (90%), CO2 (5%) and Oxygen (5%).
METHODS DETAILS
Drugs
Artesunate (AS) used in this study was obtained from Tokyo Chemical Industries Co. Ltd. The AS powder was dissolved in ethanol to make a 21 mg/mL stock, which was further diluted with water to the administrated concentration and immediately introduced into the stomach of mice with an oral gavage cannula at 64 mg/kg mouse body weight as previously described (LaCrue et al., 2011). Dihydroartemisinin (DHA) used in this study was obtained from AK Scientific, Inc. DHA was dissolved in DMSO to make a 1 mM stock. P. falciparum 3D7 culture was treated with 200 nM DHA for 6 hours, followed by magnetic column purification for 3 consecutive days to remove unaffected parasites by the drug as previously described (Teuscher et al., 2010). The in vivo half-lives of AS and DHA are within one hour (O’Neill et al., 2010). Sal was obtained from Tocris bioscience and prepared as a 100 mM stock solution in DMSO. P. berghei infected mice were intravenously injected with Sal at 2 mg/kg mouse body weight. P. falciparum culture was treated with Sal at 10 μM. The half-life of Sal in plasma is approximately 6 hours (Zhang et al., 2012b). GSK2606414 was obtained from MedChemexpress and prepared as a 100 mg/mL stock solution in DMSO. P. berghei infected mice were i.v. injected with 75 mg/kg of GSK2606414 in a solution with 0.5% hydroxypropyl methylcellulose and 0.1% Tween 80. P. falciparum culture was treated with GSK2606414 at 2 μM.
Giemsa stain to monitor recrudescence
Thin blood smears were made from P. berghei infected mice and P. falciparum culture, followed by 100% methanol fixation and 1:10 diluted Giemsa solution for 10 min. Parasitemia were calculated by counting the normal erythrocytic stages, including replicative rings, trophozoites and schizonts.
Parasite purification and SDS-PAGE
After 3 doses of 64mg/kg AS treatment, P. berghei parasitized mouse blood was collected and the blood cells were washed twice in RPMI 1640. Then the red blood cells were collected after separation by Histopaque-1077 gradient centrifugation. Collected mouse red blood cells were then lysed with 0.2 % (w/v) saponin in PBS to released parasites. Parasite pellets were lysed and resuspended in SDS-PAGE loading buffer. Parasite proteins were resolved by SDS-PAGE and transferred to PVDF membrane for immunoblot. P. falciparum infected human red blood cells were treated with 200 nM DHA for 6 hours, followed by magnetic 3-step column purification to remove unaffected parasites by the drug (Teuscher et al., 2010).
Antiserum
The C-terminus of PK4 (outside of the kinase domain) fused with GST was expressed in E. coli, and the purified protein was used to immunize BALB/c mice. Immunoblot of P. berghei parasite lysates with the mouse serum (1:3,000 dilution) revealed a major band >290 kDa (endogenous PbPK4 is 310 kDa). PfBIP is a major ER chaperone protein (Kumar et al., 1991). The rabbit anti-PfBIP serum that was generated by immunization with a polypeptide consisting of the the last 11 amino acid residues of PfBIP was used to detect PbBIP (Kumar et al., 1991). This portion of BIP is highly conserved among the Plasmodium species (Roobsoong et al., 2014). The anti-total eIF2α and anti-eIF2α-P sera were generated in the laboratory of William J. Sullivan Jr., and used in Plasmodium as previously described (Narasimhan et al., 2008a; Zhang et al., 2010; Zhang et al., 2016).
Generation of PbeIK1(−) parasites
PbeIK1 knockouts were generated using the same method that we previously described for generation of PbeIK2 (−) parasites (Zhang et al., 2010). The 3′-terminal of PbeIK1 coding sequence in the genome (PbANKA_130840, 4.5kb) is adjacent (less than 500 bp) to PbANKA_130830. We deleted the 5′-terminus (2.5-kb) of the PbeIK1 coding sequence to avoid interference with the downstream gene. The 2.5-kb of the 5′-portion of this gene encodes the entire eIF2α kinase catalytic domain (Zhang et al., 2010).
Overexpression of dominant-negative or wt PK4
The PbPK4 expression cassette contains 1.2-kb of the 5′-untranslated region (UTR), PbPK4 coding sequence, and 1.0-kb of the 3′-UTR. The sequence around the threonine 2436 in endogenous PbPK4 is caaatgattggaacccctggatataca (T2436 underlined); the sequence around the threonine 2436 in synonymous version of PbPK4 is cagatgataggtacgccaggttacact (T2436 underlined, and synonymous mutations marked in bold); the PbPK4 sequence with the alanine 2436 codon mutant is cagatgataggtgcaccaggttacact (A2436 underlined, and mutations marked in bold). Vector pUC57 encoding PbPK4 with the synonymous or T2436A codons were transfected into P. berghei ANKA mature schizonts as previously described (Janse et al., 2006). Empty vector was a control. The transfected schizonts were intravenously injected to mice. Six hour later when the parasites developed into ring stage, the recipient mice were used to analyze PK4 mRNA level, total PK4 protein level, eIF2α phosphorylation level, and protein synthesis.
In Vitro kinase Assay
GST-tagged PfPK4 kinase domain was expressed and purified from E. coli. The purified GST-PfPK4KD and PbeIF2α were incubated with 20 μCi [γ-32P]ATP in a final concentration of 50 μM and kinase buffer solution (15 mM Hepes [pH 7.5], 100 mM NaCl, 2 mM TCEP, 5 mM MgCl2) in a volume of 50 μL at 37 °C for 5–60 min. The GST-PfPK4KD protein was pre-incubated with GSK2606414, and PbeIF2α and [γ-32P]ATP were added. Proteins were then separated by SDS-PAGE, followed by autoradiography.
Glycerol-gradient sedimentation
The lysis buffer (1% Triton x 100, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 20 mM Hepes [pH 7.5]) was used to extract Plasmodium membrane proteins. Extracts were centrifuged through 20–40% glycerol gradients in polyallomer tubes of 11X 60 mm as described for the human eIF2α kinase PERK (Bertolotti et al., 2000; Ma et al., 2002). Each 4-mL gradient was divided into eight equal fractions of 500 μL. Aliquots of each fraction were subjected to SDS–PAGE after adjustment of the glycerol concentration in each fraction to 20%, and the contents of PK4 and BIP were measured by immunoblots.
Quantification and Statistical Analysis
P. berghei recrudescence in mouse
Five Swiss Webster mice per group were used to monitor parasitemia of P. berghei in Figure 1D, 2C, 2G, 4E, 5C, 5D, and 5F. Ten Swiss Webster mice per group were used to monitor parasitemia of P. berghei in Figure S1, S3C, S4B and S4C.
Giesma stains, immunoblots, immunoflorescence assay, and P. facliparum recrudescence assay
Three independent experiments were performed to confirm the reproducibility, and data are representative of 3 independent experiments.
The mRNA levels of PK4 and incorporation of [35S]Met/Cys
Two independent experiments were performed to evaluate the mRNA levels of PK4 and the incorporation of [35S]Met/Cys. Each value is the mean ± standard deviation (SD) of independent experiments (Figure 4B and 4D).
RSA survival rate
Five independent experiments were performed to evaluate RSA survival rate (Figure 6C). The normality assumption was tested using the Shapiro-Wilk test. Statistically significant differences were determined by the Student’s t-test.
Supplementary Material
Highlights.
Plasmodium eIF2α kinase PK4 is activated upon artemisinin treatment
eIF2α phosphorylation by PK4 leads to latency and recrudescence post artemisinin therapy
Inhibiting PK4 abolishes recrudescence after artemisinin therapy
Acknowledgments
This research was supported by grants from the National Institutes of Health (AI108592 to Victor Nussenzweig, AI124723 to WJS and RCW, AI070258 to MT). We thank Chandy John at Indiana University School of Medicine for providing laboratory facilities. We thank David Fidock at Columbia University for providing the parasites Dd2 and Dd2C580Y.
Footnotes
Author Contributions
M.Z. contributed design the study, performed the experiments, and wrote the manuscript. J.G. and A.R. contributed P. falciparum culture. C.F and M.T. contributed FACS. N.C.W. contributed discussion. R.C.W. contributed discussion and wrote the manuscript. V. N. contributed to the study design and wrote the manuscript. W.J.S. contributed reagent supply, discussion and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J Med Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- Babbitt SE, Altenhofen L, Cobbold SA, Istvan ES, Fennell C, Doerig C, Llinas M, Goldberg DE. Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc Natl Acad Sci U S A. 2012;109:E3278–3287. doi: 10.1073/pnas.1209823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013;369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Chen MZ, Moily NS, Bridgford JL, Wood RJ, Radwan M, Smith TA, Song Z, Tang BZ, Tilley L, Xu X, et al. A thiol probe for measuring unfolded protein load and proteostasis in cells. Nat Commun. 2017;8:474. doi: 10.1038/s41467-017-00203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, LaCrue AN, Teuscher F, Waters NC, Gatton ML, Kyle DE, Cheng Q. Fatty acid synthesis and pyruvate metabolism pathways remain active in dihydroartemisinin-induced dormant ring stages of Plasmodium falciparum. Antimicrob Agents Chemother. 2014;58:4773–4781. doi: 10.1128/AAC.02647-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ. Honoring antiparasitics: The 2015 Nobel Prize in Physiology or Medicine. Biomed J. 2016;39:93–97. doi: 10.1016/j.bj.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Kyle DE, Gatton ML. Artemisinin resistance in Plasmodium falciparum: A process linked to dormancy? Int J Parasitol Drugs Drug Resist. 2012;2:249–255. doi: 10.1016/j.ijpddr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd A, Teuscher F, Kyle DE, Cheng Q, Gatton ML. Artemisinin-induced parasite dormancy: a plausible mechanism for treatment failure. Malar J. 2011;10:56. doi: 10.1186/1475-2875-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- Dogovski C, Xie SC, Burgio G, Bridgford J, Mok S, McCaw JM, Chotivanich K, Kenny S, Gnadig N, Straimer J, et al. Targeting the cell stress response of Plasmodium falciparum to overcome artemisinin resistance. PLoS Biol. 2015;13:e1002132. doi: 10.1371/journal.pbio.1002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O’Neill PM, Bray PG, Ward SA, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- Fang X, Reifman J, Wallqvist A. Modeling metabolism and stage-specific growth of Plasmodium falciparum HB3 during the intraerythrocytic developmental cycle. Mol Biosyst. 2014;10:2526–2537. doi: 10.1039/c4mb00115j. [DOI] [PubMed] [Google Scholar]
- Fennell C, Babbitt S, Russo I, Wilkes J, Ranford-Cartwright L, Goldberg DE, Doerig C. PfeIK1, a eukaryotic initiation factor 2alpha kinase of the human malaria parasite Plasmodium falciparum, regulates stress-response to amino-acid starvation. Malar J. 2009;8:99. doi: 10.1186/1475-2875-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AR, Bushell E, Schwach F, Girling G, Anar B, Quail MA, Herd C, Pfander C, Modrzynska K, Rayner JC, et al. A genome-scale vector resource enables high-throughput reverse genetic screening in a malaria parasite. Cell Host Microbe. 2015;17:404–413. doi: 10.1016/j.chom.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobler L, Chavchich M, Haynes RK, Edstein MD, Grobler AF. Assessment of the induction of dormant ring stages in Plasmodium falciparum parasites by artemisone and artemisone entrapped in Pheroid vesicles in vitro. Antimicrob Agents Chemother. 2014;58:7579–7582. doi: 10.1128/AAC.02707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happi CT, Gbotosho GO, Sowunmi A, Falade CO, Akinboye DO, Gerena L, Kyle DE, Milhous W, Wirth DF, Oduola AM. Molecular analysis of Plasmodium falciparum recrudescent malaria infections in children treated with chloroquine in Nigeria. Am J Trop Med Hyg. 2004;70:20–26. [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Hartwig CL, Rosenthal AS, D’Angelo J, Griffin CE, Posner GH, Cooper RA. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem Pharmacol. 2009;77:322–336. doi: 10.1016/j.bcp.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc. 2006;1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- Klonis N, Xie SC, McCaw JM, Crespo-Ortiz MP, Zaloumis SG, Simpson JA, Tilley L. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc Natl Acad Sci U S A. 2013;110:5157–5162. doi: 10.1073/pnas.1217452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugasia IR, Polara FK, Assallum H. Recrudescence of Plasmodium malariae after Quinine. Case Rep Med. 2014;2014:590265. doi: 10.1155/2014/590265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Koski G, Harada M, Aikawa M, Zheng H. Induction and localization of Plasmodium falciparum stress proteins related to the heat shock protein 70 family. Mol Biochem Parasitol. 1991;48:47–58. doi: 10.1016/0166-6851(91)90163-z. [DOI] [PubMed] [Google Scholar]
- LaCrue AN, Scheel M, Kennedy K, Kumar N, Kyle DE. Effects of artesunate on parasite recrudescence and dormancy in the rodent malaria model Plasmodium vinckei. PLoS One. 2011;6:e26689. doi: 10.1371/journal.pone.0026689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Arnold K, Guo XB, Jian HX, Fu LC. Randomised comparative study of mefloquine, qinghaosu, and pyrimethamine-sulfadoxine in patients with falciparum malaria. Lancet. 1984;2:1360–1361. doi: 10.1016/s0140-6736(84)92057-9. [DOI] [PubMed] [Google Scholar]
- Li Q, Remich S, Miller SR, Ogutu B, Otieno W, Melendez V, Teja-Isavadharm P, Weina PJ, Hickman MR, Smith B, et al. Pharmacokinetic evaluation of intravenous artesunate in adults with uncomplicated falciparum malaria in Kenya: a phase II study. Malar J. 2014;13:281. doi: 10.1186/1475-2875-13-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Vattem KM, Wek RC. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem. 2002;277:18728–18735. doi: 10.1074/jbc.M200903200. [DOI] [PubMed] [Google Scholar]
- Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, Rizk SS, Njimoh DL, Ryan Y, Chotivanich K, et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683–687. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S, Ashley EA, Ferreira PE, Zhu L, Lin Z, Yeo T, Chotivanich K, Imwong M, Pukrittayakamee S, Dhorda M, et al. Drug resistance. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science. 2015;347:431–435. doi: 10.1126/science.1260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan J, Joyce BR, Naguleswaran A, Smith AT, Livingston MR, Dixon SE, Coppens I, Wek RC, Sullivan WJ., Jr Translation Regulation by Eukaryotic Initiation Factor-2 Kinases in the Development of Latent Cysts in Toxoplasma gondii. J Biol Chem. 2008a;283:16591–16601. doi: 10.1074/jbc.M800681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan J, Joyce BR, Naguleswaran A, Smith AT, Livingston MR, Dixon SE, Coppens I, Wek RC, Sullivan WJ., Jr Translation regulation by eukaryotic initiation factor-2 kinases in the development of latent cysts in Toxoplasma gondii. J Biol Chem. 2008b;283:16591–16601. doi: 10.1074/jbc.M800681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndounga M, Pembe Issamou M, Casimiro PN, Koukouikila-Koussounda F, Bitemo M, Diassivy Matondo B, Ndounga Diakou LA, Basco LK, Ntoumi F. Artesunate-amodiaquine versus artemether-lumefantrine for the treatment of acute uncomplicated malaria in Congolese children under 10 years old living in a suburban area: a randomized study. Malar J. 2015;14:423. doi: 10.1186/s12936-015-0918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- O’Brien C, Henrich PP, Passi N, Fidock DA. Recent clinical and molecular insights into emerging artemisinin resistance in Plasmodium falciparum. Curr Opin Infect Dis. 2011;24:570–577. doi: 10.1097/QCO.0b013e32834cd3ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin--the debate continues. Molecules. 2010;15:1705–1721. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. Embo Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peatey CL, Chavchich M, Chen N, Gresty KJ, Gray KA, Gatton ML, Waters NC, Cheng Q. Mitochondrial Membrane Potential in a Small Subset of Artemisinin-Induced Dormant Plasmodium falciparum Parasites In Vitro. J Infect Dis. 2015;212:426–434. doi: 10.1093/infdis/jiv048. [DOI] [PubMed] [Google Scholar]
- Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, et al. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A. 2008;105:9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, Zhang F, Herring C, Mathews MB, Qin J, Hinnebusch AG. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol Cell Biol. 1998;18:2282–2297. doi: 10.1128/mcb.18.4.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Roobsoong W, Maher SP, Rachaphaew N, Barnes SJ, Williamson KC, Sattabongkot J, Adams JH. A rapid sensitive, flow cytometry-based method for the detection of Plasmodium vivax490 infected blood cells. Malar J. 2014;13:55. doi: 10.1186/1475-2875-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaukat AM, Gilliams EA, Kenefic LJ, Laurens MB, Dzinjalamala FK, Nyirenda OM, Thesing PC, Jacob CG, Molyneux ME, Taylor TE, et al. Clinical manifestations of new versus recrudescent malaria infections following anti-malarial drug treatment. Malar J. 2012;11:207. doi: 10.1186/1475-2875-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Chaotheing S, Kaewprommal P, Piriyapongsa J, Wongsombat C, Suwannakitti N, Koonyosying P, Uthaipibull C, Yuthavong Y, Kamchonwongpaisan S. Plasmodium parasites mount an arrest response to dihydroartemisinin, as revealed by whole transcriptome shotgun sequencing (RNA-seq) and microarray study. BMC Genomics. 2015;16:830. doi: 10.1186/s12864-015-2040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solyakov L, Halbert J, Alam MM, Semblat JP, Dorin-Semblat D, Reininger L, Bottrill AR, Mistry S, Abdi A, Fennell C, et al. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat Commun. 2011;2:565. doi: 10.1038/ncomms1558. [DOI] [PubMed] [Google Scholar]
- Stocks PA, Bray PG, Barton VE, Al-Helal M, Jones M, Araujo NC, Gibbons P, Ward SA, Hughes RH, Biagini GA, et al. Evidence for a common non-heme chelatable-iron-dependent activation mechanism for semisynthetic and synthetic endoperoxide antimalarial drugs. Angew Chem Int Ed Engl. 2007;46:6278–6283. doi: 10.1002/anie.200604697. [DOI] [PubMed] [Google Scholar]
- Straimer J, Gnadig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, Dacheux M, Khim N, Zhang L, Lam S, et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347:428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DJ. Timing is everything for artemisinin action. P Natl Acad Sci USA. 2013;110:4866–4867. doi: 10.1073/pnas.1301607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan WJ, Jr, Narasimhan J, Bhatti MM, Wek RC. Parasite-specific eIF2 (eukaryotic initiation factor-2) kinase required for stress-induced translation control. Biochem J. 2004;380:523–531. doi: 10.1042/BJ20040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surolia N, Padmanaban G. Chloroquine inhibits heme-dependent protein synthesis in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1991;88:4786–4790. doi: 10.1073/pnas.88.11.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher F, Chen N, Kyle DE, Gatton ML, Cheng Q. Phenotypic changes in artemisinin514 resistant Plasmodium falciparum lines in vitro: evidence for decreased sensitivity to dormancy and growth inhibition. Antimicrob Agents Chemother. 2012;56:428–431. doi: 10.1128/AAC.05456-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. Artemisinin-induced dormancy in plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis. 2010;202:1362–1368. doi: 10.1086/656476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R, Straschil U, Bateman A, Bohme U, Cherevach I, Gong P, Pain A, Billker O. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe. 2010;8:377–387. doi: 10.1016/j.chom.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaytler P, Bertolotti A. Exploiting the selectivity of protein phosphatase 1 for pharmacological intervention. FEBS J. 2013;280:766–770. doi: 10.1111/j.1742-4658.2012.08535.x. [DOI] [PubMed] [Google Scholar]
- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- Tucker MS, Mutka T, Sparks K, Patel J, Kyle DE. Phenotypic and genotypic analysis of in vitro-selected artemisinin-resistant progeny of Plasmodium falciparum. Antimicrob Agents Chemother. 2012;56:302–314. doi: 10.1128/AAC.05540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang J, Huang L, Li J, Fan Q, Long Y, Li Y, Zhou B. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PLoS One. 2010;5:e9582. doi: 10.1371/journal.pone.0009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Pharmacokinetic and pharmacodynamic considerations in antimalarial dose optimization. Antimicrob Agents Chemother. 2013;57:5792–5807. doi: 10.1128/AAC.00287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Langer C, Goodman CD, McFadden GI, Beeson JG. Defining the timing of action of antimalarial drugs against Plasmodium falciparum. Antimicrob Agents Chemother. 2013;57:1455–1467. doi: 10.1128/AAC.01881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B, Lelievre J, Barragan MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother. 2010;54:1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World malaria report. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- Yeka A, Banek K, Bakyaita N, Staedke SG, Kamya MR, Talisuna A, Kironde F, Nsobya SL, Kilian A, Slater M, et al. Artemisinin versus nonartemisinin combination therapy for uncomplicated malaria: randomized clinical trials from four sites in Uganda. Plos Med. 2005;2:e190. doi: 10.1371/journal.pmed.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SK, Wek RC. Upstream Open Reading Frames Differentially Regulate Gene-specific Translation in the Integrated Stress Response. J Biol Chem. 2016;291:16927–16935. doi: 10.1074/jbc.R116.733899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng N, Li Y, He L, Xu X, Galicia V, Deng C, Stiles BL. Adaptive basal phosphorylation of eIF2alpha is responsible for resistance to cellular stress-induced cell death in Pten-null hepatocytes. Mol Cancer Res. 2011;9:1708–1717. doi: 10.1158/1541-7786.MCR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fennell C, Ranford-Cartwright L, Sakthivel R, Gueirard P, Meister S, Caspi A, Doerig C, Nussenzweig RS, Tuteja R, et al. The Plasmodium eukaryotic initiation factor-2alpha kinase IK2 controls the latency of sporozoites in the mosquito salivary glands. J Exp Med. 2010;207:1465–1474. doi: 10.1084/jem.20091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Joyce BR, Sullivan WJ, Jr, Nussenzweig V. Translational control in Plasmodium and toxoplasma parasites. Eukaryot Cell. 2013;12:161–167. doi: 10.1128/EC.00296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mishra S, Sakthivel R, Fontoura BM, Nussenzweig V. UIS2: A Unique Phosphatase Required for the Development of Plasmodium Liver Stages. PLoS Pathog. 2016;12:e1005370. doi: 10.1371/journal.ppat.1005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mishra S, Sakthivel R, Rojas M, Ranjan R, Sullivan WJ, Jr, Fontoura BM, Menard R, Dever TE, Nussenzweig V. PK4, a eukaryotic initiation factor 2alpha(eIF2alpha) kinase, is essential for the development of the erythrocytic cycle of Plasmodium. Proc Natl Acad Sci U S A. 2012a;109:3956–3961. doi: 10.1073/pnas.1121567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Hamamura K, Jiang C, Zhao L, Yokota H. Salubrinal promotes healing of surgical wounds in rat femurs. J Bone Miner Metab. 2012b;30:568–579. doi: 10.1007/s00774-012-0359-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


