Abstract
Objective
Women with insulin-requiring gestational diabetes mellitus (GDM) are at high risk of developing diabetes within a few years postpartum. We implemented this phase II study to test the hypothesis that vildagliptin, a dipeptidyl peptidase-4 inhibitor, is superior to placebo in terms of reducing the risk of postpartum diabetes.
Methods
Women with insulin-requiring GDM were randomized to either placebo or 50 mg vildagliptin twice daily for 24 months followed by a 12-month observation period (EudraCT: 2007-000634-39). Both groups received lifestyle counseling. The primary efficacy outcomes were the diagnosis of diabetes (American Diabetes Association (ADA) criteria) or impaired fasting glucose (IFG)/impaired glucose tolerance (IGT).
Results
Between 2008 and 2015, 113 patients (58 vildagliptin, 55 placebo) were randomized within 2.2–10.4 (median 8.6) months after delivery. At the interim analysis, nine diabetic events and 28 IFG/IGT events had occurred. Fifty-two women withdrew before completing the treatment phase. Because of the low diabetes rate, the study was terminated. Lifestyle adherence was similar in both groups. At 24 months, the cumulative probability of postpartum diabetes was 3% and 5% (hazard ratio: 1.03; 95% confidence interval: 0.15–7.36) and IFG/IGT was 43% and 22% (hazard ratio: 0.55; 95% confidence interval: 0.26–1.19) in the placebo and vildagliptin groups, respectively. Vildagliptin was well tolerated with no unexpected adverse events.
Conclusions
The study did not show significant superiority of vildagliptin over placebo in terms of reducing the risk of postpartum diabetes. However, treatment was safe and suggested some improvements in glycemic control, insulin resistance, and β-cell function. The study identified critical issues in performing clinical trials in the early postpartum period in women with GDM hampering efficacy assessments. With this knowledge, we have set a basis for which properly powered trials could be performed in women with recent GDM.
Trial registration number at ClinicalTrials.gov
Keywords: Gestational diabetes mellitus, Prevention, Dipeptidyl peptidase-4 inhibitor, Postpartum diabetes, Randomized controlled trial, Life-style
Abbreviations: DPP4 inhibitor, dipeptidyl peptidase-4 inhibitor; GDM, gestational diabetes mellitus; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, Glucagon-like peptide-1; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; ITT, intention-to-treat
Highlights
-
•
Treatment with vildagliptin suggested positive effects on β-cell function and HbA1c.
-
•
Treatment with vildagliptin was safe.
-
•
Contraindication of vildagliptin during lactation led to exclusion of women with early postpartum diabetes.
-
•
Slow enrolment and high drop-out rates are major challenges in studies of women with GDM.
1. Introduction
Gestational diabetes mellitus (GDM) occurs in about 13% of pregnancies worldwide [1], [2] and is associated with adverse short- and long-term outcomes in mothers and their offspring [3], [4]. Although it resolves soon after delivery in many cases, women with GDM are at increased risk of developing diabetes in the postpartum period [5], [6], [7]. Diet and exercise are recommended to manage postpartum diabetes risk. However, the overall adherence to diet and exercise recommendations is poor. Relatively few studies have been undertaken to examine whether it is possible to reduce the risk of postpartum diabetes in women with prior GDM through pharmacotherapy [8], [9]. However, no pharmacotherapy intervention trial has been conducted in women with GDM during the first year after delivery, when the risk of developing postpartum diabetes is greatest [7]. The pathogenesis of GDM is still unknown, but there is evidence that impaired insulin secretion contributes to hyperglycemia during pregnancy in a large proportion of women with prior GDM [10]. Therefore, we reasoned that interventions aimed at improving/preserving insulin secretion in the early postpartum period may help to prevent postpartum diabetes in women with GDM, particularly in those who required insulin during pregnancy.
Glucagon-like peptide-1 (GLP-1) is an important mediator of insulin secretion and glycemic control [11], and several dipeptidyl peptidase-4 (DPP4) inhibitors, which prevent GLP-1 degradation, have been approved for the treatment of type 2 diabetes [12]. GLP-1 analogues and DPP4 inhibitors also have beneficial effects on islet function and β-cell preservation [13], [14], [15], [16]. To date, however, no trial has assessed whether DPP4 inhibitors can prevent risk of postpartum diabetes in women with a recent history of GDM. Considering these properties of DPP4 inhibitors, we performed an investigator-initiated phase II study to test the hypothesis that administration of vildagliptin, a representative DPP4 inhibitor, within 1 year after delivery is superior to placebo in terms of reducing the risk of postpartum diabetes in women with prior insulin-requiring GDM. We chose a 2-year treatment period and a 1-year follow-up period because we found that many diabetic events in women with prior GDM occurred within 1 year of delivery in our previous study [6], and considering the cost and feasibility of longer-term studies. Both groups received repeated lifestyle counseling, including dietary and physical activity counseling, which is recommended in the postpartum follow-up of women with GDM.
2. Material and methods
2.1. Design
This study was performed as an investigator-initiated, phase II, single-center, randomized, double-blind, placebo-controlled trial, and was conducted at the Forschergruppe Diabetes e.V., Munich-Neuherberg, Germany. It was approved by the Ethikkommission der Fakultät für Medizin, Technische Universität München (project no. 1832/07). The study was registered on the European Clinical Trials Database (identifier: 2007-000634-39; https://www.clinicaltrialsregister.eu/ctr-search/trial/2007-000634-39/DE). This trial was performed in agreement with the Declaration of Helsinki. All participants provided written informed consent.
The study was designed to test the hypothesis that administration of vildagliptin 50 mg twice daily for 24 months, when first administered within 9 months after delivery, is superior to placebo in terms of reducing the proportion of women who progressed to postpartum diabetes with a total follow-up of 36 months. Based on an expected rate of postpartum diabetes of 61% over 3 years in the placebo group, as observed in the German Gestational Diabetes Study [6], we calculated that enrolling 70 participants to each group would provide 80% power to detect a 50% hazard reduction of postpartum diabetes over 3 years with a two-sided α of 0.05 and a drop-out rate of 15%. We projected to complete the enrollment of 140 women within 4 years of starting the study. Secondary objectives were to test whether vildagliptin treatment improved β-cell function and insulin sensitivity.
2.2. Eligibility criteria
Patients with insulin-treated GDM were referred for participation in the trial from hospitals or outpatient clinics in Germany. Females aged ≥18 years old, <9 months postpartum, and who had insulin-treated GDM during their most recent pregnancy were eligible for the study. Women were classified as having GDM if two of three capillary blood glucose values measured during an oral glucose tolerance test (OGTT) were >90 mg/dL (>5 mmol/L) in the fasting state before a 75-g glucose load or >180 mg/dL (>10.6 mmol/L) at 60 min after and >155 mg/dL (>8.9 mmol/L) at 120 min after the glucose load. Insulin-requiring GDM was defined according to the Diabetes and Pregnancy Study group recommendations of the German Diabetes Association [17]. Major exclusion criteria were diagnosis of diabetes at/before screening (women with IFG/IGT at the screening or baseline visit were eligible), positivity for type 1 diabetes-associated islet autoantibodies (glutamic acid decarboxylase or antibody or insulinoma antigen-2), and planning to continue lactation. Other exclusion criteria are listed in the supplementary materials.
2.3. Randomization, treatment, and clinical visits
Eligible women were centrally randomized at the baseline visit to receive either 50 mg vildagliptin or placebo, which were administered twice daily for 24 months. The study drug was provided by Novartis. The study drug could be stopped temporarily if an adverse event occurred. The participants and investigators were blinded to the allocated treatment. Patients returned to the study centre for efficacy and safety assessments at 3, 6, 9, 12, 18, 24, 30, and 36 months after randomization. OGTTs were performed at screening, 6, 12, 18, 24, 30, and 36 months with blood samples drawn at −20, 0, 30, 60, 90, and 120 min. A standard meal challenge test was performed at baseline, 24 months, and 36 months with blood samples drawn at −20, 0, 15, 30, 45, 60, 90, and 120 min. All enrolled women received once a year standard lifestyle recommendations in the form of an annual, individualized consultation based on the dietary reports provided by the women. The participants also received a brochure including general recommendations on healthy diet.
2.4. Assessment of lifestyle compliance
Compliance to lifestyle counseling was assessed at baseline, 12 months, 24 months, and 36 months. At these visits, the participants were provided a pedometer and advised to record their steps for 7 consecutive days. Dietary information was collected by a Food Frequency Questionnaire [18], reflecting the nutritional habits of the past 4 weeks. Daily energy and nutrient intake were calculated using the PRODI® software and intake of macronutrients was expressed as percentage of recommended daily intake [19].
2.5. Laboratory measurements
Laboratory tests of safety parameters were performed centrally at the Institute for Clinical Chemistry at Klinikum Schwabing (Medizet) using accredited methods. Insulin was measured at the Forschergruppe Diabetes e.V. using the Mercodia Ultrasensitive Insulin ELISA (Uppsala, Sweden). Proinsulin was measured with a quantitative ELISA (TecoMedical, Sissach, Switzerland), and glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 were measured with ELISAs (Merck KGaA, Darmstadt, Germany) according to the manufacturer's instructions.
2.6. Anthropometric measurements
Anthropometric data were collected by trained staff using standardized protocols. Maternal weight and height were measured in light clothes and without shoes and were used to calculate body mass index (BMI). Waist circumference was measured at the highest point of the iliac crest. Hip circumference was measured at the level of maximum extension of the buttocks.
2.7. Questionnaire data
Age at baseline, race, family history of diabetes, smoking behavior, and lactation duration were obtained during the baseline visit. Gestational age, child's birth weight, and delivery mode were obtained from the paediatricians' records.
2.8. Efficacy assessment
The primary efficacy outcome was the diagnosis of diabetes according to the American Diabetes Association (ADA) 1997 criteria [20] or ADA 2012 criteria [21], or the onset of IGT and/or IFG (in accordance with World Health Organization 2006 guidelines). Women who developed diabetes according to the (ADA) 1997 criteria were to discontinue treatment. Secondary efficacy outcomes were markers of β-cell function; markers of insulin resistance; areas under the concentration–time curves for plasma glucose, GLP-1, and GIP; HbA1c; BMI; waist-to-hip ratio; and blood pressure. The diagnostic criteria and calculation of indices/ratios are described in the supplementary materials.
2.9. Safety parameters
Safety parameters included laboratory tests (alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase, bilirubin, blood cell counts, creatinine, creatine kinase, sodium, potassium, urea, uric acid, urine protein), pulse rate, and pregnancy tests at baseline, and at 3, 6, 9, 12, 18, 24, 30, and 36 months. We also documented adverse events and concomitant medications.
2.10. Protocol changes
The trial protocol was amended with respect to the eligibility criteria (the maximum time between delivery and randomization was increased from 7 months to 9 months), the vildagliptin dose regimen (from 100 mg once daily to 50 mg twice daily), a change to diagnostic criteria (use of the ADA 2012 criteria [21] as an alternative primary efficacy outcome), the inclusion of IGT and/or IFG as additional efficacy outcomes, and the decision to perform an interim (futility) analysis in November 2015.
2.11. Statistical analyses
The primary efficacy variables (diabetes according to ADA 1997 and 2012 criteria, as well as IGT/IFG as a combined variable) were compared between the treatment groups using the Kaplan–Meier method and the log-rank test, as well as hazard ratios (HRs) determined by Cox proportional hazards regression. Participants who discontinued treatment due to a new pregnancy were censored at the time of their last follow-up. For secondary efficacy outcomes and lifestyle variables, the median and interquartile range were calculated for each visit, and the changes in each variable from baseline to each visit were compared between the two groups using the Wilcoxon signed-rank test. The primary and secondary outcomes were analyzed according to the intention-to-treat (ITT) principle for all randomized patients who had taken at least one dose of study drug and had some post-baseline data for the primary efficacy parameters. Safety laboratory variables were presented as the median with interquartile range, together with the number and percentage of participants with values outside normal range, which were compared between the two groups using Fisher's exact test. The numbers of women with and without adverse events likely related to the study drug were compared between the two group groups using the χ2 test. Demographic, anthropometric, and metabolic variables at baseline were compared using the Mann–Whitney U-test or the χ2 test. Missing data were not imputed, unless the value of a secondary outcome variable or safety variable was missing at screening, in which case the baseline value was used instead, or vice versa (as appropriate).
All analyses were performed on a significance level of α = 0.05 (two-sided) without correction for multiple testing, and were carried out using SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Participants
Of 155 women screened between 2008 and 2015, 113 were allocated to treatment within 2.2–10.4 (median 8.6) months after delivery, including 58 women to vildagliptin (median age at baseline visit: 35.8 years) and 55 to placebo (median age at baseline visit: 33.1 years). The median duration of follow-up was 1.92 (range: 0·18–3.31) years and 2.50 (range 0.28–3.27) years in the vildagliptin and placebo groups, respectively. Of the 113 participants, 15 (13.3%) had no post-baseline assessment of any outcome variable. Therefore, the number of women included in the analyses of each primary efficacy outcome ranged from 35 to 48 for vildagliptin and from 39 to 50 for placebo (Supplementary Figure 1).
When the interim analysis was performed after a trial duration of 7.75 years, 9 women had developed postpartum diabetes (4 by ADA 1997 criteria and 8 by ADA 2012 criteria; one had to be excluded for ADA 2012 outcome analysis due to an HbA1c value >6.5% [48 mmol/mol] at the baseline visit) and 28 had IFG/IGT. Because the rate of diabetic events was lower than anticipated, the study was deemed underpowered for assessing the null hypothesis and was terminated at the recommendation of the Data Safety Monitoring Board. By then, 63 of the 113 participants (54.0%; 30 in the vildagliptin group and 33 in the placebo group) had received the intervention for 24 months, and 45 participants (38.1%; 20 in the vildagliptin group and 25 in the placebo group) had completed the 36-month study period. Of the 50 women who discontinued the study or were lost to follow-up during the treatment period, six in the vildagliptin group and three in the placebo group did so because of a new pregnancy, while two in the vildagliptin group and one in the placebo group discontinued because they developed diabetes according to the ADA 1997 criteria (study endpoint). In the vildagliptin arm, a further patient lacked the second confirmation of diabetes as required by the ADA 1997 criteria.
Both groups were similar in terms of their demographic, anthropometric, and metabolic variables, including age and BMI at baseline, race, family history of diabetes, delivery mode, HbA1c in the last trimester and at baseline, and fasting plasma glucose at baseline. Both groups were also similar in terms of their lifestyle behavior at baseline (Supplementary Table 1).
3.2. Development of postpartum diabetes
Figure 1 shows the Kaplan–Meier curves for the development of diabetes based on the ADA 1997 (Figure 1A) and ADA 2012 (Figure 1B) criteria. The cumulative probability of developing postpartum diabetes according to ADA 1997 criteria at 24 months after randomization was 5% (95% confidence interval [CI]: 1–20%) and 3% (95% CI: 0–17%) in the vildagliptin and placebo groups, respectively. The HR for vildagliptin relative to placebo yielded 1.03 (95% CI: 0.15–7.36). When using the ADA 2012 criteria, the cumulative probability of postpartum diabetes after 24 months was 7% (95% CI: 2–21%) and 13% (95% CI: 6–28%) in the vildagliptin and placebo groups, respectively (HR: 0.59; 95% CI: 0.14–2.47).
Figure 1.
Cumulative probability of developing postpartum diabetes according to the 1997 ADA criteria (A) and 2012 ADA criteria (B) in women with a recent history of insulin-requiring GDM treated with 50 mg vildagliptin or placebo twice daily.
3.3. Development of postpartum IFG/IGT
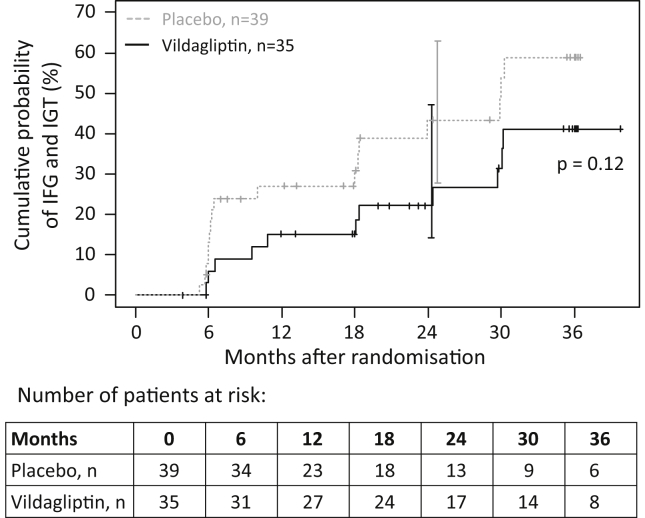
Seventy-four participants were included in the analysis of the development of IFG and/or IGT. The cumulative probability of IFG and/or IGT developing within 24 months after randomization was lower in the vildagliptin group than in the placebo group (22% [95% CI: 11–41%] vs 43% [95% CI: 28–63%]; Figure 2). However, this association was not statistically significant (HR: 0.55; 95% CI: 0.26–1.19).
Figure 2.
Cumulative probability of developing postpartum impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) in women with a recent history of insulin-requiring GDM treated with 50 mg vildagliptin or placebo twice daily.
3.4. Secondary outcomes
The secondary efficacy outcomes are presented in Figure 3, Figure 4. Women in the vildagliptin group started with a significantly higher insulinogenic index at screening/baseline (p = 0.02), but also experienced a significantly greater change between baseline and 24 months after randomization (p = 0.03) compared with the placebo group. However, there were no significant differences between the two groups in the other markers of β-cell function at baseline or during the follow-up (Figure 3A). Similarly, there were no significant differences in the screening/baseline values or the changes from baseline in markers of insulin resistance, including fasting insulin, HOMA insulin resistance or insulin-sensitivity index (Figure 3B). Regarding other efficacy measures, the vildagliptin group appeared to experience a reduction in HbA1c from screening/baseline to 12–30 months after randomization (Figure 4), which was statistically significant at 18 months (p = 0.02), but not at 12, 24, or 30 months (p ≥ 0.07). There also seemed to be a trend towards lower values of BMI and blood pressure in the vildagliptin group, but the changes in these values from screening/baseline were not significant at any time-point (Figure 4).
Figure 3.
Markers of β-cell function (A) and insulin resistance (B) in women with a recent history of insulin-requiring GDM treated with 50 mg vildagliptin or placebo twice daily. The markers of β-cell function were measured at baseline/screening and up to 36 months after randomization. Results are shown as the median (interquartile range). OGTT = oral glucose tolerance test; SMC = standard meal challenge. HOMA-IR = homeostatic model assessment of insulin resistance; ISI = insulin-sensitivity index.
Figure 4.
Other efficacy measures (glucose, HbA1c, GLP-1, GIP, BMI, waist-to-hip ratio, and blood pressure) in women with a recent history of insulin-requiring GDM treated with 50 mg vildagliptin or placebo twice daily. The efficacy variables were measured at baseline/screening and up to 36 months after randomization. Results are shown as the median (interquartile range). AUC = area under the curve; GIP = glucose-dependent insulinotropic polypeptide GLP-1 = glucagon-like peptide-1; HbA1c = hemoglobin A1c; OGTT = oral glucose tolerance test; SMC = standard meal challenge. HbA1c is expressed in %: 6.2% convert to 44 mmol/mol and 5.1% convert to 31 mmol/mol. BMI = body mass index.
3.5. Safety
Supplementary Table 2 shows the incidences of all adverse events that occurred in a frequency of more than 1% of all observed adverse events in either treatment group. No significant differences could be observed in the frequency of adverse events between the treatment groups. There was one serious adverse event (lipase increased), which has been related to vildagliptin treatment in a previous study [22], and led to discontinuation of treatment. This serious adverse event occurred in one woman in the vildagliptin group, who was found to have small gallstones, typical of cholelithiasis, on ultrasound. Therefore, the elevated lipase levels were probably related to bile duct obstruction in this woman. No other drug related serious adverse events were observed. We observed no significant differences between the two groups in the frequencies of laboratory safety parameters outside their respective normal ranges or outside 3x upper level of normal values, except for greater numbers of bilirubin levels above the normal range in the vildagliptin group and of triglyceride levels in the placebo group (Supplementary Table 3).
3.6. Lifestyle adherence
Adherence to lifestyle counseling is shown in Supplementary Figure 5. Physical activity (daily step count) and dietary intake of total energy, macronutrients, and fiber were similar in both groups throughout the study, and did not change significantly between baseline and 24 months after randomization (p = 0.45 for steps/day and p = 0.14 for energy intake/day).
4. Discussion
This study did not show significant superiority of administration of vildagliptin over placebo in the early post-delivery period in terms of reducing the risk of postpartum diabetes or abnormal glucose tolerance in women who required insulin to treat GDM. Likewise, variables related to glycemic control, insulin resistance, and β-cell function were not significantly different between the two treatment groups, although one could argue that vildagliptin promoted improvements in these variables relative to placebo. However, the study was terminated early as an interim analysis revealed that the number of diabetic events was considerably lower than anticipated and that the study was thus underpowered.
Despite that the study is not conclusive, it identified the critical issues in performing clinical trials in the early postpartum period in women with GDM hampering efficacy assessments.
The cumulative probability of developing postpartum diabetes in women with prior insulin-requiring GDM was 37% in the first 9 months postpartum in our previous GDM cohort [6], while in the present study, we excluded women who developed diabetes between delivery and the screening visit, which was conducted at a median of 8 months after delivery due to the contraindication to give vildagliptin during lactation. Because we excluded women with rapid progression to diabetes, our study might not reflect the true incidence of postpartum diabetes in women with prior GDM.
We must also acknowledge possible limitations of the study design, including the difficulty in recruiting the desired sample size. Based on our experience, only approximately 30% of women with GDM require insulin during pregnancy, which limits the potential population of eligible participants. Additionally, there was a relatively high discontinuation rate; it is possible that some women developed diabetes, IGT, or IFG after discontinuation. Finally, the study did not include a molecular mechanistic part to elucidate potential disease related pathways.
Several recent studies have provided evidence to suggest that lifestyle interventions may reduce the risk of postpartum diabetes in women with prior GDM [23], [24]. Both groups in our study received intensive diet and exercise interventions throughout the trial. This may have led to an overall reduction in the number of diabetic events thereby confounding efficacy assessments.
Although some studies have investigated pharmacological interventions to prevent the progression of pre-diabetes [8], [25], we are aware of only two such studies in women with prior GDM and neither enrolled women in the early post-delivery period to prevent postpartum diabetes. One study, the TRIPOD study [8], assessed the efficacy of troglitazone compared with placebo for the prevention of postpartum diabetes in 266 women with a history of GDM who were enrolled up to 4 years after delivery. In that study, the incidence of diabetes was reduced by 50% in the troglitazone group. However, the study was terminated early because troglitazone was removed from the market after reports of severe adverse events. The Diabetes Prevention Program [9] investigated the impact of intensive lifestyle interventions and metformin on the incidence of diabetes in people with elevated BMI, IGT and elevated fasting glucose, including 350 women with a history of GDM. In that study, participants were randomized to treatment at a mean of 12 years after delivery. Among women with prior GDM, the progression to diabetes was 35% lower in the intensive lifestyle intervention group and 40% lower in the metformin group compared with placebo suggesting that metformin may have a similar efficacy compared to intensive lifestyle interventions for reducing the risk of postpartum diabetes. Unlike the TRIPOD and Diabetes Prevention Program, we found no significant benefit of the study drug (in our case vildagliptin).
In addition to assessing the efficacy of vildagliptin, we also examined its safety in terms of adverse events and laboratory variables in this cohort of women. The safety data, including the types of adverse events, were generally consistent with the results of clinical trials of vildagliptin [26], [27] and suggest that there are no significant safety concerns associated with administering vildagliptin at a dose of 50 mg twice daily in this cohort of women.
In conclusion, in our study, administration of vildagliptin did not achieve a significant reduction in the risk of postpartum diabetes compared with placebo in women with prior insulin-requiring GDM at the time of the interim analysis. However, our study identified the critical issues in performing clinical trials in the early postpartum period in women with GDM hampering efficacy assessments. These included 1) slow enrollment, 2) high rates of discontinuation, 3) later than planned enrollment of participants because vildagliptin was contraindicated during lactation leading to the exclusion of women who developed diabetes before randomization and 4) the combination with active life style intervention reducing overall diabetes rates. Our experience in this study highlights the challenges encountered in studies of women with GDM. We believe that with this study we have set a basis for which properly powered trials could be performed in women with recent GDM.
Author contributions
SHu contributed to the lifestyle component of the study, interpreted the results, and drafted the manuscript. MP and AB undertook the statistical analyses and contributed to interpretation of the results. AH contributed to data acquisition as a study physician and critically reviewed the manuscript. DM and CP contributed to study administration and data acquisition. SHi, MB, and MHe were the study nurses and contributed to data acquisition. DK performed data management. NH performed data management and contributed to statistical analysis of the lifestyle data. JK performed measurements of incretins and critically reviewed the manuscript for intellectual content. MW, MHu, and MF contributed to protocol development and data acquisition. JH contributed to data coordination, statistical analysis, data safety monitoring, and critically reviewed the manuscript for intellectual content. AGZ is the study's principal investigator, conceived and designed the study, interpreted the results, wrote the manuscript, and critically reviewed the manuscript for intellectual content. She is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the contents of this manuscript. All authors have read and edited the manuscript, and approved the version to be published.
Funding
This study was funded by Forschergruppe Diabetes e.V., München-Neuherberg and was supported by grants from the Kompetenznetz Diabetes mellitus (Competence Network for Diabetes mellitus) funded by the Federal Ministry of Education and Research (FKZ 01GI0805-07). SHu has received funding from the European Union's HORIZON 2020 research and innovation program (grant agreement no. 633595 DynaHEALTH).
Acknowledgments
The authors thank Nicholas D. Smith, PhD, for medical writing support. Study medication was kindly provided by Novartis Pharma GmbH, Nuremberg, Germany.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.12.015.
Contributor Information
Anette-G. Ziegler, Email: anette-g.ziegler@helmholtz-muenchen.de.
PINGUIN Study Group:
Markus Walter, Heike Börschmann, Sophia Ebe, Eleni Giannopoulou, Minna Harsunen, Veronika Hofbauer, Anna Hofelich, Andrea Schuppenies, Maike Wallner, David Wiesenäcker, Stephanie Zillmer, Melanie Bunk, Melanie Herbst, Susanne Hivner, Lorenz Lachmann, Daniela Much, Claudia Peplow, Joerg Hasford, Markus Pfirrmann, Rüdiger Landgraf, Karl-Theo Maria Schneider, Elisabeth André, Viktoria Janke, Andreas Beyerlein, Sandra Hummel, Ezio Bonifacio, Martin Füchtenbusch, Michael Hummel, and Denise Kohn
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Physical activity (A) and dietary behavior (B) in women with a recent history of insulin-requiring GDM treated with 50 mg vildagliptin or placebo twice daily. Physical activity and dietary behavior were assessed at baseline/screening for up to 36 months after randomization. Black and grey bars represent the median in each group per visit.
References
- 1.International Diabetes Federation . 7th ed. International Diabetes Federation; Brussels, Belgium: 2015. IDF diabetes atlas.http://www.diabetesatlas.org [Google Scholar]
- 2.Melchior H., Kurch-Bek D., Mund M. The prevalence of gestational diabetes. Deutsches Ärzteblatt International. 2017;114(24):412–418. doi: 10.3238/arztebl.2017.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damm P., Houshmand-Oeregaard A., Kelstrup L., Lauenborg J., Mathiesen E.R., Clausen T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–1399. doi: 10.1007/s00125-016-3985-5. [DOI] [PubMed] [Google Scholar]
- 4.Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., Coustan D.R. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L., Casas J.P., Hingorani A.D., Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 6.Lobner K., Knopff A., Baumgarten A., Mollenhauer U., Marienfeld S., Garrido-Franco M. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. 2006;55(3):792–797. doi: 10.2337/diabetes.55.03.06.db05-0746. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler A.G., Wallner M., Kaiser I., Rossbauer M., Harsunen M.H., Lachmann L. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012;61(12):3167–3171. doi: 10.2337/db12-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan T.A., Xiang A.H., Peters R.K., Kjos S.L., Marroquin A., Goico J. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51(9):2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 9.Aroda V.R., Christophi C.A., Edelstein S.L., Zhang P., Herman W.H., Barrett-Connor E. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. Journal of Clinical Endocrinology & Metabolism. 2015;100(4):1646–1653. doi: 10.1210/jc.2014-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powe C.E., Allard C., Battista M.C., Doyon M., Bouchard L., Ecker J.L. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39(6):1052–1055. doi: 10.2337/dc15-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drucker D.J. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Molecular Endocrinology. 2003;17(2):161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- 12.Tuch B.E. Clinical use of GLP-1 agonists and DPP4 inhibitors. Pancreatology. 2016;16(1):8–9. doi: 10.1016/j.pan.2015.05.465. [DOI] [PubMed] [Google Scholar]
- 13.Dalle S., Burcelin R., Gourdy P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic beta-cell impairments in type 2 diabetes. Cellular Signalling. 2013;25(2):570–579. doi: 10.1016/j.cellsig.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y.J., Guo X., Li C.J., Li D.Q., Zhang J., Yang Y. Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism. 2015;64(2):226–235. doi: 10.1016/j.metabol.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Xu G., Stoffers D.A., Habener J.F., Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 16.Zander M., Madsbad S., Madsen J.L., Holst J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 17.Kleinwechter H., Schafer-Graf U., Buhrer C., Hoesli I., Kainer F., Kautzky-Willer A. Gestational diabetes mellitus (GDM) diagnosis, therapy and follow-up care: Practice Guideline of the German Diabetes Association(DDG) and the German Association for Gynaecologyand Obstetrics (DGGG) Experimental and Clinical Endocrinology & Diabetes. 2014;122(7):395–405. doi: 10.1055/s-0034-1366412. [DOI] [PubMed] [Google Scholar]
- 18.Toeller M., Frisch A., Müller-Wieland D. Fragebogen zur Erfassung der Nahrungsaufnahme in Risikogruppen (NARI) Diabetologie und Stoffwechsel. 2010;5(05):309–314. [Google Scholar]
- 19.Deutsche Gesellschaft für Ernährung, Oesterreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung, Schweizerische Vereinigung für Ernährung . 2016. Referenzwerte für die Nährstoffzufuhr. Bonn. 2. Auflage, 2. aktualisierte Ausgabe. [Google Scholar]
- 20.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. Erratum 2012;35(3):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girgis C.M., Champion B.L. Vildagliptin-induced acute pancreatitis. Endocrine Practice. 2011;17(3):e48–50. doi: 10.4158/EP10383.CR. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Ferre N., Del Valle L., Torrejon M.J., Barca I., Calvo M.I., Matia P. Diabetes mellitus and abnormal glucose tolerance development after gestational diabetes: a three-year, prospective, randomized, clinical-based, Mediterranean lifestyle interventional study with parallel groups. Clinical Nutrition. 2015;34(4):579–585. doi: 10.1016/j.clnu.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Guo J., Chen J.L., Whittemore R., Whitaker E. Postpartum lifestyle interventions to prevent type 2 diabetes among women with history of gestational diabetes: a systematic review of randomized clinical trials. Journal of Womens Health (Larchmt) 2016;25(1):38–49. doi: 10.1089/jwh.2015.5262. [DOI] [PubMed] [Google Scholar]
- 25.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomized trial. Lancet. 2002;359(9323):2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 26.Foley J.E., Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naive patients with type 2 diabetes. Hormone and Metabolic Research. 2009;41(12):905–909. doi: 10.1055/s-0029-1234042. [DOI] [PubMed] [Google Scholar]
- 27.Goke B., Hershon K., Kerr D., Calle Pascual A., Schweizer A., Foley J. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naive patients with type 2 diabetes: comparison with metformin. Hormone and Metabolic Research. 2008;40(12):892–895. doi: 10.1055/s-0028-1082334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Physical activity (A) and dietary behavior (B) in women with a recent history of insulin-requiring GDM treated with 50 mg vildagliptin or placebo twice daily. Physical activity and dietary behavior were assessed at baseline/screening for up to 36 months after randomization. Black and grey bars represent the median in each group per visit.