ABSTRACT
The detection of campylobacters in stools is performed essentially by culture, but this technique has a low sensitivity. New detection methods are now available. Among them, immunochromatography tests (ICTs) are very attractive in that they offer a result within 15 min. However, previous studies suggest that these tests have a relatively low specificity. The objective of this study was to evaluate the performance of these tests. During the study period, all patients who consulted the emergency units and had a stool culture were included. Their stool samples were tested with two ICTs, Ridaquick Campylobacter and ImmunoCard STAT! Campy. Stools were also tested by a home-made PCR and two commercially available enzyme-linked immunosorbent assays (ELISAs) when one of the ICTs was positive. The composite reference standard (CRS) was defined as positive if the culture was positive or, in case of a negative culture, if the PCR and one of the ELISAs were positive simultaneously. Three hundred and five patients were included. Among the 50 positive specimens with Ridaquick Campylobacter, 47 were considered true positives by the CRS, corresponding to a positive predictive value (PPV) of 94.0%. Among the 52 positive specimens with ImmunoCard STAT! Campy, 44 were considered true positives by the CRS, corresponding to a PPV of 84.6%. The negative predictive values were estimated at 94.9 and 92.4% for the Ridaquick Campylobacter and ImmunoCard STAT! Campy tests, respectively. ICTs appear to be very efficient and allow a very rapid detection of campylobacters, which is important for treating early campylobacter infections with an adapted antibiotherapy.
KEYWORDS: ImmunoCard STAT! Campy, Ridaquick Campylobacter, immunochromatographic tests, rapid campylobacter detection
INTRODUCTION
Campylobacters, especially Campylobacter jejuni and Campylobacter coli, are the main cause of bacterial enteric infections worldwide (1–3). These infections can also lead to extraintestinal localizations and severe long-term complications, e.g., Guillain-Barré Syndrome (4–6). Symptoms can be reduced, thanks to an adapted antibiotic treatment administered as early as possible; otherwise, the benefit of the treatment is limited (7). The problem is that campylobacters are traditionally detected by stool culture with results usually available after 48 to 72 h, which does not allow an early treatment (8). Furthermore, campylobacter culture is demanding in the laboratory: stool specimens need to be treated rapidly, and agar plates have to be incubated in a microaerobic atmosphere. For these reasons, culture, which is time-consuming, underestimates some cases, especially when culture requirements are not completely fulfilled. Other techniques have been developed and commercialized, e.g., molecular methods (real-time PCR) and enzyme-linked immunosorbent assays (ELISAs) which have a better sensitivity than culture and a good specificity. They give a result within a few hours but are also technically demanding. Recently, immunochromatographic tests (ICTs) which allow a result within a few minutes and are very easy to perform have been developed. The first evaluations of these tests showed a good sensitivity but an apparent lack of specificity (9, 10) in comparison to culture. However, this result may have been related to a suboptimal sensitivity of culture. For this reason, we used a composite reference standard (CRS) for campylobacter infection, designed to overcome this possible problem. The CRS was defined as positive when a culture was positive or, in the case of a negative culture, when both an ELISA and a PCR were positive. This was done to better estimate the specificity and the positive predictive value (PPV) of ICTs.
MATERIALS AND METHODS
Study design.
From April 2014 to October 2015, all patients with gastrointestinal symptoms, consulting the emergency units, adult or pediatric, of our hospital and for whom a stool analysis was prescribed, were included in the study. Stools were sent immediately to the Bacteriology Laboratory at room temperature without transport medium, except for a few cases for which a Cary Blair medium was used. The fresh, unpreserved stools were tested simultaneously with two ICTs, and culture was performed as soon as they arrived at the laboratory. Stools were stored at −80°C following a positive detection with one or both ICTs in the first 96 patients included. In the following 209 patients all stool specimens were also stored at −80°C regardless of their ICT result, in order to check whether the stools found negative with both ICTs were truly negative. All frozen stools were tested a second time with a PCR and two different ELISAs.
Culture.
A stool sample was directly inoculated on a Karmali agar plate (Oxoid, Basingstoke, Hampshire, United Kingdom), and the plates were incubated for a maximum of 3 days in a microaerobic atmosphere. Colonies resembling campylobacter colonies were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker Daltonics, Bremen, Germany) (11).
Rapid immunochromatographic tests.
Two different tests were used: ImmunoCard STAT! Campy (Meridian Bioscience, Inc., Cincinnati, OH) and Ridaquick Campylobacter (R-Biopharm AG, Darmstadt, Germany). Both were used according to the manufacturer's instructions.
ELISAs.
Two different tests were used: RIDAScreen Campylobacter (R-Biopharm AG) and Premier Campy (Meridian Bioscience, Inc.), both according to the manufacturer's instructions.
Real-time PCR.
DNA extractions were performed using an Arrow Stool DNA kit (DiaSorin, Cypress, CA); no extraction control was used. The real-time PCR and hybridization reactions were performed according to the method published by Ménard et al. (12) using a LightCycler thermocycler (Roche Diagnostics, Meylan, France). A Campylobacter jejuni strain was used as a positive control, and sterilized water was used as a negative control.
Composite reference standard.
In order to better assess the diagnostic accuracy of the ICTs and overcome the lack of sensitivity of stool culture as a reference standard, a composite reference standard (CRS) was used. The CRS was defined as positive if the culture was positive or, in case of a negative culture, if a PCR and one or both ELISAs were positive simultaneously.
Statistical analysis.
Statistical analyses were performed by the Clinical Epidemiology Unit of Bordeaux University Hospital (USMR). Quantitative variables were described using median and range. Qualitative variables were described using frequency and percentage. Diagnostic performance was estimated using proportions and their two-sided 95% confidence intervals (CIs) according to a binomial exact distribution. The CRS was not available for the first 96 patients because their samples were not frozen. Not taking into account these missing data leads to biases in the estimation of sensitivity, specificity and prevalence. Since they were “missing completely at random” (MCAR) data, we could correct the estimates by the standard method of inverse probability weighting (the weight of a patient in the estimation of the parameters was the inverse probability that the CRS was available for this patient) (13). Statistical analyses were performed with SAS software (v9.3; SAS Institute, Cary, NC).
RESULTS
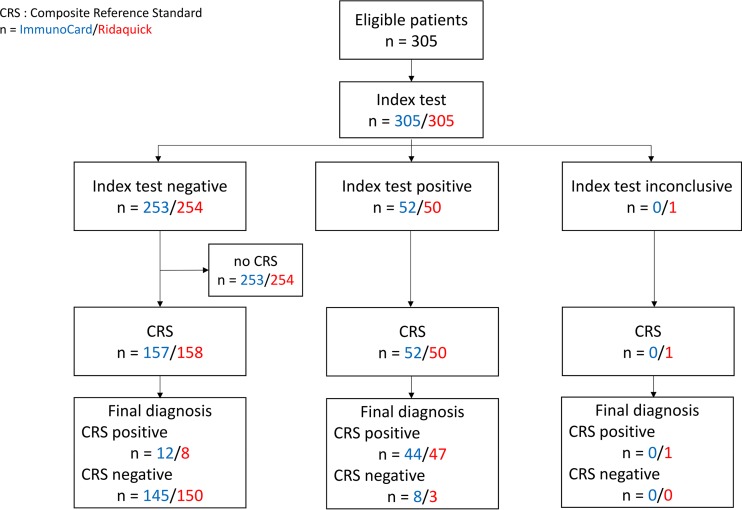
Over the 18-month period, 305 cases fulfilled the inclusion criteria and were included according to the flow chart presented in the Fig. 1.
FIG 1.
Flow chart of the study. The flow chart indicates the numbers of participants at each stage of the Campylobacter test study, i.e., the numbers of patients receiving each ICT index test (blue, ImmunoCard STAT! Campy; red, Ridaquick Campylobacter), the numbers receiving the CRS among them, and also the numbers of patients with a positive or a negative result according to the CRS.
The majority of the patients were male (57.7%), and the mean age was 21.6 years. The characteristics of the patients are presented in Table 1. A large majority of patients (64.9%) were children under 15 years of age. Among this pediatric population, 41.3% were 1 to 12 years old, and 18.0% were 1 to 12 months old.
TABLE 1.
Demographic and clinical characteristics of 305 patients enrolled in the study
| Characteristics | No. (%) or median (range) |
|---|---|
| Sex | |
| Male | 176 (57.7) |
| Female | 129 (42.3) |
| Age (yrs) | 5 (0–106) |
| Age groups | |
| Newborn (0–30 days) | 4 (1.3) |
| Infant (1–12 mos) | 55 (18) |
| Children (1–12 yrs) | 126 (41.3) |
| Teenager (13–19 yrs) | 13 (4.3) |
| Adults | 107 (35.1) |
| Temp (°C) | 38 (35–41) |
| Diarrhea | |
| No diarrhea | 85 (27.9) |
| Nonbloody diarrhea | 160 (52.4) |
| Bloody diarrhea | 60 (19.7) |
| C-reactive protein | |
| Normal (≤5 mg/liter) | 48 (22.6) |
| Abnormal (>5 mg/liter) | 164 (77.4) |
| Leukocyte quantification (109/liter) | 10 (1–80) |
The clinical symptoms observed in the patients are presented in Table 1. The majority of the patients suffered from diarrhea (72.1%) sometimes bloody diarrhea (19.7%), vomiting (30.8%), and abdominal pain (35.7%). The C-reactive protein (CRP) level was quantified in almost 70% of the patients, and it was increased in more than 75% of them. A diagnosis of campylobacter infection was more frequent in children than in other age groups (48% versus 24%, respectively). A campylobacter infection was more frequently associated with an abnormal CRP (97.3% versus 68%) and bloody diarrhea (23% versus 18%) (data not shown). Patients with a campylobacter infection had less vomiting, a slightly higher temperature, and a lower leukocyte count (median, 9 versus 11) compared to campylobacter-negative cases.
The different test result combinations are presented in Table 2. Considering the CRS, stool specimens of 56 patients were positive, corresponding to a campylobacter prevalence of 19.8% (95% CI = 14.7 to 25.9). Among these samples, 23 were positive by all of the different tests, including the CRS. The CRS was unavailable, by design, for 96 consecutive specimens for which both ICTs were negative.
TABLE 2.
Number of patients presenting with each observed profile according to the positive or negative result for every diagnostic test applied
| Composite reference standard result | Test resulta |
No. of patients | |||||
|---|---|---|---|---|---|---|---|
| ImmunoCard STAT! Campy ICT | Ridaquick ICT | Culture | PCR | Ridascreen ELISA | PremierCampy ELISA | ||
| + | |||||||
| + | + | + | ND | ND | ND | 2 | |
| − | − | + | ND | ND | ND | 1 | |
| + | NI | + | + | + | + | 1 | |
| + | + | + | + | + | + | 23 | |
| − | + | + | + | + | + | 2 | |
| − | − | + | + | + | + | 1 | |
| + | + | + | + | + | − | 4 | |
| + | + | + | + | − | + | 1 | |
| + | − | + | + | − | + | 1 | |
| + | + | + | + | − | − | 2 | |
| − | − | + | + | − | − | 1 | |
| + | + | + | − | − | − | 3 | |
| − | + | + | − | − | − | 2 | |
| + | + | − | + | + | + | 6 | |
| − | + | − | + | + | + | 1 | |
| − | − | − | + | + | + | 4 | |
| + | + | − | + | + | − | 1 | |
| − | |||||||
| − | − | − | + | − | − | 2 | |
| − | − | − | − | ND | ND | 2 | |
| − | − | − | − | + | − | 4 | |
| − | − | − | − | − | + | 1 | |
| + | + | − | − | − | − | 1 | |
| + | − | − | − | − | − | 7 | |
| − | + | − | − | − | − | 2 | |
| − | − | − | − | − | − | 133 | |
| − | − | − | ND | ND | ND | 96 | |
ND, not done; NI, not interpretable.
Culture was carried out on all of the stool samples and was positive in 44 cases. The majority of the campylobacter strains were C. jejuni (93.2%), followed by C. coli (4.5%) and Campylobacter fetus (2.3%). Three cases were positive by culture whereas neither the ImmunoCard STAT! Campy nor the Ridaquick Campylobacter ICT result was positive: in one of the cases, C. fetus was isolated. In contrast, seven positive specimens (according to the CRS) were negative by culture but positive by the two ICTs.
The Ridaquick Campylobacter ICT was positive for 50 specimens, negative for 254 specimens, and uninterpretable for 1 specimen. The ImmunoCard STAT! Campy ICT was positive for 52 specimens and negative for 253 specimens. The two ICTs were both positive for 43 specimens and negative for 246 specimens.
Table 3 presents the diagnostic classification of each ICT according to the observed CRS status. Among the 50 positive specimens with the Ridaquick Campylobacter ICT, 47 were considered true positives by the CRS corresponding to a PPV of 94.0% (95% CI = 83.5 to 98.8). Among the 52 positive specimens with the ImmunoCard STAT! Campy ICT, 44 were considered true positives by the CRS corresponding to a PPV of 84.6% (95% CI = 71.9 to 93.1). The negative predictive values (NPVs) were estimated at 94.9% (95% CI = 90.3 to 97.8) and 92.4% (95% CI = 87.0 to 96.0) for the Ridaquick Campylobacter and ImmunoCard STAT! Campy ICTs, respectively. Corrected sensitivities and specificities of both ICTs are presented in Table 4.
TABLE 3.
Results for the ImmunoCard STAT! Campy and the Ridaquick Campylobacter kits according to the composite reference standard for the study patients
| ICT | Result | Composite reference standard (no. of results) |
||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| ImmunoCard STAT! Campy | Positive | 44 | 8 | 52 |
| Negative | 12 | 145 | 157 | |
| Total | 56 | 153 | 209 | |
| Ridaquick Campylobacter | Positive | 47 | 3 | 50 |
| Negative | 8 | 150 | 158 | |
| Total | 55 | 153 | 208a | |
Inconclusive index test for one patient.
TABLE 4.
Estimation of corrected sensitivities and specificities for both ICTs for the 305 patients enrolled in the studya
| ICT | % accuracy (95% CI) |
|---|---|
| ImmunoCard STAT! Campy | |
| Sensitivity | 72.8 (57.6–84.9) |
| Specificity | 96.7 (92.5–98.9) |
| Ridaquick Campylobacter | |
| Sensitivity | 79.0 (63.4–90.2) |
| Specificity | 98.8 (9.5–99.9) |
Corrected sensitivities and specificities take into account the unavailability of the CRS in 96 consecutive patients. CI, confidence interval.
DISCUSSION
We assessed the diagnostic accuracy of two ICTs for the detection of campylobacters in patients referred for gastroenteritis to the emergency units of a university hospital. In order to overcome the lack of sensitivity of stool culture previously described (9, 10), a CRS was used, combining ELISAs and molecular tests.
The population infected by campylobacters in our study corresponds to what is usually described in terms of age and symptoms (1). Accordingly, the majority of the patients were younger than 15 years old and had diarrhea. The C-reactive protein was logically increased in the majority of the patients. The prevalence of infection in this study is quite high compared to other studies described in the literature (14). This is most certainly due to our recruitment of only emergency unit patients, reflecting a community recruitment compared to other studies in which the stools of patients hospitalized in all departments of the hospital were included (9, 10). Furthermore, stool samples were treated as soon as they arrived in the laboratory: technicians were more attentive and certainly performed the culture more quickly than usual, leading to a better performance of the culture. Given that only 11 samples were transported in Cary Blair medium, there was no influence of this transport medium on our results. Indeed, the high campylobacter prevalence obtained in our study confirms the fact that Campylobacter species are responsible for more gastroenteritis cases than salmonella (1) and should be sought in community patients with digestive symptoms.
Our study shows a very interesting performance of the ICTs in terms of PPV and NPV compared to previous studies (9, 10). One prior study evaluated the Ridaquick Campylobacter performance and obtained equivalent results in terms of specificity (97%) but reported a higher sensitivity (87%) (15). Differences can be explained by the fact that they did not use a CRS, only a molecular test when culture and the Ridaquick Campylobacter were discordant. This “discrepant analysis” has been acknowledged to provide biased estimates of accuracy (14). Another study evaluated the ImmunoCard STAT! Campy and found similar sensitivity and specificity (14). However, the prevalence observed in this last study was much lower than in our study, explaining the very low PPV that they obtained compared to our PPV.
Indeed, if in our study, only culture without any ICT had been performed to detect campylobacter-infected patients, eight cases would have been missed; furthermore, if only ICTs, without culture, had been performed, three cases would have been missed, including the C. fetus case. The hypothesis that ICTs give numerous false-positive results was not confirmed here, leading to the conclusion that ICTs can be used as a screening method on its own. Culture could be added to obtain information on antimicrobial susceptibility. A negative point is that Campylobacter species other than C. jejuni and C. coli will not be detected, but their occurrence is rare, as was the case with only one C. fetus occurrence in this study.
The strengths of the study for producing valid estimates of ICT diagnostic accuracy were as follows: (i) the inclusion of consecutive patients representative of the population who would benefit from these tests; (ii) the verification of 153 consecutive patients with negative results of both ICTs allowing valid estimates of sensitivity, specificity, and NPV; (iii) the blind evaluation of the tests comprising the CRS; (iv) the statistical correction of accuracy estimates and their 95% CI for first ICT-negative patients with missing completed CRS; and (v) the CRS aimed at correcting the imperfect sensitivity of stool culture. In contrast, the main methodological weakness was that the real diagnostic accuracy of the CRS is unknown; however, it was defined on the biological basis of improving the imperfect sensitivity of culture, which is the usual reference standard. It may be interesting to use latent class analysis models to further assess the accuracy of these tests (16). Alternatively, the paradigm of evaluation may be changed by designing a randomized clinical trial comparing the clinical outcome of patients who receive or do not receive an ICT in order to determine their management.
Furthermore, the great advantage of the ICTs is that they are very easy to use and they offer a result in less than 15 min in contrast to PCRs, even multiplex PCRs, or ELISAs, which are more time-consuming with a result obtained after more than 2 h. Consequently, the ICTs allow patients to be treated correctly and rapidly as soon as the result is available. It is indeed important to treat patients as soon as possible in order to shorten the duration of the symptoms. This is not possible with culture, since the diagnosis is usually obtained when patients are no longer symptomatic. Compared to automatized multiplex PCRs (BD Max, for example), ICTs offer a more rapid result and do not require a specific automation, which could be a limiting factor for some laboratories.
This study was conducted in partnership with clinicians who now need rapid results to meet expectations of an effective and appropriate treatment for their patients, a situation which will, in turn, relieve emergency units. Practically speaking, we recommend that campylobacters be detected in patients' stools using an ICT and, in the event of a positive result, culture should be performed in order to isolate the strain and perform antibiotic susceptibility testing.
ACKNOWLEDGMENTS
We acknowledge the Bordeaux University Hospital for financial support.
We thank R-Biopharm and Meridian for donating part of the commercialized kits. We also thank Adélaïde Doussau de Bazignan for participation in the early versions of the study protocol.
REFERENCES
- 1.Van Cauteren D, De Valk H, Sommen C, King LA, Jourdan-Da Silva N, Weill F-X, Le Hello S, Mégraud F, Vaillant V, Desenclos JC. 2015. Community incidence of campylobacteriosis and nontyphoidal salmonellosis, France, 2008-2013. Foodborne Pathog Dis 12:664–669. doi: 10.1089/fpd.2015.1964. [DOI] [PubMed] [Google Scholar]
- 2.Moore JE, Corcoran D, Dooley JSG, Fanning S, Lucey B, Matsuda M, McDowell DA, Mégraud F, Millar BC, O'Mahony R, O'Riordan L, O'Rourke M, Rao JR, Rooney PJ, Sails A, Whyte P. 2005. Campylobacter. Vet Res 36:351–382. doi: 10.1051/vetres:2005012. [DOI] [PubMed] [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wassenaar TM, Blaser MJ. 1999. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect 1:1023–1033. doi: 10.1016/S1286-4579(99)80520-6. [DOI] [PubMed] [Google Scholar]
- 5.Ajene AN, Fischer Walker CL, Black RE. 2013. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, Salmonella and Shigella-associated reactive arthritis. J Health Popul Nutr 31:299–307. doi: 10.3329/jhpn.v31i3.16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga M, Gilbert M, Takahashi M, Li J, Koike S, Hirata K, Yuki N. 2006. Comprehensive analysis of bacterial risk factors for the development of Guillain-Barre syndrome after Campylobacter jejuni enteritis. J Infect Dis 193:547–555. doi: 10.1086/499969. [DOI] [PubMed] [Google Scholar]
- 7.Salazar-Lindo E, Sack RB, Chea-Woo E, Kay BA, Piscoya ZA, Leon-Barua R, Yi A. 1986. Early treatment with erythromycin of Campylobacter jejuni-associated dysentery in children. J Pediatr 109:355–360. doi: 10.1016/S0022-3476(86)80404-8. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald C, Nachamkin I. Campylobacter and Arcobacter, p 998–1012. In Manual of clinical microbiology, 11th ed ASM Press, Washington, DC. [Google Scholar]
- 9.Floch P, Goret J, Bessède E, Lehours P, Mégraud F. 2012. Evaluation of the positive predictive value of a rapid Immunochromatographic test to detect Campylobacter in stools. Gut Pathog 4:17. doi: 10.1186/1757-4749-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessède E, Delcamp A, Sifré E, Buissonnière A, Mégraud F. 2011. New methods for detection of campylobacters in stool samples in comparison to culture. J Clin Microbiol 49:941–944. doi: 10.1128/JCM.01489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bessède E, Solecki O, Sifré E, Labadi L, Mégraud F. 2011. Identification of Campylobacter species and related organisms by matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Clin Microbiol Infect Dis 17:1735–1739. doi: 10.1111/j.1469-0691.2011.03468.x. [DOI] [PubMed] [Google Scholar]
- 12.Ménard A, Dachet F, Prouzet-Mauleon V, Oleastro M, Mégraud F. 2005. Development of a real-time fluorescence resonance energy transfer PCR to identify the main pathogenic Campylobacter spp. Clin Microbiol Infect Dis 11:281–287. doi: 10.1111/j.1469-0691.2005.01072.x. [DOI] [PubMed] [Google Scholar]
- 13.Horvitz DG, Thompson DJ. 1952. A generalization of sampling without replacement from a finite universe. J Am Stat Assoc 47:663–685. doi: 10.1080/01621459.1952.10483446. [DOI] [Google Scholar]
- 14.Fitzgerald C, Patrick M, Gonzalez A, Akin J, Polage CR, Wymore K, Gillim-Ross L, Xavier K, Sadlowski J, Monahan J, Hurd S, Dahlberg S, Jerris R, Watson R, Santovenia M, Mitchell D, Harrison C, Tobin-D'Angelo M, DeMartino M, Pentella M, Razeq J, Leonard C, Jung C, Achong-Bowe R, Evans Y, Jain D, Juni B, Leano F, Robinson T, Smith K, Gittelman RM, Garrigan C, Nachamkin I. 2016. Multicenter evaluation of clinical diagnostic methods for detection and isolation of Campylobacter spp. from Stool. J Clin Microbiol 54:1209–1215. doi: 10.1128/JCM.01925-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez-Camarasa C, Gutiérrez-Fernández J, Rodríguez-Granger JM, Sampedro-Martínez A, Sorlózano-Puerto A, Navarro-Marí JM. 2014. Evaluation of the rapid RIDAQUICK Campylobacter® test in a general hospital. Diagn Microbiol Infect Dis 78:101–104. doi: 10.1016/j.diagmicrobio.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Reitsma JB, Rutjes AWS, Khan KS, Coomarasamy A, Bossuyt PM. 2009. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 62:797–806. doi: 10.1016/j.jclinepi.2009.02.005. [DOI] [PubMed] [Google Scholar]