Abstract
In view of the observation that corticotropin-releasing factor (CRF) affects several brain functions through at least two subtypes of G protein-dependent receptors and a binding protein (CRFBP), we have developed synthetic strategies to provide enhanced binding specificity. Human/rat CRF (h/rCRF) and the CRF-like peptide sauvagine (Svg), differing in their affinities to CRFBP by two orders of magnitude, were used to identify the residues determining binding to CRFBP. By amino acid exchanges, it was found that Ala22 of h/rCRF was responsible for this peptide's high affinity to CRFBP, whereas Glu21 located in the equivalent position of Svg prevented high affinity binding to CRFBP. Accordingly, [Glu22]h/rCRF was not bound with high affinity to CRFBP in contrast to [Ala21]Svg, which exhibited such high affinity. Furthermore, the affinity of both peptides to either CRF receptor (CRFR) subtype was not reduced by these replacements, and their subtype preference was not changed. Thus, exchange of Ala and Glu and vice versa in positions 22 and 21 of h/rCRF and Svg, respectively, serves as a switch discriminating between CRFBP and CRFR. On the basis of this switch function, development of new specific CRF agonists and antagonists is expected to be facilitated. One application was the modification of the CRF antagonist astressin (Ast), whose employment in animal experiments is limited by its low solubility in cerebrospinal fluid. Introduction of Glu residues into Ast generated with [Glu11,16]Ast an acidic astressin, which efficiently antagonized in vivo the CRFR1-dependent reduction of locomotion induced by ovine CRF without detectable binding to CRFBP.
Corticotropin-releasing factor (CRF; Fig. 1) (1) represents an early chemical signal of the stress response and activates the hypothalamus–pituitary–adrenal axis in response to a stressful stimulus (2). CRF is also recognized as an important neuromodulator of memory consolidation, anxiety, locomotor activity, and food intake (3, 4). It exhibits its biologic actions through G protein-dependent receptors. To date, mainly two subtypes of CRF receptors (CRFRs) (5, 6), CRFR1 and CRFR2, have been identified. The central actions of CRF-like peptides are also modulated by the water-soluble CRF binding protein (CRFBP) (7, 8). Consistent with the results of experiments on CRFBP-deficient mice (9), the physiological role of endogenous CRFBP in the central nervous system may be at least in part to limit the availability of free ligand for CRFR-mediated actions in brain regions where CRFBP, CRFR, and CRF are colocalized. In addition, central injection of CRF antagonists such as α-helical CRF9–41 (α-hel-CRF9–41) (10), which are bound by CRFBP with high affinity, may release endogenous CRF by displacement from CRFBP (11). Therefore, strategies to synthesize CRF analogs binding selectively to CRFBP or CRFR are of great importance for the investigation of CRF receptor-mediated brain functions. In view of the distinct distribution of CRFBP in the mammalian brain (3) and the region-dependent actions of CRF (4), the design of specific CRFBP ligands may also be helpful for using endogenous CRF to strengthen differentially a CRFR subtype-specific action—for example, to enhance memory consolidation by endogenous CRF released from hippocampal CRFBP.
Figure 1.
Sequence comparison of CRF and sauvagine (Svg) analogs. A dash marks an identical amino acid residue. The ARAE motif of h/rCRF and the aligned corresponding stretches of residues of other CRF-like peptides are boxed. The site of the Glu/Ala switch is underlayed in gray. The bracket between Glu30 and Lys33 of the astressin (Ast) analogs depicts the lactam bridge connecting the side chains of Glu30 and Lys33. B, norleucine; Z, pyroglutamic acid; f, d-phenylalanine; ■, C-terminal amide.
The stretch of amino acid residues 22–25, Ala-Arg-Ala-Glu, of human/rat CRF (h/rCRF; Fig. 1), representing the ARAE motif (12), was found to be responsible for the high affinity of h/rCRF to CRFBP, in contrast to the low affinity of ovine CRF (oCRF) containing the sequence Thr-Lys-Ala-Asp instead (12). Interestingly, each residue of the ARAE sequence contributed to the high affinity of h/rCRF, as was demonstrated by single residue exchanges (12). Although sauvagine (Svg), another peptide of the CRF peptide family, binds to CRFBP with only low affinity, the Svg analog containing the ARAE sequence, instead of Glu21-Lys22-Gln23-Glu24, binds with high affinity (13). However, it has not yet been investigated whether all residues of the ARAE motif are important.
This task was undertaken here with the objective to develop strategies permitting synthesis of CRF analogs for CRFBP or CRFR without cross-reaction. We found that one residue of the ARAE motif served as a switch enhancing or preventing ligand binding to CRFBP. This knowledge can be applied to the development of peptidic CRF agonists and antagonists. The application to the development of an acidic analog of the CRF antagonist astressin (Ast) is described here.
The availability of CRF antagonists has been crucial for the investigation of the differential actions of CRFR on various biological functions. Whereas the CRF antagonist anti-Svg-30 (aSvg-30) (14) is selective for CRFR2 and useful in in vivo experiments because of its high solubility in cerebrospinal fluid (CSF), the antagonists α-hel-CRF9–41 (10) and Ast (15) are not selective, and bind with different affinities to CRFBP (13), CRFR1, and CRFR2. Additionally, Ast, which mainly binds to CRFR2 and CRFR1, is the least soluble of the three antagonists α-hel-CRF9–41, Ast, and aSvg-30 in artificial CSF (aCSF) (16). In this situation, it appeared especially important to develop a specific peptidic antagonist for CRFR1, whose significance in anxiety (17, 18), learning (4), and locomotion (19) is established. Because CRF receptor subtypes can be distinguished with differential procedures using aSvg-30 and Ast, we focused on enhancing the solubility of Ast by introduction of a Glu residue on the basis of the experiments identifying the affinity switch mentioned above.
Methods
Peptide Synthesis.
Peptides were synthesized and purified as described recently, and characterized with a BioIon-20 plasma desorption mass spectrometer (BioIon Nordic, Uppsala, Sweden) (13, 14).
Production of rCRFBP, rCRFR1, and mCRFR2β.
rCRFBP was produced in HEK-293 cells stably transfected with cDNA coding for rCRFBP C-terminally fused with a His6 sequence as described (13). rCRFR1 and mCRFR2β were obtained from membrane fractions prepared from HEK-293 cells stably transfected with cDNA coding for rCRFR1 and mCRFR2β, respectively (14).
Competition Binding Assay.
The utilization of a scintillation proximity assay (SPA) (20) by using lectin-coated neuropeptide γ receptor SPA beads (Amersham Pharmacia) for CRFR binding analysis was established in our laboratory (21).
Binding of CRF-like peptides to rCRFBP was carried out with an SPA in nickel-chelate-coated 96-well microtiter plates (Flash Plate PLUS, NEN), which bound rCRFBP tagged with a C-terminal His6 sequence (rCRFBP-His6). The scintillator beads carrying the Ni2+ ions for binding of the His tag were located at the inner surface of the wells of this microtiter plate. Unlabeled peptide (0 to maximal 3 μM), 0.1 nM radiolabeled ligand [125I-Tyr0]h/rCRF (2000 Ci/mmol; NEN; 1 Ci = 37 GBq) and cell culture medium containing rCRFBP-His6 (13) were mixed in a total volume of 200 μl of assay buffer (PBS, pH 7.5, and 0.02% nonionic detergent Nonidet P-40). The plates were sealed and incubated for 4 h at room temperature.
The radioactivities in the microtiter plates were measured in a Wallac 1450 Microbeta scintillation counter (Wallac, Gaithersburg, MD) by detection of the light emitted from the scintillator beads. In both SPA systems, the detection of the radioligand depended on its proximity to the beads containing the scintillator. When the radioligand receptor complex was bound to one bead by specific interaction of CRFR or CRFBP with the lectin (neuropeptide Y receptor SPA) or the Ni2+ ions (Flash Plate PLUS), respectively, light was emitted from the scintillator on excitation by the radiation of the radioligand. The major portion of counted radioactivity (>70%) represented specific binding that was because of radiolabeled ligand specifically bound to CRFBP or CRFR. In the same manner, radioligand nonspecifically bound to a bead could cause light emission which represented nonspecific binding (<30%). Binding data were analyzed by using the PRISM computer program (GraphPad, San Diego).
Measurement of Intracellular cAMP Accumulation.
The cells were stimulated as described (22) by using increasing concentrations of h/rCRF or Svg. Intracellular cAMP was measured with the Biotrak cAMP 125I SPA system (Amersham Pharmacia) according to the manufacturer's product manual.
Determination of the Maximum Solubility.
Peptides were dissolved in 10 mM acetic acid and mixed with 2× concentrated aCSF. The concentration of each peptide in 10 mM acetic acid was adjusted so that a pellet was observed after mixing with the same volume of 2× concentrated aCSF. The final concentration in the supernatant was determined by amino acid analysis, which was performed after hydrolysis of peptides (6 M HCl, 3 h, 150°C) in the presence of norleucine as internal standard with a Beckman HPLC Analyzer System 6300 (Beckman Coulter).
Isoelectric Focusing (IEF).
IEF was carried out with a Bio-Rad IEF cell system using Bio-Rad IEF strips (11 cm, pH range from 3 to 10). The system was cooled to 20°C. Calibration was performed with 80 μg of Sigma IEF mix 3.6–9.3 dissolved in 200 μl of water containing 0.2% Bio-Lyte 3/10 and 0.1% Nonidet P-40. Twenty-five micrograms of each peptide was dissolved in 200 μl of water containing 0.2% Bio-Lyte 3/10, 0.1% Nonidet P-40, and 10 mM DTT. IEF gels were placed in the chamber containing the peptide solution. The IEF gels were initially rehydrated for 12 h at 50 V. Focusing of the peptides was achieved by application of a linear voltage gradient starting at 250 V and reaching 8,000 V in 2.5 h. The gels were then exposed to 8,000 V for 4.5 h. Subsequently, the IEF gels were stained in the Bio-Rad IEF gel staining solution and destained in a mixture of 10% acetic acid/40% methanol/50% (vol/vol) water.
On-Line RP-HPLC-Mass Spectrometry.
On-line RP-HPLC-mass spectrometry (HPLC-MS) for the determination of the relative hydrophobicity of the CRF agonists and antagonists was performed as described (13). Mixtures of the agonists or antagonists were each separated in a single run. The peptides were identified by their molecular mass determined in the mass spectrometer.
Elevated Plus-Maze.
The elevated plus-maze behavior of C57BL/6J mice cannulated in the lateral ventricles (4) was investigated 15 min after injection of oCRF in the elevated plus-maze test (23). Peptide concentrations were determined by amino acid analysis (4). CRF antagonists in aCSF as vehicle or vehicle alone were injected 30 min before oCRF administration. The behavior of the mice was recorded by a video camera connected to a PC and analyzed by the Technical & Scientific Equipment software VIDEOMOT 2. The time spent, distance crossed, and number of entries in the open arms, closed arms, and center were recorded. The cannular placement was confirmed for each mouse by histological examination of the brains after methylene blue injection (4). The behavioral data were analyzed by t tests or ANOVA followed by post hoc Scheffé's test for multiple comparisons. Data are presented as mean ± SEM. The shift of preference from the open to the closed arms is interpreted as an increase of anxiety-like behavior. Locomotor activity is determined with this assay by the distance traveled.
Results
Pharmacological Relevance of Residue 22 of h/rCRF.
A high throughput binding assay based on the SPA principle (20) was developed for recombinant rCRFBP by employing the affinity of the His tag of CRFBP to Ni2+ ions. This assay did not require a separation of bound and free radioligand, in contrast to the charcoal precipitation assay used earlier to characterize the pharmacological profile of rCRFBP (13). The SPA was validated through the known rank order of affinity of different CRF-like peptides as determined to be h/rCRF ≥ α-hel-CRF9–41 ≫ Svg > Ast > oCRF ≫ aSvg-30 (Table 1). This order was in agreement with results obtained with the charcoal precipitation assay (13).
Table 1.
Binding of various CRF-like agonists and antagonists to rCRFBP and CRFR subtypes
| Peptide | IC50,
nM
|
||
|---|---|---|---|
| rCRFBP | rCRFR1 | mCRFR2β | |
| h/rCRF | 0.54 (0.38–0.71) | 1.6 (1.3–1.9) | 42 (25–59) |
| [Glu22]h/rCRF | 80 (70–90) | 0.61 (0.44–0.79) | 16 (15–18) |
| Svg | 57 (45–70) | 0.52 (0.29–0.74) | 0.92 (0.72–1.1) |
| [Ala21,23, Arg22]Svg | 0.84 (0.66–1.0) | 0.29 (0.06–0.51) | 0.82 (0.56–1.1) |
| [Ala21]Svg | 0.94 (0.60–1.3) | 0.32 (0.15–0.49) | 1.1 (0.89–1.3) |
| oCRF | 470 (420–530) | 1.0 (0.65–1.4) | 200 (110–300) |
| Ast | 90 (76–104) | 11 (7.7–14) | 5.2 (2.6–7.8) |
| [Glu11]Ast | NB | 1.4 (0.94–1.9) | 0.58 (0.54–0.63) |
| [Glu11,16]Ast | NB | 3.3 (2.8–3.7) | 1.1 (0.87–1.3) |
| aSvg–30 | NB | 370 (330–400) | 0.30 (0.24–1.36) |
| α-hel-CRF9–41 | 1.0 (0.9–1.0) | 61 (52–69) | 4.3 (3.3–5.3) |
IC50 values are the mean of at least four experiments performed in duplicate; 95% confidence intervals are given in parentheses; NB, no specific binding with up to 3 μM inhibitor.
rCRFBP bound h/rCRF (IC50 = 0.54 nM) and [Ala24,23,Arg22]Svg (IC50 = 0.84 nM), which contained the amino acid residues of the ARAE motif of h/rCRF, with indistinguishable affinities in contrast to the lower affinity of Svg (IC50 = 57 nM; Fig. 2; Table 1). When the relative hydrophobicities (24) and the conformational preferences (25) of amino acid residues 22–24, Ala-Arg-Ala, of h/rCRF and 21–23, Glu-Lys-Gln, of Svg were compared (Table 2), it was observed that all residues shared a high propensity for the formation of α-helical secondary structures. Major differences were found for residues Ala24 of h/rCRF and Gln23 of Svg, which differed significantly in their relative hydrophobicity. Residues Ala22 of h/rCRF and Glu21 of Svg differed in their relative hydrophobicity and in the net charge of their side chains. Therefore, it was hypothesized that amino acid residue Ala22 of h/rCRF and the corresponding residue Glu21 of Svg were responsible for the affinity difference of these peptides to CRFBP. This hypothesis was tested by the synthesis of [Glu22]h/rCRF and [Ala21]Svg (Fig. 1) and subsequent analysis of their binding to rCRFBP. The affinity of [Glu22]h/rCRF (IC50 = 80 nM) was decreased by two orders of magnitude compared with h/rCRF. Similarly, the affinity of [Ala21]Svg (IC50 = 0.94 nM) compared with Svg was enhanced by two orders of magnitude (Fig. 2). The binding affinities of Svg and [Ala21]Svg to both CRFR subtypes did not deviate significantly from one another, whereas the binding affinity of [Glu22]h/rCRF compared with that of h/rCRF was slightly enhanced (Table 1). However, the selectivity of h/rCRF favoring CRFR1 by a factor of ≈30 was not altered.
Figure 2.
Competitive binding of the CRF and Svg analogs to rCRFBP. Binding curves were normalized by total binding in the absence of competitor (B0). Data points represent pooled data from at least four independent experiments.
Table 2.
Relative hydrophobicities and conformational preferences of the differing amino acids of the ARAE motif in h/rCRF and the corresponding stretch in Svg
| Equivalent
residue
|
Rel. hydrophobicity
|
Secondary
structure propensities
|
||
|---|---|---|---|---|
| h/rCRF/Svg | h/rCRF/Svg | α-Helix (Pα) h/rCRF/Svg | β-Structure (Pβ) h/rCRF/Svg | Turn structure (Pt) h/rCRF/Svg |
| Ala22/Glu21 | 0.25/−0.62 | 1.41/1.59 | 0.72/0.52 | 0.82/1.01 |
| Arg23/Lys22 | −1.8/−1.1 | 1.21/1.23 | 0.84/0.69 | 0.90/1.07 |
| Ala24/Gln23 | 0.25/−0.69 | 1.41/1.27 | 0.72/0.98 | 0.82/0.84 |
A similar modification as described for h/rCRF was carried out for Ast. The acidic peptide [Glu11]Ast (Fig. 1) obtained was analogous to [Glu22]h/rCRF as Ast is to h/rCRF. As expected from the pharmacological profile of [Glu22]h/rCRF, no detectable specific binding of [Glu11]Ast to rCRFBP was found. The affinity of [Glu11]Ast to both CRFR subtypes was increased by one order of magnitude compared with the affinity of Ast (Table 1). The antagonistic properties of Ast and [Glu11]Ast were obtained by determination of the relative intrinsic activity and potency by using either CRFR subtype (Table 3). No significant differences between Ast and [Glu11]Ast were found (Table 3). To facilitate comparison of the most frequently used antagonists, α-hel-CRF9–41 and aSvg-30 were included in the study.
Table 3.
Relative intrinsic activities and relative potencies of various CRF-like antagonists
| Antagonist | Rel. intrinsic activity,*
%
|
Rel. potency of
antagonist,† %
|
||
|---|---|---|---|---|
| rCRFR1 | mCRFR2β | rCRFR1 | mCRFR2β | |
| Ast | 9.8 (9.3–10.3) | 1.1 (0.9–1.2) | 86 (85–87) | 99 (99–98) |
| [Glu11]Ast | 8.4 (7.9–8.8) | 0.84 (0.67–1.0) | 86 (85–86) | 98 (99–98) |
| [Glu11,16]Ast | 9.1 (8.4–9.4) | 1.0 (0.76–1.3) | 83 (82–85) | 97 (96–97) |
| aSvg-30 | 6.5 (6.1–6.8) | 1.0 (0.9–1.1) | 2.0 (0–6.9) | 99 (98–99) |
| α-hel-CRF9–41 | 19 (18–20) | 1.4 (1.2–1.5) | 14 (13–15) | 97 (96–97) |
The intrinsic activity was determined by the stimulation of rCRFR1-HEK-293 cells and mCRFR2β-HEK-293 cells with 10 pM h/rCRF and Svg, respectively. Rel. intrinsic activity (%) = [cAMP (1 μM antagonist)/cAMP (10 nM agonist)] × 100.
Rel. potency (%) = 100 − [cAMP (1 nM agonist + 1 μM antagonist)/cAMP (1 nM agonist)] × 100. Numbers in parentheses are 95% confidence intervals.
Solubility and in Vivo Potency of Ast Analogs.
The in vivo potency of the antagonists was assayed by intracerebroventricular (i.c.v.) injection of mice and subsequent behavioral analysis in the elevated plus-maze (26) for anxiety-like behavior and locomotor activity, two behavioral responses modulated by CRF (3).
It had been earlier observed that Ast preinjected i.c.v. did not prevent oCRF-induced changes of the mice's behavior in the elevated plus-maze (16). This failure of action was attributed to the limited solubility of Ast in aCSF (16).
The maximum solubility of [Glu11]Ast (cmax = 125 μM) in aCSF was found to be 16 times higher than that of Ast (Table 4). Because the replacement of a hydrophobic residue by a Glu residue enhanced the solubility, we considered increasing the solubility even more by an additional Glu residue. Therefore, Leu16 of Ast was replaced by Glu corresponding to the Svg sequence (Fig. 1). The resulting acidic Ast analog [Glu11,16]Ast (cmax = 290 μM) showed a 40 times higher maximum solubility in aCSF compared with Ast (Table 4). However, [Glu11]Ast, which was much more soluble at physiological pH than Ast, appeared to be less charged than Ast based on IEF measurements.
Table 4.
RP-HPLC retention time, isoelectric point, and maximum solubility (cmax) in aCSF of CRF-like agonists and antagonists
| Peptide | Retention time*t, min:sec | Isoelectric point,† pH | cmax, μM |
|---|---|---|---|
| Svg | 39:12 | 5.1 | ND |
| [Glu22]h/rCRF | 41:41 | 5.6 | ND |
| [Ala21]Svg | 42:32 | 7.4 | ND |
| oCRF | 42:51 | 6.4 | ND |
| h/rCRF | 47:51 | 5.9 | ND |
| aSvg-30 | 19:51 | >9.5 | >4,000‡ |
| [Glu11,16]Ast | 29:24 | 6.2 | 290 |
| [Glu11]Ast | 30:25 | 7.0 | 120 |
| Ast | 35:17 | 8.9 | 8 |
| α-hel-CRF9–41 | 42:41 | 5.3 | 1,480‡ |
ND, not determined.
Retention time for the antagonists and the agonists were determined in separate HPLC runs and cannot be compared.
Isoelectric points were determined by IEF.
Brauns et al. (16).
[Glu11,16]Ast was bound by both CRFR subtypes with higher affinity than Ast (IC50 = 3.3 nM for rCRFR1 and IC50 = 1.1 nM for mCRFR2β). No specific binding of [Glu11,16]Ast to rCRFBP was detectable.
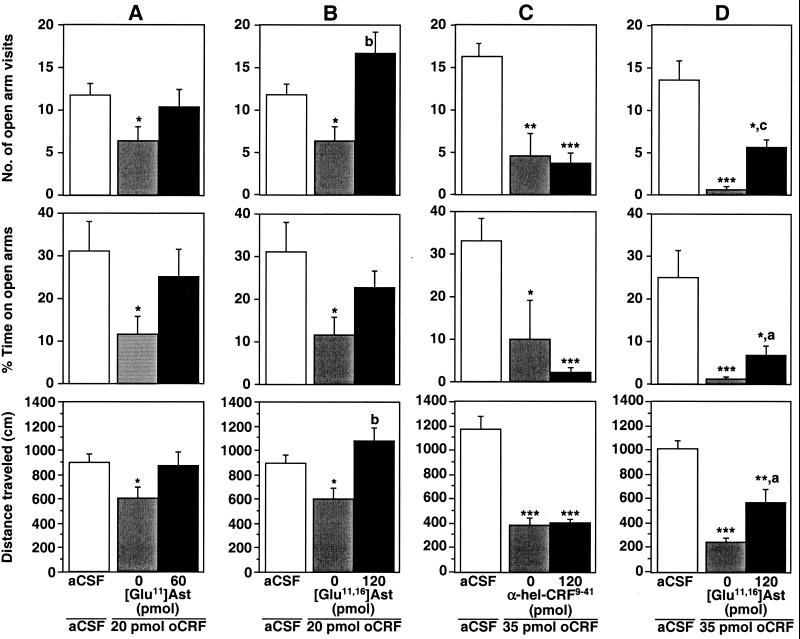
In the behavior experiments, oCRF was used because of its preference for CRFR1 (27). It was observed that locomotion of mice was markedly decreased after i.c.v. injection of 20 pmol (90 ng) of oCRF, as indicated by the total distance crossed within the tested time period (F2,21 = 3.0, P < 0.05; Fig. 3). Similarly, anxiety-like behavior was enhanced, as indicated by the reduced number of open arm visits (F2,21 = 2.6, P < 0.05) and the percentage of time spent in the open arms of the maze (F2,21 = 2.7, P < 0.05; Fig. 3). These behavior effects were significantly enhanced by the injection of 35 pmol (170 ng) of oCRF (F2,21 = 31.2, P < 0.001; F2,21 = 17.7, P < 0.001; F2,21 = 8.8, P < 0.001; Fig. 3). When 60 pmol (230 ng) of [Glu11]Ast was preinjected before the application of 20 pmol of oCRF, the oCRF-induced behavior changes were prevented (Fig. 3A). However, the dose of [Glu11]Ast was not sufficient to generate a significant difference between the behavior responses elicited by oCRF alone and oCRF in the presence of antagonist. A higher concentration of [Glu11]Ast could not be used because of solubility limitations. The introduction of the second Glu residue to generate [Glu11,16]Ast increased the solubility as mentioned above (Table 4) and permitted the employment of higher doses. It was observed that the number of open-arm visits and traveled distances of the mice treated with 20 pmol of oCRF and the mice treated with 20 pmol of oCRF and 120 pmol of [Glu11,16]Ast differed significantly from one another (Fig. 3B). The same dose of [Glu11,16]Ast injected before the application of 35 pmol of oCRF significantly attenuated the oCRF-induced behavioral effects in the plus-maze test (Fig. 3D). In contrast, no significant change of the oCRF-induced behavior effects was found when 120 pmol (450 ng) of α-hel-CRF9–41 was preinjected before the application of 35 pmol of oCRF (Fig. 3C).
Figure 3.
Potency of the antagonists [Glu11]Ast and [Glu11,16]Ast in the plus-maze behavior of C57BL/6J mice. The antagonists and agonists were injected i.c.v. 30 and 15 min, respectively, before exposure to the elevated plus-maze for 5 min. (A) Sixty picomoles (230 ng) of [Glu11]Ast, 20 pmol (90 ng) of oCRF; (B) 120 pmol (430 ng) of [Glu11,16]Ast, 20 pmol of oCRF; (C) 120 pmol (450 ng) of α-hel-CRF9–41, 35 pmol (170 ng) of oCRF; (D) 120 pmol of [Glu11,16]Ast, 35 pmol of oCRF. Statistically significant differences were determined by t tests: *, P < 0.05 vs. aCSF; **, P < 0.01 vs. aCSF; ***, P < 0.001 vs. aCSF; a, P < 0.05 vs. oCRF; b, P < 0.01 vs. oCRF; c, P < 0.001 vs. oCRF.
Discussion
The ligand requirements of CRFBP and CRFR are significantly different. It has been demonstrated that the central stretch of residues 6–33 of h/rCRF is sufficient for high-affinity binding to CRFBP, but not to CRFR (11, 12). Svg and h/rCRF differ in this central part of 28 residues by 16 residues. Most importantly, the exchange of Ala22 of h/rCRF by the corresponding Glu21 of Svg switched off the high affinity binding to CRFBP without decreasing the affinity to CRFR. Consistently, the moderate affinity of Ast to rCRFBP was abolished by the introduction of Glu in the corresponding position.
The acidic Ast analog [Glu11,16]Ast, but also [Glu11]Ast (Fig. 1), did not show specific binding to rCRFBP, whereas the affinity to either CRFR subtype was increased (Table 1). By the introduction of the two Glu residues into the Ast sequence to generate [Glu11,16]Ast, the preference of Ast for rCRFR1 over rCRFBP was enhanced by a factor of more than 100.
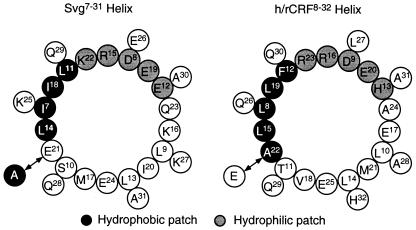
By using CD (28) and NMR (29, 30) spectroscopic methods, evidence has been provided that CRF forms an amphiphilic helix whose hydrophobic patch binds to hydrophobic surfaces (31). On the basis of the helical wheel diagrams for the central parts of the peptides Svg (28) and h/rCRF (Fig. 4), it is suggested that residues 21 and 22, respectively, were part of a hydrophobic patch composed of residues Ala22, Leu15, Leu8, Leu19, and Phe12 of h/rCRF (Fig. 4). It was demonstrated by CD that α-helical structures of h/rCRF and α-hel-CRF9–41 are involved in binding to CRFBP (32). In view of the crucial role of Ala22 for binding to CRFBP, we concluded that the hydrophobic patch may be important for binding to CRFBP. Consistently, Ala24 located on the opposite site of the helical wheel of h/rCRF was found to be not important for binding to CRFBP. Interestingly, residues 8, 12, 15, and 19 of the hydrophobic patch are highly conserved throughout the CRF peptide family. A greater variability was found for residue 22, which is replaced by Ser, Thr, and Glu. The remarkable homology of the hydrophobic patch in urocortin and Svg, which are only homologous to an extent of 44% with h/rCRF, underlines its importance.
Figure 4.
Helical wheel diagrams showing the internal amphiphilic helices of Svg and h/rCRF.
On the basis of the importance of the hydrophobic patch of h/rCRF for high-affinity binding to CRFBP, it was speculated that the crucial contribution of Ala22 in h/rCRF resulted from a direct hydrophobic interaction of the small Ala side chain with the ligand-binding site of CRFBP. This interaction may have been disturbed by the charged bulky Glu residue in [Glu22]h/rCRF as indicated by its lower affinity, whereas it may have been facilitated in [Ala21]Svg as demonstrated by the latter peptide's high affinity to CRFBP. This view is consistent with the observation that replacement of Thr22 of oCRF by an Ala residue enhanced the affinity by one order of magnitude (12). The affinity difference of two orders of magnitude between Svg and h/rCRF depended mainly on the exchange of a single amino acid, of Glu for Ala or vice versa.
From the results of IEF (Table 4), it was concluded that Ast was positively charged in aCSF, whereas [Glu11]Ast and [Glu11,16]Ast carried a negative charge. In view of the observation that the isoelectric points of [Glu11]Ast and [Glu11,16]Ast were closer to the physiological pH than the isoelectric point of Ast, the solubility of these peptides obviously did not depend mainly on electric charges. It was more likely that it depended on an increasing hydrophilicity of the side chains which was detected even under the acidic conditions of HPLC-MS (Table 4).
[Glu11,16]Ast was successfully used to prevent the oCRF-induced enhancement of anxiety-like behavior and decrease of locomotor activity of the mouse in the elevated plus-maze. The behavioral effects of [Glu11,16]Ast were probably mediated by CRFR1 in view of the observations that oCRF binds preferentially to CRFR1 (27) and that activation of CRFR1 in a novel environment results in reduction of locomotor activity (19). Alternatively, α-hel-CRF9–41 has been used for i.c.v. injection to inhibit CRFR1-mediated effects (33). Usually, doses of 260 pmol to 1.3 nmol (1 μg to 5 μg) of α-hel-CRF9–41 per mouse have been used to inhibit CRFR1-mediated effects such as reduction of the locomotor activity in the elevated plus-maze (33, 34). These reports are in agreement with our finding that 120 pmol of α-hel-CRF9–41, representing a 3-fold molar excess of the oCRF dose used, did not prevent the oCRF-induced behavior changes. In contrast, a dose of 120 pmol (430 ng) of [Glu11,16]Ast was sufficient to inhibit significantly the behavioral effects of oCRF in the elevated plus-maze. The increased in vivo potency of [Glu11,16]Ast was facilitated by the markedly higher affinity to CRFR1 compared with Ast and α-hel-CRF9–41 in combination with the lack of detectable specific binding to CRFBP. Thereby, an absorption of the antagonist by binding to CRFBP and, in addition, release of endogenous CRF from CRFBP by the antagonist was prevented.
Acknowledgments
We thank Thomas Liepold for amino acid analysis and Christina Schrick for expert technical help. For running the plasma desorption mass spectra we thank Dr. Bodo Zimmermann. Dr. Olaf Brauns, Dr. Bernhard Hofmann, and Dr. Sabine Sydow (Schering) are gratefully acknowledged for their helpful discussions.
Abbreviations
- CRF
corticotropin-releasing factor
- h/rCRF
human/rat CRF
- oCRF
ovine CRF
- CRFR
CRF receptor
- rCRFR
rat CRFR
- CRFBP
CRF binding protein
- rCRFBP
rat CRFBP
- Svg
sauvagine
- aSvg-30
anti-sauvagine-30
- Ast
astressin
- α-hel-CRF9–41
α-helical CRF9–41
- CSF
cerebrospinal fluid
- aCSF
artificial CSF
- IEF
isoelectric focusing
- i.c.v.
intracerebroventricular(ly)
- SPA
scintillation proximity assay
References
- 1.Spiess J, Rivier J, Rivier C, Vale W. Proc Natl Acad Sci USA. 1981;78:6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vale W, Spiess J, Rivier C, Rivier J. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 3.Eckart K, Radulovic J, Radulovic M, Jahn O, Blank T, Stiedl O, Spiess J. Curr Med Chem. 1999;6:1035–1053. [PubMed] [Google Scholar]
- 4.Radulovic J, Rühmann A, Liepold T, Spiess J. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiess J, Dautzenberg F M, Sydow S, Hauger R L, Rühmann A, Blank T, Radulovic J. Trends Endocrinol Metab. 1998;9:140–145. doi: 10.1016/s1043-2760(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 6.Perrin M H, Vale W W. Ann NY Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 7.Potter E, Behan D P, Fischer W H, Linton E A, Lowry P J, Vale W W. Nature (London) 1991;349:423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- 8.Kemp C F, Woods R J, Lowry P J. Peptides. 1998;19:1119–1128. doi: 10.1016/s0196-9781(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 9.Karolyi I J, Burrows H L, Ramesh T M, Nakajima M, Lesh J S, Seong E, Camper S A, Seasholtz A F. Proc Natl Acad Sci USA. 1999;96:11595–11600. doi: 10.1073/pnas.96.20.11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivier J, Rivier C, Vale W W. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- 11.Behan D P, Heinrichs S C, Troncoso J C, Liu X J, Kawas C H, Ling N, De S E B. Nature (London) 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 12.Sutton S W, Behan D P, Lahrichi S L, Kaiser R, Corrigan A, Lowry P, Potter E, Perrin M H, Rivier J, Vale W W. Endocrinology. 1995;136:1097–1102. doi: 10.1210/endo.136.3.7867564. [DOI] [PubMed] [Google Scholar]
- 13.Jahn O, Eckart K, Sydow S, Hofmann B A, Spiess J. Peptides. 2001;22:47–56. doi: 10.1016/s0196-9781(00)00356-9. [DOI] [PubMed] [Google Scholar]
- 14.Rühmann A, Bonk I, Lin C J R, Rosenfeld M G, Spiess J. Proc Natl Acad Sci USA. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulyas J, Rivier C, Perrin M, Koerber S C, Sutton S, Corrigan A, Lahrichi S L, Craig A G, Vale W W, Rivier J. Proc Natl Acad Sci USA. 1995;92:10575–10579. doi: 10.1073/pnas.92.23.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brauns O, Liepold T, Radulovic J, Spiess J. Neuropharmacology. 2001;41:507–516. doi: 10.1016/s0028-3908(01)00094-6. [DOI] [PubMed] [Google Scholar]
- 17.Smith G, Aubry J-M, Dellu F, Contarino A, Bilezikjian L, Gold L, Chen R, Marchuk Y, Hauser C, Bentley C, et al. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 18.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul J, Stalla G K, Blanquet V, Steckler T, Holsboer F, Wurst W. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 19.Contarino A, Dellu F, Koob G, Smith G, Lee K, Vale W, Gold L. Endocrinology. 2000;141:2698–2702. doi: 10.1210/endo.141.7.7653. [DOI] [PubMed] [Google Scholar]
- 20.Udenfriend S, Gerber L D, Brink L, Spector S. Proc Natl Acad Sci USA. 1985;82:8672–8676. doi: 10.1073/pnas.82.24.8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann, A., Sydow, S., Jahn, O., van Werven, L., Liepold, T., Eckart, K. & Spiess, J. (2001) Prot. Sci.10, in press. [DOI] [PMC free article] [PubMed]
- 22.Sydow S, Radulovic J, Dautzenberg F M, Spiess J. Mol Brain Res. 1997;52:182–193. doi: 10.1016/s0169-328x(97)00256-8. [DOI] [PubMed] [Google Scholar]
- 23.Radulovic J, Kammermeier J, Spiess J. Behav Brain Res. 1998;95:179–189. doi: 10.1016/s0166-4328(98)00039-4. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberg D. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 25.Williams R W, Chang A, Juretic D, Loughran S. Biochim Biophys Acta. 1987;916:200–204. doi: 10.1016/0167-4838(87)90109-9. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto T, Pearse R V, Lin C R, Rosenfeld M G. Proc Natl Acad Sci USA. 1995;92:1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behan D P, Grigoriadis D E, Lovenberg T, Chalmers D, Heinrichs S, Liaw C, De Souza E B. Mol Psychiatry. 1996;1:265–277. [PubMed] [Google Scholar]
- 28.Pallai P V, Mabilia M, Goodman M, Vale W, Rivier J. Proc Natl Acad Sci USA. 1983;80:6770–6774. doi: 10.1073/pnas.80.22.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romier C, Bernassau J, Cambillau C, Darbon H. Protein Eng. 1993;6:149–156. doi: 10.1093/protein/6.2.149. [DOI] [PubMed] [Google Scholar]
- 30.Dathe M, Fabian H, Gast K, Zirwer D, Winter R, Beyermann M, Schumann M, Bienert M. Int J Peptide Protein Res. 1996;47:383–393. doi: 10.1111/j.1399-3011.1996.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 31.Lau S H, Rivier J, Vale W, Kaiser E T, Kézdy F J. Proc Natl Acad Sci USA. 1983;80:7070–7074. doi: 10.1073/pnas.80.23.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowry P J, Koerber S C, Woods R J, Baigent S, Sutton S, Behan D P, Vale W, Rivier J. J Mol Endocrinol. 1996;16:39–44. doi: 10.1677/jme.0.0160039. [DOI] [PubMed] [Google Scholar]
- 33.Kishimoto T, Radulovic J, Radulovic M, Lin C R, Schrick C, Hooshmand F, Hermanson O, Rosenfeld M G, Spiess J. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 34.Stenzel-Poore M P, Heinrichs S C, Rivest S, Koob G F, Vale W W. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]