Abstract
Background
HER2 mutation has been found to be an oncogenic driver gene in non-small cell lung cancers(NSCLC) and HER2-directed therapies have shown promising results in this unique population, while little is known about its association with outcomes of chemotherapy. The aim of this study was to investigate the efficacy of first line chemotherapy in patients with advanced HER2-mutant lung adenocarcinomas.
Methods
Patients with advanced NSCLC(N = 1714) initially underwent testing for EGFR, KRAS, BRAF mutations and ALK, ROS1 rearrangements, and negative cases were then assessed for HER2 mutations using the method of amplification refractory mutation system(ARMS). The efficacy of first line pemetrexed-based chemotherapy was investigated in patients with HER2-mutant and those with EGFR-mutant, ALK/ROS1-rearranged and KRAS-mutant advanced adenocarcinomas.
Results
HER2 mutations were detected in 29 of 572(5.1%) specimens from a selected population of EGFR/KRAS/BRAF/ALK/ROS1 negative patients. All of them are adenocarcinomas. Among patients with HER2-mutant lung cancers, 25 received pemetrexed-based first line chemotherapy. The objective response rate(ORR) was 36.0%. Their median progression free survival(PFS) was 5.1 months, which was similar with that of KRAS-mutant group (n = 40,5.0 months, p = 0.971), numerically shorter than that of EGFR-mutant group(n = 74, 6.5 months, p = 0.247) and statistically significantly shorter than that of ALK/ROS1-rearranged group (n = 39,9.2 months, p = 0.004). Furthermore, HER2 variants subgroup analysis showed that PFS was inferior in A775_G776insYVMA group compared with other variants (4.2 vs 7.2 months, p = 0.085).
Conclusions
Patients with advanced HER2-mutant lung adenocarcinomas showed an inferior outcome of first line pemetrexed-based chemotherapy compared to those with ALK/ROS1 rearrangements, which strengthen the need for effective HER2-targeted drugs in clinical practice.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4277-x) contains supplementary material, which is available to authorized users.
Keywords: HER2 mutation, Lung adenocarcinoma, Pemetrexed
Background
Human epidermal growth factor receptor2(HER2) positivity is well-studied in breast cancer, while much less defined in lung cancer. Although anti-HER2 monoclonal antibody such as trastuzumab has been proven effective in breast cancer and gastric cancer [1, 2], the clinical trials [3, 4] of lung cancer including patients treated with trastuzumab combined with chemotherapy failed to demonstrate benefit in survival in HER2 IHC positive patients. Besides that, pan-HER TKI dacomitinib also showed no response in patients with HER2 amplifications in a phase II trial [5].
Apart from HER2 over-expression and amplification, HER2 gene mutation is a distinct entity in lung carcinogenesis with an incidence of 4.8% among EGFR wild-type lung adenocarcinoma resection samples [6]. Drugs that target HER2 gene mutations are currently being investigated. The National Comprehensive Cancer Network (NCCN) recommend trastuzumab or afatinib as potential therapy options for non-small cell lung cancers(NSCLC) patients with HER2 mutations. Several phase I/II trials [5, 7–9] is now investigating the efficacy of other irreversible pan-HER receptor family inhibitors, such as dacomitinib, neratinib and pyrotinib. Currently, HER2 mutation is emerging as a promising druggable target, while the optimal choice of targeted therapy remains poorly defined.
Chemotherapy is still the standard first-line regimen for patients with advanced NSCLC who are improper for targeted therapy. Among them, pemetrexed-based regimen has showed superior efficacy with less side effects and was recommended preferentially for patients with adenocarcinomas [10, 11]. ALK/ROS1/RET positive patients showed a superior progression free survival(PFS) after pemetrexed-based therapy than patients with KRAS mutations [12–16]. While the effects of HER2 mutation on the outcomes of pemetrexed-based chemotherapy is still unknown in patients with advanced NSCLC.
Aim to investigate the efficacy of pemetrexed-based chemotherapy in patients with HER2-mutant lung adenocarcinomas, we conducted this retrospective study in Chinese patients with 1714 advanced NSCLC. In addition, we also observed the clinicopathologic and molecular features of HER2 mutations in patients with advanced NSCLC.
Methods
Patients population
Patients with advanced NSCLC (stage IIIB/IV) and performed EGFR, ALK, ROS1, BRAF and KRAS testing at Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China from January 2015 to September 2016 were included into this study. HER2 mutations testing were performed in all these 5 genes pan-negative patients. Their clinical data were collected including age, gender, smoking status, tumor histology, performance status (PS) and the outcomes of anti-cancer therapies. Patients with HER2, EGFR or KRAS mutation or ALK or ROS1 rearrangement and received first-line pemetrexed-based chemotherapy (pemetrexed monotherapy or combination therapy with platinum) were eligible for analysis. A history of radiotherapy, first-line targeted therapy, or immune-directed therapy was exclusionary.
Molecular testing
HER2 mutation testing was performed using the method of amplification refractory mutation system(ARMS) by ADx HER2 Mutation Detection Kit (Amoy Diagnostics, Xiamen, China). Samples positive for HER2 mutations were confirmed by DNA sequencing using primers with the following sequences: 5’GCC ATG GCT GTG GTT TGT GAT AGG3’ (forward) and 5’ATC CTA GCC CCT TGT GGA CAT AGG3’, which amplified a 342-bp fragment in exon20 of the HER2 gene. The details can be referred to our previous study [6].
Similarly, EGFR, BRAF and KRAS mutation were performed using EGFR, BRAF V600 and KRAS Mutations Detection Kit (Amoy Diagnostics, Xiamen, China) respectively by ARMS method. ALK and ROS1 rearrangement testing were performed using AmoyDx EML4-ALK and ROS1 Fusions Detection Kit (Amoy Diagnostics, Xiamen, China) respectively by the method of reverse transcriptase polymerase chain reaction(RT-PCR). The details were described in our previous articles [13, 17–19].
Statistical analysis
Tumor response was evaluated every 2 cycles of chemotherapy according to response evaluation criteria in solid tumors (version 1.1). PFS was defined as the time interval from the first day of treatment to documented disease progression or death of any cause. All of the statistical tests were performed using the SPSS 19.0. Chi-square test or Fisher’s exact test was used to examine the clinicopathologic association of HER2 mutations and response rate comparison. Age differences were compared using the t test for independent samples or the one-way analysis of variance. The Kaplan–Meier method was used to estimate the PFS and the log-rank test was used to analyze PFS between the different groups. Results were considered significantly different if the p value was less than 0.05 in a two-way analysis.
Results
Patients’ characteristics
From January 2015 to September 2016, a total of 1714 patients with advanced NSCLC underwent testing for EGFR, KRAS, BRAF, ALK and ROS1.The results showed that there were 809 patients(47.2%) with EGFR mutations,149(7.8%) with KRAS mutations,19(1.1%) with BRAF mutations,106(6.2%) with ALK rearrangements, 43(2.5%) with ROS1 rearrangements, and 16 patients (0.9%) with multiple positive results. In addition, 572 pan-negative patients also have tested their HER2 status by ARMS and 29 (29/572,5.1%) were identified as HER2 mutation positive (Fig 1).
Fig. 1.
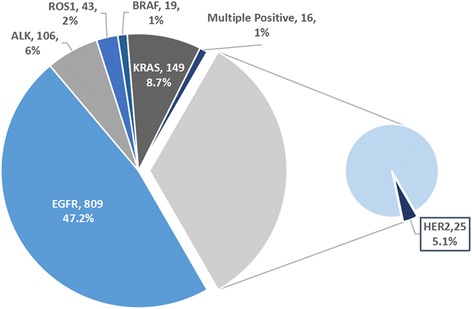
Mutations-testing results of 1714 patients with advanced non-small cell lung cancers.(multiple positive results 7EGFR&ALK, 1EGFR&ROS1, 2EGFR&KRAS, 2EGFR&BRAF, 2ALK&KRAS, 1ROS1&KRAS, 1EGFR&ROS1&KRAS)
HER2-mutant lung cancer patients had a median age of 58 (range 44–77 years) and mutations were more common in females (p<0.001),non-smokers (p = 0.034) and adenocarcinomas (p = 0.002)(Table 1). Twenty-four of 29 patients had available samples for sequencing and had the details variants of HER2 mutation including 14 with exon20 A775_G776insYVMA, 3with P780_Y781insGSP, 3with G776 > VC, 2with G776 > IC, 1with G776 > LC, and 1with G776C (Additional file 1: Figure S1).
Table 1.
Clinical characteristics of patients with HER2-mutant lung cancers
| Clinical characteristics | Total (n = 572) | HER2 negative (n = 543) | HER2 positive (n = 29) | P value |
|---|---|---|---|---|
| Age, years (median,range) | 64(27–92) | 64(27–92) | 58(44–77) | 0.017 |
| < 65 | 305(53.3%) | 283(52.1%) | 22(75.9%) | |
| ≥ 65 | 267(46.7%) | 260(47.9%) | 7(24.1%) | |
| Gender | ||||
| Male | 430(75.2%) | 417(76.8%) | 13(44.8%) | <0.001 |
| Female | 142(24.8%) | 126(23.2%) | 16(55.2%) | |
| Smoking status | ||||
| Non-smoker | 305(53.3%) | 284(52.3%) | 21(72.4%) | 0.034 |
| Smoker | 267(46.7%) | 259(47.7%) | 8(27.6%) | |
| Histology | ||||
| Adenocarcinoma | 429(75%) | 400(74.0%) | 29(100%) | 0.002 |
| Non-Adenocarcinoma | 143(25%) | 117(21.3%) | 0 | |
Outcomes of chemotherapy: Comparison among oncogenic mutations groups
Patients received first-line pemetrexed-based chemotherapy were eligible for analysis(n = 25,14 combined with carboplatin, 7 combined with cisplatin and 4 monotherapy). Since most patients with druggable mutations chose TKI as a first-line treatment, only 74 of 809 EGFR-mutant patients and 39 of 149 ALK/ROS1-rearranged patients were included. While there were a relatively large number of patients with KRAS mutation, the first 40 of KRAS identified were selected for this study.
The baseline characteristics of patients with HER2-mutant were compared with patients with EGFR-mutant, ALK/ROS1-rearranged, and KRAS-mutant lung cancers as summarized in Table 2. HER2, EGFR, KRAS mutations and ALK, ROS1 rearrangements did not co-occur with each other in individual patient samples. KRAS mutations were more frequently detected in patients with more than 65 years old, male and smokers. And comparison revealed no significant differences in terms of PS score (p = 0.269), monotherapy versus combination therapy (p = 0.570), maintenance therapy versus non-maintenance therapy(p = 0.175).
Table 2.
Baseline characteristics of patients treated with pemetrexed-containing chemotherapy
| Clinical characteristics | HER2 | EGFR | ALK/ROS1 | KRAS | P value |
|---|---|---|---|---|---|
| N | 25 | 74 | 39 | 40 | |
| Age, years (median,range) | 55(44–77) | 58(27–80) | 54(37–77) | 64.5(33–80) | 0.002 |
| < 65 | 21(84.0%) | 55(74.3%) | 28(71.8%) | 20(50.0%) | |
| ≥ 65 | 4(16.0%) | 19(25.7%) | 11(28.2%) | 20(50.0%) | |
| Gender | |||||
| Male | 12(48.0%) | 37(50.0%) | 20(51.3%) | 33(82.5%) | 0.004 |
| Female | 13(52.0%) | 37(50.0%) | 19(48.7%) | 7(17.5%) | |
| Smoking status | |||||
| Non-smoker | 18(72.0%) | 56(75.7%) | 29(74.4%) | 15(37.5%) | <0.001 |
| Smoker | 7(28.0%) | 18(24.3%) | 10(25.6%) | 25(62.5%) | |
| PS | |||||
| 0–1 | 22(88.0%) | 68(91.9%) | 36(92.3%) | 32(80.0%) | 0.269 |
| ≥ 2 | 3(12.0%) | 6(8.1%) | 3(7.7%%) | 8(20.0%) | |
| Therapy | |||||
| Monotherapy | 4(16.0%) | 9(12.2%) | 3(7.7%) | 9(22.5%) | 0.570 |
| Plus carboplatin | 14(56.0%) | 42(56.8%) | 24(61.5%) | 23(57.5%) | |
| Plus cisplatin | 7(28.0%) | 23(31.1%) | 12(30.8%) | 8(20.0%) | |
| Maintenance therapy | 7(28.0%) | 18(24.3%) | 13(33.3%) | 5(12.5%) | 0.175 |
| No maintenance | 18(72.0%) | 56(75.7%) | 26(66.7%) | 35(87.5%) | |
The response was evaluated in all 178 patients. Both the objective response rate(ORR) and the disease control rate (DCR) were not significantly different among four groups (Table 3). However, PFS was significantly different among all groups. Patients in the HER2-mutant group had a median PFS of 5.1 months (95% confidence interval [CI], 4.90–5.30) (95% CI 4.90–5.30), which was numerically shorter than that of the EGFR-mutant group (6.5 months, 95% CI 4.48–8.52, p = 0.247) and significantly shorter than that of the ALK/ROS1-rearranged (9.2 months, 95% CI 6.41–11.99, p = 0.004). Similarly, in KRAS-mutant lung cancers, PFS (5.0 months, 95% CI 3.67–6.33) was inferior compared with EGFR-mutant (6.5 months, p = 0.242) and ALK/ROS1-rearranged (9.2 months, p = 0.007) lung cancers. PFS was not significantly different between the HER2-mutant and the KRAS-mutant lung cancers groups (5.1 vs 5.0 months, p = 0.971) (Fig.2a).
Table 3.
The objective response rate(ORR)and the disease control rate (DCR) of patients treated with pemetrexed-based therapy in four groups
| HER2 | EGFR | ALK/ROS1 | KRAS | P value | |
|---|---|---|---|---|---|
| n | 25 | 74 | 39 | 40 | |
| ORR% | 36.0 | 33.8 | 41.3 | 35.0 | 0.896 |
| DCR% | 92.0 | 78.4 | 87.2 | 72.5 | 0.139 |
Fig. 2.
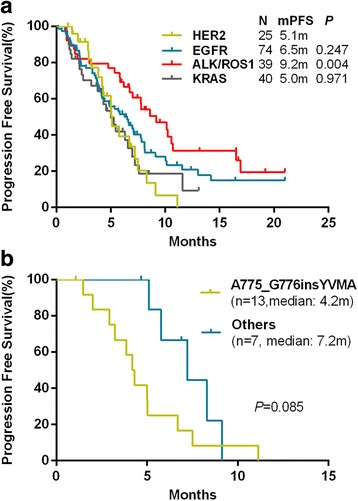
Progression-free survival time. a:Progression-free survival time of patients treated with pemetrexed-based therapy The “HER2” group were compared with the “EGFR”, “KRAS” and “ALK/ROS1” groups. b: Progression-free survival time of patients with HER2-mutant lung adenocarcinomas treated with pemetrexed-based therapy. The A775_G776insYVMA group were compared with the other variants group (n=7, 3with P780_Y781insGSP, 2with G776>IC, 1with G776>LC, and 1with G776C)
Outcomes of chemotherapy: Comparison among HER2 variants subgroups
Twenty patients of the 25 patients receiving first-line pemetrexed-based chemotherapy had known HER2 variants (Additional file 1: Figure S1). According to the frequency of the variants, they were divided into the exon20 A775_G776insYVMA group (n = 13) and the other variants group (n = 7, 3with P780_Y781insGSP, 2with G776 > IC, 1with G776 > LC, and 1with G776C). PFS has a trend to be inferior in the YVMA group, even though no statistically significant difference existed between the 2 groups (4.2 vs 7.2 months, p = 0.085) (Fig 2b).
Discussion
As far as we know, this study is the first study to compare the efficacy of pemetrexed-based chemotherapy between HER2-mutant and groups of EGFR-mutant, ALK/ROS1-rearranged and KRAS-mutant lung adenocarcinoma. We found that patients with HER2-mutant lung cancers had a PFS of 5.1 months that was similar with KRAS-mutant (5.0 months, p = 0.971) lung cancers, and numerically shorter than EGFR-mutant (6.5 months, p = 0.247) and significantly shorter than ALK/ROS1-rearranged (9.2 months, p = 0.004) lung cancers, showing that HER2-mutant lung cancer patients may have poor outcomes with chemotherapy, which strengthen the importance of developing HER2-targeted drugs in this population. We also investigate the clinicopathologic features in patients with advanced HER2-mutant lung adenocarcinomas and found that HER2 mutations were more common in younger patients, females, non-smokers and adenocarcinomas.
Different from HER2 over-expression and amplification, HER2 mutations was found to be a distinct entity in patients with NSCLC [20]. HER2 mutations are found in about 1%–2% of NSCLC [20–22]. In this study, the incidence of HER2 mutations was 5.1% in EGFR/KRAS/BRAF/ALK/ROS1 negative patients, indicating that HER2 mutations will be enriched in the population without other driver gene mutations. Consistent with our study, a study from the Memorial Sloan Kettering Cancer Center (MSKCC) group [23] showed that in a selected population with EGFR/KRAS/ALK negative, the incidence of HER2 mutations can reach up to 6%. In the early stage resection samples, our previous study [6] showed that the presence of HER2 mutations was not correlated with gender, age, or smoking status. However, another retrospective study [24] of resection samples obtained at Fudan University Shanghai Cancer Center found that the incidence of HER2 mutations can reach up to 5.94% in non-smoking patients with lung adenocarcinoma. Similarly, in biopsied samples from advanced NSCLC, our study showed that HER2 mutations were more common in non-smokers and lung adenocarcinomas. But HER2 mutations were also frequently detected in younger patients and females in our study. Furthermore, exon20 A775_G776insYVMA was the most frequently alteration.
In the era of targeted therapy, several oncogenic driver mutations were found not only could predict the efficacy of targeted therapy, but also associated with superior outcome of first line pemetrexed chemotherapy, such as ALK, ROS1 and RET [12–16]. Thus, we further investigate the association of HER2 mutation with the efficacy of pemetrexed-based chemotherapy in patients with advanced lung adenocarcinomas. We found that patients with HER2-mutant lung cancers had a PFS of 5.1 months. Similar to this study, in the EUHER2 study [25] of patients with HER2-mutant lung cancers, ORR and PFS with chemotherapy were 43.5% and 6 months in first-line and 10% and 4.3 months in second-line therapies. Our study also showed that HER2-mutant lung cancers had a similar PFS of pemetrexed-based chemotherapy with KRAS-mutant lung cancers (5.0 months), which was inferior compared with EGFR-mutant(6.5 months) and ALK/ROS1-rearranged (9.2 months), indicating that HER2 mutation might predict a poor efficacy of pemetrexed-based chemotherapy, just like KRAS mutation. Although pemetrexed-based chemotherapy had the longest duration among chemotherapies(pemetrexed/taxane/gemcitabine/vinorelbine/etoposide±platinum) for patients with HER2 mutations according to Eng et al’s study [26] and Gow et al’s study [27], outcomes of pemetrexed for HER2 were poor compared to other oncogene subgroups,such as ALK and ROS1. Furthermore, we further divide HER2 mutations into the exon20 A775_G776insYVMA group and the other variants group and it was the first time that we found that patients with YVMA insertion were associated with an inferior PFS (4.2 vs 7.2 months, p = 0.085).
Currently, NCCN guideline recommend trastuzumab and afatinib as the targeted therapeutic options for patients with advanced HER2-mutant NSCLC. While, in EUHER2 study [25], afatinib showed a modest response of 18.2% and median PFS of 3.9 months even though this drug has showed response in all 3 assessable patients with HER2-mutant adenocarcinoma in a preliminary study [28]. Meanwhile, several other studies [5, 7, 8] investigated the efficacy of other irreversible pan-HER receptor family inhibitors, dacomitinib, neratinib, or neratinib combining with mTOR inhibitors in advanced NSCLC patients harboring HER2 mutations and showed a moderate response of 12%–21%. Although these ORR or PFS are much diminished compared with those of TKIs directed at other targets in NSCLC, HER2-targeted drugs is still promising. A phase II study recently investigated a novel EGFR/HER2 inhibitor, pyrotinib, in heavily pre-treated patients with HER2-mutant adenocarcinomas and found a promising results with RR of 54.5%(6/11) and median PFS of 6.2 months [9]. Large number cohort study is still needed to validate the efficacy of pyrotinib in this setting.
Our study does have several limitations. First, it was a retrospective study with limited patients number(n = 25), while this study presented the real world nature in Chinese population. Second, HER2 mutation testing was performed using the method of ARMS, thus some rare mutations might be missed in our population. Next generation sequencing (NGS), which allows for simultaneous testing for multiple mutations using one platform and one sample, is emerging as an important method for identification of gene mutations in NSCLC, but single-gene sequencing is still more widely used. Thirdly, a substantial part of the patients with HER2 mutations also participant into the previous clinical trial of HER2-targeted drugs [9], thus the overall survival might be heavily influenced by the subsequent therapy.
Conclusions
In conclusion, HER2 mutations were more frequent happened in younger patients, females, non-smokers and adenocarcinomas of advanced NSCLC. Patients with HER2-mutant lung adenocarcinomas, especially YVMA insertion, showed poor response to pemetrexed-based chemotherapy. Thus, developing HER2-targeted drugs to improve their poor prognosis is urgently needed for this population.
Additional file
Figure S1. Study flow chart. (PDF 160 kb)
Acknowledgments
This study was supported in part by grants from projects of the Science and Technology Commission of Shanghai Municipality (No.16411964600), and Shanghai Municipal Education Commission (No.16SG18).
Funding
The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- ARMS
Amplification refractory mutation system
- HER2
Human epidermal growth factor receptor2
- NCCN
The National Comprehensive Cancer Network.
- NSCLC
Non-small cell lung cancers
- ORR
The objective response rate
- PFS
Progression free survival
- RT-PCR
Reverse transcriptase polymerase chain reaction
Authors’ contributions
YW and SZ contributed equally in preparing and conducting this research. FW, JZ, XL and CZ provided the patient information and followed the patient survival data. SR and CZ designed and coordinated the research in the whole process. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Ethical Review Committee of the Shanghai Pulmonary Hospital(No.K16–223-1) and informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest were disclosed.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4277-x) contains supplementary material, which is available to authorized users.
Contributor Information
Yan Wang, Email: wangyan093289@tongji.edu.cn.
Shijia Zhang, Email: 996757211@qq.com.
Fengying Wu, Email: fywu@163.com.
Jing Zhao, Email: zhaojing626@163.com.
Xuefei Li, Email: bug_lily2003@163.com.
Shengxiang Ren, Email: harry_ren@126.com.
Caicun Zhou, Email: caicunzhoudr@163.com.
References
- 1.Plosker GL, Keam SJ. Spotlight on Trastuzumab in the management of HER2-positive metastatic and early-stage breast cancer. BioDrugs. 2006;20(4):259–262. doi: 10.2165/00063030-200620040-00007. [DOI] [PubMed] [Google Scholar]
- 2.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 3.Gatzemeier U, Groth G, Butts C, Van Zandwijk N, Shepherd F, Ardizzoni A, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol. 2004;15:19–27. doi: 10.1093/annonc/mdh031. [DOI] [PubMed] [Google Scholar]
- 4.Krug LM, Miller VA, Patel J, Crapanzano J, Azzoli CG, Gomez J, et al. Randomized phase II study of weekly docetaxel plus trastuzumab versus weekly paclitaxel plus trastuzumab in patients with previously untreated advanced nonsmall cell lung carcinoma. Cancer. 2005;104:2149–2155. doi: 10.1002/cncr.21428. [DOI] [PubMed] [Google Scholar]
- 5.Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O'Connell J, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann of Oncol. 2015;26:1421–1427. doi: 10.1093/annonc/mdv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Zhao C, Su C, Ren S, Chen X, Zhou C. Epidemiological study of HER-2 mutations among EGFR wild-type lung adenocarcinoma patients in China. BMC Cancer. 2016;16:828. doi: 10.1186/s12885-016-2875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi L, Bahleda R, Tolaney SM, Kwak EL, Cleary JM, Pandya SS, et al. I Phase study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol 2014;32(January (2)):68–75. [DOI] [PubMed]
- 8.Besse B, Soria J-C, Yao B. Neratinib with or without temsirolimus in patients with non-small cell lung cancer carrying HER2 somatic mutations: an inter- national randomized phase II study. ESMO Cong 2014. Abstract LBA39 PR.
- 9.Ren S, Gao G, Wu F, Su C, Chen X, He Y, et al. Preliminary Results of a Phase II Study about the Efficacy and Safety of Pyrotinib in Patients with HER2 Mutant Advanced NSCLC. WCLC 2016.Abstract MA 0403.
- 10.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus Pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung Cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Oh I, Kim K, Jang T, Choi Y, Kim Y, et al. A randomized phase iii study of docetaxel plus cisplatin versus pemetrexed plus cisplatin in first line non-squamous non-small cell lung cancer (NSQ-NSCLC). Ann Oncol (2014) 25 (suppl 4; abstr LBA41_PR).
- 12.Lee JO, Kim TM, Lee SH, Kim DW, Kim S, Jeon YK, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of Pemetrexed in patients with non-small cell lung Cancer. J Thorac Oncol. 2011;6(9):1474–1480. doi: 10.1097/JTO.0b013e3182208fc2. [DOI] [PubMed] [Google Scholar]
- 13.Ren S, Chen X, Kuang P, Zheng L, Su C, Li J, et al. Association of EGFR mutation or ALK rearrangement with expression of DNA repair and synthesis genes innever-smoker women with pulmonary adenocarcinoma. Cancer. 2012;118:5588–5594. doi: 10.1002/cncr.27603. [DOI] [PubMed] [Google Scholar]
- 14.Chen YF, Hsieh MS, Wu SG, Chang YL, Yu CJ, Yang JC, et al. Efficacy of Pemetrexed-based chemotherapy in patents with ROS1 fusion-positive lung adenocarcinoma compared with in patients harboring other driver mutations in east Asian populations. J Thorac Oncol. 2016;11(7):1140–1152. doi: 10.1016/j.jtho.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Jiang T, Zhao C, Li W, Li X, Zhao S, et al. Efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement. Oncotarget. 2016;7(46):75145–75154. doi: 10.18632/oncotarget.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drilon A, Bergagnini I, Delasos L, Sabari J, Woo KM, Plodkowski A, et al. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol. 2016;27:1286–1291. doi: 10.1093/annonc/mdw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren S, Kuang P, Zheng L, Su C, Li J, Li B, et al. Analysis of driver mutations in femalenon-smoker Asian patients with pulmonary adenocarcinoma. Cell Biochem Biophys. 2012;64:155–160. doi: 10.1007/s12013-012-9384-8. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhang J, Gao G, Li X, Zhao C, He Y, et al. EML4-ALK fusion detected by RT-PCR confers similar response to crizotinib as detected by FISH in patients with advanced NSCLC. J Thorac Oncol. 2015;10:1546–1552. doi: 10.1097/JTO.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Liu Y, Zhao C, Li X, Wu C, Hou L, et al. Feasibility of cytological specimens for ALK fusion detection in patients with advanced NSCLC using the method of RT-PCR. Lung Cancer. 2016;94:28–34. doi: 10.1016/j.lungcan.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, et al. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016;11(3):414–419. doi: 10.1016/j.jtho.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazières J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 22.Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French cooperative thoracic intergroup (IFCT) Lancet. 2016;387(10026):1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 23.Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18(September (18)):4910–4918. [DOI] [PMC free article] [PubMed]
- 24.Li C, Fang R, Sun Y, Han X, Li F, Gao B, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from east Asian never smokers. PLoS One. 2011;6:e28204. doi: 10.1371/journal.pone.0028204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazières J, Barlesi F, Filleron T, Besse B, Monnet I, Beau-Faller M, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27(2):281–286. doi: 10.1093/annonc/mdv573. [DOI] [PubMed] [Google Scholar]
- 26.Eng J, Hsu M, Chaft JE, Kris MG, Arcila ME, Li BT. Outcomes of chemotherapies and HER2 directed therapies in advanced HER2-mutant lung cancers. Lung Cancer. 2016;99:53–56. doi: 10.1016/j.lungcan.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gow CH, Chang HT, Lim CK, Liu CY, Chen JS, Shih JY. Comparable clinical outcomes in patients with HER2-mutant and EGFR-mutant lung adenocarcinomas. Genes Chromosomes Cancer. 2017;56(5):373–381. doi: 10.1002/gcc.22442. [DOI] [PubMed] [Google Scholar]
- 28.De Grève J, Teugels E, Geers C, Decoster L, Galdermans D, De Mey J, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 2012;76(April (1)): 123–127. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flow chart. (PDF 160 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


