Abstract
We aimed to evaluate the diagnostic accuracy of liver stiffness measurement (LSM) in 188 chronic hepatitis B (CHB) patients with alanine transaminase (ALT) ≤ twice the upper limit of normal (ULN). Liver fibrosis was staged using METAVIR scoring system. Define significant fibrosis as F2-F4, severe fibrosis as F3-F4, and cirrhosis as F4. To predict F2-F4, the AUROC of LSM was higher than that of APRI (0.86 vs 0.73, p = 0.001) and FIB-4 (0.86 vs 0.61, p < 0.001). To predict F4, the AUROC of LSM was also higher than that of APRI (0.93 vs 0.77, p = 0.012) and FIB-4 (0.93 vs 0.64, p < 0.001). Patients with ALT levels 1–2 ULN had higher cut-off values than patients with normal ALT levels for the diagnosis of F2-F4 (6.5 vs 6 kPa) and F4 (10.2 vs 7.8 kPa). Using cut-off values regardless of ALT levels, the diagnostic accuracy of LSM was 81% for F2-F4, and 89% for F4. Applying ALT-stratified cut-off values, the diagnostic accuracy of LSM was 82% for F2-F4, and 86% for F4. In conclusion, LSM is a reliable noninvasive test for the diagnosis of liver fibrosis. Applying ALT-stratified cut-off values did not enhance diagnostic accuracy of LSM in CHB patients with ALT ≤ 2 ULN.
Introduction
Hepatitis B virus (HBV) infection is still a major public health burden. In China, the prevalence of hepatitis B surface antigen (HBsAg) is 9.75% in 1992, and 7.18% in 20061. A study published in 2016 showed that the HBsAg positive rate was 6.0% in men aged 21–49 years in rural China2. Another study showed that the HBsAg positive rate was 6.1% in Northeastern China in 20163. In the last decade, the prevalence of HBV in China has changed from highly endemic to intermediate endemic2. However, in China, the absolute number of patients with HBV infection is still large because of its vast denominator.
The assessment of the severity of liver fibrosis is important to identify patients for treatment and hepatocellular carcinoma (HCC) surveillance. Liver biopsy has traditionally been considered the reference method for evaluation of liver fibrosis. However, liver biopsy is a costly and invasive procedure, carrying potential complications. Therefore, non-invasive diagnostic method would be more acceptable to patients. Liver stiffness measurement (LSM) has been introduced as a new, non-invasive method for the diagnosis of liver fibrosis. Many studies found that LSM could predict liver fibrosis accurately in patients with chronic hepatitis C (CHC)4–7. In recent years, several studies have been performed to apply LSM to patients with chronic hepatitis B (CHB)6,8–10. However, these studies were mainly performed in European and United States, and the results cannot be extrapolated to Chinese patients with CHB. First, HBV genotype A is highly prevalent in Europe and United States, while genotypes B and C are common in China. Second, most CHB patients are HBeAg positive and high HBV DNA levels in China, while most CHB patients are HBeAg negative and low HBV DNA levels in Europe and United States11,12. Therefore, further studies should be performed in Chinese patients with CHB.
According to EASL clinical guidelines, patients with HBV DNA > 20,000 IU/ml and ALT > 2 ULN should start antiviral therapy regardless of the degree of fibrosis13. For these patients, liver fibrosis assessment may provide additional useful information, but it does not usually change the decision for treatment. In patients with ALT ≤ 2 ULN, liver fibrosis assessment should be used for decision on treatment indications. Patients with at least significant fibrosis should be treated13. Thus, patients with ALT ≤ 2 ULN have more needs for liver fibrosis assessment than those with ALT > 2 ULN. In this study, we aimed to: (1) assess the diagnostic accuracy of LSM in Chinese patients with CHB; (2) compare the diagnostic accuracy of LSM with serum fibrosis models (APRI and FIB-4); (3) evaluate the impact of ALT levels on LSM in patients with ALT ≤ 2 ULN.
Material and Method
Study population
A total of 305 consecutive CHB patients from the Ruian people’s hospital, Wenzhou, Zhejiang, China, between July 2013 and July 2015, were retrospectively analyzed. Inclusion criteria: (1) the persistent presence of serum HBsAg for more than 6 months; (2) ATL ≤ 2 ULN (the ULN is 40 IU/L); (3) had liver biopsy, LSM, and routine laboratory tests. Exclusion criteria: (1) significant alcohol consumption (>20 g/day) (n = 24); (2) co-infection with HCV or HDV (n = 10); (3) accompanied with autoimmune liver disease (ALD) or nonalcoholic fatty liver disease (NAFLD) (n = 14); (4) prior or current antiviral therapy (n = 32); (5) body mass index (BMI) >28 kg/m2 (n = 13); (6) inappropriate biopsy samples (n = 8); (7) unreliable LSM values (n = 16). Finally, 188 patients were included in this study. Figure 1 summarized the flow diagram of the study population.
Figure 1.
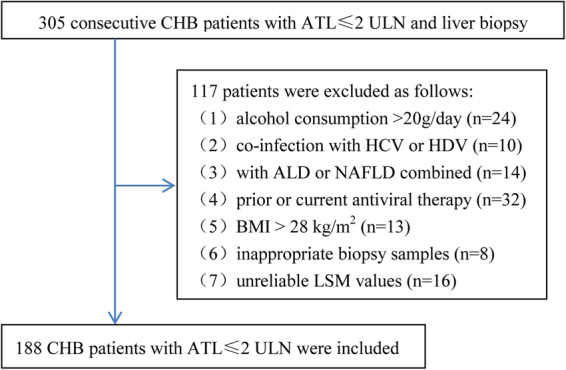
Flow diagram of the study population and reasons for exclusion. CHB, chronic hepatitis B; ALT, alanine transaminase; ULN, upper limit of normal; HCV, hepatitis C virus; HDV, hepatitis D virus; ALD, autoimmune liver disease; NAFLD, non-alcoholic fatty liver disease; BMI, body mass index; LSM, liver stiffness measurement.
All patients signed the informed consent before liver biopsy, and all clinical procedures were in accordance with the Helsinki declaration. The ethics committee of Ruian people’s hospital approved the study protocol. All experiments were performed in accordance with relevant guidelines and regulations13,14.
Liver histological assessment
Percutaneous liver biopsy was performed. Liver samples were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. A minimum of 15 mm of liver tissue with at least 6 portal tracts was considered suitable for histological scoring14. The biopsy samples were assessed by two independent pathologists blinded to the results of non-invasive fibrosis tests. Discordant cases were reviewed by a third highly experienced liver pathologist. The METAVIR scoring system was used to determine liver fibrosis grade15: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; and F4, cirrhosis. We defined significant fibrosis as F2-F4, severe fibrosis as F3-F4, and cirrhosis as F4.
Liver stiffness measurement
LSM was performed by operators trained according to the manufacturers’ recommendations using FibroScan (Echosens; Paris, France) equipped with the M probe (3.5 MHz transducer, measurement of liver stiffness take place between 25 and 65 mm) within one week of liver biopsy. Briefly, LSM was performed following an overnight period of fasting. Mild amplitude and low-frequency vibrations were transmitted to the liver of each patient, inducing an elastic shear wave propagating through the underlying liver tissue. The velocity of the wave directly correlated to the tissue stiffness. The LSM values may be considered reliable when 10 valid measurements are obtained, with a success rate of ≥60% and an interquartile range/median LSM ≤30%16,17.
Routine laboratory tests
Fasting blood samples were obtained, and routine laboratory tests were performed within one week of liver biopsy. Serum HBsAg was detected using the enzyme-linked immunosorbent assay kit (Wanti BioPharm, Inc., Beijing, China). Serum HBV DNA was measured using the kit for PCR (ABI 7500; Applied Biosystems, Foster City, USA) with a limit detection of 500 copies/ml. Serum biochemical parameters including ALT were measured using full automated biochemistry analyzer (AU2700; Olympus Corporation, Tokyo, Japan).
Serum fibrosis models calculation
APRI = (AST (IU/L)/ULN of AST)/platelet count (109/L) × 100; ULN of AST = 40 IU/L.
FIB-4 = (age (years) × AST (IU/L))/(platelet count (109/L) × (ALT (IU/L))1/2).
Statistical analysis
The Kolmogorov-Smirnov test was used to verify the normal assumption of quantitative data. The baseline data was presented as follows: normal distribution data as mean ± standard deviation, non-normal distribution continuous data as median (interquartile range (IQR)), and categorical variables as number (percentage). The t-test (for normal distribution variables), Mann-Whitney test (for non-normal distribution continuous variables), and Chi-squared test (for categorical variables), respectively, were performed to identify the statistical differences between two groups. The correlation analysis was performed using the Spearman test. The diagnostic performance was assessed using the receiver operating characteristic (ROC) curves. The area under ROC curves (AUROCs) were compared using Z-test18. The optimal cut-off was obtained by maximizing Youden index (sensitivity + specificity-1). Diagnostic performance was evaluated by sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic accuracy (DA). All significance tests were two tailed, and p < 0.05 was considered statistically significant. All statistical analyses were carried out using SPSS statistical software version 15.0 (SPSS Inc. Chicago, IL, USA) and MedCalc Statistical Software version 16.1 (MedCalc Software bvba, Ostend, Belgium).
Results
Baseline characteristics of patients
The baseline characteristics of patients were shown in Table 1. The majority of patients were male (62.8%), HBeAg positive (66.0%), and middle-aged (37 ± 10 years). The median BMI, HBV DNA, AST, GGT, total bilirubin, and albumin were 22.5 kg/m2 (IQR = 20.4–24.2), 5.4 log10 copies/ml (IQR = 3.2–7.5), 26 IU/L (IQR = 21–34), 17 IU/L (IQR = 13–29), 13 μmol/L (IQR = 10–17), and 46 g/L (IQR = 44–48), respectively. The mean ALT and platelet count levels were 39 IU/L and 196 × 109/L, respectively. The Median LSM value, APRI, and FIB-4 scores were 5.8 kPa (IQR = 3.7–8.5), 0.40 (IQR = 0.29–0.60), and 1.0 (IQR = 0.7–1.5), respectively. The METAVIR inflammation grades were as follows: A0 = 17 (9%); A1 = 76 (40.4%); A2 = 61 (32.4%); and A3 = 34 (18.9%). The METAVIR fibrosis grades were as follows: F0 = 11 (5.9%); F1 = 83 (44.1%); F2 = 54 (28.7%); F3 = 12 (6.4%); and F4 = 28 (14.9%).
Table 1.
Characteristics of the study subjects.
| Characteristic | All patients (n = 188) | F0-F1 (n = 94) | F2-F4 (n = 94) | p Value |
|---|---|---|---|---|
| Male sex (n, %) | 118 (62.8%) | 59 (62.8%) | 59 (62.8%) | |
| Age (years) | 37 ± 10 | 34 ± 9 | 40 ± 10 | <0.001 |
| BMI (kg/m2) | 22.5 (20.4–24.2) | 22.3 (20.1–23.8) | 22.6 (20.8–24.6) | 0.962 |
| HBeAg positive (n, %) | 124 (66.0%) | 37 (39.4%) | 87 (60.6%) | <0.001 |
| HBV-DNA (log10 copies/ml) | 5.4 (3.2–7.5) | 3.2 (2.7–4.1) | 7.5 (4.9–7.7) | <0.001 |
| ALT (IU/L) | 39 ± 16 | 35 ± 16 | 44 ± 16 | <0.001 |
| AST (IU/L) | 26 (21–34) | 25 (21–31) | 28 (23–37) | 0.003 |
| GGT (IU/L) | 17 (13–29) | 19 (13–34) | 16 (13–26) | 0.173 |
| Platelet count (109/L) | 196 ± 52 | 197 ± 54 | 196 ± 51 | 0.884 |
| Total Bilirubin (μmol/L) | 13 (10–17) | 14 (10–17) | 13 (10–17) | 0.260 |
| Albumin (g/L) | 46 (44–48) | 47 (44–48) | 46 (44–48) | 0.418 |
| LSM value (kPa) | 5.8 (3.7–8.5) | 4.2 (2.8–5.5) | 8.4 (6.6–10.8) | <0.001 |
| APRI | 0.40 (0.29–0.60) | 0.33 (0.27–0.42) | 0.50 (0.34–0.76) | <0.001 |
| FIB-4 | 1.0 (0.7–1.5) | 0.9 (0.6–1.3) | 1.1 (0.7–2.1) | 0.010 |
| METAVIR inflammation grade (A0/A1/A2/A3) | 17 (9%)/76 (40.4%)/61 (32.4%)/34 (18.9%) | |||
| METAVIR fibrosis grade (F0/F1/F2/F3/F4) | 11 (5.9%)/83 (44.1%)/54 (28.7%)/12 (6.4%)/28 (14.9%) | |||
BMI, body mass index; ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; LSM, liver stiffness measurement; APRI, AST to platelet ratio index; FIB-4, fibrosis index based on the 4 factors.
Of 188 patients, 94 (50%) have F2-F4, 40 (21.3%) have F3-F4, and 28 (14.9%) have F4, respectively. Patients with F2-F4 had higher age (40 vs 34 years, p < 0.001), proportion of HBeAg-positive (60.6% vs 39.4%, p < 0.001), HBV DNA (7.5 vs 3.2 log10 copies/mL, p < 0.001), ALT (44 vs 35 IU/L, p < 0.001), AST (28 vs 25 IU/L, p = 0.003), LSM value (8.4 vs 4.2 kPa, p < 0.001), APRI (0.50 vs 0.33, p < 0.001), and FIB-4 (1.1 vs 0.9, p = 0.010) than patients with F0-F1. No significant differences were seen in sex, BMI, GGT, platelet count, total bilirubin, and albumin.
Correlation between noninvasive fibrosis tests and METAVIR fibrosis stages
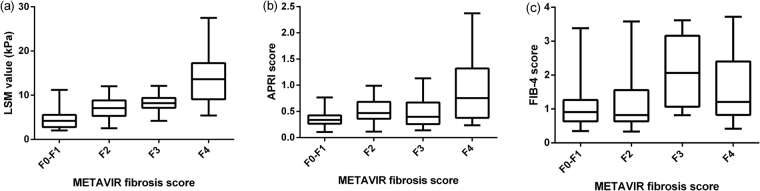
The association between METAVIR fibrosis stages and noninvasive fibrosis tests was presented in Table 2 and Fig. 2. The METAVIR fibrosis stages were positive correlated with LSM values (r = 0.72, p < 0.001), APRI (r = 0.43, p < 0.001), and FIB-4 (r = 0.27, p < 0.001). LSM values, APRI, and FIB-4 tended to increase with the increased METAVIR fibrosis stages (Fig. 2).
Table 2.
Correlations between noninvasive fibrosis tests and METAVIR fibrosis stages.
| Variables | Spearman’s r | p value |
|---|---|---|
| LSM | 0.72 | <0.001 |
| APRI | 0.43 | <0.001 |
| FIB-4 | 0.27 | <0.001 |
LSM, liver stiffness measurement; Spearman’s r, correlation coefficient.
Figure 2.
Association between METAVIR fibrosis scores and noninvasive fibrosis tests. LSM, liver stiffness measurement; APRI, aspartate transaminase to platelet ratio index; FIB-4, fibrosis index based on the 4 factors.
Diagnostic performances of noninvasive fibrosis tests
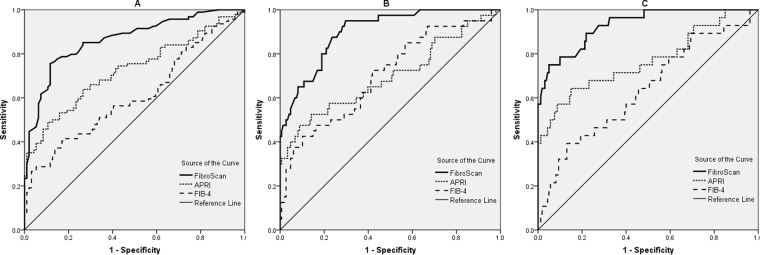
The ROC curves of noninvasive fibrosis tests were shown in Fig. 3. The AUROCs of noninvasive fibrosis tests were shown in Table 3. To predict F2-F4, the AUROC of LSM was higher than that of APRI (0.86 vs 0.73, p = 0.001) and FIB-4 (0.86 vs 0.61, p < 0.001). To predict F3-F4, the AUROC of LSM was also higher than that of APRI (0.90 vs 0.70, p < 0.001) and FIB-4 (0.90 vs 0.70, p < 0.001). To predict F4, the AUROC of LSM was significantly higher than that of APRI (0.93 vs 0.77, p = 0.012) and FIB-4 (0.93 vs 0.64, p < 0.001).
Figure 3.
ROC curves for significant fibrosis (A), severe fibrosis (B), and cirrhosis (C). APRI, aspartate transaminase to platelet ratio index; FIB-4, fibrosis index based on the 4 factors.
Table 3.
The AUROCs for noninvasive fibrosis tests in predicting liver fibrosis and cirrhosis.
| Significant fibrosis | Severe fibrosis | Cirrhosis | ||||
|---|---|---|---|---|---|---|
| AUROC | (95% CI) | AUROC | (95% CI) | AUROC | (95% CI) | |
| FibroScan | 0.86 | (0.81–0.91) | 0.90 | (0.85–0.94) | 0. 93 | (0.89–0.96) |
| APRI | 0.73 | (0.66–0.79) | 0.70 | (0.63–0.76) | 0.77 | (0.70–0.83) |
| FIB-4 | 0.61 | (0.53–0.68) | 0.70 | (0.63–0.77) | 0.64 | (0.56–0.71) |
| AUROC Comparison | ||||||
| FibroScan and APRI | p = 0.001 | p < 0.001 | p = 0.012 | |||
| FibroScan and FIB-4 | p < 0.001 | p < 0.001 | p < 0.001 | |||
| APRI and FIB-4 | p = 0.009 | p = 0.926 | p = 0.015 | |||
AUROC, area under the receiver operating characteristic curve; CI, confidence interval; Significant fibrosis, METAVIR F2-F4; Severe fibrosis, METAVIR F3-F4; Cirrhosis, METAVIR F4.
Diagnostic thresholds of noninvasive fibrosis tests
The diagnostic thresholds of noninvasive fibrosis tests were presented in Table 4. Maximizing Youden index, the cut-off values of LSM were 6.5 kPa, 7.4 kPa, and 9.5 kPa, for predicting F2-F4, F3-F4, and F4, respectively. The cut-offs of APRI were 0.52 for predicting F2-F4, and 0.62 for predicting F4. The optimal cut-off of FIB-4 was 1.8 for predicting F3-F4.
Table 4.
Diagnostic thresholds of noninvasive fibrosis tests.
| Cut-offs | Se (%) | Sp (%) | PPV (%) | NPV (%) | PLR | NLR | DA (%) | |
|---|---|---|---|---|---|---|---|---|
| LSM value (kPa) | ||||||||
| F2-F4 | ≥6.5 | 76 | 88 | 87 | 78 | 6.45 | 0.28 | 81 |
| F3-F4 | ≥7.4 | 88 | 76 | 50 | 96 | 3.70 | 0.16 | 78 |
| =F4 | ≥9.5 | 75 | 95 | 72 | 96 | 15 | 0.26 | 89 |
| APRI | ||||||||
| F2-F4 | ≥0.52 | 50 | 89 | 82 | 64 | 4.6 | 0.57 | 69 |
| =F4 | ≥0.62 | 64 | 85 | 43 | 93 | 4.29 | 0.42 | 81 |
| FIB-4 | ||||||||
| F3-F4 | ≥1.80 | 46 | 90 | 53 | 85 | 4.19 | 0.64 | 79 |
Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; DA, diagnostic accuracy; LSM, liver stiffness measurement; Significant fibrosis, METAVIR F2-F4; Severe fibrosis, METAVIR F3-F4; Cirrhosis, METAVIR F4.
The impact of ALT levels on the diagnostic performances and cutoff values of LSM
To assess the impact of ALT levels on LSM, we stratified the 188 patients into two categories: 107 patients had normal ALT levels, and 81 patients had mildly elevated ALT levels (ULN < ALT ≤ 2 ULN) (Table 5). For predicting significant fibrosis, the AUROC of LSM was 0.86 in patients with normal ALT levels, and 0.84 in patients with mildly elevated ALT levels. For predicting cirrhosis, the AUROC of LSM was 0.88 in patients with normal ALT levels, and 0.98 in patients with mildly elevated ALT levels.
Table 5.
The AUROCs and optimal cut-offs for LSM according to ALT levels.
| ALT levels | AUROC (95% CI) | Cut off (kPa) | Se (%) | Sp (%) | PPV (%) | NPV (%) | PLR | NLR | DA (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Significant fibrosis | ≤1 ULN (n = 107) | 0.86 (0.78–0.92) | 6 | 83 | 88 | 81 | 89 | 6.91 | 0.20 | 82 |
| 1–2 ULN (n = 81) | 0.84 (0.74–0.91) | 6.5 | 74 | 89 | 93 | 63 | 6.67 | 0.29 | ||
| Cirrhosis | ≤1 ULN (n = 107) | 0.88 (0.80–0.93) | 7.8 | 75 | 82 | 35 | 96 | 4.19 | 0.30 | 86 |
| 1–2 ULN (n = 81) | 0.98 (0.93–1.00) | 10.2 | 94 | 95 | 83 | 98 | 20.31 | 0.07 |
AUROC, area under the receiver operating characteristic curve; LSM, liver stiffness measurement; ALT, alanine transaminase; CI, confidence interval; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; DA, diagnostic accuracy; Significant fibrosis, METAVIR F2-F4; Cirrhosis, METAVIR F4.
Patients with mildly elevated ALT levels had higher cut-off values than patients with normal ALT levels for predicting F2-F4 (6.5 vs 6 kPa) and F4 (10.2 vs 7.8 kPa). Using cut-offs regardless of ALT levels, the diagnostic accuracy of LSM was 81% for F2-F4, and 89% for F4 (Table 4). Applying ALT-stratified cut-off values, the diagnostic accuracy of LSM was 82% for predicting F2-F4, and 86% for predicting F4 (Table 5).
Discussion
In this study, we compared the diagnostic performance of LSM with that of serum fibrosis models (APRI and FIB-4). LSM showed significantly higher diagnostic performance than APRI and FIB-4 for the diagnosis of significant fibrosis, severe fibrosis, and cirrhosis. Previous studies had also evaluated the diagnostic performance of LSM in Chinese chronic HBV-infected patients with normal or mildly elevated ALT levels, yet none has compared LSM with serum fibrosis models19–21. The advantages of this study include comparison with serum fibrosis models and using liver biopsy as reference.
We confirmed the good performance of LSM to predict significant fibrosis with an AUROC of 0.86, and predict cirrhosis with an AUROC of 0.93 in Chinese CHB patients with ALT ≤ 2 ULN. The results are consistent with European studies22,23. A prospective study included 202 CHB patients found that the AUROC of LSM is 0.81 for predicting significant fibrosis, and 0.93 for predicting cirrhosis22. Another study included 125 European patients found that the AUROC of LSM is 0.85 for predicting significant fibrosis, and 0.90 for predicting cirrhosis23. In a Korea study, the diagnostic performances of LSM were better than our results, with AUROC of 0.94 for predicting significant fibrosis, and 0.96 for predicting cirrhosis24. The different histological scoring systems between this study (METAVIR scoring systems) and the Korea study (Batts scoring system) might be a reason for the difference.
The optimal cutoff values of LSM in this study (6.5 kPa for significant fibrosis and 9.5 kPa for cirrhosis) were lower than that reported by Marcellin et al. (7.2 kPa for significant fibrosis and 11 kPa for cirrhosis)22, and Jia et al. (7.3 kPa for significant fibrosis and 10.7 kPa for cirrhosis)25. A meta-analysis found that the optimal cutoff values of LSM were 7.9 kPa for significant fibrosis and 11.7 kPa for cirrhosis26. Obviously, the LSM cutoff values in this study were lower than previous studies. Three possible reasons are as follows. First, this study was performed in patients with ALT ≤ 2 ULN, while previous studies were performed in general CHB patients including ALT > 2 ULN. As elevated ALT levels were associated with higher LSM value, the cutoff values of LSM in patients with ALT ≤ 2 ULN were lower than general patients including ALT > 2 ULN. Second, the LSM test can be biased by high levels of liver inflammation (ALT > 2 ULN) rather than normal or mildly liver inflammation (ALT ≤ 2 ULN). Moreover, the differences in prevalence of significant fibrosis and cirrhosis might be the third explanation for the reason why the cut-offs presented by this study were not in line with the previously published data22,25.
Several studies have showed the impact of ALT levels on LSM value. Chan et al. founded that elevated ALT levels were associated with higher LSM value (r = 2.8, p < 0.001), and proposed various optimal cut-offs depending on magnitude of ALT elevation19. Arena et al. also found a positive correlation between ALT levels and LSM values at the onset of acute viral hepatitis (r = 0.53, p = 0.02)27. Wong et al. suggested that serum ALT levels should always be taken into account when interpreting results from LSM, especially in patients who might have HBV flares28. However, applying ALT-related cut-off values did not improve the diagnostic accuracy of LSM in this study. Our results is well-supported by other studies29,30. Cardoso et al. have first challenged the approach of using ALT guided cut-offs for LSM in patients with CHB29. Although Cardoso et al. found a positive correlation between ALT levels and LSM values (r = 0.365, p < 0.001), ALT specific cut-offs did not enhance diagnostic performance in patients with CHB29. Seo et al. also found that mildly elevated ALT levels did not influence the diagnostic performance of LSM30. We concluded that LSM mainly was influenced by acute viral hepatitis, HBV flares, or significantly elevated ALT levels (ALT > 2 ULN) rather than mildly elevated ALT levels.
For predicting cirrhosis, the AUROC of LSM in patients with mildly elevated ALT levels is higher than that in patients with normal ALT levels (0.98 vs 0.88). The difference may be related to difference in cirrhosis prevalence in the studied populations, known as the spectrum bias31,32. In this study, the prevalence of cirrhosis in patients with mildly elevated ALT levels is higher than patients with normal ALT levels (19.6% vs 11.2%).
Based on evidence from the systematic review, the WHO guidelines recommended that LSM and APRI were the most useful tests for the assessment of cirrhosis in resource-limited settings33. Although APRI had been recommended for the assessment of cirrhosis, our results suggest that APRI and FIB-4 are significantly inferior to LSM. Based on our results, we recommended that LSM should be considered as the preferred noninvasive fibrosis tests, and APRI should be considered when LSM is unavailable. Liver biopsy remains within the armamentarium of hepatologists when there are discordances between clinical symptoms and the extent of fibrosis assessed by non-invasive approaches.
This study has several limitations. First, the retrospective analysis might have caused selective bias resulting in underestimated sensitivity and overestimated specificity of non-invasive fibrosis diagnostic models. Second, this study might be not timely. This cohort included 188 patients who had liver biopsies and LSM values between July 2013 and July 2015. Data since July 2015 were lacking.
In conclusion, our study assessed the accuracy of LSM for predicting significant fibrosis and cirrhosis in Chinese CHB patients with ALT ≤ 2 ULN. LSM showed higher diagnostic performances than APRI and FIB-4. For patients with ALT ≤ 2 ULN, ALT levels did not affect the diagnostic performance of LSM, and ALT-stratified cut-off values did not enhance diagnostic accuracy of LSM in this specific population.
Acknowledgements
This study was supported by grant No. SHDC12015129 from the ShenKang development center of Shanghai, and grant No. 17411969700 from the science and technology commission of Shanghai. The funding organizations are public institutions and had no role in the design and conduct of the study; collection, management, and analysis of the data; or preparation, review, and approval of the manuscript.
Author Contributions
Study concept and design: Qiang Li, Liang Chen, and Yu Zhou. Analysis and interpretation of data: Qiang Li. Drafting of the manuscript: Qiang Li. Critical revision of the manuscript for important intellectual content: Liang Chen and Yu Zhou.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liang Chen, Email: chenliang@shaphc.org.
Yu Zhou, Email: zhouyu8088vv@163.com.
References
- 1.Yonghao G, et al. An epidemiological serosurvey of hepatitis B virus shows evidence of declining prevalence due to hepatitis B vaccination in central China. Int J Infect Dis. 2015;40:75–80. doi: 10.1016/j.ijid.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, et al. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: a population-based, cross-sectional study. Lancet Infect Dis. 2016;16:80–86. doi: 10.1016/S1473-3099(15)00218-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, et al. Epidemiology of Hepatitis B and Hepatitis C Infections and Benefits of Programs for Hepatitis Prevention in Northeastern China: A Cross-Sectional Study. Clin Infect Dis. 2016;62:305–312. doi: 10.1093/cid/civ859. [DOI] [PubMed] [Google Scholar]
- 4.Castera L, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Lucidarme D, et al. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1083–1089. doi: 10.1002/hep.22748. [DOI] [PubMed] [Google Scholar]
- 6.Arena U, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288–1293. doi: 10.1136/gut.2008.149708. [DOI] [PubMed] [Google Scholar]
- 7.Zarski JP, et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol. 2012;56:55–62. doi: 10.1016/j.jhep.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Zeng X, et al. The Diagnostic Value of FibroScan in Assessing Significant Liver Fibrosis in Patients with Chronic Hepatitis B. West Indian Med J. 2015;65:106–110. doi: 10.7727/wimj.2014.150. [DOI] [PubMed] [Google Scholar]
- 9.Goyal R, et al. Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B. J Gastroenterol Hepatol. 2013;28:1738–1745. doi: 10.1111/jgh.12318. [DOI] [PubMed] [Google Scholar]
- 10.Afdhal NH, et al. Accuracy of fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: a United States multicenter study. Clin Gastroenterol Hepatol. 2015;13:772–779. doi: 10.1016/j.cgh.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Kurbanov F, Tanaka Y, Mizokami M. Geographical and genetic diversity of the human hepatitis B virus. Hepatol Res. 2010;40:14–30. doi: 10.1111/j.1872-034X.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu CJ, Kao JH. Global perspective on the natural history of chronic hepatitis B: role of hepatitis B virus genotypes A to J. Semin Liver Dis. 2013;33:97–102. doi: 10.1055/s-0033-1345716. [DOI] [PubMed] [Google Scholar]
- 13.EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 67, 370–398 (2017). [DOI] [PubMed]
- 14.EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 63, 237–264 (2015). [DOI] [PubMed]
- 15.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 16.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich, C. F. et al. EFSUMB Guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultrasound Int Open38, e16–e47 (2017). [DOI] [PubMed]
- 18.Albeck MJ, Borgesen SE. [ROC-curve analysis. A statistical method for the evaluation of diagnostic tests] Ugeskr Laeger. 1990;152:1650–1653. [PubMed] [Google Scholar]
- 19.Chan HL, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36–44. doi: 10.1111/j.1365-2893.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang R, et al. Fibroscan improves the diagnosis sensitivity of liver fibrosis in patients with chronic hepatitis B. Exp Ther Med. 2016;11:1673–1677. doi: 10.3892/etm.2016.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie QX, et al. Role of FibroScan in liver fibrosis evaluation in patients with chronic hepatitis B virus infection and related influencing factors. Zhonghua Gan Zang Bing Za Zhi. 2016;24:659–664. doi: 10.3760/cma.j.issn.1007-3418.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcellin P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242–247. doi: 10.1111/j.1478-3231.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 23.Verveer C, et al. Evaluation of transient elastography for fibrosis assessment compared with large biopsies in chronic hepatitis B and C. Liver Int. 2012;32:622–628. doi: 10.1111/j.1478-3231.2011.02663.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim BK, et al. Prospective validation of ELF test in comparison with Fibroscan and FibroTest to predict liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One. 2012;7:e41964. doi: 10.1371/journal.pone.0041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia J, et al. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:756–762. doi: 10.1111/jgh.12840. [DOI] [PubMed] [Google Scholar]
- 26.Chon YE, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. doi: 10.1371/journal.pone.0044930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arena U, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380–384. doi: 10.1002/hep.22007. [DOI] [PubMed] [Google Scholar]
- 28.Wong GL, et al. Clinical factors associated with liver stiffness in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Gastroenterol Hepatol. 2009;7:227–233. doi: 10.1016/j.cgh.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Cardoso AC, et al. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012;32:612–621. doi: 10.1111/j.1478-3231.2011.02660.x. [DOI] [PubMed] [Google Scholar]
- 30.Seo YS, et al. Accuracy of transient elastography in assessing liver fibrosis in chronic viral hepatitis: A multicentre, retrospective study. Liver Int. 2015;35:2246–2255. doi: 10.1111/liv.12808. [DOI] [PubMed] [Google Scholar]
- 31.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299:926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
- 32.Poynard T, et al. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53:1615–1622. doi: 10.1373/clinchem.2007.085795. [DOI] [PubMed] [Google Scholar]
- 33.Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. (World Health Organization, Geneva, 2015). [PubMed]