Abstract
Darunavir/ritonavir (DRV/r) is a second-generation protease inhibitor used in treatment-naïve and -experienced HIV-positive adult patients. To evaluate efficacy and safety in these patient settings, we performed a meta-analysis of randomized controlled trials. We considered eight studies involving 4240 antiretroviral treatment (ART)-naïve patients and 14 studies involving 2684 ART-experienced patients. Regarding efficacy in the ART-naive patients, the virological response rate was not significantly different between DRV/r and the comparator. For the ART-experienced failing patients, the virological response rate was significantly higher with DRV/r than with the comparator (RR 1.45, 95% CI: 1.01–2.08); conversely, no significant differences were found between the treatment-experienced and virologically controlled DRV/r and comparator groups. Regarding safety, the discontinuation rates due to adverse events (AEs) and DRV/r-related serious adverse events (SAEs) did not significantly differ from the rates in the comparator group (RR 0.84, 95% CI: 0.59–1.19 and RR 0.78, 95% CI: 0.57–1.05, respectively). Our meta-analysis indicated that DRV/r-based regimens were effective and tolerable for both types of patients, which was consistent with published data.
Introduction
Darunavir (DRV; TMC114; Prezista®) is a second-generation non-peptidomimetic protease inhibitor (PI) that was approved in 2007 in Italy for use in combination with ritonavir booster (DRV/r). DRV is used in combination with other antiretroviral (ARV) drugs for the treatment of human immunodeficiency virus (HIV) type 1 infection at two dosage regimens [800 mg once daily (OD) and 600 mg twice daily (both co-administered with ritonavir)]1,2. These regimens allow treatment of the entire setting of HIV-positive patients, from treatment-naive to highly experienced subjects and even those harboring HIV resistance mutations3.
The efficacy and tolerability of DRV/r have been evaluated in registrative randomized controlled clinical trials (RCT) in treatment-naïve4,5 and treatment-experienced6–9 patients with HIV-1 infection, with documented long-term efficacy and tolerability7,10–12. These results have been confirmed by real world evidence from observational studies13.
A once-daily co-formulation of DRV 800 mg plus a new booster, cobicistat 150 mg (Rezolsta®), is currently available. This fixed-dose combination (FDC) allows replacement of ritonavir as a booster for the treatment of both naïve and treatment-experienced adults14. The safety and efficacy of a single tablet regimen (STR) of darunavir/cobicistat/tenofovir alafenamide/emtricitabine (D/C/F/TAF) is being evaluated in two large phase III trials in treatment-naive and virologically suppressed patients (NCT02431247 and NCT02269917, respectively). The results of studies using cobicistat as a booster for darunavir showed no difference in efficacy from the use of ritonavir as a booster; therefore, the results of the present meta-analysis can be considered of interest even in this changing environment.
Current Italian15 (with some restrictions), European16, British17 and DHHS18 HIV/AIDS guidelines recommend the use of darunavir boosted with ritonavir or cobicistat as the only boosted protease inhibitor (bPI) (alongside other options, including integrase inhibitors and rilpivirine) as one preferred third agent in addition to a nucleoside reverse transcriptase inhibitor backbone, including tenofovir fumarate or tenofovir alafenamide and emtricitabine18.
Hence, the primary purpose of the present meta-analysis was to evaluate the efficacy, safety and tolerability of DRV/r-based regimens for treatment-naive HIV-1-infected patients or ART-experienced patients using reported RCTs.
Results
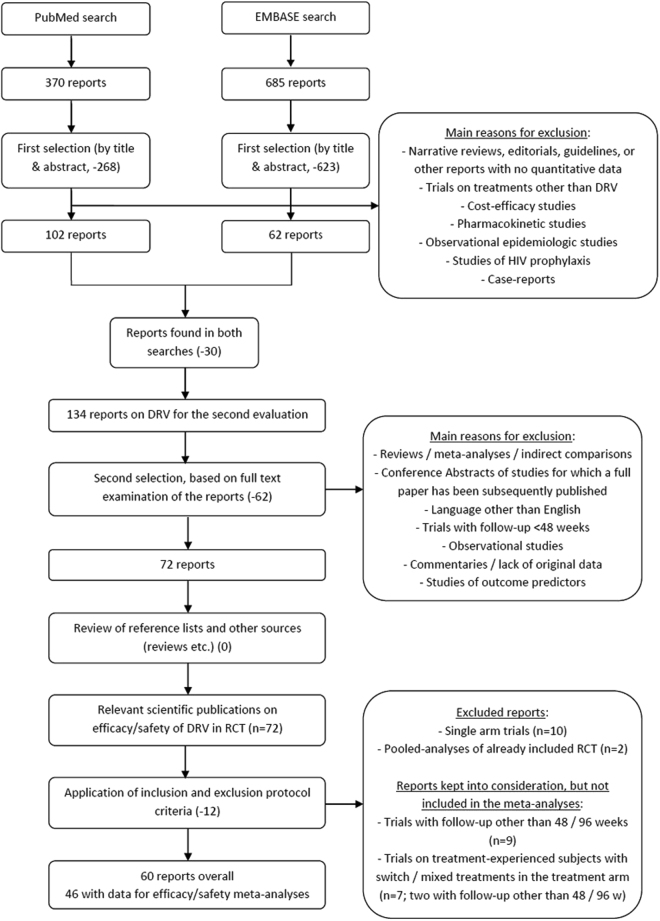
A search of electronic medical databases retrieved a total of 1055 articles. After title and abstract screening, we excluded 891 articles mainly because the authors did not report original data (i.e., narrative reviews, editorials, guidelines, or case reports) or the studies were designed as pharmaco-economic evaluations. After removal of duplicates using the Endnote X7 software, 134 articles on DRV were considered in-depth, and all full texts were downloaded and screened for final inclusion. After cross-checking for additional potentially missed references, 46 original articles with data on efficacy and safety were included in the present meta-analysis (Fig. 1). We considered three groups of studies based on the features of the enrolled patients: ART-naïve, ART-experienced failing and ART-experienced virologically controlled subjects. From a statistical perspective, we considered only studies with 48 and 96 weeks of follow-up (FU) to obtain sufficient subjects to conduct a meta-analysis. The main characteristics of the design and the baseline characteristics of the enrolled patients in the studies included in this analysis are summarized in Table 1 (ART-naïve adult patients) and Table 2 (ART-experienced adult patients). The results of the individual study quality assessments are reported and summarized in Supplementary Table 1. The study protocols were obtained where available to assess selective outcomes reports. The included studies achieved adequate sequence generation, but allocation concealment was not reported in all studies. All studies reported statistical analyses of the outcomes and addressed any incomplete data, such as loss to follow-up. All RCTs included were open-label; therefore, the two domains of performance bias and attrition bias were deemed to have a high risk of bias (Supplementary Table 1).
Figure 1.
Flow-chart describing the literature search and study selection processes.
Table 1.
Main characteristics of trials considering ART-naïve adult patients.
| Trial | Reference(s) | Enrollment period | Geographic area | No. of enrolled subjects (treated:control) | Patient characteristics at baseline: -Median/mean age - Cutoff for plasma viral load (copies/ml) - Cutoff for CD4 cell count |
Duration of follow-up (weeks) | DRV group regimen | Control group regimen |
|---|---|---|---|---|---|---|---|---|
| ART - naïve patients (8 studies ; 4568 total patients enrolled) | ||||||||
| ACTG 5257 | Lennox JL37, Ofotokun I43 |
2009–2011 | US & Puerto Rico | 1809 (601:605:603) | -37 y (median) - pVL > 1000 -CD4 not limited | 96 | DRV/r (800 mg/d) |
Two groups: 1) ATV/r 2) RAL |
| ARTEMIS | Ortiz R4 | 2005–2008 | US, UK, Thailand, Argentina, France, Australia | 689 (343:346) |
-36 y (mean) in DRV/r and 35 (mean) in LPV/r -pVL ≥ 5000 -CD4 not limited |
48 | DRV/r (800 mg/d) |
LPV/r |
| Mills AM5 | 96 | |||||||
| Lathouwers E12 | 96 | |||||||
| Orkin C10 | 192 | |||||||
| ATADAR | Martinez E44 | 2011 | Spain | 178 (88:90) | -35 y (mean) treat vs 37 y (mean) control - pVL ≥ 1000 -CD4 not limited |
96 | DRV/r (800 mg/d) |
ATV/r |
| FLAMINGO | Clotet B20 | 2011–2012 | Europe, US and South America | 484 (242:242) |
-Adult -34 y (median age) - pVL > 1000 - CD4 not limited |
48 | DRV/r (800 mg/d) |
DTG |
| Molina JM45 | 96 | |||||||
| IMEA 040 DATA trial | Slama L19 | 2011–2013 | France | 120 (61:59) | -Adult -43 y (median) - pVL > 1000 - CD4 < 200 |
48 | DRV/r (800 mg/d) |
ATV/r |
| METABOLIK | Aberg JA46 | NA | US | 65 (34:31) | -36.5 y (median age) in the study group and 35.0 y in the control group -pVL > 1000 - CD4 not limited |
48 | DRV/r (800 mg/d) |
ATV/r |
| NEAT001/ ANRS143 |
Raffi F47 | 2010–2011 | Europe | 805 (401:404) |
-37 y (median age) in the RAL group and 39 y (median) in the TDF-FTC group -pVL > 1000 - CD4 < 500 |
96 | DRV/r (800 mg/d) + TDF/FTC | RAL + DRV/R (800 mg/d) |
| OPTIPRIM-ANRS 147 | Chéret A48 | 2010–2011 | France | 90 (45:45) |
-35 y (median age) - pVL not limited - CD4 < 500 |
96 | DRV/r (800 mg/d) + TDF/FTC | DRV/r (800 mg/d) + RAL/MVC + TDF/FTC |
Table 2.
Main characteristics of trials considering ART treatment-experienced adult patients.
| Trial | Reference(s) | Enrollment period | Geographic area | No. of enrolled subjects (treated:control) | Reason for discontinuation of earlier treatments | Patient characteristics at baseline: - Median/mean age - Median/mean time since treatment started - Cutoff for plasma viral load (copies/ml) - Cutoff for CD4 cell count |
Duration of follow-up (weeks) | DRV group regimen | Control group regimen |
|---|---|---|---|---|---|---|---|---|---|
| Treatment-experienced failing subjects, DRV 600 mg BID (3 studies; 1440 total patients enrolled) | |||||||||
| ODIN | Cahn P8 | NA | North, Central and South America, Europe, Australia and Asia | 590 (294:296) |
Treatment simplification | -40.2 y (mean age) in the study group and 40.7 y (mean) in the control group - pVL > 1000 - CD4 < 50 |
48 | DRV/r (600 mg BID) | DRV/r (800 mg OD) |
| POWER (1–2) |
Clotet B22 | 2005 | Multicentric | 255 (131:124) |
Increase in drug resistance | -43.9 y (mean age) in the study group and 44.4 y (mean) in the control group - pVL > 1000 - at least one primary PI mutation |
48 | DRV/r (4 dosages; only 600 mg BID was included in the meta-analysis) | Control PI |
| TITAN | Madruga JV9 | 2005–2007 | Multicentric | 595 (298:297) |
DRV experienced in the border range | - 40 y (mean age) - 9.1 y (mean duration of infection) - pVL > 1000 - CD4 not limited |
48 | DRV/r (600 mg BID) + OBR | LPV/r + OBR |
| Banhegyi D11 | 96 | ||||||||
| Treatment-experienced, virologically controlled subjects, DRV 800 mg/d (11 studies; 1046 total patients enrolled) | |||||||||
| 2PM STUDY | Gianotti N49 | 2013–2014 | Italy | 43 (15:13:15) |
NA | -Adult - 46 y (median age) - pVL < 50 - CD4 > 200 |
48 | DRV/r (800 mg) |
1) LPV/r 2) Triple |
| DRIVESHAFT | Huhn GD50 | NA | NA | 60 (30:30) |
NA | - median age and previous ART duration are NA - pVL < 40 - CD4 not limited |
48 | DRV/r (800 mg OD) |
DRV/r (600 mg BID) |
| DRV600 | Moltó J51 | 2012–2013 | Spain | 100 (50:50) |
NA | - 45.2 y (mean age) - 8.5 y (mean time since diagnosis) - pVL < 50 - CD4 not limited |
48 | DRV/r (800 mg) |
DRV/r (600 mg) |
| LOPIDAR | Santos J R52 | NA | Spain | 75 (40:33) |
Treatment simplification | - 43 y (median age) - 108 w (median HIV diagnosis) - pVL < 50 CD4nadir > 100 |
48 | DRV/r (800 mg) | LPV/r |
| MIDAS | Hamzah L53 | NA | NA | 64 (32:32) |
Side effects | -age NA - pVL < 50 - CD4 not limited |
48 | DRV/r (800 mg) | TDF/FTC/EFV |
| MONARCH | Guaraldi G54 | NA | Italy | 30 (15:15) |
NA | - 45 y (median age) in the study group and 43 y (median) in the control group -pVL < 50 - CD4 > 200 - CD4nadir > 100 |
48 48 |
DRV/r (800 mg) + NRTIs (triple) |
DRV/r (800 mg) monotherapy |
| MONET | Arribas JR28 | 2007–2008 | Europe | 256 (127:129) |
NA | - 44 y (median age) - 7.4 y (mean) in art in the study group and 5.9 y in the control group - pVL < 50 - CD4 > 200 |
48 | DRV/r (800 mg) + NRTIs | DRV/r (800 mg) monotherapy |
| Clumeck N55 | 96 | ||||||||
| PROBE | Maggiolo F56 | 2014 | Italy | 60 (30:30) |
Avoid drawbacks and toxicities due to the nucleoside backbone | - 49 y (median) in the DRV group and 48 y in the control group - 93 m (median previous art) in the DRV group and 98 m in the control group - pVL < 50 - CD4 not limited - negative HBV |
48 | DRV/r (800 mg) + RPV | Triple |
| PROTEA | Antinori A29 | NA | Europe and Israel | 273 (137:136) |
NA | - 42 y (mean age) -pVL < 50 for the previous 48 w - CD4 > 200 |
48 | DRV/r (800 mg) + 2NRTIs (triple) |
DRV/r (800 mg) monotherapy |
| Girard PM57 | 96 | ||||||||
| SPARE | Nishijima T58 | 2011 | Japan | 58 (28:30) |
NA | - 44 y (median age) in the study group and 39 y in the control group - pVL < 50 CD4 not limited |
48 | DRV/r (800 mg) + RAL | LPV/r + TVD |
| Treatment-experienced subjects, mixed/other combinations (1 study; 225 total patients enrolled) | |||||||||
| MONOI | Katlama C30 | 2007–2008 | France | 225 (112:113) |
NA | - 46 y (median age) in the study group and 45 y in the control group -pVL < 50 -pVL < 400 for > 18 m |
48 | DRV/r (600 mg BID, switched to 800 mg OD if pVL < 50 at w48) + NRTIs (triple) |
DRV/r (600 mg BID, switched to 800 mg OD if pVL < 50 at w48) monotherapy |
| Valantin MA21 | 96 | ||||||||
NA: Not applicable
Efficacy
Efficacy was defined as the virological response rate (viral load < 50 copies/ml) at 48 and 96 weeks for the ART-naïve adult patients and at 48 weeks for the ART-experienced patients.
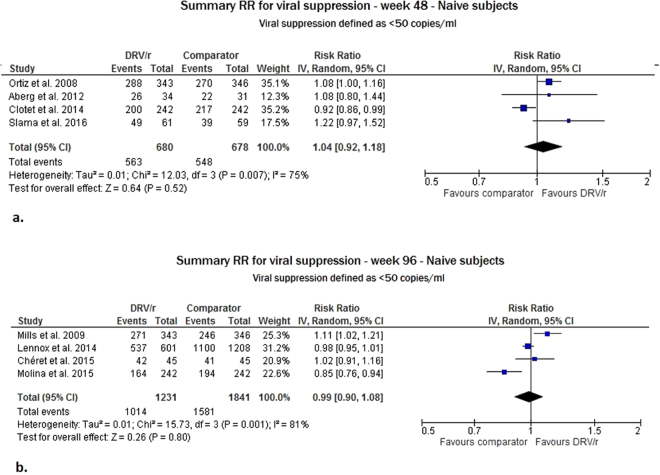
For the ART-naïve patients, we included eight studies in this meta-analysis covering a total of 4430 adult patients evaluated (four with 48 weeks of FU and four with 96 weeks of FU). In the intention-to-treat (ITT) analysis, the virological response rate with DRV/r was not significantly different from the comparator at weeks 48 and 96, with risk ratio (RR) values equal to 1.04 (95% confidence interval (CI): 0.92–1.18) and 0.99 (95% CI: 0.90–1.08), respectively. A high degree of heterogeneity emerged between the RR estimates at week 48 (heterogeneity test I2 = 75%, p = 0.007) and week 96 (I2 = 81%, p = 0.001) (Fig. 2).
Figure 2.
Meta-analysis of viral suppression for ART-naïve adult subjects at 48 (Panel a) and 96 (Panel b) weeks of follow-up.
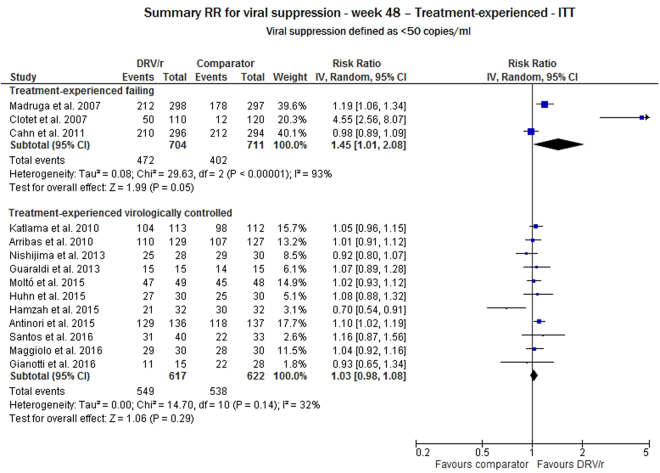
For the ART-experienced patients, data were available from three studies for failing subjects (a total of 1440 adult patients evaluated) and from 11 studies for virologically controlled subjects (a total of 1553 adult patients evaluated). At week 48, the ITT analysis of the treatment-experienced failing subjects showed that the virological response rate was significantly higher for DRV/r than for the comparator group (RR 1.45, 95% CI: 1.01–2.08), but the heterogeneity test showed high variability among the studies (p < 0.0001). Conversely, for the treatment-experienced virologically controlled DRV/r group, no significant difference was found between the DRV/r and comparator groups (RR 1.03, 95% CI: 0.98–1.08), and the variability of the study estimate was low (I2 = 32%, p = 0.14) (Fig. 3).
Figure 3.
Meta-analysis of viral suppression for ART-experienced adult subjects at 48 weeks of follow-up.
In the sensitivity analyses conducted in naïve subjects at week 48, we calculated pooled RRs after excluding the studies one by one. No study had a notable influence on the overall estimate, because the pooled RRs varied between 1.01 (when excluding the IMEA19 study) and 1.09 (when excluding the FLAMINGO20 study). The same result was obtained for the treatment-experienced virologically controlled subjects. No evidence of publication bias was detected.
Safety
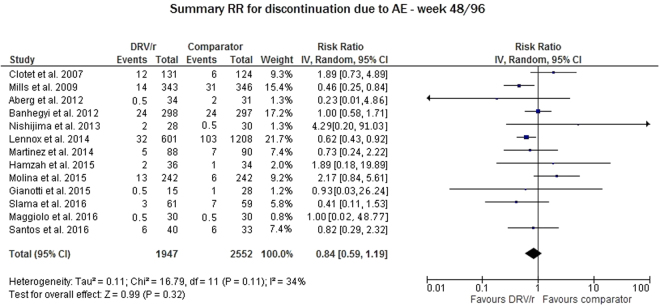
We evaluated the discontinuation rate due to adverse events (AEs) related to DRV/r for 13 studies and pooled the results for weeks 48 and 96. The DRV/r safety profile was not significantly different from that of the comparator (RR 0.84, 95% CI: 0.59–1.19); this result was supported by the low variability between studies (I2 = 34%, p = 0.11), as shown in Fig. 4.
Figure 4.
Meta-analysis of studies reporting data on treatment discontinuation due to adverse events and any serious adverse event related to the administered treatment.
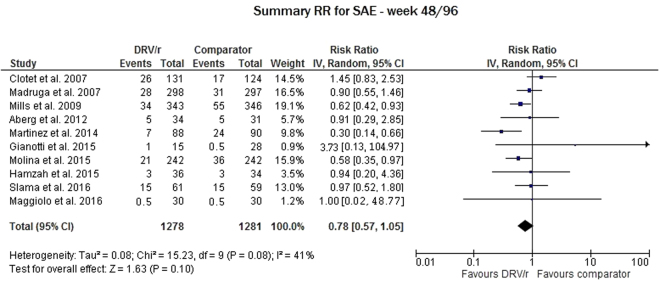
Regarding the discontinuation rate due to serious adverse events (SAEs) related to DRV/r, we evaluated 10 studies and pooled the results for weeks 48 and 96. In this analysis, the difference between the DRV/r and the comparator was also not significant (RR 0.78, 95% CI: 0.57–1.05), and low-to-moderate variability was found between the study RRs (I2 = 41%, p = 0.08) (Fig. 5).
Figure 5.
Meta-analysis of studies reporting data on any serious adverse events (SAEs).
Notably, cardiovascular (CV) events were analyzed for all of the studies included in this meta-analysis. In the 19 trials (including a total of 4992 subjects), seven non-specified CV events were reported in the MONOI21 trial, one stroke was reported in the DRV/r arm and one myocardial infarction (MI) in the lopinavir/ritonavir (LPV/r) arm in the ARTEMIS10 trial, one MI was reported in the DRV/r arm and one cardiomyopathy in the dolutegravir (DTG) arm in the FLAMINGO20 trial, and one case of pericarditis was reported in the atazanavir (ATV) arm in the IMEA19 trial. When publications were available, CV events were also evaluated at the longest follow-up time point (Table 3). The proportion of CV events in the DRV/r-treated patients was 0.18% (9/4992). For DRV/r, the incidence rate (IR) was 1.44 per 1000 person-years.
Table 3.
Cardiovascular events reported in clinical trials containing darunavir.
| Author | Study | Weeks considered in the meta-analysis | CV AE/SAE | Other weeks in the same study | CV AE/SAE |
|---|---|---|---|---|---|
| Aberg46 | METABOLIK | 48 | no CV AE/SAE | ||
| Mills5 | ARTEMIS | 96 | no CV AE/SAE | 192 | 1 stroke in the DRV arm; 1 MI in the LPV/r arm |
| Hamzah53 | MIDAS | 48 | no CV AE/SAE | ||
| Gianotti49 | 2PM | 48 | no CV AE/SAE | ||
| Maggiolo56 | PROBE | 48 | no CV AE/SAE | ||
| Raffi47 | NEAT 001 | 96 | no CV AE/SAE | ||
| Clotet22 | POWER 1–2 | 48 | no CV AE/SAE | 96* (1-2-3) | no CV AE/SAE |
| Molina45 | FLAMINGO | 96 | no CV AE/SAE | 48 | 1 MI in the DRV/r arm; 1 cardiomyopathy in the DTG arm |
| Martinez44 | ATADAR | 48 | no CV AE/SAE | ||
| Madruga9 | TITAN | 48 | no CV AE/SAE | ||
| Slama19 | IMEA | 48 | no CV AE/SAE | 1 pericarditis in the ATV arm | |
| Chéret48 | OPTIPRIM | 96 | no CV AE/SAE | ||
| Lennox37 | ATG5257 | 96 | no CV AE/SAE | ||
| Huhn50 | DRIVESHAFT | 48 | no CV AE/SAE | ||
| Clumeck55 | MONET | 96 | no CV AE/SAE | 144 | no CV AE/SAE |
| Valantin21 | MONOI | 96 | 4 CV SAE | 48 | 3 CV AE grades 3-4 |
| Guaraldi54 | MONARCH | 48 | no CV AE/SAE | ||
| Santos52 | LOPIDAR | 48 | no CV AE/SAE | ||
| Nishijima58 | SPARE | 48 | no CV AE/SAE | ||
| Cahn8 | ODIN | 48 | no CV AE/SAE | ||
| Girard57 | PROTEA | 96 | no CV AE/SAE |
*Publication at 96 weeks including POWER Studies-1-2-3
Abbreviations: CV = cardiovascular; AE = adverse event; ATV = atazanavir; DTG = dolutegravir; DRV = darunavir; LPV = lopinavir; MI = Myocardial Infarction; SAE = serious adverse event
Mono vs triple therapy
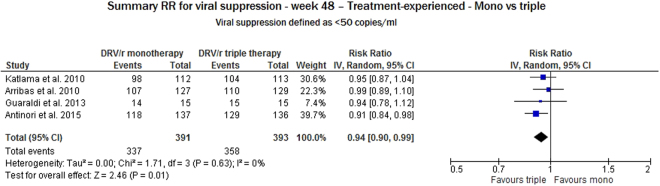
To evaluate the sole impact of DRV/r on safety, we compared the results of monotherapy with those of triple therapy in the studies reporting on DRV/r in treatment-experienced, virologically controlled subjects. The monotherapy arm of the trials was taken as a comparator. We considered four studies reporting endpoints of viral suppression at week 48. DRV/r was significantly better in triple therapy than in monotherapy (RR 0.94, 95% CI: 0.90–0.99). No heterogeneity was found between the estimates (I2 = 0%, p = 0.63) (Fig. 6).
Figure 6.
Meta-analysis of viral suppression for ART-experienced subjects at 48 weeks of follow-up considering monotherapy vs triple therapy.
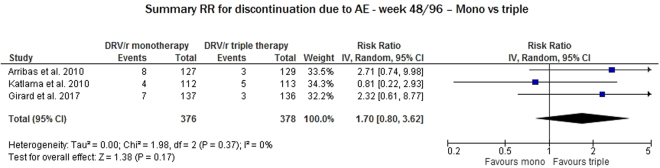
We evaluated three studies to assess discontinuation due to AEs at week 48. This variable did not significantly differ between DRV/r in monotherapy and DRV/r in triple therapy (RR 1.70, 95% CI: 0.80–3.62) in the absence of heterogeneity between RRs (I2 = 0%, p = 0.37) (Fig. 7).
Figure 7.
Meta-analysis of studies reporting data on any serious adverse events (AEs) considering monotherapy vs triple therapy* - *Favors triple indicates that a higher number of AEs was reported in the triple therapy arms.
Discussion
Nineteen RCTs were included in this meta-analysis. The first RCT was published in 2007 and described treatment-experienced subjects, and the most recent trials were published in 2016 and involved naive subjects.
In the ITT analysis of the ART-naïve subjects, the virological response rate did not differ between the DRV/r and the comparator arms, at both 48 and 96 weeks, despite the wide variability of the studies. Heterogeneity can be explained by the baseline characteristics of the subjects included in studies, such as ARTEMIS10 and IMEA19 compared to FLAMINGO20. The subjects were more advanced in ARTEMIS10 and IMEA19 than in FLAMINGO20, with higher viral loads and lower CD4+ cell counts.
In the ITT analysis at week 48 of the ART-experienced failing subjects, the virological response rate was significantly higher for DRV/r than for the comparator drugs, regardless of the previous clinical and treatment history and despite the wide heterogeneity of the studies. To date, DRV/r is the only antiretroviral drug which have been studied in highly pretreated subjects, and this population has not been enrolled in any subsequent study. In a pooled analysis of POWER studies22, DRV/r provided a sustained virological response in patients with reverse transcriptase and protease resistance-associated mutations at baseline22. This finding shows the high potency and high genetic barrier of DRV/r23 and its efficacy against resistant viruses. These results are in line with the well-known genetic barrier of DRV/r and its proven efficacy against resistant viruses. Furthermore, the DRV genetic barrier is still unequalled with respect to both other PIs and to inhibitors of strand transfer (INSTIs).
In the ITT analysis at week 48 of the ART-experienced virologically controlled subjects, the virological response rate was comparable to that of the comparator group (I2 = 34.6%, p = 0.122). In four of these studies, DRV/r was used as a monotherapy, and its potency in reaching viral undetectability was confirmed, as was its good penetration in HIV reservoirs21,23–25. These results were achieved in clinical practice in both naïve and highly experienced patients, the latter of whom had approximately seven years of FU, as reported in an Italian observational cohort (the TMC114HIV4042 study13, registered in ClinicalTrials.gov under the identifier NCT01375881).
The safety profile of DRV/r was similar to that of the comparator irrespective of the dosage and the comparator used. In this analysis, pooling the naïve and experienced subjects could have introduced bias, because the naïve subjects had never taken DRV/r. Notably, in the FLAMINGO trial, significantly more SAEs occurred in the DTG arm than in the DRV/r arm (RR 0.58; 95% CI: 0.35–0.97)20.
The safety data were also confirmed in the TMC114IHIV4042 study13, where the DRV/r-based treatment was well tolerated, with only 3.0% of the treatment discontinuations due to AEs. Notably, no differences were observed in the AE/SAE types and/or frequencies in this study compared to those reported in the DRV/r RCTs1,2,13.
Moreover, following the recently presented D:A:D cohort data on cardiovascular risk in HIV-positive subjects treated with DRV/r-based regimen26, we showed that the cardiovascular events rates in all studies included in this meta-analysis were low, even though the observational period was approximately three years compared to the more than six-year observation period included in the D:A:D26.
Triple therapy proved to be superior in efficacy (defined as viral suppression) to monotherapy. Patient characteristics (i.e., residual viremia and a nadir CD4+ count <100 cells/μL) should be taken into account when establishing a monotherapy regimen, as highlighted by Gianotti et al.27, who reported selection criteria for entry of candidate virologically suppressed HIV-positive individuals into DRV/r monotherapy27. Following this scoring system, DRV/r monotherapy and standard therapy “could be equally effective” with the same virological failure rate as standard triple therapy27.
No mutations associated with DRV resistance were reported for monotherapy based on DRV/r, and sensitivity to DRV was maintained28–30. To date, no INSTI drug has shown the same genetic barrier: INSTI resistance-associated mutations have been found in failing monotherapy31. In terms of safety, adverse events leading to therapy discontinuation were relatively rare and were even rarer in the monotherapy studies28–30.
Limitations
The limitations of this meta-analysis include the use of different comparators in the studies, inhomogeneity in the study duration, the use of different timepoints for the efficacy/safety assessments, the wide timespan of the studies considered and the inclusion of only English-language publications. All the RCTs included were open-label; therefore, the risk of performance bias was increased. However, the outcomes evaluated were objective measures, which might have decreased the risk of bias. Furthermore, this analysis only included studies using DRV boosted with the pharmaco-enhancer ritonavir. However, the results of two recent registrative studies conducted with naïve and virologically suppressed, experienced patients taking ART based on DRV boosted with the new pharmaco-enhancer cobicistat have been published32,33. Further research including those data are recommended.
Strenghts
The main strength of this meta-analysis is the comprehensive search for published clinical studies from multiple electronic databases using a cross-checking strategy for additional potentially missed articles. The meta-analytic approach allowed us to obtain more precise estimates of the pooled results, which can provide clinicians with suggestions for use in clinical practice, as previous meta-analyses have done34,35. Furthermore, the studies considered here were conducted in different years; therefore, the patient characteristics differed greatly among the studies (in previous years, the patients were more advanced). Nevertheless, the results shown in response to DRV treatment were consistent and confirmed its well-known efficacy and safety profile; thus, this treatment remains an effective option for current patients.
Using this meta-approach, we re-analyzed study-level data; however, additional original studies involving a longer follow-up period and patients enrolled in real-life settings are required to better understand the efficacy, effectiveness and safety of DRV/r.
Conclusion
The evidence shown in this analysis confirms that DRV/r is an effective regimen for ART-naive and ART-experienced subjects, with no differences from the comparator arms detected. DRV/r was safe and well-tolerated in every group of subjects. The good safety profile of DRV when used in monotherapy is highlighted.
Methods
Search strategy
A systematic literature search of clinical trials including DRV use in HIV-positive patients was conducted in September 2016 using the Medline and EMBASE databases. No data were generated in this work, which analyzed publicly available publications. We did not prepare a specific review protocol for this project. We adopted a wide-ranging search strategy using a predefined generic search string with no temporal restrictions and no search filters whenever possible. This strategy was finalized to minimize the probability of excluding relevant papers from the present meta-analysis. The Medline/Pubmed search string was as follows: “(darunavir OR prezista OR tmc114) AND trial”. A similar combination of keywords was used in the EMBASE search; however, that search was restricted to clinical trials using the “study types” filter. A cross-check for additional articles that were potentially missed during the main search process was conducted by exploring the Cochrane Register of Controlled Trials (CENTRAL) and Google Scholar (using the same keywords and reviewing the first 150 papers according to their relevance) and performing thorough searches of the reference lists of relevant reviews and the papers selected for inclusion. Figure 1 provides a flow-chart with detailed information on the search and selection processes.
Inclusion and exclusion criteria
The identified publications were considered for inclusion in the meta-analysis if the following criteria were met: randomized clinical trials with at least 48 weeks of follow-up and with DRV use in at least one study arm. Observational studies, interventions other than DRV use, reviews, meta-analyses, indirect comparisons, commentaries and other articles lacking original data were excluded. Single-arm trials and pooled analyses were also excluded after careful consideration. Conference abstracts were included, whereas unpublished studies and articles in languages other than English were excluded. No studies were excluded a priori for weakness of design or data quality.
Study selection, data extraction and risk of bias assessment
Two researchers independently examined the articles retrieved from the Medline/PubMed and EMBASE databases. Discrepancies between the researchers’ results were discussed and resolved. In the first selection step, the articles were evaluated based on their titles and abstracts. After merging the publications from the PubMed and EMBASE searches, a total of 134 unique publications remained. The second and third selection steps were based on full-text examinations of the retrieved articles. Sixty articles reporting data on the efficacy or safety of DRV in HIV-positive patients from RCTs with at least 48 weeks of follow-up were retained. Fourteen of these studies were included in the tables but were not used in the meta-analyses due to the relatively small number of studies with their specific characteristics (i.e., they reported results for follow-up periods other than 48 or 96 weeks or they reported results from trials on treatment-experienced subjects with switched or mixed treatments).
Two researchers reviewed the selected studies and extracted relevant information. In particular, the extracted data included the trial name, enrollment period, geographic area, number of patients included and treatment regimen in each study arm, the reason for discontinuation of earlier treatments (for studies with treatment-experienced patients), the patient characteristics at baseline, and the follow-up duration. This information was organized in two tables that separated the trials with treatment-naïve and treatment-experienced patients. The latter patients were further divided into subgroups representing trials of (i) treatment-experienced failing subjects treated with a DRV 600 mg BID regimen compared with another regimen, (ii) treatment-experienced virologically controlled subjects treated with a DRV 800 mg regimen compared with another regimen, and (iii) treatment-experienced subjects treated with a mixed/other DRV regimen. The main results for the efficacy (i.e., viral suppression defined as <50 copies/ml) and safety outcomes (i.e., treatment discontinuation due to adverse events or serious adverse events) were also extracted into spreadsheets for subsequent meta-analyses. Whenever available, we extracted the results from the intention-to-treat analysis. Discrepancies between researchers were checked in the original reports and resolved.
The risk of bias in the included studies was assessed by three authors using the Cochrane risk of bias tool36. Discrepancies between the researchers were discussed and resolved through discussion with a senior reviewer.
Statistical analyses
In the efficacy outcome analyses, the results obtained at weeks 48 and 96 and for the treatment-naïve and treatment-experienced patients were always analyzed separately. However, for the safety outcomes, all trials were jointly analyzed using the results for the longest follow-up time when several results were available from the same trial. The risk ratios for each study were pooled. When the risk ratio was not provided but sufficient data were available in the publication to compute this measure, we calculated unadjusted risk ratios and their 95% CIs from the outcome distributions of subjects in the treatment and control arms. When more than one publication reported results from the same study (i.e., with extended follow-up periods), we included the earliest publication in the meta-analysis because the completion rate was higher and the endpoint was more similar to those of the other studies. The ACTG5257 study37 was a three-arm trial. Therefore, we pooled data from the ATV and RAL arms to compute a single risk ratio for each efficacy and safety outcome. These ratios were included in the meta-analysis.
We computed summary risk ratios (RR) for each efficacy and safety outcome for the patients treated with DRV compared to other treatments using random-effects models (i.e., as weighted averages using the inverse of the sum of the variance of the log (risk ratio) and using the moment estimator of the variance between studies as the weight)38,39. Heterogeneity between trials was assessed using the χ2 test (defined as a p-value less than 0.10), and inconsistency was measured using the I2 statistic, which describes the percentage of total variation across studies due to heterogeneity rather than chance40. Values of the I2 statistic of approximately 25%, 50% and 75% are indicative of low, moderate and high heterogeneity, respectively40. The presence of publication bias was assessed based on a visual examination of the funnel plots and by applying the tests proposed by Begg and Mazumdar41 and Egger42. We conducted sensitivity analyses by excluding each study one by one from the meta-analysis. No other sub-group analyses were planned. All statistical analyses were performed using the RevMan software (version 5.3 for Windows).
Electronic supplementary material
Acknowledgements
We thank Dr. Carlotta Galeone (senior epidemiologist/biostatistician, ScD, PhD) from the University of Milan for her fundamental contribution in reviewing all of the statistical analyses and Dr. Claudio Citterio for his work in the search and selection of the bibliography. Statinfo, a statistical consultant for Janssen-Cilag SpA, provided the statistical analyses. We thank also Dr. Chiara Formigoni for manuscript review. The present study was supported by Janssen-Cilag SpA.
Author Contributions
Andrea Antinori (A.A.), Adriano Lazzarin (A.L.), Alessia Uglietti (A.U.), Maria Palma (M.P.), Daniela Mancusi (D.M.) and Roberta Termini (R.T.) reviewed and the statistical analyses, and wrote and reviewed the manuscript.
Competing Interests
Andrea Antinori (A.A.) has received honoraria for consultancies with Gilead Sciences, ViiV Healthcare, Merck Sharp & Dohme, Janssen-Cilag, Abbvie, and Bristol-Myers Squibb and has also received research grants from Gilead Sciences, Bristol-Myers Squibb, Janssen-Cilag, and ViiV Healthcare; Adriano Lazzarin (A.L.) has received fees for advisory board participation and conference talks from BMS, ViiV, Gilead, MSD, Mylan, Abbvie, Janssen Cilag, and Teva; Alessia Uglietti (A.U.), Maria Palma (M.P.), Daniela Mancusi (D.M.) and Roberta Termini (R.T.) are employees of Janssen-Cilag SpA, Italy.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23375-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prezista 800®. Summary of Product Characteristics (SmPC). Available from: http://www.ema.europa.eu/docs/it_IT/document_library/EPAR__Product_Information/human/000707/WC500041756.pdf, Accessed March 21, 2017.
- 2.Prezista 600®. Summary of Product Characteristics (SmPC). Available from: http://www.ema.europa.eu/docs/it_IT/document_library/EPAR__Product_Information/human/000707/WC500041756.pdf, Accessed March 21, 2017.
- 3.Deeks ED. Darunavir: a review of its use in the management of HIV-1 infection. Drugs. 2014;74:99–125. doi: 10.1007/s40265-013-0159-3. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz R, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22:1389–1397. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 5.Mills AM, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23:1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 6.Pozniak A, Opravil M, Beatty G, Hill A, de Bethune MP, Lefebvre E. Effect of baseline viral susceptibility on response to darunavir/ritonavir versus control protease inhibitors in treatment-experienced HIV type 1-infected patients: POWER 1 and 2. AIDS Res Hum Retroviruses. 2008;24:1275–1280. doi: 10.1089/aid.2007.0275. [DOI] [PubMed] [Google Scholar]
- 7.Arasteh K, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antivir Ther. 2009;14:859–864. doi: 10.3851/IMP1301. [DOI] [PubMed] [Google Scholar]
- 8.Cahn P, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011;25:929–939. doi: 10.1097/QAD.0b013e328345ee95. [DOI] [PubMed] [Google Scholar]
- 9.Madruga JV, et al. TITAN Study Group. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 10.Orkin C, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14:49–59. doi: 10.1111/j.1468-1293.2012.01060.x. [DOI] [PubMed] [Google Scholar]
- 11.Banhegyi D, Katlama C, da Cunha CA, et al. Week 96 efficacy, virology and safety of darunavir/r versus lopinavir/r in treatment-experienced patients in TITAN. Curr HIV Res. 2012;10:171–181. doi: 10.2174/157016212799937218. [DOI] [PubMed] [Google Scholar]
- 12.Lathouwers E, et al. Virological characterization of patients failing darunavir/ritonavir or lopinavir/ritonavir treatment in the ARTEMIS study: 96-week analysis. Antivir Ther. 2011;16:99–108. doi: 10.3851/IMP1719. [DOI] [PubMed] [Google Scholar]
- 13.Antinori A, et al. Effectiveness, durability, and safety of darunavir/ritonavir in HIV-1-infected patients in routine clinical practice in Italy: a postauthorization noninterventional study. Drug Des Devel Ther. 2016;10:1589–1603. doi: 10.2147/DDDT.S104875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capetti A, Cossu MV, Rizzardini G. Darunavir/cobicistat for the treatment of HIV-1: a new era for compact drugs with high genetic barrier to resistance. Expert Opin Pharmacother. 2015;16:2689–2702. doi: 10.1517/14656566.2015.1109632. [DOI] [PubMed] [Google Scholar]
- 15.Ministero della Salute [webpage on the Internet]. Linee Guida Italiane sull’utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV 2016. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2545_allegato.pdf. Accessed March 21, 2017.
- 16.European AIDS Clinical Society [webpage on the Internet]. EACS Guidelines Version 8.2 2017. http://www.eacsociety.org/files/guidelines_8.2-english.pdf, Accessed March 21, 2017.
- 17.BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update) [webpage on the Internet] http://www.bhiva.org/documents/Guidelines/Treatment/2016/treatment-guidelines-2016-interim-update.pdf. [DOI] [PubMed]
- 18.AIDS info [webpage on the Internet] Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. DHHS Guidelines 2016. Accessed March 21, 2017.
- 19.Slama L, et al. Efficacy and safety of once-daily ritonavir-boosted atazanavir or darunavir in combination with a dual nucleos(t)ide analogue backbone in HIV-1-infected combined ART (cART)-naive patients with severe immunosuppression: a 48 week, non-comparative, randomized, multicentre trial (IMEA 040 DATA trial) J Antimicrob Chemother. 2016;71:2252–2261. doi: 10.1093/jac/dkw103. [DOI] [PubMed] [Google Scholar]
- 20.Clotet B, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383:2222–2231. doi: 10.1016/S0140-6736(14)60084-2. [DOI] [PubMed] [Google Scholar]
- 21.Valantin MA, et al. Body fat distribution in HIV-infected patients treated for 96 weeks with darunavir/ritonavir monotherapy versus darunavir/ritonavir plus nucleoside reverse transcriptase inhibitors: the MONOI-ANRS136 substudy. HIV Med. 2012;13:505–515. doi: 10.1111/j.1468-1293.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- 22.Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 23.Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. doi: 10.1016/j.meegid.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calcagno A, et al. Cerebrospinal fluid inhibitory quotients of antiretroviral drugs in HIV-infected patients are associated with compartmental viral control. Clin Infect Dis. 2015;60:311–317. doi: 10.1093/cid/ciu773. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher CV, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryom, L. et al. Association between cardiovascular disease and contemporarily used protease inhibitors. CROI 2017, February 13–16, Seattle, Abs 128LB.
- 27.Gianotti N, et al. Refining criteria for selecting candidates for a safe lopinavir/ritonavir or darunavir/ritonavir monotherapy in HIV-infected virologically suppressed patients. PLoS One. 2017;12:e017161. doi: 10.1371/journal.pone.0171611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arribas JR, et al. The MONET trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV RNA below 50 copies/ml. AIDS. 2010;24:223–230. doi: 10.1097/QAD.0b013e3283348944. [DOI] [PubMed] [Google Scholar]
- 29.Antinori A, Clarke A, Svedhem-Johansson V, et al. Week 48 efficacy and central nervous system analysis of darunavir/ritonavir monotherapy versus darunavir/ritonavir with two nucleoside analogues. AIDS. 2015;29:1811–1820. doi: 10.1097/QAD.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 30.Katlama C, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24:2365–2374. doi: 10.1097/QAD.0b013e32833dec20. [DOI] [PubMed] [Google Scholar]
- 31.Wijting, I et al. Dolutegravir as maintenance monotherapy for HIV-1: A randomized clinical trial. Abstract presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 13–16, 2017; Seattle, WA. Abstract 451LB.
- 32.Orkin C, et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5:e23–e34. doi: 10.1016/S2352-3018(17)30179-0. [DOI] [PubMed] [Google Scholar]
- 33.Eron, J. et al. Week 48 results of AMBER: A Phase 3, randomised, double-blind trial in antiretroviral treatment-naïve HIV-1-infected adults to evaluate the efficacy and safety of the once-daily, single-tablet regimen of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) versus darunavir/cobicistat plus emtricitabine/tenofovir disoproxil fumarate. Milan, Italy EACS Conference 2017. Abstract PS8/2.
- 34.Menshawy A, et al. Efficacy and safety of atazanavir/ritonavir-based antiretroviral therapy for HIV-1 infected subjects: a systematic review and meta-analysis. Arch Virol. 2017;162:2181–2190. doi: 10.1007/s00705-017-3346-9. [DOI] [PubMed] [Google Scholar]
- 35.Baril J, et al. A meta-analysis of the efficacy and safety of unboosted atazanavir compared with ritonavir-boosted protease inhibitor maintenance therapy in HIV-infected adults with established virological suppression after induction. HIV Medicine. 2014;15:301–310. doi: 10.1111/hiv.12118. [DOI] [PubMed] [Google Scholar]
- 36.Higgins, J. P. T. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: http://handbook.cochrane.org, Accessed January 25, 2018.
- 37.Lennox JL, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. (ACTG5257) Ann Intern Med. 2014;161:461–467. doi: 10.7326/M14-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 39.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 40.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 42.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ofotokun I, Na LH, Landovitz RJ, et al. Comparison of the metabolic effects of ritonavir-boosted darunavir or atazanavir versus raltegravir, and the impact of ritonavir plasma exposure: ACTG 5257. Clin Infect Dis. 2015;60:1842–1851. doi: 10.1093/cid/civ193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez E, et al. Early lipid changes with atazanavir/ritonavir or darunavir/ritonavir. HIV Med. 2014;15:330–338. doi: 10.1111/hiv.12121. [DOI] [PubMed] [Google Scholar]
- 45.Molina JM, Clotet B, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet. 2015;2:e127–e136. doi: 10.1016/S2352-3018(15)00027-2. [DOI] [PubMed] [Google Scholar]
- 46.Aberg JA, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses. 2012;28:1184–1195. doi: 10.1089/aid.2011.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raffi F, et al. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet. 2014;384:1942–1951. doi: 10.1016/S0140-6736(14)61170-3. [DOI] [PubMed] [Google Scholar]
- 48.Chéret A, et al. Intensive five-drug antiretroviral therapy regimen versus standard triple-drug therapy during primary HIV-1 infection (OPTIPRIM-ANRS 147): a randomised, open-label, phase 3 trial. Lancet Infect Dis. 2015;15:387–396. doi: 10.1016/S1473-3099(15)70021-6. [DOI] [PubMed] [Google Scholar]
- 49.Gianotti N, et al. Monotherapy with darunavir/ritonavir or lopinavir/ritonavir versus standard antiretroviral therapy: a randomized clinical trial (2pm Study) New Microbiologica. 2016;39:290–294. [PubMed] [Google Scholar]
- 50.Huhn GD, Sigman A, Livak B. Simplification from twice-daily to once-daily darunavir/ritonavir in a randomized trial among HIV-infected persons with HIV-1 RNA suppression on antiretroviral therapy. Antivir Ther. 2015;20:849–854. doi: 10.3851/IMP2962. [DOI] [PubMed] [Google Scholar]
- 51.Moltó J, et al. Reduced darunavir dose is as effective in maintaining HIV suppression as the standard dose in virologically suppressed HIV-infected patients: a randomized clinical trial. J Antimicrob Chemother. 2015;70:1139–45. doi: 10.1093/jac/dku516. [DOI] [PubMed] [Google Scholar]
- 52.Santos JR, et al. Efficacy and Safety of Treatment Simplification to Lopinavir/Ritonavir or Darunavir/Ritonavir Monotherapy: A Randomized Clinical Trial. AIDS Res Hum Retroviruses. 2016;32:452–455. doi: 10.1089/aid.2015.0248. [DOI] [PubMed] [Google Scholar]
- 53.Hamzah L, et al. Effects on vitamin D, bone and the kidney of switching from fixed-dose tenofovir disoproxil fumarate/emtricitabine/efavirenz to darunavir/ritonavir monotherapy: a randomized, controlled trial (MIDAS) Antivir Ther. 2016;21:287–96. doi: 10.3851/IMP3000. [DOI] [PubMed] [Google Scholar]
- 54.Guaraldi G, et al. Randomized trial to evaluate cardiometabolic and endothelial function in patients with plasma HIV-1 RNA suppression switching to darunavir/ritonavir with or without nucleoside analogues. HIV Clin Trials. 2013;14:140–8. doi: 10.1310/hct1404-140. [DOI] [PubMed] [Google Scholar]
- 55.Clumeck N, et al. 96 week results from the MONET trial: a randomized comparison of darunavir/ritonavir with versus without nucleoside analogues, for patients with HIV RNA <50 copies/ml at baseline. J Antimicrob Chemother. 2011;66:1878–1885. doi: 10.1093/jac/dkr199. [DOI] [PubMed] [Google Scholar]
- 56.Maggiolo F, Di Filippo E, Valenti D, Ortega PS, Callegaro A. NRTI Sparing Therapy in Virologically Controlled HIV-1 Infected Subjects: Results of a Controlled, Randomized Trial (Probe) J Acquir Immune Defic Syndr. 2016;72:46–51. doi: 10.1097/QAI.0000000000000966. [DOI] [PubMed] [Google Scholar]
- 57.Girard PM, et al. Week 96 efficacy and safety of darunavir/ritonavir monotherapy vs. darunavir/ritonavir with two nucleoside reverse transcriptase inhibitors in the PROTEA trial. HIV Med. 2017;18:5–12. doi: 10.1111/hiv.12386. [DOI] [PubMed] [Google Scholar]
- 58.Nishijima T, et al. Switching tenofovir/emtricitabine plus lopinavir/r to raltegravir plus Darunavir/r in patients with suppressed viral load did not result in improvement of renal function but could sustain viral suppression: a randomized multicenter trial. PLoS One. 2013;8:e73639. doi: 10.1371/journal.pone.0073639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.