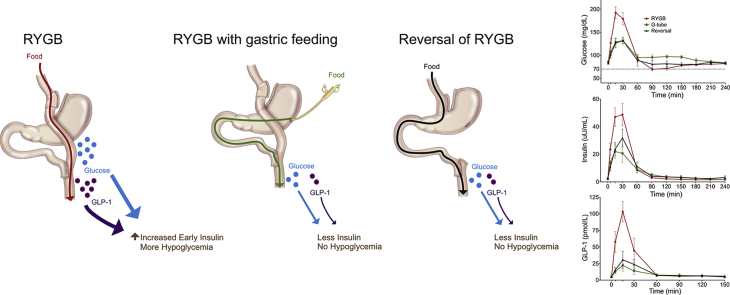
Abstract
Objective
Postprandial hypoglycemia is an infrequent but disabling complication of Roux-en-Y gastric bypass (RYGB) surgery. Controversy still exists as to whether the postprandial hyperinsulinemia observed is due to inherent changes in pancreatic β-cell mass or function or to reversible alterations caused by RYGB anatomy. We aimed to determine if gastric feeding or reversal of RYGB would normalize postprandial glucose and hormone excursions in patients with symptomatic hypoglycemia.
Methods
We completed a prospective study of six patients with severe symptomatic RYGB hypoglycemia who underwent RYGB reversal. An additional subject without hypoglycemia who underwent RYGB reversal was also studied prospectively. Mixed meal tolerance testing (MTT) was done orally (RYGB anatomy), via gastrostomy tube in the excluded stomach in the setting of RYGB, and several months after RYGB reversal.
Results
All subjects reported symptomatic improvement of hypoglycemia after reversal of RYGB. Weight gain after reversal was moderate and variable. Postprandial glucose, insulin, and GLP-1 excursions were significantly diminished with gastric feeding and after reversal. Insulin secretion changed proportional to glucose levels and insulin clearance increased after reversal. Glucagon/insulin ratios were similar throughout study. We further compared the impact of modified sleeve gastrectomy reversal surgery to those with restoration of complete stomach and found no significant differences in weight regain or in postprandial glucose or hormone levels.
Conclusions
Reversal of RYGB is an effective treatment option for severe postprandial hypoglycemia. The pathophysiology of this disorder is primarily due to RYGB anatomy resulting in altered glucose, gut, and pancreatic hormone levels and decreased insulin clearance, rather than inherent β-cell hyperplasia or hyperfunction.
Keywords: Hypoglycemia, Insulin, Glucagon-like peptide 1, Roux en Y gastric bypass, Gastric bypass reversal, Bariatric surgery
Abbreviations: AUC, area under the curve; G-tube, gastrostomy tube; GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide 1; GTT, glucose tolerance testing; HOMA-IR, Homeostatic model assessment-insulin resistance index; MTT, mixed meal tolerance test; PP, pancreatic polypeptide; PYY, Peptide YY; RYGB, Roux-en-Y gastric bypass
Graphical abstract
Highlights
-
•
Prospective study of gastric feeding and bypass reversal in hypoglycemic subjects.
-
•
Altered gut anatomy is the main driver of postmeal glucose and hormone excursions.
-
•
Beta-cell function is not inherently altered in gastric-bypass related hypoglycemia.
-
•
Bypass reversal is effective therapy for gastric-bypass related hypoglycemia.
1. Introduction
Roux-en-Y gastric bypass (RYGB) surgery is the most commonly performed bariatric procedure worldwide and has dramatic effects on weight loss and diabetes remission [1], [2]. Postprandial hypoglycemia is recognized as a late complication of RYGB. The prevalence of this complication in patients after RYGB remains unclear. The prevalence has been estimated as low as 0.1%, based on self-reporting of hypoglycemic episodes or hospital admissions for hypoglycemia or related symptoms [3], [4]. However, another study identified much higher prevalence of 34% based on self-reported symptoms consistent with hypoglycemia [5]. Direct testing of RYGB patients with glucose tolerance tests or continuous glucose monitoring has found highly variable rates of hypoglycemia, ranging from 10 to 70% [6], [7], [8], [9]. It appears that symptomatic hypoglycemia occurs in a relatively small subset of patients after RYGB, and most of these can be adequately treated with dietary therapy. However, some patients develop severe post-prandial hypoglycemia after RYGB, resulting in multiple daily episodes of hypoglycemia, seizures, loss of consciousness and a significant decrease in quality of life and safety. Therefore, identifying the cause and effective treatments for this disorder is essential.
The initial report of hypoglycemia after RYGB suggested that the etiology was related to nesidioblastosis, or overgrowth of the pancreatic β-cells, leading to excessive insulin secretion [10]. However, subsequent analysis of the same pancreatic samples found no evidence of β-cell hyperplasia [11]. Despite these conflicting reports, partial pancreatectomy has been advocated as a treatment for these patients. Of patients who underwent partial pancreatectomy, 90% experienced recurrent symptoms and 25% experienced no improvement in quality of life [12]. Therefore, this invasive procedure has a low success rate, further raising the question whether excess β-cell mass or function truly contributes to the etiology of this disorder.
We hypothesized that the pathophysiology of this syndrome is not due to inherent changes in pancreatic β-cell mass or function, but due to reversible alterations caused by RYGB anatomy. Thus, RYGB reversal would be an effective treatment approach for patients with RYGB hypoglycemia. We previously published the surgical approach and described symptomatic improvement in four of these patients after laparoscopic RYGB reversal [13]. In this study, we provide detailed metabolic and hormonal profiling after mixed meal tolerance tests (MTT) in six subjects with RYGB hypoglycemia, prospectively comparing responses to oral intake, intake through a gastrostomy tube (G-tube) inserted into the excluded stomach, and oral intake after RYGB reversal. In this way, we can identify factors that vary dependent on the route of food delivery in the same patient, rather than comparisons of different patients with and without RYGB and hypoglycemia. The use of each patient as their own internal control is a unique characteristic of our study that provides key insights into the causality of hypoglycemia in RYGB. We are also able to provide information on metabolic outcomes after RYGB reversal to normal anatomy or sleeve gastrectomy.
2. Materials and methods
2.1. Subjects
The University of Wisconsin (UW) Institutional Review Board approved the study. Written informed consent was obtained for MTT and subsequent hormonal analysis. G-tube placement and RYGB reversal surgery were performed independent of the research protocol as clinically indicated for the treatment of complications of RYGB. Hypoglycemic subjects had the G-tube placement before reversal in order to evaluate glucose control while feeding through the excluded stomach. Subjects were recruited from clinical practice in UW Endocrinology or Bariatric Surgery clinics with a history of RYGB and frequent episodes of symptomatic postprandial hypoglycemia. All six symptomatic subjects had a clinical diagnosis of post-RYGB hypoglycemia, based on obtaining history of Whipple's triad (hypoglycemia with venous glucose values less than 55 mg/dL occurring with symptoms and resolved by food intake) and ruling out alternate causes of hypoglycemia. Home blood glucose monitoring during symptoms with either glucometer or continuous glucose monitor similarly confirmed hypoglycemia with frequent readings below 55 mg/dL. Fasting glucose, insulin, C-peptide, early morning cortisol, and thyroid stimulating hormone were assessed in all patients and were normal. All subjects had at least 2 hypoglycemic episodes (glucose <60 mg/dL) per week on home capillary glucose readings or continuous glucose monitoring. Oral glucose tolerance testing (GTT) was performed as part of diagnostic workup and all hypoglycemic subjects were symptomatic with glucose ≤ 60 mg/dL during the GTT. Importantly, the diagnosis of hypoglycemia was not based solely on results of the GTT, this was simply an adjunct to other clinical information and diagnostic workup. Several subjects had developed some degree of hypoglycemic unawareness by the time the decision was made to proceed to reversal surgery. Dietary therapy and medical therapy with acarbose (titrated to maximally tolerated dose or 100 mg with each meal) were attempted in all subjects. A subset of subjects had additional medical therapy trials of somatostatin analogue, diazoxide, prednisone, and/or calcium channel blockers. Side effects of some medical therapies resulted in discontinuation. Not all subjects were able to fully comply with dietary recommendations. Despite best attempts at dietary and medical therapy, all subjects had persistent, frequent episodes of hypoglycemia. An additional patient was recruited with a history of RYGB surgery, but no symptomatic hypoglycemia. All patients were candidates for RYGB reversal based on improved symptoms with G-tube feeding. Patients were offered to have the G-tube remain in place for feeding and possible sole therapy; however, none chose that option. Further details of the individual subjects are provided in Table 1. The surgical technique for reversal has been previously described [13], but essentially the gastric pouch was reconnected to the stomach and the alimentary limb was excised.
Table 1.
Clinical characteristics of subjects.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Average (±SD) |
|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | F | F | F | F | |
| Age at RYGB (yrs) | 46 | 35 | 40 | 28 | 32 | 37 | 25 | 35 ± 7 |
| BMI before RYGB (kg/m2) | 38 | 50 | 50 | 37 | 47 | 49 | 54 | 46 ± 7 |
| Preop DM? | no | no | no | no | no | prediabetes | no | |
| Onset of sxs (months post bypass) | 12 | 19 | 6 | 56 | 100 | 32 | asymptomatic | 37.5 ± 36 |
| Other problems | hypopara-thyroid | nausea/vomiting | reflux | anemia | hypopara-thyroid | |||
| Complications of hypoglycemia | car accident, LOC | car accident | Hand callous/inability to monitor glucose | seizures | severe headaches | seizures | n/a | |
| Nadir glucose with OGTT (mg/dL) | 44 | 44 | 38 | 60 | 46 | 54 | 64 | 50 ± 9.5 |
| HOMA-IR at diagnosis (from OGTT) | 2.08 | 0.85 | 0.61 | 1.58 | 1.12 | 1.54 | 0.92 | 1.2 ± 0.5 |
| Hemoglobin A1C at diagnosis | 5.70% | 5.50% | 5.50% | 5.60% | 5.00% | 5.60% | n/a | 5.48 ± 0.2% |
| Treatment modalities implemented prior to reversal | Dietary, acarbose, verapamil | Dietary, acarbose, diazoxide, verapamil, octreotide | Dietary, acarbose, diazoxide | Dietary, acarbose | Dietary, acarbose, prednisone | Dietary, acarbose | n/a | |
| BMI at reversal (kg/m2) | 27.5 | 24 | 33 | 28 | 30 | 23 | 28 | 27 ± 3.5 |
| Reversal surgery date (months post bypass) | 39 | 41 | 48 | 93 | 122 | 55 | 33 | 62 ± 33 |
| Reversal anatomy | normal | sleeve gastrectomy | sleeve gastrectomy | sleeve gastrectomy | sleeve gastrectomy | normal | normal | |
| Weight change from G-tube MTT to post op MTT (lbs/BMI in kg/m2) | 21 lbs +3.2 BMI |
−21 lbs −3.9 BMI |
28 lbs +3.2 BMI |
0 lbs | −22 lbs −3.5 BMI |
17 lbs +3.1 BMI |
−14 lbs −2.4 BMI |
2 ± 19 lbs 0 ± 3.2 BMI |
| Total weight change with reversal (lbs/BMI in kg/m2) | 38 lbs +5.8 BMI |
11 lbs +2 BMI |
128 lbs +16.4 BMI |
37 lbs +5.1 BMI |
2 lbs +0.3 BMI |
19 lbs +3.5 BMI |
13 lbs +2.2 BMI |
35 ± 43 lbs 5 ± 5.3 BMI |
| BMI at last follow up after reversal (kg/m2) | 33.3 | 26.3 | 49.2 | 33 | 30.3 | 26.2 | 30 | 33 ± 8 |
| Months of followup after reversal | 47 | 18 | 34 | 20 | 9 | 3 | 11 | 20 ± 15 |
2.2. Procedures
All subjects had a G-tube placed in the excluded stomach. MTT was delayed until at least 4 weeks after G-tube insertion. Subjects underwent three separate MTTs. First, they consumed the meal orally, which resulted in absorption via the RYGB anatomy. They completed a second MTT within 1–3 days (except for one patient who did not complete for ∼3 months) via G-tube. The G-tube test is intended to mimic the effects of RYGB reversal. Finally, the subjects returned an average of 4 months after they had RYGB reversal surgery for a final MTT (range 3–6.9 months). The final meal was consumed orally, with nutrient delivery through the reversal anatomy (esophagus, stomach, pylorus, duodenum, and into small bowel).
After a 10 h fast, the liquid meal (Ensure (Abbott Laboratories); 250 kcal in 237 mL, 23 g simple sugars in a total of 40 g carbohydrate, 6 g of fat, and 9 g of protein) was consumed (orally or via G-tube administration) within 15 min. Samples were collected at baseline (fasting) and 5, 15, 30, 60, 90, 120, 150, 180, 210, and 240 min after completion of the meal. All blood samples were collected into lavender top blood collection tubes containing dipeptidyl peptidase 4 (DPP4) inhibitor (Millipore), aprotinin (Sigma), and AEBSF (4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride) (Sigma), mixed and stored on ice until serum was collected, aliquoted, and frozen at −80 °C.
2.3. Assays
Glucose was analyzed at University of Wisconsin Hospital Clinical Lab. Analysis of hormone levels was done with the following ELISA kits from Millipore: Human Insulin-# EZHI-14K, Human C-peptide-# EZHCP-20K, Glucagon-Like Peptide-1 (active)-#EGLP-35K, Human GIP (total)-# EZHGIP-54K, Human Pancreatic Polypeptide (PP)-# EZHPP40K, Human PYY (total)-# EZHPYYT66K. Glucagon was measured with an RIA kit from Millipore: Glucagon-#GL-32K. Some analyses of GIP, PP, and PYY were done with Millipore Milliplex multi-analyte profiling #HGT69K. Internal quality control ensured that samples run with Milliplex vs. single ELISA assay gave similar results. The samples run with Milliplex were equally distributed throughout the groups (RYGB vs. G-tube vs. Reversal).
2.4. Analysis
Rates of rise or fall in glucose or insulin values were calculated as the slope (difference in value divided by time) from baseline (time = 0) value to peak value (rise) or from peak value to nadir value (fall). Pre-hepatic insulin secretion rates (ISR) were calculated from deconvolution of C-peptide measurements using the Insulin SECretion (ISEC) program [14]. Insulin clearance was calculated as the incremental area under the curve (iAUC) (0–150 min) of pre-hepatic insulin secretion/iAUC (0–150 min) of systemic insulin. AUCs were calculated with time zero value as a baseline using the trapezoidal model. Homeostatic model assessment-insulin resistance index (HOMA-IR) was calculated as fasting glucose × fasting insulin/405. Data were analyzed with GraphPad Prism and SPSS software. Paired t-tests using Wilcoxon signed rank tests were used to compare AUC, to preserve intra-individual comparisons. Pearson correlations were used to assess linear association between insulin secretion rate and glucose or GLP-1 values.
3. Results
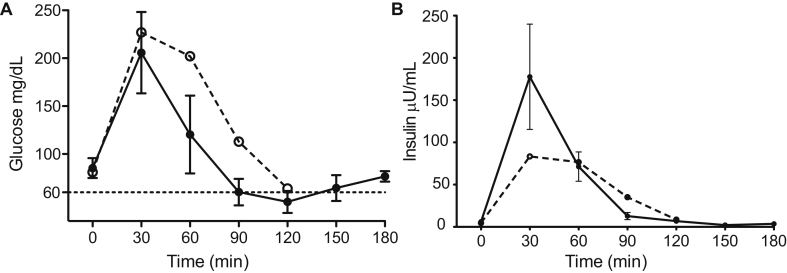
We recruited seven subjects with a clinical indication for RYGB reversal. Six of the subjects had RYGB hypoglycemia that was persistent and severe despite dietary and medical management. The seventh subject did not have symptomatic hypoglycemia but had recurrent hypocalcemia secondary to hypoparathyroidism that was the result of inadvertent parathyroidectomy during a thyroidectomy. The clinical characteristics of the subjects are detailed in Table 1. Hypoglycemic symptoms developed an average of 3 years after RYGB. None had a prior diagnosis of diabetes, and all had normal hemoglobin A1C levels at the time of diagnosis of hypoglycemia. A 75 g oral GTT confirmed the diagnosis and all hypoglycemic subjects reached a nadir glucose of ≤60 mg/dL between 90 and 120 min after glucose challenge with symptoms of hypoglycemia (Figure 1A). A pattern of rapid and robust insulin secretion after glucose administration was seen, as has been previously described in RYGB hypoglycemia [15] (Figure 1B). Notably, the rise in insulin was blunted in the one asymptomatic patient, despite a significant early rise in glucose levels. Patients underwent laparoscopic reversal of RYGB, to either “normal” anatomy or with a modified sleeve gastrectomy, as previously described [13]. The average BMI at the time of reversal was 27, and the reversal surgery was an average of 5 years after the original RYGB surgery. Insulin sensitivity, as measured by HOMA-IR, did not change significantly in the months between initial MTT and reversal MTT (0.41 vs. 0.49, p = 0.38), and no subjects had a HOMA-IR value greater than 1 at the time of reversal MTT, indicating no significant insulin resistance had developed despite some weight gain. On average, there was no weight change from the time of G-tube MTT to the post-reversal MTT, although individuals ranged from 22 pounds weight loss (or −3.5 BMI units) to 28 pounds weight gain (or 3.2 BMI units) (Table 1). At mean follow-up of 20 months (range 3–47) after RYGB reversal, no recurrent episodes of neuroglycopenia were reported and all subjects reported significant improvement in severity and frequency of hypoglycemic episodes. As previously reported, in four of six patients, the average number of hypoglycemic episodes per week decreased from 18.5 ± 12.4 to 1.5 ± 1.9 after reversal (P = .05) [13].
Figure 1.
Glucose (A) and Insulin (B) responses during 75 g oral glucose tolerance testing (GTT) via the RYGB anatomy in 6 symptomatic (solid line and closed circle) and 1 asymptomatic (dashed line and open circle) subject. Error bars represent standard error of the mean.
3.1. MTT: glucose and insulin
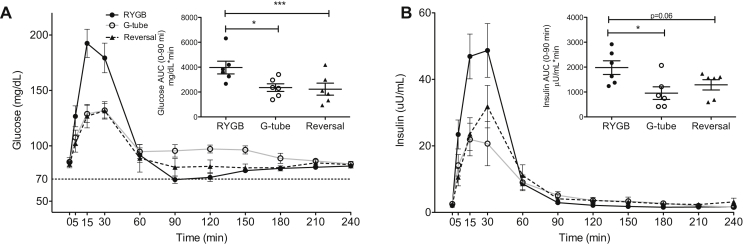
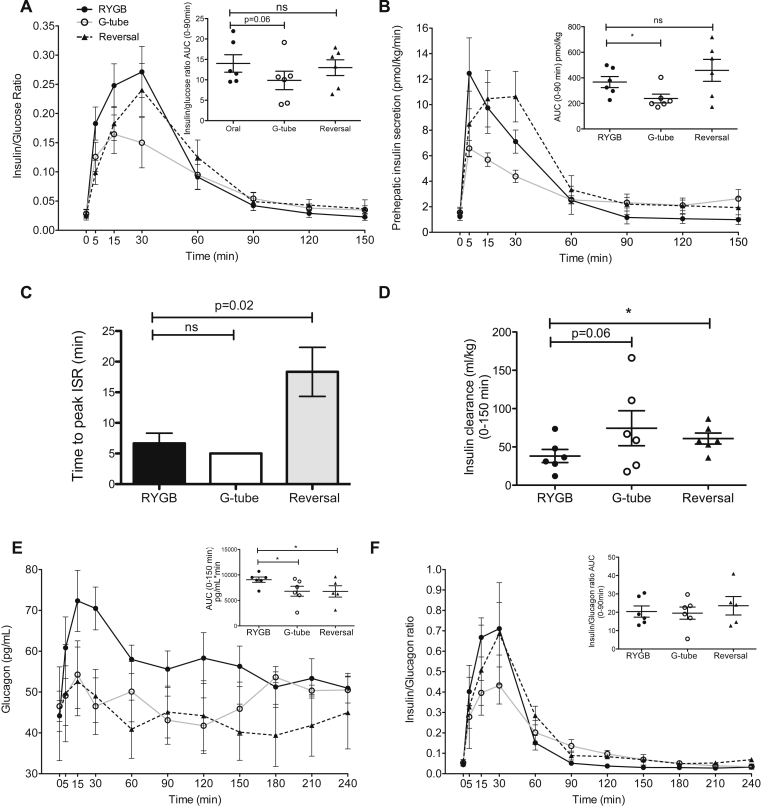
We measured glucose and insulin levels during the meal tests. Data in Figure 2, Figure 3, Figure 4 are from six symptomatic hypoglycemic patients only. Fasting glucose and fasting insulin levels did not differ across the duration of the study. There was a dramatic difference in glucose excursion comparing RYGB meal to either G-tube or reversal (Figure 2A). The rate of glucose rise and fall with RYGB meal was increased compared to G-tube or post-reversal (glucose rise: 6.3 vs. 2.6 vs. 2.9 mg/dl/min, p = 0.03, glucose fall: 1.9 vs. 0.75 vs 0.9 mg/dl/min, p = 0.06). Nadir glucose was lower in RYGB compared to G-tube (67 vs. 87 mg/dL, p = 0.03), even though frank hypoglycemia did not occur during MTT. Peak glucose was also higher after RYGB feeding (193 vs. 135 vs. 133 mg/dL, p = 0.03). These changes in glucose were paralleled by a similar pattern for insulin, with higher insulin levels in RYGB feeding during the first 90 min (Figure 2B). We also found a more rapid rise and fall in insulin levels with RYGB feeding compared to G-tube feeding (insulin rise: 2.59 vs. 1.15 μU/mL/min, p = 0.03; insulin fall: 0.36 vs. 0.13 μU/mL/min, p = 0.03). Notably, G-tube feeding essentially normalized postprandial glucose and insulin levels. There were no overall differences in insulin to glucose ratio, suggesting that the insulin response is proportional to the concurrent glucose (Figure 3A). Overall pre-hepatic insulin secretion was reduced with G-tube feeding compared to RYGB feeding, consistent with the reduction in overall insulin levels (Figure 3B). Pre-hepatic insulin secretion was not different overall after reversal, compared to RYGB, however there was a left shift in the RYGB insulin secretion compared to reversal (time to peak insulin secretion rate shorter in RYGB (6.7 min) vs. Reversal (18.3 min), p = 0.02) (Figure 3C). We also calculated an empirical measure of β-cell function using the ratio of the integral of the glucose concentration to the integral of the insulin secretion rate over the first 150 min [16] and found that there was no evidence of increased β-cell function in the RYGB MTT, and in fact there was a trend toward an increase after reversal (0.034 vs. 0.032 vs. 0.049, all differences non-significant). Insulin clearance trended higher with gastric feeding and was increased after reversal (Figure 3D).
Figure 2.
Glucose (A) and Insulin (B) responses to mixed meal tolerance testing (MMT) orally via the RYGB anatomy (black solid line and closed circle), via G-tube (black dashed line and triangle) and orally after reversal (gray solid line and open circle), *P < .05, **P < .01, ***P < .0001. Error bars represent standard error of the mean.
Figure 3.
Insulin and glucagon dynamics during MTT. Insulin:glucose ratio (A), pre-hepatic insulin secretion rates (ISR) (B), time to peak of ISR (C), insulin clearance (D), glucagon (E) and insulin:glucagon ratio (F) during mixed meal testing. Oral via the RYGB anatomy (black solid line and closed circle, black bar), via G-tube (black dashed line and triangle, white bar) and orally after reversal (gray solid line and open circle, gray bar), ns not significant, *P < .05. Error bars represent standard error of the mean.
Figure 4.
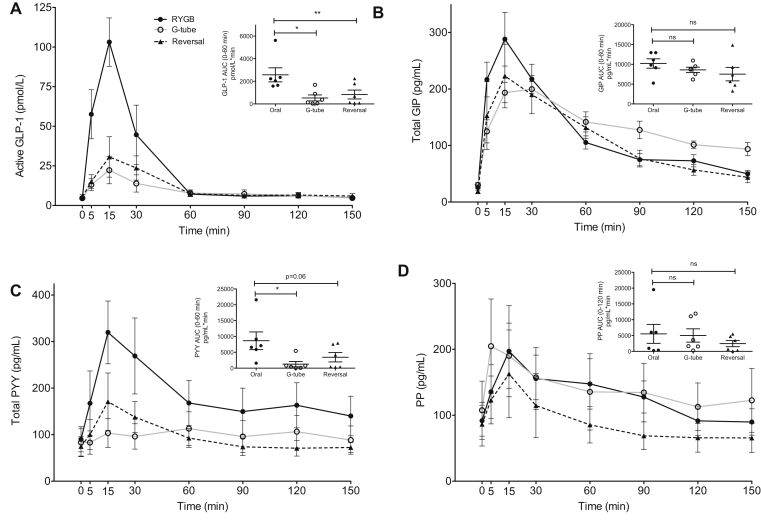
Gut hormone profiles during MTT. Active glucagon-like peptide 1 (GLP-1) (A), total glucose-dependent insulinotropic peptide (GIP) (B), total peptide YY (PYY) (C), and pancreatic polypeptide (PP) (D) responses during MTT. Orally via the RYGB anatomy (black solid line and closed circle), via G-tube (black dashed line and closed triangle) and orally after reversal (gray solid line and open circle), ns not significant, *P < .05, **P < .01. Error bars represent standard error of the mean.
3.2. MTT: glucagon, GLP-1, GIP and PYY
Postprandial glucagon levels were higher with RYGB feeding (Figure 3E), yet there were no differences in insulin to glucagon ratios (Figure 3F).
Gastric feeding and RYGB reversal dramatically lowered post-prandial GLP-1 levels, compared to RYGB (Figure 4A). There was no significant difference in gastric inhibitory peptide (GIP) levels across the different studies (Figure 4B). Peptide YY (PYY) levels were also decreased with G-tube feeding and trended toward a decrease after reversal (Figure 4C). Pancreatic polypeptide (PP) levels were not significantly changed across the study (Figure 4D).
3.3. Drivers of insulin secretion
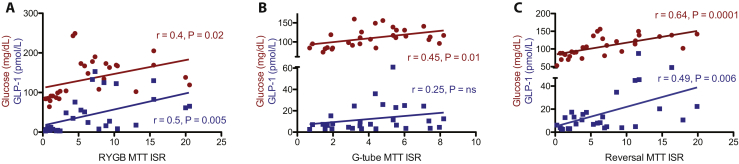
To determine the predominant drivers of insulin secretion, we assessed correlations between glucose and GLP-1 values with the insulin secretion rate over the first 60 min of the MTT. Linear correlations are shown in Figure 5. In RYGB MTT, the strongest correlation to insulin secretion rate (ISR) was with GLP-1 (r = 0.5, p = 0.005), although glucose was also positively correlated (r = 0.4, p = 0.02). However, with G-tube MTT, only glucose correlated with ISR (r = 0.45, p = 0.01). During the reversal MTT, glucose had the strongest correlation with ISR (r = 0.64, p = 0.0001), although GLP-1 was also positively correlated (r = 0.49, p = 0.006).
Figure 5.
Correlations between glucose (red) or glucagon-like peptide 1 (GLP-1) (blue) with insulin secretion rate (ISR) over the first 60 min of mixed meal tolerance testing (MTT) orally via the RYGB anatomy (RYGB), via G-tube and orally after reversal (Reversal). Pearson correlation r values and p values are shown on each graph in the corresponding color.
3.4. Outcomes in subject without symptomatic hypoglycemia
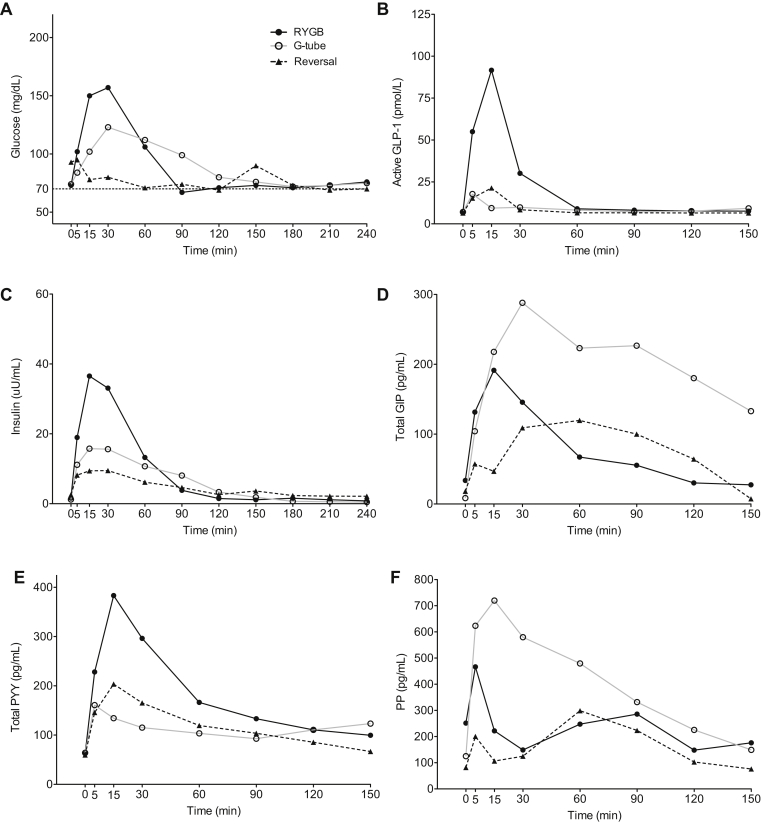
Finally, we present the results of our single patient without symptomatic hypoglycemia (Table 1, subject 7 and Figure 6) who also underwent RYGB reversal for the indication of hypoparathyroidism. This patient demonstrated similar responses to gastric feeding and RYGB reversal as the hypoglycemic patients, with reductions in postprandial glucose, insulin, GLP-1, and PYY. This patient did have a slightly different pattern of GIP response, with the most GIP production occurring in the G-tube MTT. This patient also had a very robust PP response in the G-tube MTT. The implications of these differences in a single patient compared to average responses in the symptomatic group are limited.
Figure 6.
Glucose (A), active glucagon-like peptide 1 (GLP-1) (B), insulin (C), total GIP (D), total peptide YY (PYY) (E), and pancreatic polypeptide (PP) (F) responses in the single asymptomatic patient during mixed meal testing orally via the RYGB anatomy (black solid line and closed circle), via G-tube (black dashed line and closed triangle) and orally after reversal (gray solid line and open circle).
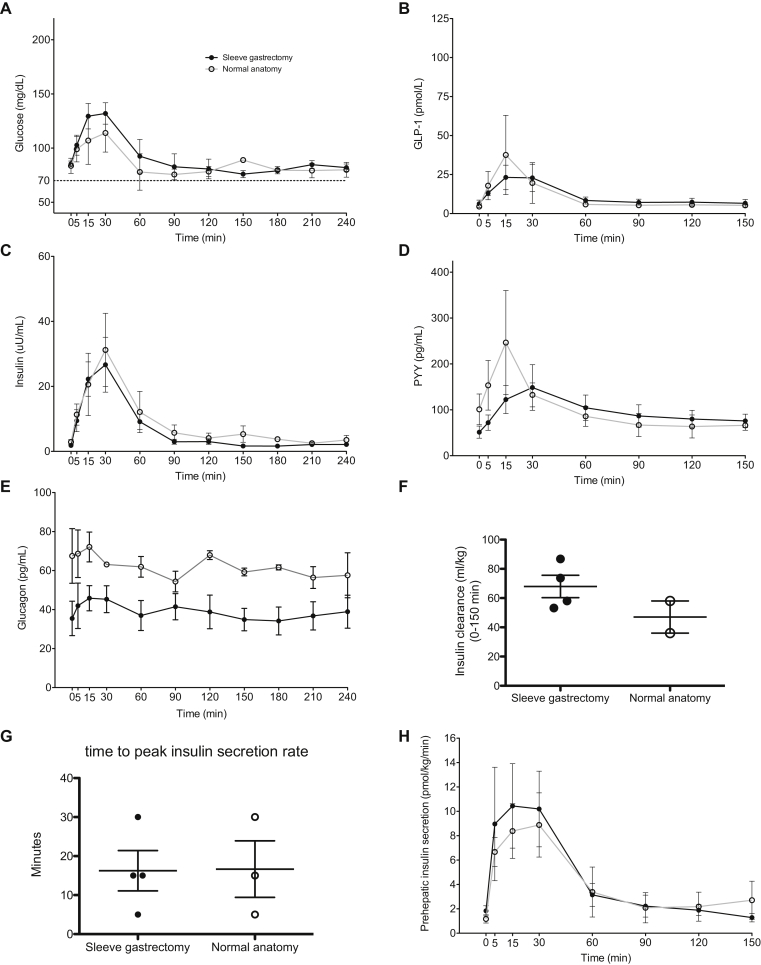
3.5. Reversal to modified sleeve gastrectomy versus normal anatomy
As some of our subjects received a modified sleeve gastrectomy upon RYGB reversal, we wanted to examine whether this had an impact on post-reversal response to mixed meal. Figure 7 shows the post-reversal MTT results when comparing those subjects with a sleeve gastrectomy (n = 4) to those with “normal” anatomy (n = 3) after reversal. Although there was slightly increased glucose excursion after the meal in the sleeve gastrectomy patients, this was not statistically significant as measured by AUC over 90 min (p = 0.67) (Figure 7A). There were no significant differences in postprandial insulin or GLP-1 levels (Figure 7B–C). Postprandial PYY levels appeared slightly higher in “normal” anatomy, however this was not statistically significant (p = 0.84) due to high variability. Fasting glucagon levels averaged higher in the patients with “normal” anatomy after reversal, but there was no dramatic change in glucagon levels with meal intake in either group after reversal (Figure 7E). We also saw no difference in insulin clearance between these groups (Figure 7F). We also saw no difference in insulin secretion (Figure 7G) or the time to reach peak insulin secretion rate (Figure 7H). Additionally, we saw no dramatic difference in the clinical outcome of recurrent hypoglycemia, with all subjects having minimal to no recurrent hypoglycemia on clinical follow up. We included the subject without pre-reversal hypoglycemia in this analysis as she increased the sample size of those with “normal” anatomy. None of the above findings differed based on inclusion of this asymptomatic subject.
Figure 7.
Comparison of sleeve gastrectomy to normal anatomy. Glucose (A), glucagon-like peptide 1 (GLP-1) (B), insulin (C), peptide YY (PYY) (D), glucagon (E), insulin clearance (F), time to peak insulin secretion (G) and prehepatic insulin secretion (H) during mixed meal testing after reversal to sleeve gastrectomy (black line and closed circles) and normal anatomy (gray line and open circles). Error bars represent standard error of the mean.
3.6. Longer term weight changes
While weight gain after reversal was variable over an average of 20 months of follow-up (35 ± 43 lbs, 5 ± 5.3 BMI units), only one patient regained a dramatic amount of weight (128 lbs), despite receiving a sleeve gastrectomy (Table 1). This subject (subject 3 in Table 1) was noncompliant with dietary advice for weight maintenance and was regaining weight even before the reversal surgery. At final follow up, all subjects had a BMI that was less than their original BMI prior to RYGB surgery (Table 1). Weight change did not differ when comparing those with sleeve gastrectomy on reversal compared to reversal to “normal” anatomy (44.5 pounds (5.95 BMI units) gain sleeve vs. 28.5 pounds (3.83 BMI units) gain “normal”, p = 0.57 (lbs) and 0.65 (BMI)).
4. Discussion
Hypoglycemia after RYGB surgery remains a poorly understood phenomenon with limited published information on effective therapy. We present here a study of six subjects with recalcitrant, symptomatic hypoglycemia after RYGB and show that RYGB reversal was an effective treatment and did not lead to dramatic weight regain in the majority of patients. Our study was unique in that it evaluated these patients prospectively with MTTs at baseline, with a G-tube into the excluded stomach, and after RYGB reversal. We found that gastric feeding and RYGB reversal dramatically reduced postprandial glucose, insulin, and GLP-1 excursions. Because the altered feeding route alone is sufficient to normalize postprandial response and we see a consistent correlation between glucose levels and insulin secretion rates, we conclude that there is no overgrowth of the pancreatic β-cells or inherent hyperfunction of the pancreatic β-cells, as has been suggested as a potential etiology of this disorder [10], [17]. Instead, we conclude that alteration in the timing and location of carbohydrate absorption with RYGB leads to increased postprandial glucose concentrations triggering robust, early insulin secretion. This can lead to overcorrection of glucose levels resulting in symptomatic hypoglycemia. However, these alterations in insulin secretion and subsequent hypoglycemia are reversible with alternate feeding routes via G-tube or reversal anatomy.
Our conclusions are consistent with a recent study by Patti et al. that found increased β-cell function in RYGB-hypoglycemic patients compared to non-hypoglycemic RYGB patients with oral MTT, but not after intravenous glucose administration. Due to the lack of response to intravenous glucose, their study also concludes that there is no inherent difference in β-cell function, but rather that gut-derived factors contribute to the enhanced post-prandial insulin secretion in hypoglycemic patients [18]. We also saw a shorter time to maximal insulin secretion with RYGB feeding (Figure 3C). A similar left shift in insulin secretion has been noted when comparing RYGB patients to controls in other studies [19]. Overall, this implies that insulin is secreted earlier, in response to the rapid rise in serum glucose at 5–15 min after the RYGB meal, but overall β-cell function does not differ.
Similar to our findings, a single case report described dramatic improvement in severe post-RYGB hypoglycemia when the patient had a G-tube placed in the remnant stomach for a small bowel obstruction [20]. In this patient, similar reductions in postprandial glucose, insulin, GLP-1, and glucagon were seen with G-tube versus oral feeding. Another single case reported improved postprandial symptoms (not hypoglycemia) and similar changes in glucose, insulin, and insulin secretion rate with G-tube feeding in a RYGB patient. However, GLP-1 levels did not decrease with G-tube feeding in this single subject [21]. Additionally, a few small case series have looked at gastric feeding in RYGB subjects without hypoglycemia [22], [23], [24] and similarly describe reduced postprandial glucose, insulin, and GLP-1 compared to oral feeding. Taken together, these case reports or small case series are consistent with our findings of normalized post-prandial responses with gastric feeding.
Two recent single case reports describe similar improvements in hypoglycemia after reversal of RYGB [25], [26]. However, in contrast, a case series of two hypoglycemic patients concluded that RYGB reversal was not effective therapy, as both had persistent hypoglycemia during MTT [27]. Although the surgical technique for reversal was generally the same as in our study, there were some notable differences in the results of MTT in these subjects compared to our study. First, both subjects had much higher postprandial insulin levels after reversal, despite reduced postprandial glucose. Therefore, the preserved insulin to glucose ratio that we observed (Figure 3A) was not seen in these subjects. Second, both subjects demonstrated a dramatic increase in postprandial GIP levels after reversal, while we saw no significant difference in GIP. The only consistent finding in this study was the reduction in postprandial GLP-1 with RYGB reversal. We note that the GLP-1 and GIP levels described in their study are markedly lower than those reported here and by others [15], and there was a significantly delayed insulin, GIP, and GLP-1 secretion profile of one of the subjects. While we cannot fully explain the differences between these subjects and those in our study, we postulate that at least one appears to be an outlier due to delayed postprandial responses perhaps from delayed gastric emptying after reversal. This subject also had a history of partial pancreatectomy prior to RYGB reversal, which may have contributed to altered responses. Notably both subjects in this study reported at least minimal improvement in hypoglycemic symptoms after RYGB reversal; while all of our patients reported symptomatic improvement in hypoglycemia after reversal and no further severe episodes of hypoglycemia occurred over long term follow up. Most recently, a retrospective case series described subjective clinical improvement in hypoglycemia in 6 of 8 subjects who underwent reversal, with and without sleeve gastrectomy [28].
These differences highlight the need for detailed attention to definitions when reporting on these complex and relatively rare patients, as varied definitions have been used to describe hyperinsulinemic hypoglycemia after RYGB and the outcomes in response to gastric feeding and/or reversal. Our report is on a selected group of patients with documented, recurrent, symptomatic hypoglycemia with associated hyperinsulinemia that remained uncontrolled despite dietary or drug therapy. We did not rely only on biochemical detection of hypoglycemia after an OGTT to diagnose patients. Up to 72% of patients with RYGB will have measured hypoglycemia after an OGTT, although often asymptomatic [7], [29]. We also used both objective and subjective long-term clinical follow up to define a successful outcome from the reversal, and did not rely solely on response to the MTT [13]. Other important unresolved issues are whether variations in the technique at index RYGB and/or at reversal surgery may impact hypoglycemia events and the ability to recover from hypoglycemia. Two of these unexplored technical issues are 1) the impact of disrupting vagal innervation at index RYGB (that may impact portal vein glucose sensing and liver and intestinal gluconeogenesis capacity) [30] or 2) whether the excision of the alimentary limb (by reducing the amount of L cells available to secrete GLP-1) may then contribute to the glucose and hormonal improvements [28]. Lastly, we and others have also reported that a proportion of patients that had reversal may experience perioperative complications or side effects associated to reversal surgery, such as recurrent or de novo gastroesophageal reflux disease, or other gastrointestinal symptoms [13], [28], [31]. Despite some of the differences to previous reports noted above, our study is unique in that all subjects are studied prospectively under all three conditions, and we are not describing case reports of 1 or 2 patients. Our findings in RYGB hypoglycemic subjects prior to reversal are consistent with postprandial responses seen throughout the literature, suggesting that we do not have any significant outliers in our group. Finally, our sample size, while still small, is large enough to make statistically significant comparisons.
A failure to secrete adequate glucagon in response to hypoglycemia could explain the etiology of RYGB hypoglycemia, although previous studies have actually shown increased postprandial glucagon secretion after RYGB [32], [33], [34]. A recent study suggested that enhanced postprandial glucagon secretion may contribute to the increased insulin levels in RYGB hypoglycemia due to glucagon's ability to potentiate insulin secretion in the presence of high levels of GLP-1 [35], [36]. We also found higher postprandial glucagon levels with RYGB compared to gastric feeding or reversal (Figure 3E). However, glucagon remained elevated in the setting of the most dramatic drop in glucose (RYGB at 60–90 min). We do not see any evidence that impaired glucagon production can explain postprandial hypoglycemia; however, we also do not see any rise in glucagon levels after the nadir glucose levels are reached. We also do not see any correlation of glucagon levels to insulin secretion rate in the RYGB MTT (Pearson r = 0.06, p = ns, data not shown) to suggest that glucagon is a predominant driver of insulin secretion. The insulin to glucagon ratios (Figure 3F) demonstrate that insulin predominates in the first 90 min and glucagon predominates thereafter in all three meal tests, indicating there is not an inherent imbalance between insulin and glucagon levels. The role of glucagon in the etiology of RYGB hypoglycemia remains unclear, but our data do not suggest that it plays a predominant role in the alterations in glucose or insulin levels in our patients.
Reduced hepatic insulin clearance is another proposed mechanism for RYGB-associated hypoglycemia [19], with resultant increased post-hepatic insulin levels contributing to the hypoglycemia. We found that RYGB reversal increased insulin clearance (Figure 3D). This is somewhat contradictory to other studies showing an increase in insulin clearance in RYGB patient compared to controls [32], [33]. However, one study found reduced insulin clearance as a feature of hypoglycemic RYGB subjects compared to asymptomatic RYGB subjects and postulated that this is a key pathophysiologic feature of this disorder [19]. Our results suggest that this reduced insulin clearance rate is not a fixed, inherent feature of these hypoglycemic patients but rather is a dynamic variable that can change in response to altered feeding routes and with RYGB reversal.
GLP-1 is dramatically elevated after RYGB and has been implicated as a causal factor in RYGB-associated hypoglycemia [15], [32]. Treatment with a GLP-1 receptor antagonist corrected postprandial hypoglycemia and reduced postprandial insulin secretion in subjects with RYGB hypoglycemia [32], [37]. Interestingly, benefits have also been noted with treatment with the GLP-1 receptor agonist, liraglutide [38]. It is possible that the continuous activation of GLP-1 receptors during long-acting agonist therapy blunts the impact of acute endogenous GLP-1 excursions after meals. Our results also suggest an important role for GLP-1, as the postprandial GLP-1 levels are dramatically reduced with gastric feeding or bypass reversal (Figure 4A). We also find that GLP-1 is a strong contributor to the enhanced insulin secretion rate in the RYGB, and its role in insulin secretion diminishes with G-tube or post-reversal feeding (Figure 5). Therefore, our findings are consistent with a contributory role for GLP-1 in the enhanced postprandial insulin secretion with RYGB, likely in combination with the rapid and large rise in glucose concentrations.
We also found a decrease in PYY levels with gastric feeding and reversal surgery. (Figure 4C). The role of PYY in RYGB hypoglycemia is unknown. Postprandial PYY levels are known to increase after RYGB [39]. However, no difference has been found in PYY levels when comparing hypoglycemic RYGB subjects to non-hypoglycemic RYGB controls [15]. PYY is secreted by enteroendocrine cells in the gut and can delay gastric emptying and promote satiety. PYY had initially been thought to stimulate postprandial insulin secretion, although recent studies find no significant impact of PYY on insulin secretion [40], [41], [42]. At this time, it is unclear if PYY is contributing to, or simply correlated with, the resolution of postprandial hyperinsulinemia and hypoglycemia in our study.
Sleeve gastrectomy was performed in several of our subjects at the time of RYGB reversal. We found similar improvements in hypoglycemic symptoms and no significant difference between the two post-reversal groups in glucose or insulin levels during MTT (Figure 7A,C). Additionally, we did not find a significant difference in postprandial GLP-1 when comparing the subjects with sleeve gastrectomy after reversal to those with “normal” anatomy after reversal (Figure 7B). This is consistent with evidence that sleeve gastrectomy does not lead to the dramatic elevations in GLP-1 seen in RYGB [43]. Post-prandial hypoglycemia after sleeve gastrectomy is not as well recognized clinically as after RYGB. After GTT, hypoglycemia has been reported as high as 33% in one study 12 months after sleeve gastrectomy [44]; however, the prevalence of self-reported hypoglycemia symptoms in sleeve gastrectomy is significantly lower than with RYGB [5]. Although our intention in performing sleeve gastrectomy upon RYGB reversal was to limit future weight gain, we did not find any significant difference in post-reversal weight gain dependent on the type of procedure (Table 1). Our results suggest that sleeve gastrectomy may be performed during RYGB-reversal and post-prandial hypoglycemia will be effectively ameliorated in these patients.
Many studies have compared the postprandial responses of symptomatic hypoglycemic RYGB subjects to asymptomatic RYGB subjects, and some subtle differences have been identified [15], [18], [19], [32]. However, pre-operative insulin sensitivity and β-cell responsiveness may be larger contributors to predicting which patients will develop symptomatic hypoglycemia after RYGB [45]. Our study was not designed to examine differences between asymptomatic and symptomatic hypoglycemia in RYGB. However, we present data from a single asymptomatic patient who also underwent RYGB reversal. The changes in postprandial hormone responses with gastric feeding and after reversal in this patient were very similar to those with symptomatic hypoglycemia (Figure 6), with the exception of higher GIP and PP levels after G-tube feeding. The results from this asymptomatic patient suggest that postprandial glucose, insulin, and GLP-1 responses are an inherent feature of the route of feeding, and are not highly specific for those suffering from RYGB-related hypoglycemia.
The treatment of RYGB-related hypoglycemia remains a challenge, particularly in severely affected patients as described in this study. Current treatment paradigms primarily rely on use of low carbohydrate diets and acarbose to limit postprandial glucose excursions [46], [47], [48]. Other traditional medical therapies to inhibit insulin secretion have been used, including somatostatin analogues, verapamil, and diazoxide, although these are not particularly effective and/or have significant side effects [49], [50]. Newer studies have examined the use of GLP-1 receptor antagonists and agonists as potential treatment strategies for this disorder [37], [38] and several other medical therapies are currently in development [51]. We propose that reversal of gastric bypass is an effective and safe treatment for RYGB-related hypoglycemia; however, it should only be considered in patients with severe symptoms that cannot be managed with dietary modification or available medical therapies. It may also be a useful option in patients with other malabsorptive complications from RYGB, who would have additional benefit from reversal surgery.
In conclusion, the pathophysiology of postprandial hypoglycemia seems to be primarily due to RYGB anatomy resulting in altered glucose, gut and pancreatic hormone levels, and decreased insulin clearance rather than inherent β-cell hyperplasia or hyperfunction. Furthermore, RYGB reversal is an effective treatment option in select patients with severe hypoglycemia.
Acknowledgements
DBD has been supported by the NIDDK (DK083442) and the University of Wisconsin Department of Medicine. GMC has been supported by the University of Wisconsin Department of Surgery. The project was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Science (NCATS) grant UL1TR000427. The William S. Middleton Memorial Veterans Hospital provided resources and use of facilities. The content is solely the responsibility of the authors and does not represent the views of the NIH, Department of Veterans Affairs, or the United States Government.
Contributor Information
Dawn Belt Davis, Email: dbd@medicine.wisc.edu.
Guilherme M. Campos, Email: guilherme.campos@vcuhealth.org.
Disclosure summary
The authors have nothing to disclose.
Author contributions
DBD was involved in study concept and design, acquisition of data, analysis and interpretation of data and writing of manuscript; JK was involved in acquisition of data, analysis and interpretation of data and critical revision of manuscript; MZ, SS, and JH were involved in acquisition of data and data analysis; GMC was involved in study concept and design, analysis and interpretation of data, writing and critical revision of the manuscript.
Conflict of interest
None declared.
References
- 1.Zimmet P.P., Alberti K.G.M.M.K. Surgery or medical therapy for obese patients with type 2 diabetes? New England Journal of Medicine. 2012;366(17):1635–1636. doi: 10.1056/NEJMe1202443. [DOI] [PubMed] [Google Scholar]
- 2.Schauer P.R., Bhatt D.L., Kirwan J.P., Wolski K., Aminian A., Brethauer S.A. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. New England Journal of Medicine. 2017;376(7):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsk R., Jonas E., Rasmussen F., Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53(11):2307–2311. doi: 10.1007/s00125-010-1798-5. [DOI] [PubMed] [Google Scholar]
- 4.Sarwar H., Chapman W.H., Pender J.R., Ivanescu A., Drake A.J., Pories W.J. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obesity Surgery. 2014;24(7):1120–1124. doi: 10.1007/s11695-014-1260-8. [DOI] [PubMed] [Google Scholar]
- 5.Lee C.J., Clark J.M., Schweitzer M., Magnuson T., Steele K., Koerner O. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity. 2015;23(5):1079–1084. doi: 10.1002/oby.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pigeyre M., Vaurs C., Raverdy V., Hanaire H., Ritz P., Pattou F. Increased risk of OGTT-induced hypoglycemia after gastric bypass in severely obese patients with normal glucose tolerance. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2015;11(3):573–577. doi: 10.1016/j.soard.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Roslin M.S., Oren J.H., Polan B.N., Damani T., Brauner R., Shah P.C. Abnormal glucose tolerance testing after gastric bypass. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2013;9(1):26–31. doi: 10.1016/j.soard.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamsson N., Edén Engström B., Sundbom M., Karlsson F.A. Hypoglycemia in everyday life after gastric bypass and duodenal switch. European Journal of Endocrinology. 2015;173(1):91–100. doi: 10.1530/EJE-14-0821. [DOI] [PubMed] [Google Scholar]
- 9.Kefurt R., Langer F.B., Schindler K., Shakeri-Leidenmühler S., Ludvik B., Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2015;11(3):564–569. doi: 10.1016/j.soard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Service G.J., Thompson G.B., Service F.J., Andrews J.C., Collazo-Clavell M.L., Lloyd R.V. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. New England Journal of Medicine. 2005;353(3):249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 11.Meier J.J., Butler A.E., Galasso R., Butler P.C. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care. 2006;29(7):1554–1559. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 12.Vanderveen K.A., Grant C.S., Thompson G.B., Farley D.R., Richards M.L., Vella A. Outcomes and quality of life after partial pancreatectomy for noninsulinoma pancreatogenous hypoglycemia from diffuse islet cell disease. Surgery. 2010;148(6):1237–1245. doi: 10.1016/j.surg.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos G.M., Ziemelis M., Paparodis R., Ahmed M., Davis D.B. Laparoscopic reversal of Roux-en-Y gastric bypass: technique and utility for treatment of endocrine complications. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2014;10(1):36–43. doi: 10.1016/j.soard.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hovorka R., Soons P.A., Young M.A. ISEC: a program to calculate insulin secretion. Computer Methods and Programs in Biomedicine. 1996;50(3):253–264. doi: 10.1016/0169-2607(96)01755-5. [DOI] [PubMed] [Google Scholar]
- 15.Goldfine A.B., Mun E.C., Devine E., Bernier R., Baz-Hecht M., Jones D.B. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. Journal of Clinical Endocrinology & Metabolism. 2007;92(12):4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 16.Mari A., Schmitz O., Gastaldelli A., Oestergaard T., Nyholm B., Ferrannini E. Meal and oral glucose tests for assessment of beta -cell function: modeling analysis in normal subjects. American Journal of Physiology - Endocrinology and Metabolism. 2002;283(6):E1159–E1166. doi: 10.1152/ajpendo.00093.2002. [DOI] [PubMed] [Google Scholar]
- 17.Vella A., Service F.J. Incretin hypersecretion in post-gastric bypass hypoglycemia primary problem or red herring? Journal of Clinical Endocrinology & Metabolism. 2007;92(12):4563–4565. doi: 10.1210/jc.2007-2260. [DOI] [PubMed] [Google Scholar]
- 18.Patti M.-E., Li P., Goldfine A.B. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity. 2015;23(4):798–807. doi: 10.1002/oby.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salehi M., Gastaldelli A., D'Alessio D.A. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. The Journal of Clinical Endocrinology and Metabolism. 2014;99(6):2008–2017. doi: 10.1210/jc.2013-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin T., Peck M., Holst J., Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. The Journal of Clinical Endocrinology and Metabolism. 2010;95(4):1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 21.Salehi M., Gastaldelli A., D'Alessio D.A. Evidence from a single individual that increased plasma GLP-1 and GLP-1-stimulated insulin secretion after gastric bypass are independent of foregut exclusion. Diabetologia. 2014;57(7):1495–1499. doi: 10.1007/s00125-014-3258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindqvist A., Spégel P., Ekelund M., Mulder H., Groop L., Hedenbro J. Effects of ingestion routes on hormonal and metabolic profiles in gastric-bypassed humans. The Journal of Clinical Endocrinology and Metabolism. 2013;98(5):E856–E861. doi: 10.1210/jc.2012-3996. [DOI] [PubMed] [Google Scholar]
- 23.Pournaras D.J., Aasheim E.T., Bueter M., Ahmed A.R., Welbourn R., Olbers T. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2012;8(4):371–374. doi: 10.1016/j.soard.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Hansen E.N., Tamboli R.A., Isbell J.M., Saliba J., Dunn J.P., Marks-Shulman P.A. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. AJP: Gastrointestinal and Liver Physiology. 2011;300(5):G795–G802. doi: 10.1152/ajpgi.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao B.B., Click B., Eid G., Codario R.A. Management of refractory noninsulinoma pancreatogenous hypoglycemia syndrome with gastric bypass reversal: a case report and review of the literature. Case Reports in Endocrinology. 2015;2015 doi: 10.1155/2015/384526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qvigstad E., Gulseth H.L., Risstad H., le Roux C.W., Berg T.J., Mala T. A novel technique of Roux-en-Y gastric bypass reversal for postprandial hyperinsulinemic hypoglycaemia: a case report. International Journal of Surgery Case Reports. 2016;21:91–94. doi: 10.1016/j.ijscr.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C.J., Brown T., Magnuson T.H., Egan J.M., Carlson O., Elahi D. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. The Journal of Clinical Endocrinology and Metabolism. 2013;98(7):E1208–E1212. doi: 10.1210/jc.2013-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arman G.A., Himpens J., Bolckmans R., Van Compernolle D., Vilallonga R., Leman G. Medium-Term Outcomes after Reversal of Roux-en-Y Gastric bypass. Obesity Surgery. 2017 doi: 10.1007/s11695-017-2928-7. [DOI] [PubMed] [Google Scholar]
- 29.Roslin M., Damani T., Oren J., Andrews R., Yatco E., Shah P. Abnormal glucose tolerance testing following gastric bypass demonstrates reactive hypoglycemia. Surgical Endoscopy. 2011;25(6):1926–1932. doi: 10.1007/s00464-010-1489-9. [DOI] [PubMed] [Google Scholar]
- 30.Qiu N.-C., Zhang Q., Song X., Liu M.-E., Li X.-K., Shan C.-X. Impact of the hepatic branch of the vagus and Roux-en-Y gastric bypass on the hypoglycemic effect and glucagon-like peptide-1 in rats with type 2 diabetes mellitus. The Journal of Surgical Research. 2014;191(1):123–129. doi: 10.1016/j.jss.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 31.Shoar S., Nguyen T., Ona M.A., Reddy M., Anand S., Alkuwari M.J. Roux-en-Y gastric bypass reversal: a systematic review. Surgery for Obesity and Related Diseases : Official Journal of the American Society for Bariatric Surgery. 2016;12(7):1366–1372. doi: 10.1016/j.soard.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Salehi M., Gastaldelli A., D'Alessio D.A. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3) doi: 10.1053/j.gastro.2013.11.044. 669–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campos G.M., Rabl C., Havel P.J., Rao M., Schwarz J.-M., Schambelan M. Changes in post-prandial glucose and pancreatic hormones, and steady-state insulin and free fatty acids after gastric bypass surgery. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2014;10(1):1–8. doi: 10.1016/j.soard.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salehi M., Prigeon R.L., D'Alessio D.A. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tharakan G., Behary P., Wewer Albrechtsen N.J., Chahal H., Kenkre J., Miras A.D. Roles of increased glycaemic variability, GLP-1 and glucagon in hypoglycaemia after Roux-en-Y gastric bypass. European Journal of Endocrinology. 2017;177(6):455–464. doi: 10.1530/EJE-17-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan T.M., Field B.C.T., McCullough K.A., Troke R.C., Chambers E.S., Salem V. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62(4):1131–1138. doi: 10.2337/db12-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craig C.M., Liu L.-F., Deacon C.F., Holst J.J., McLaughlin T.L. Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia. 2017;60(3):531–540. doi: 10.1007/s00125-016-4179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrahamsson N., Engström B.E., Sundbom M., Karlsson F.A. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? European Journal of Endocrinology. 2013;169(6):885–889. doi: 10.1530/EJE-13-0504. [DOI] [PubMed] [Google Scholar]
- 39.Korner J., Inabnet W., Febres G., Conwell I.M., McMahon D.J., Salas R. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. International Journal of Obesity (2005) 2009;33(7):786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witte A.B., Grybäck P., Holst J.J., Hilsted L., Hellström P.M., Jacobsson H. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regulatory Peptides. 2009;158(1–3):57–62. doi: 10.1016/j.regpep.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Sloth B., Holst J.J., Flint A., Gregersen N.T., Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. American Journal of Physiology - Endocrinology and Metabolism. 2007;292(4):E1062–E1068. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 42.Tan T.M., Salem V., Troke R.C., Alsafi A., Field B.C.T., De Silva A. Combination of peptide YY3-36 with GLP-1(7-36) amide causes an increase in first-phase insulin secretion after IV glucose. The Journal of Clinical Endocrinology and Metabolism. 2014;99(11):E2317–E2324. doi: 10.1210/jc.2014-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yousseif A., Emmanuel J., Karra E., Millet Q., Elkalaawy M., Jenkinson A.D. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obesity Surgery. 2014;24(2):241–252. doi: 10.1007/s11695-013-1066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papamargaritis D., Koukoulis G., Sioka E., Zachari E., Bargiota A., Zacharoulis D. Dumping symptoms and incidence of hypoglycaemia after provocation test at 6 and 12 months after laparoscopic sleeve gastrectomy. Obesity Surgery. 2012;22(10):1600–1606. doi: 10.1007/s11695-012-0711-3. [DOI] [PubMed] [Google Scholar]
- 45.Nannipieri M., Belligoli A., Guarino D., Busetto L., Moriconi D., Fabris R. Risk factors for spontaneously self-reported postprandial hypoglycemia after bariatric surgery. The Journal of Clinical Endocrinology and Metabolism. 2016;101(10):3600–3607. doi: 10.1210/jc.2016-1143. [DOI] [PubMed] [Google Scholar]
- 46.Kellogg T.A., Bantle J.P., Leslie D.B., Redmond J.B., Slusarek B., Swan T. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Soard. 2008;4(4):492–499. doi: 10.1016/j.soard.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Botros N., Rijnaarts I., Brandts H., Bleumink G., Janssen I., de Boer H. Effect of carbohydrate restriction in patients with hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. Obesity Surgery. 2014;24(11):1850–1855. doi: 10.1007/s11695-014-1319-6. [DOI] [PubMed] [Google Scholar]
- 48.Valderas J.P., Ahuad J., Rubio L., Escalona M., Pollak F., Maiz A. Acarbose improves hypoglycaemia following gastric bypass surgery without increasing glucagon-like peptide 1 levels. Obesity Surgery. 2012;22(4):582–586. doi: 10.1007/s11695-011-0581-0. [DOI] [PubMed] [Google Scholar]
- 49.Myint K.S., Greenfield J.R., Farooqi I.S., Henning E., Holst J.J., Finer N. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. European Journal of Endocrinology. 2012;166(5):951–955. doi: 10.1530/EJE-11-1065. [DOI] [PubMed] [Google Scholar]
- 50.Spanakis E., Gragnoli C. Successful medical management of status post-Roux-en-Y-gastric-bypass hyperinsulinemic hypoglycemia. Obesity Surgery. 2009;19(9):1333–1334. doi: 10.1007/s11695-009-9888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yaqub A., Smith E.P., Salehi M. 2017. Hyperinsulinemic hypoglycemia after gastric bypass surgery: what“s up and what”s down? International Journal of Obesity. 2005;74:579. doi: 10.1038/ijo.2017.257. [DOI] [PMC free article] [PubMed] [Google Scholar]