Abstract
Background
Malaria causes significant morbidity and mortality worldwide. There are several preventive measures that are currently employed, including insecticide-treated nets (ITNs, including long-lasting insecticidal nets and insecticidal-treated bed nets), indoor residual spraying (IRS), prophylactic drugs (PD), and untreated nets (UN). However, it is unclear which measure is the most effective for malaria prevention. We therefore undertook a network meta-analysis to compare the efficacy of different preventive measures on incidence of malaria infection.
Methods
A systematic literature review was undertaken across four medical and life sciences databases (PubMed, Cochrane Central, Embase, and Web of Science) from their inception to July 2016 to compare the effectiveness of different preventive measures on malaria incidence. Data from the included studies were analysed for the effectiveness of several measures against no intervention (NI). This was carried out using an automated generalized pairwise modeling (GPM) framework for network meta-analysis to generate mixed treatment effects against a common comparator of no intervention (NI).
Results
There were 30 studies that met the inclusion criteria from 1998–2016. The GPM framework led to a final ranking of effectiveness of measures in the following order from best to worst: PD, ITN, IRS and UN, in comparison with NI. However, only ITN (RR: 0.49, 95% CI: 0.32–0.74) showed precision while other methods [PD (RR: 0.24, 95% CI: 0.004–15.43), IRS (RR: 0.55, 95% CI: 0.20–1.56) and UN (RR: 0.73, 95% CI: 0.28–1.90)] demonstrating considerable uncertainty associated with their point estimates.
Conclusion
Current evidence is strong for the protective effect of ITN interventions in malaria prevention. Even though ITNs were found to be the only preventive measure with statistical support for their effectiveness, the role of other malaria control measures may be important adjuncts in the global drive to eliminate malaria.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2783-y) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Preventive measures, Meta-analysis, Efficacy
Background
Malaria imposes a great health and socio-economic burden on humanity, with an estimated 3.2 billion people at risk of being infected with malaria [1]. In 2016, there were approximately 216 million cases with 445,000 deaths, most of which were in children aged under 5 years in Africa [1]. Between 2000 and 2015, it has been estimated that there was a 37% global reduction in malaria incidence [2]. This improvement was likely made possible by economic development and urbanization in many endemic countries [3] as well as a substantial increase in investment in tackling malaria [4], leading to an increase in preventative activities, and improved diagnostics and treatment. The Global Technical Strategy for Malaria 2016–2030 (GTS) has a target to eliminate malaria in at least ten countries by 2020, 20 countries by 2025, and 30 countries by 2030 [2, 5].
Vector control remains an essential component of malaria control and elimination. The capacity of vectors to transmit parasites and their vulnerability to vector control measures vary by mosquito species and are influenced by local environmental factors. Personal preventive measures that prevent contact between the adult mosquitoes and human beings are the main methods of prevention currently in practice. These include insecticide-treated nets (ITNs), and indoor residual spraying (IRS) [6]. ITNs are of two types: long-lasting insecticidal nets (LLINs) that have the insecticide incorporated into fibers during the manufacturing process, which leads to a longer duration of effectiveness and insecticide-treated nets (ITNs) which are impregnated with insecticides every six months. Indoor residual spraying (IRS) involves spraying insecticides on the walls of the houses. Additionally, antimalarial chemoprophylaxis is used for prevention of malaria in children and pregnant women. The commonly used prophylactic drugs (PD) are sulphadoxine-pyrimethamine (SP), mefloquine (MQ), amodiaquine (AQ), dihydroartemisinin-peperaquine (DP) and artesunate (AS). The main advantage of using PD is that they only require a single dose to achieve a full prophylactic effect [7, 8]. However, the most common PD is SP and it is becoming less effective due to resistance [9–13]. As a result, other drugs such as MQ and AQ are increasingly being used as a substitute for or in combination with SP [12, 14]. MQ provides a longer period of prophylaxis but side effects (agranulocytosis in 1 per 2000 patients) [15] are the main problem [16, 17]. Similarly, AQ has been used in combination with SP but AQ is not well tolerated [14]. Many other less commonly utilized measures include insecticide-treated curtains (ITC), mosquito coils, insecticide-treated hammocks, and insecticide-treated tarpaulins.
There has been a decrease in malaria incidence worldwide, but what remains unclear is which of the common preventive interventions is the most effective for prevention of malaria infection. This knowledge may help prioritise resourcing of these interventions. There has been one comparative study of preventive efficacy that compares mortality across ITN, IRS and PD and this study demonstrated that the impact of IRS is equal to that of ITN on reducing malaria-attributable mortality in children [18]. There have also been several systematic reviews and meta-analyses focusing on single preventive measures. These reviews of existing data suggest that PD [19–22], is effective in preventing malaria infection in children when treated on a monthly basis with no protection when given three-monthly. The reviews of both ITN [23–25] and IRS [26, 27] provide support for their effectiveness as malaria preventive measures, but there is no data on the effectiveness of one measure over another.
Therefore, this study aims to present an up-to-date comparison of the effectiveness of the four common malaria preventive measures (ITNs, UNs, IRS and PDs) for which data are readily available and compare these against no intervention [NI, defined as no intervention or placebo or a study group with standard care (any intervention given to all participants)]. A network meta-analysis methodology was chosen to pool the data as it allows comparisons of multiple preventive measures simultaneously and allows comparisons across preventive measures not directly tested in the included trials (indirect comparisons across a pair of studies that share a common comparator). In addition, this method allows ranking of the effectiveness of these measures for decision making.
Methods
Search strategy and eligibility criteria
A systematic literature review was undertaken using four medical and life sciences databases (PubMed, Cochrane Central, Embase and Web of Science). They were searched from their inception to March 2016 for trials that compared the effectiveness of malaria preventive measures. Search terms included were “malaria”, “Plasmodium falciparum”, “Plasmodium vivax”, “bed net”, “mosquito control”, “antimalarial”, and “insecticides”; the specific keywords and connectors for each database are listed in the Additional file 1: Table S1.
The inclusion of studies were restricted to (i) interventional studies; (ii) conducted in humans (with no restriction of age or sex); (iii) that compared two or more of the following malaria preventive measures: ITN, UN, IRS, PD or NI; and (iv) reported the number of new malaria cases diagnosed through microscopy or rapid diagnostic tests (RDT) after each intervention compared amongst a population at risk over time. Exclusion criteria included: (i) non-intervention studies; (ii) conference abstracts; and (iii) other less commonly utilized malaria preventive measures including ITC, mosquito coils, insecticide-treated hammocks and insecticide-treated tarpaulins. No language restrictions were imposed. Since we used a generalized pairwise modeling approach (see below), odd numbers of treatments (e.g. three treatment arms) required selection of a pair for inclusion in this study and we therefore excluded the arm that had the most available data in this synthesis [(i) arms that we excluded do not make a difference, (ii) concurrent interventions and no effect modification].
Study selection and data extraction
The citation search was developed and executed by JC, followed by selection of citations by title and abstract independently by two researchers (KW and LFK). The selected studies underwent a full-text review for all potentially relevant studies. Data from the included studies were then independently extracted in a spreadsheet by the same two researchers. The extracted data included: (i) the country of study; (ii) year(s) when the study was conducted; (iii) study design; (iv) study population characteristics; (v) preventive measures employed in the trial; and (vi) the number of new cases of malaria and person-months at risk. The extracted data were then cross-checked by the two researchers and any discrepancies during the selection of studies or data extraction were resolved through discussion and consensus following independent evaluation by another author (SARD).
Statistical analysis
The outcome of interest was the rate ratio (RR) of new malaria cases in intervention-A vs intervention-B following the implementation of different preventative measures. An automated generalized pairwise modeling (GPM) framework [28] was used to generate mixed treatment effects against a common comparator (NI). This framework is an extension of the Bucher method [29] that automates the single three-treatment loop method. This analysis starts by pooling effect sizes based on direct comparisons between any two interventions using meta-analytic methods. The indirect comparison was then performed by automated generation of all possible closed loops of three-treatments such that one of them was common to the two studies and formed the node where the loop began and ended but where the common node was never NI, while one of the other nodes was always NI. Finally, the mixed effects (multiple direct/indirect effects) were pooled using the same meta-analysis model as used for pooling direct effects. The analysis therefore led to a final mixed treatment effect estimate for different interventions versus NI. Estimates of preventive effectiveness were then ranked by their point estimates. It should be pointed out that it is common for network plots based on Bayesian methods to rank treatments by the surface under the cumulative ranking curve (SUCRA). From our frequentist perspective, treatment effects are thought of as fixed parameters and thus, strictly speaking SUCRA does not apply. A frequentist alternative called the P-score has been proposed but SUCRA or P-scores have no major advantage compared to what we have done, i.e. ranking treatments by their point estimates [30].
All direct estimates were pooled using the inverse variance heterogeneity (IVhet) model [31] as were all mixed estimates, but this synthesis process was also repeated using the random effects model for comparison (the random effects analysis was undertaken under the GPM framework as well as under the frequentist multivariate meta-analysis framework for comparison (see Additional file 1: Tables S2 and S3 for details).
Cluster randomized controlled trials (RCT) were combined with other study types after accounting for clustering using the design effect (DEFF). The DEFF was calculated as follows:
where ρ is the intra-class correlation for the statistic in question and c is the average size of the cluster. We then divided the numbers in each 2 × 2 table of the study by the DEFF to calculate a corrected sample size, which was then utilized in the meta-analysis. Different units of clusters such as villages and households were used in different studies. The intra-class correlation coefficient (ρ) was provided only in one study [32], and this (ρ = 0.048) was used for calculation of the DEFF for other cluster RCT studies.
Statistical heterogeneity across direct effects pooled in the meta-analysis were assessed by the Cochran’s Q and the H index which is the square root of H2, the estimated residual variance from the regression of the standardized treatment effect estimates against the inverse standard error in each direct meta-analysis. H was computed as follows:
where n is the number of study estimates pooled and Q represents the Chi squared from Cochran’s Q.
Transitivity was assessed statistically by looking at inconsistency across the network as a whole using the weighted pooled H index () which was computed as follows from the Cochran’s Q statistic for the k final comparisons:
where n is the number of estimates pooled across each comparison and s is the number comparisons (out of k) were n = 1. The minimum value H or can take is 1, it is not influenced by n, and was taken to be minimal inconsistency based on our simulations of H in homogenous direct meta-analyses [28].
Sensitivity analyses were undertaken through limiting the network to (i) studies conducted in children or (ii) studies including only Plasmodium falciparum infection and then re-running the GPM analysis.
Publication bias was assessed using a ‘comparison adjusted’ funnel plot where on the horizontal axis the difference of each study’s observed ln(RR) from the comparison’s mean ln(RR) obtained from the pairwise fixed effect meta-analysis was plotted. In the absence of small-study effects, we expect the studies to form an inverted funnel centred at zero [33]. All the analyses involved in the generalised pairwise modelling (GPM) framework for multiple indirect and mixed effects were conducted using MetaXL v5.2 (EpiGear International, Sunrise Beach, Australia) [28]. Funnel and network plots were produced using Stata version 13 (Stata Corporation, College Station, TX, USA).
Quality assessment
The quality of the included studies was assessed using a modification of a quality checklist used in another study by one of the authors [34]. The studies were assessed on inclusion of safeguards relating to study design, selection, information, blinding of study assessors, and analytical biases. There were 12 questions with a possible maximum count of 17 safe-guards (Additional file 1: Table S4).
Results
Data extraction
The search strategy identified 7940 citations (Cochrane Central = 353, PubMed = 2698, Web of Science = 1534 and Embase = 3355). After deleting duplicate citations, a total of 4941 citations were retrieved for the initial screening. Of these, 4692 citations were excluded based on title only. Records of 249 citations were screened and 161 citations were excluded based on the title and abstract. Eighty eight articles were assessed for eligibility, of which 58 articles were excluded (Additional file 1: Table S5). Thirty citations fulfilled eligibility criteria and were included in the meta-analysis (Fig. 1). Data from the included studies were extracted and summarized in a spreadsheet (Table 1).
Fig. 1.
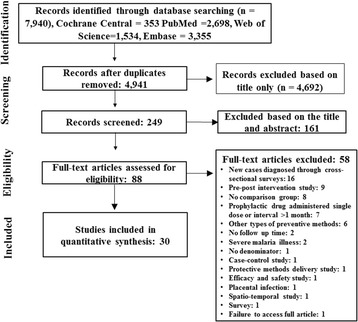
Search flowchart. Note: details of excluded studies in Additional file 1: Table S5
Table 1.
Characteristics of included studies
| Reference | Study area, year of trial | Study design | Study population | Diagnosis | I1 | Malaria incidence/person months | I2 | Malaria incidence/ person months | I3 | Malaria incidence/ person months | I4 | Malaria incidence/ person months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beach et al., 1993 [35] | Kenya, 1990 | Quasi-experimental- with control group | Children < 6 years in 6 villages | Microscopy | ITNb | 82/195 | NIb | 136/193 | ITC | 170/224 | – | – |
| Charlwood et al., 2001 [36] | Eastern Sudan, 1997 | Cluster RCT | All ages | PCR and microscopy | IRS | 46/418 | NI | 38/451 | – | – | – | – |
| Cisse et al., 2006 [37] | Sahel and sub-Sahel region, 2002–03 | RCT | Children 2–59 months | Microscopy | PD | 39/1315 | NI | 222/1321 | – | – | – | – |
| Dicko et al., 2011 [38] | Mali, 2008 | RCT | Children 3–59 months | Microscopy | PD | 149/4346 | NI | 832/4148 | – | – | – | – |
| Fraser-Hurt, 1999 [39] | Tanzania, 1996 | RCT | Children 5–24 months | Microscopy | ITN | 173/360 | NI | 234/360 | – | – | – | – |
| Hale, 2003 [40] | Ghana, 1998 | RCT | Adults | Microscopy | PD | 6/131 | NI | 86/117 | – | – | – | – |
| Hamel, 2011 [41] | Kenya, 2000 | Quasi-experimental-control group | All ages > 6 months | RDT | IRS+ITN | 114/7424 | ITN | 251/6840 | – | – | – | – |
| Hill et al., 2014 [62] | Yunnan, China, 2007 | RCT | All ages | Microscopy and RDT | ITN | 12/10,854 | NI | 73/10,857 | – | – | – | – |
| Kamol-Ratanakul et al., 1992 [53] | Thailand, 1987–88 | RCT | Adults | Microscopy | ITN | 28/1026 | UN | 51/1099 | – | – | – | – |
| Konate et al., 2011 [42] | Ghana, 2004 | RCT | Children < 6 years | Microscopy | ITN | 332/4564 | NI | 982/3504 | – | – | – | – |
| Kroeger et al., 1995 [63] | South America, 1991–94 | Quasi-experimental-control group | All ages | Microscopy and RDT | ITN | 899/38,260 | NI | 1617/40,668 | – | – | – | – |
| Kweku et al., 2008 [43] | Ghana, 2005–06 | RCT | Children 3–59 months | Microscopy | PD (monthly)b | 44/3756 | PD (bimonthly) | 112/3678 | PD (monthly) | 109/3372 | NIb | 183/3900 |
| Luxemburger et al., 1994 [54] | Thailand-Burma, 1990–91 | RCT | School children 4–15 years | Microscopy | ITN | 29/933 | UN | 52/939 | – | – | – | – |
| Lwin et al., 2012 [59] | Thailand, 2006–08 | RCT | Adults | Microscopy | PD | 5/2000 | NI | 69/845 | – | – | – | – |
| Marbiah et al., 1998 [44] | Sierra Leone, 1992–93 | Cluster RCT | Children 3 months to 6 years | Microscopy | ITN | 48/590 | NI | 90/559 | – | – | – | – |
| Mwangi et al., 2003 [45] | Kenya, 2000 | Quasi-experimental-control group | Children < 19 years | Microscopy | UNb | 62/638 | UNa | 22/42 | NIb | 58/383 | – | – |
| Nankabirwa et al., 2014 [46] | Uganda, 2011–12 | RCT | Children 6–14 years | Microscopy | PD (monthly)b | 3/2886 | PD (school term) | 81/2857 | NIb | 83/2912 | – | – |
| Nevill et al., 1988 [47] | Kenya, NR | RCT | Children 6–18 years | Microscopy | UNb | 1/130 | PDb | 8/123 | NI | 35/123 | – | – |
| Odhiambo et al., 2010 [48] | Kenya, 2004–08 | RCT | Infants 5–16 weeks | Microscopy | PDe | 2/334 | PD | 2/334 | NIe | 17/325 | – | – |
| Pinder et al., 2015 [49] | Gambia, 2010–11 | Cluster RCT | Children 6 months to 14 years | Microscopy and RDT | IRS | 15/886 | NI | 17/899 | – | – | – | – |
| Rowland et al., 1996 [55] | Pakistan, 1991 | Quasi-experimental- control group | All ages | Microscopy | ITN | 235/8388 | NI | 541/8364 | – | – | – | – |
| Sahu et al., 2003 [57] | India, 1999 | Cluster RCT | All ages | Microscopy | ITN | 3/551 | NI | 9/565 | – | – | – | – |
| Sesay et al., 2011 [50] | Gambia, 2008 | RCT | Children < 5 years | Microscopy | PD | 1/2248 | NI | 3/2279 | – | – | – | – |
| Sexton et al., 1990 [51] | Kenya, 1988 | RCT | All ages | Microscopy | ITNe | 55/339 | ITC | 34/323 | NIe | 75/322 | – | – |
| Shah et al., 2013 [61] | India, 2006–11 | Quasi-experimental- control group | All ages | Microscopy | ITN | 124/34,272 | NI | 45/18,624 | – | – | – | – |
| Sharma et al., 2009 [58] | India, 2006–07 | Cluster RCT | All ages | Microscopy | ITNe | 16/23,436 | UNb | 48/24,228 | NI | 50/12696 | – | – |
| Smithuis et al., 2013 [32] | Myanmar, 1998 | Cluster RCT | Children < 10 years | Microscopy | ITN | 351/3989 | NI | 713/4053 | – | – | – | – |
| Snow et al., 1988 [52] | Gambia, 1986 | Cluster RCT | Children 1–9 years | Microscopy | UN | 22/435 | NI | 20/303 | – | – | – | – |
| Soleimani-Ahmad et al., 2012 [60] | Iran, 2009–10 | Cluster RCT | All ages | Microscopy | ITN | 17/7008 | NI | 35/7145 | – | – | – | – |
| Taylor et al., 1999 [56] | Indonesia, 1996-97 | RCT | Adult (non-pregnant) 18–55 years | Microscopy | PDb | 28/467 | PD | 3/265 | NIe | 56/142 | – | – |
Abbreviations: I1 intervention 1, I2 intervention 2, I3 intervention 3, I4 intervention 4, MI Malaria infection, ITC insecticide-treated curtain, ITN insecticide-treated net, PD prophylactic drug, NI no intervention, RDT rapid diagnostic test, RCT randomized clinical trial, IRS indoor residual spraying, UN untreated nets
aUntreated net torn
bIncluded arms in network meta-analysis if more than two arms
Characteristics of included studies
The literature search on malaria control and preventive measures led to the identification of the five treatment groups across the studies (ITN, UN, PD, IRS and NI). A total of 30 studies were included in the current meta-analysis. These studies were conducted from 1988 to 2015. Eighteen studies were conducted in Africa [35–52], 11 studies were from Asia [32, 53–62] and one study from South America [63]. Ten studies did not restrict study participants to any age [36, 41, 51, 55, 57, 58, 60–63], four studies only included adults as study participants [40, 53, 56, 59], and the rest of the studies (16) were conducted in children and adolescents (0–19 years) [32, 35, 37–39, 42–50, 52, 54]. There were 21 studies that reported P. falciparum infection rates separately [32, 35, 37–43, 45–48, 50–52, 54, 55, 58, 60, 62]. The latter two groups were used in a sensitivity analysis (see below). The most common study design was the RCT with 16 studies [37–40, 42, 43, 46–48, 50, 51, 53, 54, 56, 59, 62], eight studies were cluster RCT [32, 36, 44, 49, 52, 57, 58, 60], and the rest (6) were quasi-experimental studies with a control group [35, 41, 45, 55, 61, 63]. Twenty one studies had two arms [32, 36–42, 44, 49, 50, 52–55, 57, 59–63], eight studies had three arms, [35, 45–48, 51, 56, 58] and one had four arms [43]. Of those with three arms we dropped the curtain arm in two studies [31, 51] (not part of this review) and one of the PD arms in four other studies [43, 46, 48, 56] that reported PD comparisons at different dosages or intervals. Microscopy was used for detection of Plasmodium parasites in 25 studies [32, 35, 37–40, 42–48, 50–61], three studies used both microscopy and RDTs [49, 62, 63], and one study each used RDT [41] and polymerase chain reaction (PCR) and microscopy [36] for diagnosis (Table 1).
Interventions utilized across studies
Twenty five studies had a NI arm [32, 35–46, 48–52, 55–57, 59, 61–63], and seven studies had a UN arm [45, 47, 52–54, 58, 60]. Of the eleven studies that used a PD arms [37, 38, 40, 42, 43, 46–48, 50, 56, 59], the regimens were all different (Additional file 1: Table S6). Fourteen studies reported the use of ITN in one arm [32, 35, 39, 44, 51, 53–55, 57, 58, 60–63]. In these studies, different types of nets and insecticides for treating such nets were used (Additional file 1: Table S7). The insecticides used for IRS in the three studies of this intervention were also different and are listed in (Additional file 1: Table S8) [36, 41, 49].
Quality assessment
The quality of the studies including types of study, randomization and other characteristics was assessed through 17 safeguards against bias as outlined in the supplementary material. They were combined into a univariate overall quality score consisting of counts of safeguards ranging between 7 and 17 out of a maximum possible of 17. The ranges of the scores were 10–17, 7–14, 9–16, 10–16, and 8–11 in PD vs NI, ITN vs NI, IRS vs NI, ITN vs UN, and UN vs NI studies, respectively. The most common safeguards missing were consideration of confounders such as socio-economic status, owning LLINs, malaria prevalence and blinding of assessors in between 46.7–93.3% of studies (Additional file 1: Table S9).
Quantitative synthesis
Seven direct estimates based on head-to-head comparison within 30 studies, which included 60 treatment groups, were available (Table 2 and Fig. 2). In these direct comparisons, PD (RR: 0.21, 95% confidence interval [CI] 0.13–0.33), ITN (RR: 0.57, 95% CI: 0.41–0.81), and UN (RR: 0.67, 95% CI: 0.49–0.92) were significantly better than NI. Similarly, UN (0.12, 95% CI: 0.01–0.94) was better as compared to PD, and IRS (RR: 0.55, 95% CI: 0.20–1.56) was not significantly different from NI.
Table 2.
Direct, indirect and final results from comparison of different preventive measures
| ID | Comparison | Active | Control | RR | 95% LCI | 95% HCI | K a | H |
|---|---|---|---|---|---|---|---|---|
| Direct estimates | ||||||||
| 1 | UN-PD | UN | PD | 0.12 | 0.01 | 0.94 | 1 | 1 |
| 2 | ITN-UN | ITN | UN | 0.56 | 0.40 | 0.76 | 4 | 1.11 |
| 3 | ITN-NI | ITN | NI | 0.57 | 0.41 | 0.81 | 10 | 2.76 |
| 4 | PD-NI | PD | NI | 0.21 | 0.13 | 0.33 | 10 | 2.49 |
| 5 | IRS-NI | IRS | NI | 0.55 | 0.20 | 1.56 | 3 | 3.41 |
| 6 | UN-NI | UN | NI | 0.67 | 0.49 | 0.92 | 2 | 1 |
| Indirect estimates (source IDs) | ||||||||
| 7 | Indirect UN vs NI (1, 4) | UN | NI | 0.02 | 0.003 | 0.21 | 2 | 3.01 |
| 8 | Indirect ITN vs NI (2, 6) | ITN | NI | 0.37 | 0.24 | 0.58 | 2 | 1.85 |
| 9 | Indirect PD vs NI (1, 6) | PD | NI | 5.70 | 0.70 | 46.58 | 2 | 3.01 |
| 10 | Indirect UN vs NI (2, 3) | UN | NI | 1.03 | 0.64 | 1.66 | 2 | 1.85 |
| Final estimates from all evidence (source IDs) | ||||||||
| PD (4, 9) | PD | NI | 0.24 | 0.004 | 15.43 | 2 | 3.00 | |
| ITN (3, 8) | ITN | NI | 0.49 | 0.32 | 0.74 | 2 | 1.85 | |
| IRS (5) | IRS | NI | 0.55 | 0.20 | 1.56 | 1 | 1 | |
| UN (6, 7, 10) | UN | NI | 0.73 | 0.28 | 1.90 | 3 | 2.59 | |
| Network H = 2.21 | ||||||||
Abbreviations: ITC insecticide-treated curtain, ITN insecticide-treated net, PD prophylactic drug, NI no intervention, IRS indoor residual spraying, UN untreated nets, RR rate ratio, LCI lower confidence interval, HCI higher confidence interval
aNumber of studies
Fig. 2.

Network plot showing the comparison groups. The circle size is proportional to the number of studies including that intervention while line width is proportional to the number of comparisons. Abbreviations: ITN, insecticide-treated nets; UN, untreated net; IRS, indoor residual spraying; NI, no intervention; PD, prophylactic drug
The indirect estimate for ITN (RR: 0.37, 95% CI: 0.24–0.58) was consistent with the direct estimate, while that for PD (RR: 5.70, 95% CI: 0.70–46.58) demonstrated an inconsistent and very uncertain effect as opposed to the direct estimate. UN had two indirect estimates possible and both were inconsistent with the direct effect but in opposite directions with either a grossly positive effect (RR: 0.02, 95% CI: 0.003–0.21) or a negative effect (RR: 1.03, 95% CI: 0.64–1.66) (Table 2).
The final estimates were based on all evidence for these interventions in comparison with NI and results showed that, PD (RR: 0.24, 95% CI: 0.004–15.43), ITN (RR: 0.49, 95% CI: 0.32–0.74), IRS (RR: 0.55, 95% CI: 0.20–1.56), and UN (RR: 0.73, 95% CI: 0.28–1.90) were all less likely to be associated with incident infection as compared to participants using no preventive measure (NI). However, only ITN demonstrated a statistically significant effect (Table 2 and Fig. 3).
Fig. 3.

Results of network meta-analysis of 30 studies comparing listed interventions against NI. Only the PD-NI mixed effects showed modest inconsistency and this is reflected in the marked uncertainty (wide 95% confidence intervals) of the effect estimate
There was overall minimal statistical network inconsistency (over comparisons despite the inconsistent direct and indirect effects, because of the huge uncertainty associated with indirect effects possibly reflecting heterogeneity in terms of the geographical locations and population characteristics of studies. One final effect (PD-NI) demonstrated modest inconsistency (H = 3.0) while the rest demonstrated minimal to no inconsistency (H < 3, see Table 2), again because of uncertainty around the individual mixed effects.
Sensitivity analysis and publication bias
Heterogeneity was evident when selection criteria were modified to include only children or only P. falciparum infections respectively (with at 2.35 and 2.29, respectively (Additional file 2: Figure S1; Additional file 3: Figure S2). However, the rank of effectiveness of different preventive measures remained unchanged in both analyses except that ITN was less effective than IRS in only P. falciparum and effects were now less precise because numbers of studies were lower.
The comparison-adjusted funnel plot demonstrated little evidence of asymmetry except for the PD-NI comparison, which was in keeping with the fact that there was both considerable heterogeneity and inconsistency across this comparison (Additional file 4: Figure S3).
Discussion
This meta-analysis showed that only ITNs had a significant effect in protection against malaria infection. While the effect size for PD was larger, the uncertainty was high, thus making the impact of this intervention uncertain. These findings confirm that impregnated insecticides on ITNs offers better protection than UNs in preventing mosquitoes from taking a blood meal from the host through its excito-repellency effect [23, 64–70]. The insecticides on ITNs may also inhibit mosquitoes from entering a house similar to the effect of IRS. Mortality of mosquitoes in the range of 25–75% has been observed after they enter huts in search of blood meals irrespective of the various different pyrethroids used in ITNs [67]. Individual studies on efficacy of this intervention have shown that the risk of malaria infection due to ITN use can reduce by up to 39–62% and child mortality by 14–29% [24, 71]. Interestingly, the impact of ITNs on child mortality and morbidity have been reported to extend out from areas with the actual ITN use to neighbouring areas because of the impact of the insecticidal nets on the entomological inoculation rate (EIR) of the local vector population [72–74]. Similarly, mathematical modelling has shown that ITNs can even protect against mosquitoes that feed outdoors [75]. ITNs have also been reported to protect women in pregnancy and in reducing placental malaria, anaemia, stillbirths and abortions [65]. Of note, the combination of IRS and ITN has been shown to offer better protection as compared to ITNs alone [27, 41, 76–79]. Only one of the latter studies was included in our synthesis which compared IRS vs NI where both arms were also given ITNs and the RR was 0.42 (95% CI: 0.34–0.52) suggesting that the effects are independent and additive on malaria prevention [41]. The other studies did not meet our inclusion criteria because these studies were cross-sectional and pre-post interventional studies but evidence from them was also supportive of this conclusion.
Despite reports of pyrethroid resistance in parts of the world including Africa [80–85], ITNs treated with pyrethroids continue to provide significant protection against malaria [69, 71, 86, 87]. ITNs of the LLIN type have insecticides impregnated in the fibres of nets, which are wash resistant for the four- to five-year lifespan of the ITNs. ITBN types of ITNs require insecticides to be impregnated every six months. Due to reduced costs and ease of implementation, the LLINs have gained huge popularity in recent years and given their superiority to IRS in this analysis as well as in previous studies [27, 88], this would represent a strong choice in terms of malaria prevention.
The biggest effect size was for PD. This intervention prevents or reduces the incidence of malaria primarily through clearing existing parasitaemia (or reducing it to a level below the fever threshold) and preventing new infections [8, 89, 90]. In our analysis however, we found the least precision for the effect estimate and the most inconsistency, suggesting that the effects varied widely across studies. Whilst the effectiveness of prophylactic drugs has been documented in children and pregnant women in sub-Saharan Africa, it has not been substantiated in other parts of the world [38, 42, 91, 92], possibly because of the limited ability of drugs to prevent relapse in P. vivax infection [8, 93, 94]. Nevertheless, in our analysis restricted to P. falciparum, the same uncertainty was observed for PDs as in the full dataset. There are other concerns apart from preventive efficacy with the use of drugs as they can also result in impairment of natural immunity, and rebound infections of the children who received chemo-prophylaxis for 1–5 years [95–98]. The widespread use of chemoprophylaxis in children and pregnant women could possibly increase the rate of spread of drug resistance [99].
The preventive measure with the next highest effect estimate was IRS, a critical component of the WHO’s Global Malaria Eradication Program from 1955–1969 and the main intervention attributed to the elimination or dramatic reduction of malaria in parts of Europe, Asia and Latin America [27]. The basic principle of IRS in vector control is that IRS protects inhabitants against mosquito bites by killing the blood-fed females who rest on the walls after feeding and also protect inhabitants against mosquito bites by diverting the vector from entering a sprayed house an effect known as excito-repellency [100, 101]. If the mosquito does enter the house, after biting, the female mosquito eventually rests on sprayed surfaces, where it picks up a lethal dose of insecticide, thus preventing transmission of the parasite to others. In a village with a high percentage coverage of houses with IRS, the mean age of the village mosquito population is expected to be reduced and very few mosquitoes will survive the approximately 12 days required for sporozoite maturation to be able to transmit the parasites [71]. Thus, IRS reduces malaria transmission at the community level by reducing mosquito longevity and abundance, but it has also been reported to provide household-level protection [27]. Studies have shown that IRS was more effective with high initial prevalence, multiple rounds of spraying and in regions with a combination of P. falciparum and P. vivax [26]. Despite all the advantages of IRS, our analysis suggested a consistently better (or at worst equivalent) efficacy for ITNs compared to IRS. Mosquito mortality has been shown to decline after the third month following IRS and by the fifth month, effectiveness reduces by 12% [102]. Efficacy might wane if walls are replastered or painted following implantation of IRS, and mosquito resistance to insecticides can emerge. In addition, there is a need for trained personnel for application of insecticides, which means IRS might not always be done effectively.
Untreated nets were the least effective in preventing malaria infection as compared to other preventive measures. UNs can offer a barrier against the bite of mosquitoes; however, mosquitoes can rest on the UNs while seeking opportunities to feed on the hosts sleeping under the nets, which can be presented when any part of a host’s body comes in contact with the nets. This happens often when hosts are in a deep sleep, especially under inadequately spaced or small nets. Untreated nets can even offer resting places to mosquitoes in an IRS-sprayed house and thus cannot be recommended given the other alternatives that exist. Finally, torn untreated nets have been shown to offer no additional protection as compared to not using nets [45, 103].
A key strength of this analysis is the use of the GPM framework which avoids approximations and assumptions that are not stated explicitly or verified when the method is applied. On the contrary, the multivariate frequentist framework assumes that if there is no common comparator in the network, this then has to be handled by augmenting the dataset with fictional arms with high variance. This is not very objective and requires a decision as to what constitutes a sufficiently high variance [104]. Another alternative, the Bayesian framework, also has its problems such as requiring prior distributions to be specified for a number of unknown parameters and choices regarding over-dispersed starting values for a number of independent chains so that convergence can be assessed. While we have several choices for the meta-analytic framework, this choice may be less important than other choices regarding the modelling of effects [105]. Indeed, we were able to use the inverse variance heterogeneity model for direct estimates which has correct error estimation when compared with the random effects model [31]. Results from a random effects model (using both the multivariate meta-analysis framework as well as the GPM framework) differ slightly from our main results, especially regarding PD, which has spuriously precise estimates using this approach (Additional file 1: Tables S2, S3).
There are limitations of this study worth noting. Even though clinical and statistical significance was found for ITNs, in reality the effectiveness of interventions (ITN) are dependent on a number of extrinsic factors such as population behaviour and vector aetiology. Studies have shown that ITN use is influenced by social behaviour including education, level of knowledge on malaria, and ease of use [106, 107]. In addition, other socio-economic factors such as working and staying overnight in the forest decreases protection despite high proportion of coverage by ITNs [108–110]. Secondly, different insecticides being used for IRS and ITN over the study period would have impacted the findings of this study and the development of insecticide resistance would undermine the effectiveness of ITNs in preventing malaria. Thirdly, the methods of diagnosis of incident malaria were different in the studies. Since most of these studies were conducted in intense malaria transmission areas, this effect is however likely to be minimal. Fourthly, the vectors were different depending on the region of the study; for instance, the commonest malaria vectors in the Asian region including Anopheles dirus, An. baimaii and An. minimus [111, 112], are able to avoid indoor sprayed surfaces because of their exophilic and exophagic characteristics [113–115] rendering most domicile-based interventions, like ITNs and IRS less effective [114, 116]. Of the three main vectors in the African region: An. arabiensis, An. funestus and An. gambiae [113, 117], only An. arabiensis shows feeding preferences for both indoors and outdoors while the other two are indoor-feeders [117]. Other challenges include insecticide resistance [118]. Finally, the drug types and regimens varied between studies. All of these limitations have the potential to increase heterogeneity between the included studies and make it more difficult to estimate the effects of the different interventions more precisely than what we have reported.
Conclusions
Even though ITNs were found to be the only preventive measure with statistical support for its effectiveness in this study, the role of all malaria control measures are important in the global drive to eliminate malaria. However, when a choice needs to be made for resource allocation, the results reported here tend to favour the use of ITNs.
Additional files
Table S1. Search Strategy. Table S2. Meta-analysis of different control measures against NI using the random effects model under the generalized pairwise modeling (GPM) framework in MetaXL. Table S3. Meta-analysis of different control measures against NI using the random effects model under the frequentist multivariate meta-analysis framework (mvmeta) in Stata. Table S4. Quality scale. Table S5. Summary of the excluded studies. Table S6. Drugs used in the included studies. Table S7. Description of ITN’s used across the studies. Table S8. Description of IRS treatments used across the included studies. Table S9. Quality assessment scores of included studies. (DOCX 56 kb)
Figure S1. Results of network meta-analysis of 21 studies with children as a study population. (TIFF 624 kb)
Figure S2. Results of network meta-analysis of 28 studies with incidence of Plasmodium falciparum. (TIFF 631 kb)
Figure S3. Funnel plot depicting asymmetry for the PD-NI comparison. (TIFF 1482 kb)
Acknowledgements
The authors pay tribute to the late Jan Barendregt of Epigear International Pty Ltd, who passed away during preparation of this work.
Funding
LFK is funded by an Endeavour Postgraduate Scholarship (#3781_2014), an Australian National University Higher Degree Scholarship, and a Fondo para la Innovación, Ciencia y Tecnología Scholarship (#095-FINCyT-BDE-2014).
Availability of data and materials
The datasets for the current study are available from the corresponding author upon request.
Abbreviations
- AQ
Amodiaquine
- AS
Artesunate
- CI
Confidence interval
- DEFF
Design effect
- DP
Dihydroartemisinin-peperaquine
- EIR
Entomological inoculation rate
- GPM
Generalized pairwise modelling
- GTS
Global Technical Strategy for Malaria 2016–2030
- HCI
Higher confidence interval
- IRS
Indoor residual spraying
- ITC
Insecticide-treated curtain
- ITN
Insecticide-treated net
- IVhet
Inverse variance heterogeneity
- LCI
Lower confidence interval
- LLIN
Long-lasting insecticidal nets
- MI
Malaria infection
- MQ
Mefloquine
- NI
No intervention
- PD
Prophylactic drugs
- RCT
Randomized controlled trials
- RDT
Rapid diagnostic tests
- RR
Rate ratio
- SP
Sulphadoxine-pyrimethamine
- SUCRA
Surface under the cumulative ranking curve
- UN
Untreated nets
- WHO
World Health Organization
Authors’ contributions
KW, LFK, SARD and ACAC conceived the idea. JC developed citation search and executed it. KW and LFK carried out the data extraction. KW, LFK, SARD and JB carried out data analysis. KW drafted the manuscript. SARD and ACAC helped in the interpretation of the findings and critical revision of manuscript. LFK, JC, JB, MLG, CB and GK were involved in revision of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Jan J Barendregt deceased 3rd June 2017
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2783-y) contains supplementary material, which is available to authorized users.
Contributor Information
Kinley Wangdi, Email: kinley.wangdi@anu.edu.au.
Luis Furuya-Kanamori, Email: Luis.Furuya-Kanamori@anu.edu.au.
Justin Clark, Email: jclark@bond.edu.au.
Michelle L. Gatton, Email: m.gatton@qut.edu.au
Cathy Banwell, Email: cathy.banwell@anu.edu.au.
Gerard C. Kelly, Email: gerardckelly@gmail.com
Suhail A. R. Doi, Email: Suhail.doi@gmx.net
Archie C. A. Clements, Email: director.rsph@anu.edu.au
References
- 1.WHO . World Malaria Report. Switzerland: World Health Organization, Geneva; 2017. [Google Scholar]
- 2.WHO . World Malaria Report. Switzerland: World Health Organization, Geneva; 2015. [Google Scholar]
- 3.Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382(9895):900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379(9814):413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 5.WHO . Global Technical Strategy for Malaria 2016–2030. World Health Organization, Geneva. Switzerland: WHO Library Cataloguing-in-Publication Data; 2015. [Google Scholar]
- 6.WHO. WHO recommendations for achieving universal coverage with long-lasting insecticidal nets in malaria control . World Health Organization. Switzerland: Geneva; 2014. [Google Scholar]
- 7.Greenwood B. Anti-malarial drugs and the prevention of malaria in the population of malaria endemic areas. Malar J. 2010;9(Suppl. 3):S2. [DOI] [PMC free article] [PubMed]
- 8.White NJ. Intermittent presumptive treatment for malaria. PLoS Med. 2005;2(1):e3. doi: 10.1371/journal.pmed.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronn AM, Msangeni HA, Mhina J, Wernsdorfer WH, Bygbjerg IC. High level of resistance of Plasmodium falciparum to sulfadoxine-pyrimethamine in children in Tanzania. Trans R Soc Trop Med Hyg. 1996;90(2):179–181. doi: 10.1016/s0035-9203(96)90129-7. [DOI] [PubMed] [Google Scholar]
- 10.Mugittu K, Abdulla S, Falk N, Masanja H, Felger I, Mshinda H, et al. Efficacy of sulfadoxine-pyrimethamine in Tanzania after two years as first-line drug for uncomplicated malaria: assessment protocol and implication for treatment policy strategies. Malar J. 2005;4:55. doi: 10.1186/1475-2875-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305(5687):1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 12.Gosling RD, Gesase S, Mosha JF, Carneiro I, Hashim R, Lemnge M, et al. Protective efficacy and safety of three antimalarial regimens for intermittent preventive treatment for malaria in infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9700):1521–1532. doi: 10.1016/S0140-6736(09)60997-1. [DOI] [PubMed] [Google Scholar]
- 13.Tan KR, Katalenich BL, Mace KE, Nambozi M, Taylor SM, Meshnick SR, et al. Efficacy of sulphadoxine-pyrimethamine for intermittent preventive treatment of malaria in pregnancy, Mansa, Zambia. Malar J. 2014;13:227. doi: 10.1186/1475-2875-13-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clerk CA, Bruce J, Affipunguh PK, Mensah N, Hodgson A, Greenwood B, Chandramohan D. A randomized, controlled trial of intermittent preventive treatment with sulfadoxine-pyrimethamine, amodiaquine, or the combination in pregnant women in Ghana. J Infect Dis. 2008;198(8):1202–1211. doi: 10.1086/591944. [DOI] [PubMed] [Google Scholar]
- 15.Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27(1):25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55(Suppl. 1):33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- 17.Briand V, Bottero J, Noel H, Masse V, Cordel H, Guerra J, et al. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine-pyrimethamine with mefloquine. J Infect Dis. 2009;200(6):991–1001. doi: 10.1086/605474. [DOI] [PubMed] [Google Scholar]
- 18.Eisele TP, Larsen D, Steketee RW. Protective efficacy of interventions for preventing malaria mortality in children in Plasmodium falciparum endemic areas. Int J Epidemiol. 2010;39(Suppl. 1):i88–101. doi: 10.1093/ije/dyq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson AL. A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc) PLoS One. 2011;6(2):e16976. doi: 10.1371/journal.pone.0016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meremikwu MM, Donegan S, Sinclair D, Esu E, Oringanje C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst Rev. 2012;2:Cd003756. doi: 10.1002/14651858.CD003756.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meremikwu MM, Donegan S, Esu E. Chemoprophylaxis and intermittent treatment for preventing malaria in children. Cochrane Database Syst Rev. 2008;2:Cd003756. doi: 10.1002/14651858.CD003756.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Matangila JR, Mitashi P, Inocencio da Luz RA, Lutumba PT, Van Geertruyden JP. Efficacy and safety of intermittent preventive treatment for malaria in schoolchildren: a systematic review. Malar J. 2015;14:450. doi: 10.1186/s12936-015-0988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi HW, Breman JG, Teutsch SM, Liu S, Hightower AW, Sexton JD. The effectiveness of insecticide-impregnated bed nets in reducing cases of malaria infection: a meta-analysis of published results. Am J Trop Med Hyg. 1995;52(5):377–382. doi: 10.4269/ajtmh.1995.52.377. [DOI] [PubMed] [Google Scholar]
- 24.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:Cd000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Van Remoortel H, De Buck E, Singhal M, Vandekerckhove P, Agarwal SP. Effectiveness of insecticide-treated and untreated nets to prevent malaria in India. Trop Med Int Health. 2015;20(8):972–982. doi: 10.1111/tmi.12522. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Fedak K, Kramer R. Reduction of malaria prevalence by indoor residual spraying: A meta-regression analysis. Am J Trop Med Hyg. 2012;87(1):117–124. doi: 10.4269/ajtmh.2012.11-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:Cd006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi SA, Barendregt JJ. A generalized pairwise modelling framework for network meta-analysis. Int J Evid Based Healthc. 2018;27 [Epub ahead of print] [DOI] [PubMed]
- 29.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 30.Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp Clin Trials. 2015;45(Pt A):130–138. doi: 10.1016/j.cct.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Smithuis FM, Kyaw MK, Phe UO, van der Broek I, Katterman N, Rogers C, et al. The effect of insecticide-treated bed nets on the incidence and prevalence of malaria in children in an area of unstable seasonal transmission in western Myanmar. Malar J. 2013;12:363. doi: 10.1186/1475-2875-12-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods. 2012;3(2):161–176. doi: 10.1002/jrsm.57. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Clark J, Siskind D, Williams GM, Byrne G, Yang JL. SA. A systematic review and meta-analysis of the effects of Qigong and Tai Chi for depressive symptoms. Complement Ther Med. 2015;23(4):516–534. doi: 10.1016/j.ctim.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Beach RF, Ruebush TK, 2nd, Sexton JD, Bright PL, Hightower AW, Breman JG, et al. Effectiveness of permethrin-impregnated bed nets and curtains for malaria control in a holoendemic area of western Kenya. Am J Trop Med Hyg. 1993;49(3):290–300. doi: 10.4269/ajtmh.1993.49.290. [DOI] [PubMed] [Google Scholar]
- 36.Charlwood JD, Qassim M, Elnsur EI, Donnelly M, Petrarca V, Billingsley PF, et al. The impact of indoor residual spraying with malathion on malaria in refugee camps in eastern Sudan. Acta Trop. 2001;80(1):1–8. doi: 10.1016/s0001-706x(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 37.Cisse B, Sokhna C, Boulanger D, Milet J, Ba el H, Richardson K, et al. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367(9511):659–667. doi: 10.1016/S0140-6736(06)68264-0. [DOI] [PubMed] [Google Scholar]
- 38.Dicko A, Diallo AI, Tembine I, Dicko Y, Dara N, Sidibe Y, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8(2):e1000407. doi: 10.1371/journal.pmed.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser-Hurt N, Felger I, Edoh D, Steiger S, Mashaka M, Masanja H, et al. Effect of insecticide-treated bed nets on haemoglobin values, prevalence and multiplicity of infection with Plasmodium falciparum in a randomized controlled trial in Tanzania. Trans R Soc Trop Med Hyg. 1999;93(Suppl. 1):47–51. doi: 10.1016/s0035-9203(99)90327-9. [DOI] [PubMed] [Google Scholar]
- 40.Hale BR, Owusu-Agyei S, Fryauff DJ, Koram KA, Adjuik M, Oduro AR, et al. A randomized, double-blind, placebo-controlled, dose-ranging trial of tafenoquine for weekly prophylaxis against Plasmodium falciparum. Clin Infect Dis. 2003;36(5):541–549. doi: 10.1086/367542. [DOI] [PubMed] [Google Scholar]
- 41.Hamel MJ, Otieno P, Bayoh N, Kariuki S, Were V, Marwanga D, et al. The combination of indoor residual spraying and insecticide-treated nets provides added protection against malaria compared with insecticide-treated nets alone. Am J Trop Med Hyg. 2011;85(6):1080–1086. doi: 10.4269/ajtmh.2011.10-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konate AT, Yaro JB, Ouedraogo AZ, Diarra A, Gansane A, Soulama I, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8(2):e1000408. doi: 10.1371/journal.pmed.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kweku M, Liu D, Adjuik M, Binka F, Seidu M, Greenwood B, Chandramohan D. Seasonal intermittent preventive treatment for the prevention of anaemia and malaria in Ghanaian children: a randomized, placebo controlled trial. PLoS One. 2008;3(12):e4000. doi: 10.1371/journal.pone.0004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marbiah NT, Petersen E, David K, Magbity E, Lines J, Bradley DJ. A controlled trial of lambda-cyhalothrin-impregnated bed nets and/or dapsone/pyrimethamine for malaria control in Sierra Leone. Am J Trop Med Hyg. 1998;58(1):1–6. doi: 10.4269/ajtmh.1998.58.1. [DOI] [PubMed] [Google Scholar]
- 45.Mwangi TW, Ross A, Marsh K, Snow RW. The effects of untreated bednets on malaria infection and morbidity on the Kenyan coast. Trans R Soc Trop Med Hyg. 2003;97(4):369–372. doi: 10.1016/s0035-9203(03)90056-3. [DOI] [PubMed] [Google Scholar]
- 46.Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, et al. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis. 2014;58(10):1404–1412. doi: 10.1093/cid/ciu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nevill CG, Watkins WM, Carter JY, Munafu CG. Comparison of mosquito nets, proguanil hydrochloride, and placebo to prevent malaria. BMJ. 1988;297(6645):401–403. doi: 10.1136/bmj.297.6645.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Odhiambo FO, Hamel MJ, Williamson J, Lindblade K, ter Kuile FO, Peterson E, et al. Intermittent preventive treatment in infants for the prevention of malaria in rural western Kenya: a randomized, double-blind placebo-controlled trial. PLoS One. 2010;5(4):e10016. doi: 10.1371/journal.pone.0010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinder M, Jawara M, Jarju LB, Salami K, Jeffries D, Adiamoh M, et al. Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: a cluster-randomised controlled trial. Lancet. 2015;385(9976):1436–1446. doi: 10.1016/S0140-6736(14)61007-2. [DOI] [PubMed] [Google Scholar]
- 50.Sesay S, Milligan P, Touray E, Sowe M, Webb EL, Greenwood BM, Bojang KA. A trial of intermittent preventive treatment and home-based management of malaria in a rural area of The Gambia. Malar J. 2011;10:2. doi: 10.1186/1475-2875-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sexton JD, Ruebush TK, 2nd, Brandling-Bennett AD, Breman JG, Roberts JM, Odera JS, Were JB. Permethrin-impregnated curtains and bed-nets prevent malaria in western Kenya. Am J Trop Med Hyg. 1990;43(1):11–18. doi: 10.4269/ajtmh.1990.43.11. [DOI] [PubMed] [Google Scholar]
- 52.Snow RW, Rowan KM, Lindsay SW, Greenwood BM. A trial of bed nets (mosquito nets) as a malaria control strategy in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1988;82(2):212–215. doi: 10.1016/0035-9203(88)90414-2. [DOI] [PubMed] [Google Scholar]
- 53.Kamol-Ratanakul P, Prasittisuk C. The effectiveness of permethrin-impregnated bed nets against malaria for migrant workers in eastern Thailand. Am J Trop Med Hyg. 1992;47(3):305–309. doi: 10.4269/ajtmh.1992.47.305. [DOI] [PubMed] [Google Scholar]
- 54.Luxemburger C, Perea WA, Delmas G, Pruja C, Pecoul B, Moren A. Permethrin-impregnated bed nets for the prevention of malaria in schoolchildren on the Thai-Burmese border. Trans R Soc Trop Med Hyg. 1994;88(2):155–159. doi: 10.1016/0035-9203(94)90273-9. [DOI] [PubMed] [Google Scholar]
- 55.Rowland M, Bouma M, Ducornez D, Durrani N, Rozendaal J, Schapira A, Sondorp E. Pyrethroid-impregnated bed nets for personal protection against malaria for Afghan refugees. Trans R Soc Trop Med Hyg. 1996;90(4):357–361. doi: 10.1016/s0035-9203(96)90505-2. [DOI] [PubMed] [Google Scholar]
- 56.Taylor WR, Richie TL, Fryauff DJ, Picarima H, Ohrt C, Tang D, et al. Malaria prophylaxis using azithromycin: a double-blind, placebo-controlled trial in Irian Jaya, Indonesia. Clin Infect Dis. 1999;28(1):74–81. doi: 10.1086/515071. [DOI] [PubMed] [Google Scholar]
- 57.Sahu SS, Jambulingam P, Vijayakumar T, Subramanian S, Kalyanasundaram M. Impact of alphacypermethrin treated bed nets on malaria in villages of Malkangiri District, Orissa, India. Acta Trop. 2003;89(1):55–66. doi: 10.1016/j.actatropica.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Sharma SK, Tyagi PK, Upadhyay AK, Haque MA, Mohanty SS, Raghavendra K, Dash AP. Efficacy of permethrin treated long-lasting insecticidal nets on malaria transmission and observations on the perceived side effects, collateral benefits and human safety in a hyperendemic tribal area of Orissa, India. Acta Trop. 2009;112(2):181–187. doi: 10.1016/j.actatropica.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Lwin KM, Phyo AP, Tarning J, Hanpithakpong W, Ashley EA, Lee SJ, et al. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother. 2012;56(3):1571–1577. doi: 10.1128/AAC.05877-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soleimani-Ahmadi M, Vatandoost H, Shaeghi M, Raeisi A, Abedi F, Eshraghian MR, et al. Field evaluation of permethrin long-lasting insecticide treated nets (Olyset(R)) for malaria control in an endemic area, southeast of Iran. Acta Trop. 2012;123(3):146–153. doi: 10.1016/j.actatropica.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Shah NK, Tyagi P, Sharma SK. The impact of artemisinin combination therapy and long-lasting insecticidal nets on forest malaria incidence in tribal villages of India, 2006–2011. PLoS One. 2013;8(2):e56740. doi: 10.1371/journal.pone.0056740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill N, Zhou HN, Wang P, Guo X, Carneiro I, Moore SJ. A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long-lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China. Malar J. 2014;13:208. doi: 10.1186/1475-2875-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kroeger A, Mancheno M, Alarcon J, Pesse K. Insecticide-impregnated bed nets for malaria control: varying experiences from Ecuador, Colombia, and Peru concerning acceptability and effectiveness. Am J Trop Med Hyg. 1995;53(4):313–323. doi: 10.4269/ajtmh.1995.53.313. [DOI] [PubMed] [Google Scholar]
- 64.Maxwell CA, Rwegoshora RT, Magesa SM, Curtis CF. Comparison of coverage with insecticide-treated nets in a Tanzanian town and villages where nets and insecticide are either marketed or provided free of charge. Malar J. 2006;5:44. doi: 10.1186/1475-2875-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gamble C, Ekwaru JP, ter Kuile FO. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane Database Syst Rev. 2006;2:Cd003755. doi: 10.1002/14651858.CD003755.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sutanto I, Freisleben HJ, Pribadi W, Atmosoedjono S, Bandi A. Purnomo. Efficacy of permethrin-impregnated bed nets on malaria control in a hyperendemic area in Irian Java, Indonesia: influence of seasonal rainfall fluctuations. Southeast Asian J Trop Med Pub Hlth. 1999;30(3):432–439. [PubMed] [Google Scholar]
- 67.Maxwell CA, Myamba J, Njunwa KJ, Greenwood BM, Curtis CF. Comparison of bednets impregnated with different pyrethroids for their impact on mosquitoes and on re-infection with malaria after clearance of pre-existing infections with chlorproguanil-dapsone. Trans R Soc Trop Med Hyg. 1999;93(1):4–11. doi: 10.1016/s0035-9203(99)90158-x. [DOI] [PubMed] [Google Scholar]
- 68.Sharma SK, Upadhyay AK, Haque MA, Tyagi PK, Mohanty SS, Raghavendra K, Dash AP. Field evaluation of Olyset nets: a long-lasting insecticidal net against malaria vectors Anopheles culicifacies and Anopheles fluviatilis in a hyperendemic tribal area of Orissa, India. J Med Entomol. 2009;46(2):342–350. doi: 10.1603/033.046.0220. [DOI] [PubMed] [Google Scholar]
- 69.Curtis CF, Jana-Kara B, Maxwell CA. Insecticide-treated nets: impact on vector populations and relevance of initial intensity of transmission and pyrethroid resistance. J Vector Borne Dis. 2003;40(1–2):1–8. [PubMed]
- 70.Wangdi K, Gatton ML, Kelly GC, Clements AC. Prevalence of asymptomatic malaria and bed net ownership and use in Bhutan, 2013: a country earmarked for malaria elimination. Malar J. 2014;13:352. doi: 10.1186/1475-2875-13-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curtis CF, Maxwell CA, Magesa SM, Rwegoshora RT, Wilkes TJ. Insecticide-treated bed-nets for malaria mosquito control. J Am Mosq Control Assoc. 2006;22(3):501–506. doi: 10.2987/8756-971X(2006)22[501:IBFMMC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 72.Gimnig JE, Vulule JM, Lo TQ, Kamau L, Kolczak MS, Phillips-Howard PA, et al. Impact of permethrin-treated bed nets on entomologic indices in an area of intense year-round malaria transmission. Am J Trop Med Hyg. 2003;68(Suppl. 4):16–22. [PubMed] [Google Scholar]
- 73.Hawley WA, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, et al. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68(Suppl. 4):121–127. [PubMed] [Google Scholar]
- 74.Larsen DA, Hutchinson P, Bennett A, Yukich J, Anglewicz P, Keating J, Eisele TP. Community coverage with insecticide-treated mosquito nets and observed associations with all-cause child mortality and malaria parasite infections. Am J Trop Med Hyg. 2014;91(5):950–958. doi: 10.4269/ajtmh.14-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Govella NJ, Okumu FO, Killeen GF. Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg. 2010;82(3):415–419. doi: 10.4269/ajtmh.2010.09-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.West PA, Protopopoff N, Wright A, Kivaju Z, Tigererwa R, Mosha FW, et al. Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: a cluster randomised trial in Tanzania. PLoS Med. 2014;11(4):e1001630. doi: 10.1371/journal.pmed.1001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.West PA, Protopopoff N, Wright A, Kivaju Z, Tigererwa R, Mosha FW, et al. Enhanced protection against malaria by indoor residual spraying in addition to insecticide treated nets: is it dependent on transmission intensity or net usage? PLoS One. 2015;10(3):e0115661. doi: 10.1371/journal.pone.0115661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fullman N, Burstein R, Lim SS, Medlin C, Gakidou E. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malar J. 2013;12:62. doi: 10.1186/1475-2875-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleinschmidt I, Schwabe C, Shiva M, Segura JL, Sima V, Mabunda SJ, Coleman M. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg. 2009;81(3):519–524. [PMC free article] [PubMed] [Google Scholar]
- 80.Glunt KD, Abilio AP, Bassat Q, Bulo H, Gilbert AE, Huijben S, et al. Long-lasting insecticidal nets no longer effectively kill the highly resistant Anopheles funestus of southern Mozambique. Malar J. 2015;14:298. doi: 10.1186/s12936-015-0807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chandre F, Manguin S, Brengues C, Dossou Yovo J, Darriet F, Diabate A, et al. Current distribution of a pyrethroid resistance gene (kdr) in Anopheles gambiae complex from west Africa and further evidence for reproductive isolation of the Mopti form. Parassitologia. 1999;41(1–3):319–322. [PubMed] [Google Scholar]
- 82.Etang J, Fondjo E, Chandre F, Morlais I, Brengues C, Nwane P, et al. First report of knockdown mutations in the malaria vector Anopheles gambiae from Cameroon. Am J Trop Med Hyg. 2006;74(5):795–797. [PubMed] [Google Scholar]
- 83.Stump AD, Atieli FK, Vulule JM, Besansky NJ. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg. 2004;70(6):591–596. [PubMed] [Google Scholar]
- 84.Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14(2):181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 85.Hargreaves K, Hunt RH, Brooke BD, Mthembu J, Weeto MM, Awolola TS, Coetzee M. Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol. 2003;17(4):417–422. doi: 10.1111/j.1365-2915.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 86.Wangdi K, Banwell C, Gatton ML, Kelly GC, Namgay R, Clements AC. Malaria burden and costs of intensified control in Bhutan, 2006–14: an observational study and situation analysis. Lancet Glob Health. 2016;4(5):e336–e343. doi: 10.1016/S2214-109X(16)00083-8. [DOI] [PubMed] [Google Scholar]
- 87.Wamae PM, Githeko AK, Otieno GO, Kabiru EW, Duombia SO. Early biting of the Anopheles gambiae s.s. and its challenges to vector control using insecticide treated nets in western Kenya highlands. Acta Trop. 2015;150:136–142. doi: 10.1016/j.actatropica.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Hii JLK, Kanai L, Foligela A, Kan SKP, Burkot TR, Wirtz RA. Impact of permethrin-impregnated mosquito nets compared with DDT house-spraying against malaria transmission by Anopheles farauti and An. punctulatus in the Solomon Islands. Med Vet Entomol. 1993;7(4):333–338. doi: 10.1111/j.1365-2915.1993.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 89.Cairns M, Carneiro I, Milligan P, Owusu-Agyei S, Awine T, Gosling R, et al. Duration of protection against malaria and anaemia provided by intermittent preventive treatment in infants in Navrongo, Ghana. PLoS One. 2008;3(5):e2227. doi: 10.1371/journal.pone.0002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Menendez C, Schellenberg D, Macete E, Aide P, Kahigwa E, Sanz S, et al. Varying efficacy of intermittent preventive treatment for malaria in infants in two similar trials: public health implications. Malar J. 2007;6:132. doi: 10.1186/1475-2875-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mace KE, Chalwe V, Katalenich BL, Nambozi M, Mubikayi L, Mulele CK, et al. Evaluation of sulphadoxine-pyrimethamine for intermittent preventive treatment of malaria in pregnancy: a retrospective birth outcomes study in Mansa, Zambia. Malar J. 2015;14(1):576. doi: 10.1186/s12936-015-0576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dicko A, Sagara I, Sissoko MS, Guindo O, Diallo AI, Kone M, et al. Impact of intermittent preventive treatment with sulphadoxine-pyrimethamine targeting the transmission season on the incidence of clinical malaria in children in Mali. Malar J. 2008;7:123. doi: 10.1186/1475-2875-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ranque S, Badiaga S, Delmont J, Brouqui P. Triangular test applied to the clinical trial of azithromycin against relapses in Plasmodium vivax infections. Malar J. 2002;1:13. doi: 10.1186/1475-2875-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pukrittayakamee S, Chantra A, Simpson JA, Vanijanonta S, Clemens R, Looareesuwan S, White NJ. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother. 2000;44(6):1680–1685. doi: 10.1128/aac.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greenwood BM, David PH, Otoo-Forbes LN, Allen SJ, Alonso PL, Armstrong Schellenberg JR, et al. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans R Soc Trop Med Hyg. 1995;89(6):629–633. doi: 10.1016/0035-9203(95)90419-0. [DOI] [PubMed] [Google Scholar]
- 96.Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350(9081):844–850. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 97.Mockenhaupt FP, Reither K, Zanger P, Roepcke F, Danquah I, Saad E, et al. Intermittent preventive treatment in infants as a means of malaria control: a randomized, double-blind, placebo-controlled trial in northern Ghana. Antimicrob Agents Chemother. 2007;51(9):3273–3281. doi: 10.1128/AAC.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chandramohan D, Owusu-Agyei S, Carneiro I, Awine T, Amponsa-Achiano K, Mensah N, et al. Cluster randomised trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana. BMJ. 2005;331(7519):727–733. doi: 10.1136/bmj.331.7519.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greenwood B. The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg. 2004;70(1):1–7. [PubMed] [Google Scholar]
- 100.Hamusse SD, Balcha TT, Belachew T. The impact of indoor residual spraying on malaria incidence in East Shoa Zone, Ethiopia. Glob Health Action. 2012;5:11619. doi: 10.3402/gha.v5i0.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Indoor residual spraying WHO. Use of indoor residual spraying for scaling up global malaria control and elimination. Geneva: World Health Organization; 2006.
- 102.Bradley J, Matias A, Schwabe C, Vargas D, Monti F, Nseng G, Kleinschmidt I. Increased risks of malaria due to limited residual life of insecticide and outdoor biting versus protection by combined use of nets and indoor residual spraying on Bioko Island, Equatorial Guinea. Malar J. 2012;11:242. doi: 10.1186/1475-2875-11-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lines JD, Myamba J, Curtis CF. Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1987;1(1):37–51. doi: 10.1111/j.1365-2915.1987.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 104.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 105.Senn S, Gavini F, Magrez D, Scheen A. Issues in performing a network meta-analysis. Stat Methods Med Res. 2013;22(2):169–189. doi: 10.1177/0962280211432220. [DOI] [PubMed] [Google Scholar]
- 106.Samadoulougou S, Pearcy M, Ye Y, Kirakoya-Samadoulougou F. Progress in coverage of bed net ownership and use in Burkina Faso 2003–2014: evidence from population-based surveys. Malar J. 2017;16(1):302. doi: 10.1186/s12936-017-1946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Russell CL, Sallau A, Emukah E, Graves PM, Noland GS, Ngondi JM, et al. Determinants of bed net use in southeast Nigeria following mass distribution of LLINs: implications for social behavior change interventions. PLoS One. 2015;10(10):e0139447. doi: 10.1371/journal.pone.0139447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Bortel W, Trung HD, Hoi le X, Van Ham N, Van Chut N, Luu ND, et al. Malaria transmission and vector behaviour in a forested malaria focus in central Vietnam and the implications for vector control. Malar J. 2010;9:373. doi: 10.1186/1475-2875-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh N, Singh OP, Sharma VP. Dynamics of malaria transmission in forested and deforested regions of Mandla District, central India (Madhya Pradesh) J Am Mosq Control Assoc. 1996;12(2 Pt 1):225–234. [PubMed] [Google Scholar]
- 110.Grietens KP, Xuan XN, Ribera J, Duc TN, Bortel W, Ba NT, et al. Social determinants of long-lasting insecticidal hammock use among the Ra-glai ethnic minority in Vietnam: implications for forest malaria control. PLoS One. 2012;7(1):e29991. [DOI] [PMC free article] [PubMed]
- 111.Dev V, Manguin S. Biology, distribution and control of Anopheles (Cellia) minimus in the context of malaria transmission in northeastern India. Parasit Vectors. 2016;9:585. doi: 10.1186/s13071-016-1878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang S, Guo S, Feng X, Afelt A, Frutos R, Zhou S, Manguin S. Anopheles vectors in mainland China while approaching malaria elimination. Trends Parasitol. 2017;33(11):889–900. doi: 10.1016/j.pt.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 113.Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trung HD, Bortel WV, Sochantha T, Keokenchanh K, Briet OJ, Coosemans M. Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: a challenge for vector control. Trop Med Int Health. 2005;10(3):251–262. doi: 10.1111/j.1365-3156.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- 115.Manh CD, Beebe NW, Van VN, Quang TL, Lein CT, Nguyen DV, et al. Vectors and malaria transmission in deforested, rural communities in north-central Vietnam. Malar J. 2010;9:259. doi: 10.1186/1475-2875-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Somboon P, Lines J, Aramrattana A, Chitprarop U, Prajakwong S, Khamboonruang C. Entomological evaluation of community-wide use of lambdacyhalothrin-impregnated bed nets against malaria in a border area of north-west Thailand. Trans R Soc Trop Med Hyg. 1995;89(3):248–254. doi: 10.1016/0035-9203(95)90525-1. [DOI] [PubMed] [Google Scholar]
- 117.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dhiman S, Goswami D, Rabha B, Gopalakrishnan R, Baruah I, Singh L. Malaria epidemiology along Indo-Bangladesh border in Tripura State, India. Southeast Asian J Trop Med Public Health. 2010;41(6):1279–1289. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy. Table S2. Meta-analysis of different control measures against NI using the random effects model under the generalized pairwise modeling (GPM) framework in MetaXL. Table S3. Meta-analysis of different control measures against NI using the random effects model under the frequentist multivariate meta-analysis framework (mvmeta) in Stata. Table S4. Quality scale. Table S5. Summary of the excluded studies. Table S6. Drugs used in the included studies. Table S7. Description of ITN’s used across the studies. Table S8. Description of IRS treatments used across the included studies. Table S9. Quality assessment scores of included studies. (DOCX 56 kb)
Figure S1. Results of network meta-analysis of 21 studies with children as a study population. (TIFF 624 kb)
Figure S2. Results of network meta-analysis of 28 studies with incidence of Plasmodium falciparum. (TIFF 631 kb)
Figure S3. Funnel plot depicting asymmetry for the PD-NI comparison. (TIFF 1482 kb)
Data Availability Statement
The datasets for the current study are available from the corresponding author upon request.


