ABSTRACT
Buruli ulcer is caused by Mycobacterium ulcerans. This neglected disease occurs in scattered foci around the world, with a higher concentration of cases in West Africa. The mycobacteria produce mycolactones that cause tissue necrosis. The disease presents as a painless skin nodule that ulcerates as necrosis expands. Finding acid-fast bacilli in smears or histopathology, culturing the mycobacteria, and performing M. ulcerans PCR in presumptive cases confirm the diagnosis. Medical treatment with oral rifampin and intramuscular streptomycin or oral treatment with rifampin plus clarithromycin for 8 weeks is supported by the World Health Organization. This review summarizes the epidemiology, pathogenesis, clinical presentation, diagnostic tests, and advances in treatment.
KEYWORDS: Buruli ulcer, Mycobacterium ulcerans, neglected disease, skin mycobacteria
INTRODUCTION
Buruli ulcer (BU), a disease caused by Mycobacterium ulcerans, occurs in scattered foci around the world, with a higher concentration of cases in remote areas of West Africa where patients have no access to care (1). Together with leprosy, BU is one of the most frequent skin mycobacterial diseases worldwide (2). The disease starts as a nodule that undergoes necrosis, producing an ulcer that keeps expanding but is painless and has no systemic symptoms. Without treatment, the disease resolves in some patients, while in others it leads to contractures that cause disfigurement, long-term disability, and social stigmatization, thus being known as “bankruptcy wound” (2). The World Health Organization (WHO) considers BU one of the 20 neglected diseases and has called for increased surveillance, control, and research (1, 3, 4).
EPIDEMIOLOGY
In the late 1800s, Sir Albert Cook described cases of chronic disfiguring skin ulcers in Uganda (3). In 1948, MacCallum et al. linked these chronic skin ulcers to a mycobacterium in six cases from rural Australia (5). These skin ulcers are known by many names (Buruli ulcer, Bairnsdale ulcer, Daintree ulcer, Mossman ulcer, Kumasi ulcer, or Searls ulcer), mostly depending on the geographic area where they are found (6). “Buruli ulcer,” the most frequently known name, refers to a Ugandan region in the southern bank of the Victoria Nile river (7). Until now, cases have been found in at least 33 countries in tropical, subtropical, and temperate climates in Asia (Malaysia, Papua New Guinea, and Sri Lanka), Western Pacific regions (Australia), the Americas (Guyana, Mexico, and Peru), and Africa, where the highest concentrations of cases occur in Benin, Cameroon, Cote d'Ivoire, Democratic Republic of the Congo, and Ghana (1). In 2015, 13 countries reported a total of 2,037 new cases (1). However, not all countries have health care systems that can detect and diagnose BU, so underreporting of cases is likely. For example, the sharp increase in the number of BU cases in Japan around 2009 to 2010 was mostly secondary to increased physician awareness and availability of diagnostic techniques (8).
The exact mode of transmission of BU is not known; however, cases occur in areas around bodies of water that are stagnant or slow moving, with many cases occurring during the rainy season. Many of these bodies of water have been subject to environmental changes such as floods, building of dams, or irrigation for agricultural purposes, as well as surrounding deforestation (9). During an outbreak in Australia, a PCR study of environmental sources found M. ulcerans in swamp water that was used for irrigation of a golf course (10). In areas of endemicity, reservoirs of the mycobacterium appear to be in biofilms in water sources, aquatic insects, mollusks, and fish (9, 11). Mosquito bites have been suggested as a mode of transmission based on epidemiologic research on Australian outbreaks as well as animal studies (12). The disease has been observed in mammals such as koalas and opossums, but the question of how animals and humans acquire the infection remains (3). It is still not known whether this is by insect bites, by injuries that occur in water sources that have M. ulcerans, or even by aerosols (9).
In Africa, around 50% of the infections occur in exposed body areas (arms or legs) of children under 15 years of age (1). In Australia and Japan, the majority of infections occur in adults. There is equal distribution between males and females, and the disease can cluster in families (3). However, there has been no documentation of human-to-human transmission, and thus BU is not considered a contagious disease (9). Finally, several studies have documented lower odds of acquiring the disease when using cloth barriers (long pants and long-sleeved shirts), using rubbing alcohol or washing minor wounds immediately after these occur, and using insect repellents.
PATHOGENESIS
The genome of M. ulcerans is approximately 5,000 kb, with about 10% of its DNA being sequences that apparently have no other function but to make copies of themselves (13). These sequences are 1 to 5 kb and are present through the mycobacterial genome. The genome of M. ulcerans is 98% identical to that of Mycobacterium marinum, and genomic analysis suggests that M. ulcerans evolved from M. marinum. This evolution included the acquisition of a large virulence plasmid (pMUM001) and insertion of the IS2404 and IS2606 genes in multiple copies (9). The plasmid contains DNA sequences that encode polyketide synthetases which are responsible for producing the toxins known as mycolactones. In addition to M. ulcerans, a few other mycobacteria produce mycolactones, including M. marinum, M. pseudoshottsii, M. liflandii, and M. xenopi (11).
Mycolactones are heat-stable polyketide macrolides that are synthesized by polyketide synthases encoded by three genes (mlsA1, mlsA2, and mlsB) (13, 14). The major and most potent mycolactones are a mixture of cis-trans isomers (mycolactones A and B). These toxins diffuse away from where the bacteria are present and accumulate in fibroblasts and macrophages (14). Mycolactones have two main targets: scaffolding proteins such as Wiskott-Aldrich syndrome protein (WASP) and cotranslational translocation proteins such as Sec61 (14). Binding of mycolactones to WASP and other scaffolding proteins disrupts the cell's actin cytoskeleton, which leads to lack of cell adhesion, poor movement of cells, and cell death. Binding of mycolactones to Sec61 and other proteins that translate RNA and translocate the protein from the endoplasmic reticulum to the cytosol results in many proteins being degraded. This in turn produces local apoptosis and necrosis of many human cells (adipocytes, fibroblasts, and leukocytes) (14). Thrombosis due to mycolactone-associated vascular damage also contributes to tissue necrosis beyond where the mycobacteria are located. In addition, in a mouse model, mycolactones activate type 2 angiotensin II receptors, which leads to hypoesthesia due to potassium-dependent hyperpolarization of neurons (15). Based on the functions of mycolactones, the immune responses of macrophages and other phagocytic cells are altered, as they will have difficulty moving toward their target and production of cytokines such as interferon gamma will be downregulated. The type of T-helper response varies depending on the lesion stage; for example, in nodules, the ratio of interferon gamma to interleukin-10 (IL-10) was higher than that in ulcerative lesions, suggesting that the T-helper 1 response is downregulated in early lesions (3). In late stages, when neutrophils are present in the lesions, there is increased production of IL-8. The T-helper 1 response eventually progresses to granuloma formation. When a protein (burulin) derived from M. ulcerans was used experimentally in the burulin skin test, cell-mediated immunity was decreased in the early stages and appeared to improve in later stages (3). Hong et al. studied the pharmacodistribution of mycolactones in a mouse model and found the toxin in mononuclear cells in spleen and lymph nodes, supporting the concept of systemic immunosuppressive effects in patients with BU (16). It should be noted that the immune response to M. ulcerans is primarily cellular; however, patients with BU produce antibodies against the heat shock protein of M. ulcerans (17).
Molecular typing of M. ulcerans from different parts of the world shows that there are differences in the strains isolated, including different combinations of the mycolactones (13). Strains from Africa tend to produce mycolactones A and B, which are more potent in causing necrosis. In contrast, strains from Asia and Australia tend to produce mostly mycolactone C with lesser amounts of mycolactones A and B, resulting in a less virulent mycobacterium. In addition to mycolactones, researchers have found that certain human polymorphisms in the SLC11A1 gene (which encodes a metal cation transport protein) confer increased susceptibility to BU, similar to what happens with the same gene for tuberculosis and leprosy (18).
CLINICAL PRESENTATION AND DIAGNOSIS
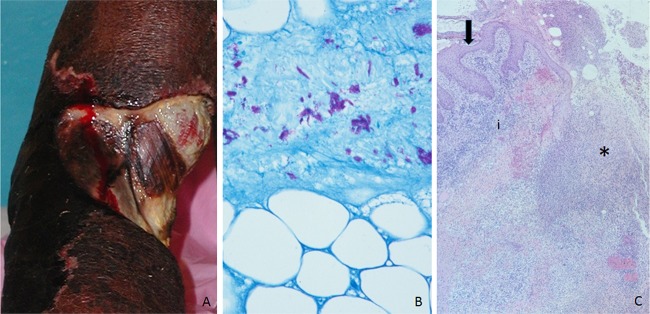
The description by Clancey et al. in 1961 of BU clinical features and disease progression in Ugandan patients has not changed over the years (7). Most of the patients were children who were in good health and recalled an induration in the extremities where the ulcer later developed. The indurated nodules may look like insect bites but are painless and cold (not inflamed). Within weeks, these lesions grow in diameter and depth, producing edematous plaques with ill-defined borders (1). On occasion there is no nodule or plaque and only diffuse swelling. Eventually the indurated, necrotic skin sloughs off, creating a painless ulcer with irregular borders (Fig. 1). The ulcer grows rapidly with the advancing necrotic subcutaneous tissue border extending several centimeters beyond the intact epidermal ulcer edge, which leads to the classic description of undermined borders. Evolution through the clinical stages takes between 3 weeks and 1 year (6). The ulcers can be very deep, exposing tendons and bone and leading to osteomyelitis. The surrounding skin can show edema and changes in pigmentation. Most of the time there is a single lesion; however, small satellite lesions can be present. In many instances the ulcer acquires secondary bacterial infections, giving it a foul smell.
FIG 1.
(A) Category 3 ulcer in the upper extremity, revealing the patient's tendons and muscle. (B) Ziehl-Neelsen stain demonstrating abundant clusters of acid-fast bacilli in the necrotic adipose tissue. This was a tissue sample from a nodule. The photograph was taken under oil immersion. (C) Histopathology of an ulcer, showing hyperplastic epidermis (arrow), necrosis (*), and inflammation (i). The photograph was taken at low magnification (4× objective). (All photographs were obtained while the author worked for the Centers for Disease Control and Prevention.)
Clancey et al. (7) documented three possible outcomes when patients presented to the hospital with an ulcer that had been present for at least 6 months: the ulcers could remain small and persist for several years, there could be an advancing edge of the ulcer while the other edge appeared to be healing, or the ulcer could extend widely, creating deformities, contractures, and amputations that lead to major disabilities. With any course, the patients maintained general good health.
The World Health Organization has classified BU lesions into three categories (1, 19). The first includes lesions that measure less than 5 cm in diameter, while the second is comprised of nonulcerative (plaque and edematous) lesions and ulcers that measure between 5 and 15 cm. The third category includes those lesions larger than 15 cm or those that involve critical sites, including eyes, genitals, breasts, bone (osteitis or osteomyelitis), and joints, or are disseminated. The third category is further subdivided into three groups (3a, single lesion with osteomyelitis; 3b, lesions at critical sites; and 3c, multiple small lesions). In Africa, around 30% of patients present in each category, while in Australia, most of the lesions are diagnosed as category one.
The differential diagnosis will vary geographically depending on the frequency of other diseases that cause skin nodules or ulcers in that particular region (1, 3). For example, in Africa, onchocerciasis should be considered in the differential diagnosis of nodules, but this is not true in Australia. The differential diagnosis for nodules in the extremities includes sebaceous cysts, lipoma, insect bites, other mycobacterial skin infections, and causes of enlarged lymph nodes. For plaque lesions, the differential diagnosis includes cellulitis and fungal infections. Finally, for ulcers the differential diagnosis includes leishmaniasis, ulcerative yaws, ulcerated squamous cell carcinoma, and ulcers caused by Haemophilus ducreyi or secondary to diabetes or arterial or venous insufficiency.
The priority is to identify patients with WHO category 1 BU lesions, as this is where medical treatment has the most effect. Programs involving training and community-based surveillance by volunteers in Ghana have resulted in increased detection of category 1 lesions in up to 70% of cases (19). The WHO recommends the use of two tests to confirm the diagnosis, though in areas of endemicity it may only be necessary to use one test based on the predictive value of positive results. In areas where BU is not endemic, case diagnosis is difficult; thus, confirmation using several diagnostic methods is frequently necessary at any stage. Cases that are imported to areas where the disease is not endemic, such as the United States, end up with delayed diagnoses.
DIAGNOSTIC TESTS
Confirmation of BU is done with four main methods: microscopic detection of acid-fast bacilli (AFB), cultures, PCR targeting specific M. ulcerans genes, and histopathology (11). Table 1 presents the advantages and disadvantages of the different methods. Since the mycobacteria are unevenly distributed in the lesions, the manner in which samples are collected is of paramount importance to obtaining an adequate result (20): To maximize the possibility of positive results when using swabs, the person obtaining the sample should swab the entire undermined edge of the ulcer (as this is where bacilli are usually present) and preferably take two collections per lesion. When obtaining material with fine-needle aspirates (FNA), these should be done from the least indurated portion of the lesion. Finally, when obtaining biopsy specimens, these should be taken below the undermined edge and include necrotic material. In the case of nodules, the biopsy specimen should be obtained from the center of the lesion and should include adipose tissue.
TABLE 1.
Diagnostic methods for Buruli ulcer
| Method | Advantages | Disadvantages |
|---|---|---|
| Acid-fast stain microscopy | 1. Performed in many countries in the world for diagnosis of tuberculosis | 1. Least sensitive and specific of the methods (around 30–40%); however, in patients with classic presentation in an area with high prevalence, a positive result is strongly suggestive of Buruli ulcer |
| 2. Can be performed in swabs and fine needle aspirates | ||
| 3. Requires minimal equipment and reagents | ||
| 4. Requires the least amt of technical expertise compared to the other methods | ||
| Culture | 1. Reference standard with highest specificity | 1. Takes 9 to 12 wk to grow even under optimal conditions (temp, 29–33°C; oxygen concn, 2.5–5%) |
| 2. Can be performed with swabs, fine-needle aspirates, and tissue | ||
| 3. Used in cases that do not respond to treatment or when there is a relapse | 2. Once growth is present, there is need for use of PCR or MALDI-TOF MS for identification | |
| PCR | 1. Most sensitive method for Buruli ulcer cases (detects 54–84% of cases depending on the series) | 1. No standardized protocols (mostly laboratory-developed assays) |
| 2. Can be performed with swabs, fine-needle aspirates, and tissue | 2. Requires specialized equipment and, for the most part, a cold chain for reagents | |
| 3. Very specific when targeting the IS2404 gene | 3. Requires trained personnel performing the assays | |
| 4. May detect nonviable organisms | ||
| Histopathology | 1. Best at finding other potential causes of nodules and ulcers (differential diagnosis) | 1. Requires obtaining tissue (biopsy or resection) |
| 2. Requires a pathology laboratory (ability to paraffin embed, cut, and stain the tissue) | ||
| 3. Needs highly trained personnel (physician trained in pathology) to read the biopsy result |
Microscopic study of smears made from swabs or FNA specimens that have been stained with Ziehl-Neelsen stain (an acid-fast stain), similar to what is used for tuberculosis, is used in many parts of Africa (20). A study in Ghana showed that this technique has a diagnostic sensitivity of 40% for nodules, which decreases to 30% for ulcers (21). Fluorescence microscopy after auramine staining of these smears has also been used to detect the mycobacterium. Of note, other mycobacteria such as M. marinum can cause skin lesions, and this diagnostic method will not be able differentiate between the diverse mycobacteria. The Australian consensus on diagnosis suggests that the presence of acid-fast bacilli in a microscopic study when the clinical picture is concordant with BU is strongly in favor of the diagnosis (22).
Cultures are the reference standard for diagnosis of many infectious diseases, including BU. However, their use for diagnostic and treatment purposes is complicated, as M. ulcerans requires 9 to 12 weeks of incubation under optimal conditions (temperature of 29 to 33°C and 2.5 to 5% oxygen concentration) to grow (1). M. ulcerans grows in Lowenstein-Jensen, Middlebrook 7H10, and Middlebrook 7H11 with oleic acid-albumin-dextrose-catalase enrichment agars and in Middlebrook 7H9 broth. Sample decontamination methods have shown detrimental effects on the recovery of this mycobacterium, particularly for environmental specimens (6). Cultures are of use when there are relapses or the ulcer does not heal (20). Once the mycobacterium has grown in culture, PCR is useful to define the species. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has been successfully used to differentiate colonies of M. marinum from those of M. ulcerans (23).
Real-time or single-step gel-based PCRs using the IS2404, 16S rRNA, or hsp-65 gene have been developed for diagnosis of M. ulcerans (20). The IS2404 gene is present in at least 50 copies per genome and it has become the standard for detection in environmental and animal samples (9, 24). Upon review of multiple series from diverse world regions, the number of PCR-positive cases is found to vary from 54 to 84% (21, 25). The variability depends on many factors, including the type of lesion, where in the lesion and how the sample was obtained, how the sample was sent to the laboratory, the amount and preparation of the sample, and the type of PCR assay, as these are mostly laboratory-developed assays (26). The consensus council in Australia on diagnosis and treatment states that testing a swab by using PCR targeting IS2404 approaches 100% sensitivity. Thus, when the PCR is negative but the smear is AFB positive, this should prompt alternative diagnoses (22). Also, both PCR and AFB microscopy being negative makes the diagnosis of BU extremely unlikely. Since most of BU cases are in Africa, methods that use stable reagents for M. ulcerans PCR need to be developed and tested. A pilot of a PCR assay with dry (lyophilized) reagents has taken place in Ghana, though further standardization for reliable use needs to occur (27). Similarly, loop-mediated isothermal amplification has been used as an alternative (11). Finally, it needs to be realized that DNA can persist in lesions, and detection does not necessarily indicate that there are live mycobacteria; thus, PCR cannot be used for follow-up of patients with recurrences.
Clancey et al. were the first to describe the histopathology of BU in human cases (7). Although this diagnostic modality requires specialized trained personnel (a pathologist) and is often not available in Africa, it has the advantage of providing alternative diagnoses when the lesions biopsied are not BU (some examples of diagnoses for nodules are keratin cysts, subcutaneous fungal infections, filarial nodules, or abscess, and diagnoses for ulcers include squamous cell carcinoma) (28). With the routine stain (hematoxylin and eosin), nodules show a characteristic necrotic center that involves deep dermis and subcutaneous adipose tissue, with little inflammation and psoriasiform hyperplasia of the epidermis (28). Ziehl-Neelsen stain or other stains for acid-fast bacilli stain show extracellular clusters of bacilli in the necrotic material (Fig. 1) and rare macrophages with intracellular mycobacteria in the periphery. When ulcers are sampled, similar changes are observed (necrosis of deep dermis and subcutaneous fat as well as epidermal hyperplasia) (Fig. 1); however, granulomatous inflammation is more frequent in the advancing ulcer border. In general, bacilli are present in ulcerated lesions but usually in lower numbers than in preulcerative lesions (nodules and plaques). Inflammation around blood vessels accompanied by thrombosis is a frequent occurrence in both ulcerative and preulcerative lesions. A study of 4 cases in which histopathology and PCR were performed in different regions of the lesion showed the presence of bacterial DNA beyond the area where bacilli were observed with acid-fast stains (26).
Alternative methods such as serology and chromatography have been studied (20). Serologic detection of antibodies to specific M. ulcerans antigens has shown mixed results, as in addition to detecting BU patients, the tests have been positive in healthy household contacts (17). Fluorescent thin-layer chromatography for mycolactone using a variety of samples from the lesions (swabs, FNA, and biopsy specimens) has shown a sensitivity of 73% and a specificity of 86%; however, this method is in early stages of development as a diagnostic tool (29).
TREATMENT
Medical treatment using daily oral rifampin and intramuscular streptomycin for 8 weeks was introduced in the early 2000s (22) and is recommended by the WHO (1). A review of treatment studies performed in eight African countries and one in Australia showed that this regimen achieved on average a 50% cure rate (30). This antibiotic regimen is highly effective for lesions that are less than 10 cm in diameter, as these patients may not require surgery. A retrospective study of Ghanaian patients who did not complete treatment demonstrated that shorter regimens (4 weeks) can be effective (31). The combination of rifampin plus clarithromycin, ciprofloxacin, or moxifloxacin for 3 months has been used in Australia and is endorsed by WHO (1, 22). In settings where rifampin is contraindicated, clarithromycin and moxifloxacin have been used. Oral combination treatment is advantageous in many parts of the world where obtaining antibiotic injections may be difficult. Intravenous treatment using amikacin for 4 to 8 weeks with oral rifampin is reserved for patients with severe, extensive disease, disease that involves tendons, nerves, joints, bone (osteomyelitis), major relapses, or areas such as the face in order to minimize surgery (22). In many parts of the world, tuberculosis, HIV, and BU are prevalent in the same population. When treating these infections, drug interactions and responses to treatment of each of these infections have to be taken into consideration to define the best order in which to treat these concurrent infections and which medications to use (32). As a general rule, screening for these infections should be performed, preventive treatment with co-trimoxazole should be given, and treatment for BU should be started before that for HIV.
In addition to antibiotic treatment, patients with ulcers should receive wound care, which may be needed daily for severe lesions (2). Physiotherapy should also be included to promote healing and prevent disabilities. Continuous rehabilitation is usually needed for those patients with disabilities. A study in Ghana that looked into patients' experiences regarding treatment showed the difficulties patients have in reaching health care centers to get antibiotic injections and in changing dressings and that it is important to explain consequences of treatment, such as the urine turning red with use of rifampin (2).
In approximately 20% of patients, transient clinical deterioration occurs 3 to 12 weeks after medical treatment has started (33). In some instances, lesions in other parts of the body are noted. Although paradoxical reactions can occur for all patients independent of stage, they tend to be more frequent in patients who have large lesions, lesions found in the trunk, and edematous lesions and in adults. These paradoxical reactions are thought to be an exaggerated immune response to mycobacterial antigens that remain in the tissues after mycolactone with its immunosuppressive effects is no longer present. Since polymorphisms in the gene SLC11A1 increase susceptibility to BU, these same polymorphisms are hypothesized to be associated with increases in paradoxical reactions after treatment (33).
The second mainstay of BU treatment is surgery as debridement with wide margins and grafting continue to be performed for large lesions (34). Surgery is also used to treat complications such as releasing contractures. The primary role of surgery is to prevent disability. Surgery has been recommended after 4 weeks of treatment with antibiotics, though currently, there are no set guidelines for surgical interventions. Debridement should spare important deep structures such as nerves, blood vessels, tendons, and joint capsules. The consensus council conference in Australia suggested surgical treatment for small lesions (22). They recommend removing all necrotic tissue and a small margin of normal tissue and closing either primarily or by grafting. The histopathologic presence of granulomas and acid-fast bacilli in the margin predicts recurrence and requires antibiotic treatment.
PREVENTION
The use of long sleeves and trousers and repellents when outdoors is recommended for people living in areas of endemicity (22). When skin abrasions occur, these have to be cleaned promptly and covered. Programs that detect disease in the early stages are needed in areas of endemicity (2).
Mycobacterium bovis BCG vaccination seems to offer some protection against disseminated disease; however, this protection appears to be short-lived (13). Vaccines specific for M. ulcerans are being tested. These vaccines target a mycolyl transferase (antigen 85A), which is an enzyme required for the integrity of the mycobacterial cell wall that induces T-cell proliferation and production of interferon gamma.
CONCLUSIONS
BU is a neglected skin infectious disease caused by M. ulcerans that has been found in at least 33 countries in tropical and subtropical regions in Africa, the Americas, and Western Pacific regions. The infection evolves from a painless skin nodule to an ulcer, usually in the extremities; it leads to contractures that cause disfigurement and long-term disability and has a high social stigma. In the last decade, major advances have included deciphering the pathogenic mechanism of the toxins produced by M. ulcerans and obtaining proof that BU can be treated medically. Nonetheless, the WHO has established the following research priorities: (i) mapping of cases in the different regions; (ii) developing diagnostic techniques that can be taken to the field and can help find cases at early stages; (iii) establishing the mode of transmission, as this remains unknown; (iv) finding treatment modalities that can be used in the rural communities where BU cases occur; (v) developing vaccines; and (vi) performing social and economic studies.
REFERENCES
- 1.World Health Organization. 2017. Buruli ulcer (Mycobacterium ulcerans infection). WHO, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs199/en/ Accessed 3 January 2018.
- 2.Velink A, Woolley R, Phillips R, Abass K, van der Werf T, Agumah E, de Zeeuw J, Klis S, Stienstra Y. 2016. Former Buruli ulcer patients' experiences and wishes may serve as a guide to further improve Buruli ulcer management. PLoS Negl Trop Dis 10:e0005261. doi: 10.1371/journal.pntd.0005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Werf T, Stienstra Y, Johnson R, Phillips R, Adjei O, Fleischer B, Wansbrough-Jones M, Johnson P, Portaels W, van der Graaf W, Asiedu K. 2005. Mycobacterium ulcerans disease. Bull World Health Organ 83:785–791. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2018. Neglected tropical diseases. World Health Organization, Geneva, Switzerland. http://www.who.int/neglected_diseases/diseases/en/ Accessed 2 January 2018.
- 5.MacCallum P, Tolhurst J, Buckle G, Sissons H. 1948. A new mycobacterial infection in man. J Pathol Bacteriol 60:93–122. [PubMed] [Google Scholar]
- 6.Zingue D, Bouam A, Tian R, Drancourt M. 2018. Buruli ulcer, a prototype for ecosystem-related infection, caused by Mycobacterium ulcerans. Clin Microbiol Rev 31:e00045-17. doi: 10.1128/CMR.00045-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancey J, Dodge O, Lunh H, Oduori M. 1961. Mycobacterial skin ulcers in Uganda. Lancet 7209:951–954. doi: 10.1016/S0140-6736(61)90793-0. [DOI] [PubMed] [Google Scholar]
- 8.Yotsu R, Nakanaga K, Hoshino Y, Suzuki K, Ishii N. 2012. Buruli ulcer and current situation in Japan: a new emerging cutaneous Mycobacterium infection. J Dermatol 39:587–593. doi: 10.1111/j.1346-8138.2012.01543.x. [DOI] [PubMed] [Google Scholar]
- 9.Merritt R, Walker E, Small P, Wallace J, Johnson P, Benbow M, Boakye D. 2010. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis 4:e911. doi: 10.1371/journal.pntd.0000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross B, Johnson P, Oppedisano F, Marino L, Sievers A, Stinear T, Hayman J, Veitch M, Robins-Browne R. 1997. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl Environ Microbiol 63:4135–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narh C, Mosi L, Quaye C, Tay S, Bonfoh B, Souza Dd. 2014. Genotyping tools for Mycobacterium ulcerans—drawbacks and future prospects. Mycobact Dis 4:1000149. doi: 10.4172/2161-1068.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace J, Mangas K, Porter J, Marcsisin R, Pidot S, Howden B, Omansen T, Zeng W, Axford J, Johnson P, Stinear T. 2017. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis 11:e0005553. doi: 10.1371/journal.pntd.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Werf T, Stinear T, Stienstra Y, van der Graaf W, Small P. 2003. Mycolactones and Mycobacterium ulcerans disease. Lancet 362:1062–1064. doi: 10.1016/S0140-6736(03)14417-0. [DOI] [PubMed] [Google Scholar]
- 14.Sarfo S, Phillips R, Wansbrough-Jones M, Simmonds R. 2016. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol 18:17–29. doi: 10.1111/cmi.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marion E, Song O, Christophe T, Babonneau J, Fenistein D, Eyer J, Letournel F, Henrion D, Clere N, Paille V, Guérineau N, André JS, Gersbach P, Altmann K, Stinear T, Comoglio Y, Sandoz G, Preisser L, Delneste Y, Yeramian E, Marsollier L, Brodin P. 2014. Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. Cell 157:1565–1576. doi: 10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Hong H, Coutanceau E, Leclerc M, Caleechurn L, Leadlay P, Demangel C. 2008. Mycolactone diffuses from Mycobacterium ulcerans-infected tissues and targets mononuclear cells in peripheral blood and lymphoid organs. PLoS Negl Trop Dis 2:e325. doi: 10.1371/journal.pntd.0000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz D, Döbeli H, Yeboah-Manu D, Mensah-Quainoo E, Friedlein A, Soder N, Rondini S, Bodmer T, Pluschke G. 2006. Use of the immunodominant 18-kilodalton small heat shock protein as a serological marker for exposure to Mycobacterium ulcerans. Clin Vaccine Immunol 13:1314–1321. doi: 10.1128/CVI.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stienstra Y, van der Werf T, Oosterom E, Nolte I, van der Graaf W, Etuaful S, Raghunathan P, Whitney E, Ampadu E, Asamoa K, Klutse E, te Meerman G, Tappero J, Ashford D, van der Steege G. 2006. Susceptibility to Buruli ulcer is associated with the SLC11A1 (NRAMP1) D543N polymorphism. Genes Immun 7:185–189. doi: 10.1038/sj.gene.6364281. [DOI] [PubMed] [Google Scholar]
- 19.Abass K, van der Werf T, Phillips R, Sarfo F, Abotsi J, Mireku S, Thompson W, Asiedu K, Stienstra Y, Klis S. 2015. Short report: Buruli ulcer control in a highly endemic district in Ghana: role of community-based surveillance volunteers. Am J Trop Med Hyg 92:115–117. doi: 10.4269/ajtmh.14-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakyi S, Aboagye S, Darko-Otchere I, Yeboah-Manu D. 2016. Clinincal and laboratory diagnosis of Buruli ulcer disease: a systematic review. Can J Infect Dis Med Microbiol 2016:5310718. doi: 10.1155/2016/5310718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bretzel G, Siegmund V, Nitschke J, Herbinger K, Thompson W, Klutse E, Crofts K, Massavon W, Etuaful S, Thompson R, Asamoah-Opare K, Racz P, Vloten F, van Berberich C, Kruppa T, Ampadu E, Fleischer B, Adjei O. 2007. A stepwise approach to the laboratory diagnosis of Buruli ulcer disease. Trop Med Int Health 12:89–96. [DOI] [PubMed] [Google Scholar]
- 22.Johnson P, Hayman J, Quek T, Fyfe J, Jenkin G, Buntine J, Athan E, Birrell M, Graham J, Lavender C, Mycobacterium ulcerans Study Team. 2007. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Med J Aust 186:64–68. [DOI] [PubMed] [Google Scholar]
- 23.Zingue D, Flaudrops C, Drancourt M. 2016. Direct matrix assited laser desorption ionization time-of-flight mass spectrometry identification of mycobacteria from colonies. Eur J Clin Microbiol Infect Dis 35:1983–1987. doi: 10.1007/s10096-016-2750-5. [DOI] [PubMed] [Google Scholar]
- 24.Ross B, Marino L, Oppedisano F, Edwards R, Robins-Browne R, Johnson P. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol 35:1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mensah-Quainoo E, Yeboah-Manu D, Asebi C, Patafuor F, Ofori-Adjei D, Junghanss T, Pluschke G. 2008. Diagnosis of Mycobacterium ulcerans infection (Buruli ulcer) at a treatment centre in Ghana: a retrospective analysis of laboratory results of clinically diagnosed cases. Trop Med Int Health 13:191–198. doi: 10.1111/j.1365-3156.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- 26.Rondini S, Horsfield C, Mensah-Quainoo E, Junghanss T, Lucas S, Pluschke G. 2006. Contiguous spread of Mycobacterium ulcerans in Buruli ulcer lesions analysed by histopathology and real-time PCR quantification of mycobacterial DNA. J Pathol Bacteriol 208:119–128. [DOI] [PubMed] [Google Scholar]
- 27.Siegmund V, Adjei O, Nitschke J, Thompson W, Klutse E, Herbinger K, Thompson T, van Vloten F, Racz P, Fleischer B, Loescher T, Bretzel G. 2007. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clin Infect Dis 45:68–75. doi: 10.1086/518604. [DOI] [PubMed] [Google Scholar]
- 28.Guarner J, Bartlett J, Whitney E, Raghunathan P, Stienstra Y, Asamoa K, Etuaful S, Klutse E, Quarshie E, van der Werf T, van der Graaf W, King C, Ashford D. 2003. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis 9:651–656. doi: 10.3201/eid0906.020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadagni A, Frimpong M, Phanzu D, Ablordey A, Kacou E, Gbedevi M, Marion E, Xing Y, Babu V, Phillips R, Wansbrough-Jones M, Kishi Y, Asiedu K. 2015. Simple, rapid Mycobacterium ulcerans disease diagnosis from clinical samples by fluorescence of mycolactone on thin layer chromatography. PLoS Negl Trop Dis 9:e0004247. doi: 10.1371/journal.pntd.0004247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vouking M, Tamo V, Tadenfok C. 2013. Clinical efficacy of rifampicin and streptomycin in combination against Mycobacterium ulcerans infection: a systematic review. Pan Afr Med J 15:155. doi: 10.11604/pamj.2013.15.155.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klis S, Kingma R, Tuah W, van der Werf T, Stienstra Y. 2016. Clinical outcomes of Ghanaian Buruli ulcer patients who defaulted from antimicrobial therapy. Trop Med Int Health 21:1191–1196. doi: 10.1111/tmi.12745. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien D, Ford N, Vitoria M, Christinet V, Comte E, Caknt A, Stienstra Y, Eholie E, Aseidu K. 2014. Management of BU-HIV co-infection. Trop Med Int Health 19:1040–1047. doi: 10.1111/tmi.12342. [DOI] [PubMed] [Google Scholar]
- 33.Barogui Y, Klis S, Johnson R, Phillips R, van der Veer E, van Diemen C, van der Werf T, Stienstra Y. 2016. Gene susceptibility and predictors of paradoxical reactions in Buruli ulcer. PLoS Negl Trop Dis 10:e0004594. doi: 10.1371/journal.pntd.0004594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adu E. 2013. Management of complications of Mycobacterium ulcerans disease: a three year review. Int J Mycobacteriol 2:206–210. doi: 10.1016/j.ijmyco.2013.08.003. [DOI] [PubMed] [Google Scholar]