ABSTRACT
The clinical utility of the QuantiFERON-CMV (QFN-CMV) assay in heart transplant recipients was assessed. Forty-four cytomegalovirus (CMV)-seropositive patients were enrolled: 17 received antiviral prophylaxis, and 27 were managed preemptively. CMV-DNAemia monitoring was performed by the use of a quantitative real-time PCR assay. The QFN-CMV assay was retrospectively performed on blood samples collected at five posttransplant time points. A higher proportion of patients with an indeterminate QFN-CMV result after the suspension of prophylaxis than of patients who showed a global T-cell responsiveness developed CMV infection (P = 0.036). Patients who reconstituted a CMV-specific response following the first CMV-DNAemia-positive result (42.9%) showed a median CMV-DNAemia peak 1 log of magnitude lower than that seen with patients with indeterminate results, and all controlled viral replication spontaneously. The 25% of patients with an indeterminate result developed CMV disease. In the preemptive strategy group, no differences in the development of subsequent infection, magnitude of viral load, and viral control were observed on the basis of QFN-CMV measurements performed before and after the first CMV-DNAemia-positive result. Considering both CMV prevention strategies, viral relapse was associated with the failure to reconstitute CMV-specific cell-mediated immunity (CMI) after the resolution of the first episode of CMV infection (P = 0.032). QFN-CMV measurements can be a useful tool for identifying patients (i) at higher risk of developing infection after discontinuing antiviral prophylaxis, (ii) with late CMV infection who would benefit from appropriate antiviral interventions, and (iii) at higher risk of viral relapses. QFN-CMV measurements taken within 1 month posttransplantation (early period) are not revealing.
KEYWORDS: cytomegalovirus, heart transplant, cytomegalovirus prevention strategies, cell-mediated immunity, CMV DNAemia
INTRODUCTION
Cytomegalovirus (CMV) infection is the most common viral complication after solid-organ transplantation, occurring mostly within the first 6 months posttransplantation (1, 2). The strategies for the prevention of CMV include universal prophylaxis and preemptive therapy, but significant variation in their clinical application among centers has been reported (3). In the absence of appropriate prophylaxis, up to 80% of solid-organ recipients may experience CMV infection that may result in asymptomatic viral replication, viral syndrome, or tissue-invasive disease (3, 4). The CMV-specific T-cell responses have been identified as an essential host factor in the control of CMV infection, and increasing interest in the development of immune monitoring techniques allowing the identification of infectious risk in patients who have undergone solid-organ transplantation has been observed in recent years (5–7). Moreover, the potential clinical applications of immune monitoring combined with virological monitoring in predicting infection or disease have been studied, although few prospective interventional studies have been reported (3, 7, 8). Currently, there are different CMV-specific T-cell assays available and the majority of those available rely on the detection of gamma interferon (IFN-γ) after stimulation of peripheral blood mononuclear cells or whole blood (WB) with viral antigens (3). The QuantiFERON-CMV (QFN-CMV) assay (Qiagen, Hilden, Germany) is the first standardized, commercially available T-cell assay that detects secretion of IFN-γ produced by CMV-specific CD8+ T cells after ex vivo stimulation with various CMV T-cell epitopes from proteins that are associated with several common HLA-I haplotypes, including pp65, pp50, immediate early antigen-1 (IE-1), IE-2, and glycoprotein B (9). The present study retrospectively evaluated the clinical utility of QFN-CMV assay in the management of posttransplant CMV infection in heart transplant (HT) recipients; both antiviral prophylaxis and preemptive therapy approaches were taken into account.
MATERIALS AND METHODS
Study patients.
Forty-four adult patients who underwent HT at the Heart and Lung Transplant Centre of the St. Orsola-Malpighi Polyclinic in Bologna, Italy, between May 2009 and February 2014 were included in the study. These patients represent a subgroup of the 51 patients enrolled in the randomized study PROTECT (Prevention of Transplant Atherosclerosis with Everolimus and Anti-cytomegalovirus Therapy; ClinicalTrials registration no. NCT00966836). QFN-CMV measurements were not available for 7 patients because the assay was not initially planned in the study protocol and because the decision was made to introduce it after the first four patients had been enrolled. In addition, 3 patients died postoperatively, after signing informed consent. In the PROTECT study, CMV-seropositive HT recipients were allocated to receive either 3 months of valganciclovir prophylaxis or preemptive therapy in order to directly compare the two different CMV prevention strategies. The population consisted of 34 males and 10 females, with a mean age of 54.6 years (range, 19 to 68 years). The most common indication for HT was dilated cardiomyopathy (n = 31; 70.5%), followed by hypertrophic cardiomyopathy (n = 6; 13.6%), restrictive cardiomyopathy (n = 4; 9.1%), valvular cardiomyopathy (n = 2; 4.5%), and congenital cardiomyopathy (n = 1; 2.3%). Induction therapy using thymoglobulin at a cumulative dose of 1 to 4 mg/kg of body weight was administered to 34 (85%) patients. In most cases, the postoperative immunosuppressive treatment consisted of a combination of CsA (cyclosporine), mycophenolate mofetil, and prednisone (PRED) (n = 23; 52.3%), followed by a combination of CsA, everolimus, and PRED (n = 15; 34.1%), CsA and PRED (n = 5; 11.3%), and, in one case, PRED and tacrolimus (n = 1; 2.3%). At the time of transplant, all patients were CMV seropositive; the CMV donor/recipient serostatus (D/R) data were as follows: D+/R+ (n = 40; 90.9%) and D−/R+ (n = 4; 9.1%).
After HT, 17 (38.6%) patients received oral valganciclovir CMV prophylaxis (450 mg twice daily, renal function adjusted) for a median time of 58 days (range, 7 to 94 days); not all patients received 3 months CMV prophylaxis as scheduled, due to drug toxicity. The remaining 27 (61.4%) patients were managed preemptively.
According to the center clinical practice, antiviral therapy (oral valganciclovir given at 900 mg twice daily, renal function adjusted) was usually administered to all patients when CMV DNA levels were higher than 4,600 IU/ml WB or a rapid increase of blood viral load was detected; the therapy was stopped when at least two consecutive blood samples tested by real-time PCR assay gave CMV DNA-negative results.
Study design.
Virological monitoring was performed on WB samples by the use of a commercial quantitative real-time PCR assay twice a week during the first month posttransplant, every week until the fourth month, twice a month until the sixth month, and monthly until the twelfth month. Afterwards, blood samples were processed if clinically indicated. Immunological monitoring using the QFN-CMV assay was retrospectively performed on blood samples collected at five fixed posttransplant time points: on the occasion of the first biopsy (i.e., 15 days posttransplant; Tbio) and at 1 (T1), 3 (T3), 6 (T6), and 12 (T12) months after HT.
In the prophylaxis and preemptive strategy groups, the occurrence of CMV infection and immune system reconstitution in the first year posttransplant were evaluated. Furthermore, the relationship between the QFN-CMV results and both the incidence of posttransplant CMV infection and viral control was analyzed. The viral relapse according to cell-mediated immunity (CMI) was also investigated.
CMV infection (reactivation/reinfection), defined as the detection of CMV DNA in WB (CMV DNAemia) at greater than the lower limit of quantification of the molecular assay, i.e.103 IU/ml, was detected in at least two consecutive samples. The definitions of CMV disease (viral syndrome and tissue-invasive disease) and late CMV infection/disease adopted in this study are those in the international consensus guidelines on the management of CMV in solid-organ transplantation reported by Kotton and colleagues (3). Specifically, CMV disease was defined as the evidence of CMV infection with attributable symptoms and late CMV infection/disease as infection/disease occurring after the discontinuation of antiviral prophylaxis (3).
Posttransplant time points selected in data analysis.
In order to analyze the relationship between the QFN-CMV results and the incidence of posttransplant CMV infection, the QFN-CMV measurements obtained at the time point closest to the suspension of prophylaxis (i.e., T1 or T3) and prior to the onset of CMV infection were taken into account in the prophylaxis and preemptive strategy groups, respectively. In particular, the Tbio time point was taken into consideration for the preemptive strategy group, given that all the preemptively managed patients developed CMV infection within 1 month posttransplant. Furthermore, to evaluate the relationship between the QFN-CMV assay results and viral control, the QFN-CMV results obtained following the first detection of CMV DNAemia were analyzed in both groups. Finally, viral relapse according to CMI was investigated among the patients who developed CMV infection, who were considered a unique population. The QFN-CMV measurements obtained at the time point after the resolution of the first episode of CMV infection were taken into account in the last analysis.
Procedures.
DNA extraction from WB samples collected in EDTA-anticoagulated tubes was performed using a QIAsymphony SP instrument (Qiagen, Hilden, Germany). Quantification of CMV DNA was performed using a real-time PCR assay (CMV ELITe MGB kit; ELITech Group, Italy) and an ABI Prism 7500 real-time PCR system (PE Applied Biosystems, Foster City, CA, USA). The extraction and amplification protocols were previously described (10). The analytical sensitivity of the assay is 10 copies of target DNA per amplification reaction. The lower limit of quantification of the assay is 103 IU/ml WB.
CMV-specific CD8+ T cell responses were assessed by the QFN-CMV assay (9). This method uses three specialized collection tubes, i.e., a tube containing a pool of 22 defined CD8+ viral epitopes (CMV tube), a mitogen tube (positive control), and a nil tube (negative control); 1 ml WB was collected into each tube. The assay and the interpretation of IFN-γ responses were performed per the manufacturer's instructions. A positive QFN-CMV result ([CMV-nil] = ≥0.2 IFN-γ IU/ml) identified a patient with detectable CMV-specific CMI; a negative result ([CMV-nil] = <0.2 IFN-γ IU/ml and [mitogen-nil] = ≥0.5 IFN-γ IU/ml) identified a patient without CMV-specific CMI but with global T-cell responsiveness. Finally, results were reported as indeterminate in nonresponders with respect to both CMV and mitogen stimulation ([CMV-nil] = <0.2 IFN-γ IU/ml and [mitogen-nil] = <0.5 IFN-γ IU/ml) in identifying patients without any CMI.
Statistical methods.
Given the small population study size, all comparisons of subgroups were carried out using nonparametric tests; specifically, the chi-square test was used to compare categorical variables and the Mann-Whitney test to compare continuous variables. Stata version 13.1 (StataCorp LP, College Station, TX) was used for all analyses.
Ethics.
The study was approved by the Independent Hospital Ethics Committee of St. Orsola-Malpighi Polyclinic in Bologna, and all patients provided informed consent.
RESULTS
CMV infection.
The 44 adult HT recipients enrolled were monitored for CMV infection for a median time of 13.7 months posttransplantation (range, 12 to 26 months). Twenty-four patients (24/44; 54.6%) developed CMV infection as follows: 7 patients in the antiviral prophylaxis strategy group (7/17; 41.2%) and 17 patients in the preemptive therapy group (17/27; 63%). The rates of CMV infection were not significantly different between the two groups (P = 0.158). The median time at which CMV DNAemia was detected in the antiviral prophylaxis strategy group was 86 days (range, 53 to 88 days) posttransplant. CMV infection occurred in all patients after the suspension of the antiviral prophylaxis, and one patient (1/7, 14.3%) developed CMV disease, i.e., CMV syndrome, at 88 days posttransplant. With regard to the preemptive strategy group, the median time at which CMV DNAemia was first detected was 25 days (range, 2 to 28 days) posttransplant. The difference in the times of onset of CMV infection between the two groups was statistically significant (86 versus 25 days, P < 0.001). CMV preemptive therapy was administered to 11 (11/17; 64.7%) patients, and no patient developed CMV disease.
Cell-mediated immunity.
The patterns of immune reconstitution during the first year posttransplant were similar for the two CMV prevention strategies (Fig. 1). In particular, a high incidence of indeterminate QFN-CMV results was observed in both groups of patients in the immediate posttransplant period (i.e., up to 88.2% of patients did not demonstrate a global cell-mediated immune response within the first 15 days posttransplant). The number of indeterminate QFN-CMV results decreased in the next time points, and the immune response became more sustained over time. At 12 months posttransplant, the proportion of patients with CMV-specific CMI was appreciably higher in the preemptive group than in the prophylaxis group (74.1% versus 47.1%; P = 0.077).
FIG 1.
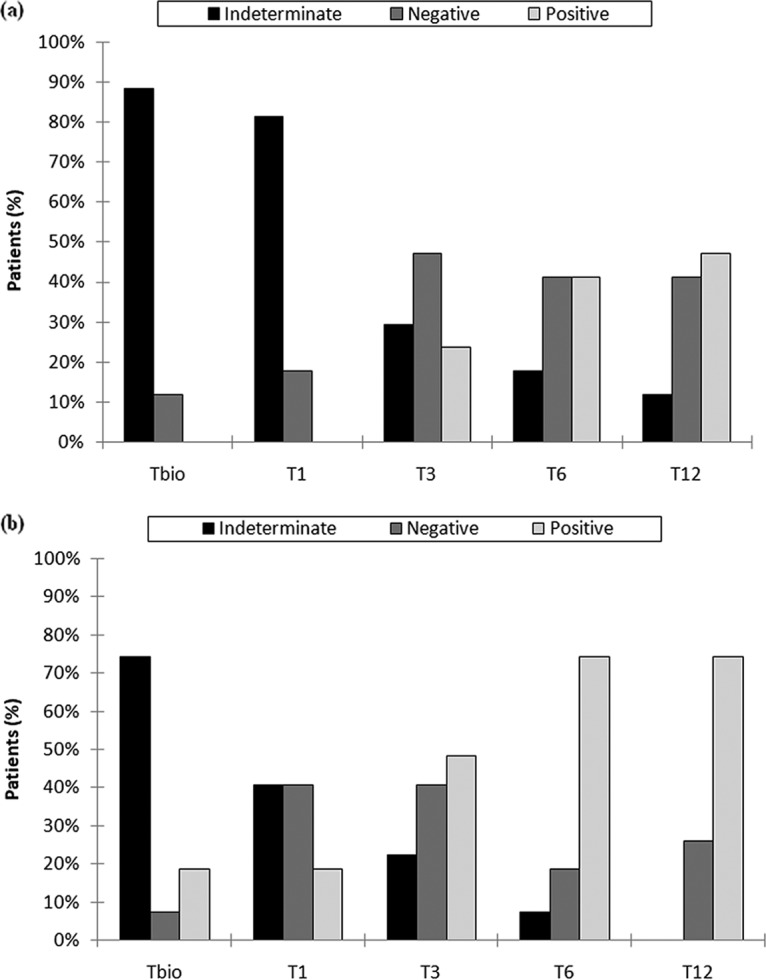
Cell-mediated immunity results at different time points during the 1-year post-HT in the prophylaxis group (a) and preemptive therapy group (b). Tbio, 15 days posttransplant; T1, 1 month posttransplant; T3, 3 months posttransplant; T6, 6 months posttransplant; T12, 12 months posttransplant.
Cell-mediated immunity and CMV infection.
The QFN-CMV results at the selected time points were available for 16/17 (94.1%) patients in the prophylaxis strategy group and for 24/27 (88.9%) preemptively managed patients (Table 1). In the prophylaxis strategy group, a higher (66.7%) proportion of patients with an indeterminate QFN-CMV result at T1/T3 developed a posttransplant CMV infection than of patients who showed global T-cell responsiveness (14.3%). This difference was statistically significant (P = 0.036). In the preemptive strategy group, in contrast, no significant difference with respect to the proportions of posttransplant CMV infection was observed between the patients without and with detectable CMI at Tbio (55.6% and 66.7%, respectively; P = 0.633) (Table 1).
TABLE 1.
Cell-mediated immunity at the time point after the suspension of antiviral prophylaxis (1 or 3 months posttransplant) and before the onset of CMV infection (15 days posttransplant [Tbio]) in the preemptive strategy groupa
| Infection status | No. (%) of patients with indicated QFN-CMV results |
|||||||
|---|---|---|---|---|---|---|---|---|
| Prophylaxis strategy group |
Preemptive strategy group |
|||||||
| Indeterminate | Negative/positive | NA | Total | Indeterminate | Negative/Positive | NA | Total | |
| Patients who developed CMV infection | 6 (66.7) | 1 (14.3) | 0 (0.0) | 7 (41.2) | 10 (55.6) | 4 (66.7) | 3c (100.0) | 17 (63.0) |
| Patients who did not develop CMV infection | 3 (33.3) | 6 (85.7) | 1b (100.0) | 10 (58.8) | 8 (44.4) | 2 (33.3) | 0 (0.0) | 10 (37.0) |
| Total | 9 | 7 | 1 | 17 | 18 | 6 | 3 | 27 |
The immunological results were retrospectively analyzed in relation to the development of posttransplant CMV infection. NA, not available.
Blood sample was not collected.
Patients developed CMV infection within 6 days posttransplant.
Cell-mediated immunity and viral control.
Among the seven patients who developed CMV infection in the prophylaxis strategy group (Table 1), the QFN-CMV assay following the first CMV DNAemia gave positive results in three (42.9%) and indeterminate results in the remaining four (57.1%) patients (Table 2). All three of the patients who showed reconstituted CMV-specific CMI controlled CMV replication spontaneously and showed a median peak viral load that was 1 log of magnitude lower than that seen with the patients who did not show global T-cell responsiveness (1,058 IU/ml versus 13,259 IU/ml WB, respectively). In contrast, all four of the patients without CMV-specific CMI received antiviral therapy and one (25%) of those patients developed CMV syndrome; the patients achieved CMV DNA negativity 42 days (median time; range, 28 to 53 days) after the start of therapy. Regarding the 17 patients who developed CMV infection in the preemptive strategy group (Table 1), the results seen with the QFN-CMV assay following the first detection of CMV DNAemia were indeterminate, negative, and positive in 7 (41.2%), 6 (35.3%), and 4 (23.5%) patients, respectively (Table 3). Of note, two patients who had had a positive QFN-CMV result before the onset of CMV infection gave negative results, showing no stable CMV-specific CMI. No remarkable difference between the patients with and without CMV-specific CMI was observed regarding the median peak viral load detected (4,978 IU/ml and 5,810 IU/ml WB, respectively) and the number of patients who received antiviral therapy (2/4 patients [50%] and 9/13 patients [69.2%], respectively).
TABLE 2.
Immunological, virological, and clinical findings for the 7 patients with CMV infection in the prophylaxis strategy groupa
| QFN-CMV result (time point following suspension of CMV prophylaxis; T1) | QFN-CMV result (time point following first CMV DNAemia; T3) | First CMV DNAemia (no. of days post-Tx) | Peak CMV DNAemia in IU/ml WB (no. of days post-Tx) | Antiviral therapyb | CMV disease |
|---|---|---|---|---|---|
| Indeterminate | Positive | 86 | 1,058 (107) | No | No |
| Indeterminate | Positive | 75 | 966 (110) | No | No |
| Negative | Positive | 53 | 3,723 (104) | No | No |
| Indeterminate | Indeterminate | 84 | 10,394 (110) | Yes | No |
| Indeterminate | Indeterminate | 88 | 7,818 (119) | Yes | Syndrome |
| Indeterminate | Indeterminate | 87 | 16,124 (118) | Yes | No |
| Indeterminate | Indeterminate | 86 | 376,971 (120) | Yes | No |
Pt, patient; Tx, transplant; WB, whole blood; T1, 1 month posttransplant; T3, 3 months posttransplant.
Valganciclovir at 900 mg twice daily (renal function adjusted) until at least two consecutive whole-blood samples gave CMV DNA-negative results.
TABLE 3.
Immunological, virological, and clinical findings for the 17 patients with CMV infection in the preemptive strategy groupa
| QFN-CMV result (time point before the onset of CMV infection) | QFN-CMV result (time point following first CMV DNAemia) | First CMV DNAemia (day post-TX) | Peak CMV DNAemia (IU/ml WB) | Antiviral therapyb | CMV disease |
|---|---|---|---|---|---|
| Indeterminate | Indeterminate | 23 | 3,333 | Yes | No |
| Indeterminate | Indeterminate | 27 | 5,810 | Yes | No |
| Indeterminate | Indeterminate | 21 | 3,229 | No | No |
| Indeterminate | Indeterminate | 28 | 54,226 | Yes | No |
| Indeterminate | Indeterminate | 24 | 6,146 | Yes | No |
| NA | Indeterminate | 6 | 2,483 | No | No |
| NA | Indeterminate | 6 | 7,458 | Yes | No |
| Indeterminate | Negative | 28 | 22,484 | Yes | No |
| Indeterminate | Negative | 18 | 64,277 | Yes | No |
| Indeterminate | Negative | 16 | 2,278 | No | No |
| Positive | Negative | 27 | 6,941 | Yes | No |
| Positive | Negative | 28 | 2,174 | Yes | No |
| NA | Negative | 2 | 2,199 | No | No |
| Indeterminate | Positive | 26 | 1,173 | No | No |
| Indeterminate | Positive | 25 | 3,857 | Yes | No |
| Positive | Positive | 27 | 6,099 | No | No |
| Positive | Positive | 28 | 103,794 | Yes | No |
Pt, patient; TX, transplant; NA, not available.
Valganciclovir at 900 mg twice daily (renal function adjusted) until at least two consecutive whole-blood samples gave CMV DNA-negative results.
Cell-mediated immunity and viral relapse.
Considering all 24 patients with CMV infection (Table 4), none of the nine patients who showed a positive QFN-CMV result after the resolution of the first episode of CMV infection developed a second episode of CMV DNAemia, while 2/8 (25%) and 4/7 (57.1%) of the patients with negative and indeterminate QFN-CMV results developed a second infection episode, respectively. This represented a statistically significant difference (P = 0.032).
TABLE 4.
CMV-specific cell-mediated immunity and incidence of a second episode of CMV infectiona
| QFN-CMV result (time point after the resolution of the first episode of CMV infection) | No. of patients (n = 24) | No. (%) of patients with second episode of CMV infectionb |
|---|---|---|
| Positive | 9 | 0 (0) |
| Negative | 8 | 2 (25) |
| Indeterminate | 7 | 4 (57.1) |
The patients were not grouped according to the CMV prevention strategy but were considered a unique population.
P value, 0.032 (Pearson's chi-square test).
Clinical outcome.
No patients died during the 12-month study period. Three patients (3/44, 6.8%) died at a mean time of 3.7 years (range, 1.2 to 5.5 years) posttransplant; the causes of death were neoplastic disease (n = 2) and sepsis (n = 1). At the time of writing, the remaining 41 (93.2%) patients were alive and well.
DISCUSSION
As reported in the recent consensus guidelines on the management of CMV infection in solid-organ transplant, viral load testing is the cornerstone of diagnosis and monitoring of CMV infection and disease. Furthermore, it was previously reported that immunological monitoring can be used as an adjunct tool to predict risk of infection and disease (3). Nevertheless, the applicability of the immunological assays to routine clinical practice is still to be assessed (7).
In this study, retrospective immunological monitoring of CMV infection was performed in HT recipients with the aim of evaluating the utility of the QFN-CMV assay in the clinical posttransplant setting. In agreement with other studies (11, 12), our results showed that CMV infection is a frequent event in the first year post-HT. However, a low (4.2%) incidence of CMV disease was observed, and this may reflect the strict adherence to the close virological monitoring schedule and the prompt clinical management of the infection applied in our study.
Prophylactic treatment was associated with a lower but not statistically significant incidence of CMV infection, while it had an impact on the time of infection development. In fact, in all cases the first CMV DNAemia detection occurred after the discontinuation of prophylactic therapy (at a median time of 58 days posttransplant), while all preemptively managed patients experienced CMV infection in the early posttransplant period (i.e., within 1 month posttransplant). These findings are in accordance with those reported by other authors (3, 13). However, to date there have not been any published randomized trials that directly compared preemptive therapy and prophylaxis in nonrenal solid-organ transplant recipients and there are no available reports of studies that clearly indicate which strategy is superior (3, 13).
With regard to posttransplant cell-mediated immunity, the complete absence of IFN-γ responses observed in a high number of cases in the early posttransplant phase could have been due to the immunosuppressive treatment used in the study population. In fact, it was reported that the T lymphocyte-depleting antibodies commonly used for induction therapy may dampen and delay reconstitution of T-cell responses (1). Besides T cell anergy, technical issues (e.g., inadequate mixing/handling of blood collection tubes) and lymphocytopenia (i.e., absolute lymphocyte count of <500 cells/μl) may also represent possible explanations for indeterminate QFN-CMV results. In fact, one of the limitations of the QFN-CMV assay is its sensitivity to lymphopenia (14), which raises issues about the clinical significance of the indeterminate results. In this regard, large perspective studies are needed to establish the threshold for the minimum number of lymphocytes able to generate protective cellular responses. However, we can also speculate with respect to the relevance of the QFN-CMV assay for indeterminate results. Indeed, even if only very few responding lymphocytes were present in these patients, they would probably not have been enough to mediate a protective immune response.
The preemptively managed patients experienced faster CMV-specific CMI reconstitution than the patients in the antiviral prophylaxis group, showing a higher proportion of positive QFN-CMV results at each time point. Of note, no patients in the antiviral prophylaxis group had a positive QFN-CMV result within 1 month posttransplant. This pattern probably reflects the different times of onset of CMV infection, which occurred earlier in the preemptive strategy group. In fact, it has been previously suggested that the occurrence of CMV DNA positivity may trigger the response of the patient's immune system (1). By analyzing the relationship between immune reconstitution and the development of CMV infection, patients without detectable CMI at the suspension of antiviral prophylaxis showed a higher incidence of subsequent CMV infection than those who had detectable T-cell responses. These results suggest that the QFN-CMV assay could be performed at the time point of the suspension of the antiviral prophylaxis in order to identify patients who do not have detectable global CMI and who could then benefit from either closer virological monitoring or a reduction of immunosuppression and/or maintenance of antiviral prophylaxis. Furthermore, in the prophylaxis strategy group, the analysis of the QFN-CMV results in the patients who developed CMV infection, specifically in relation to viral load, showed that the development of CMV-specific CMI was crucial in controlling viral replication. This finding suggests that the QFN-CMV assay could be performed in patients with late CMV infection to identify those who may not be able to control CMV replication spontaneously and who could then benefit from appropriate antiviral interventions. In contrast, in the preemptive strategy group, the QFN-CMV results obtained before and following the first appearance of CMV DNAemia were not revealing. It is important that the preemptively managed patients developed CMV infection in the early posttransplant phase (<1 month posttransplant), during which, as previously reported, a high net state of immunosuppression was observed. Furthermore, among the patients in the former group, stable CMV-specific CMI was not attained in two cases. Indeed, the same finding was obtained in the hematopoietic stem cell transplant setting by other authors who reported that the reconstitution of CMV-specific CMI was highly dynamic at the early posttransplant stages (15). Heart transplant patients are intensely immunosuppressed compared with other organ transplant patients. Finally, our results showed that a second episode of CMV infection was associated with the failure to reconstitute CMV-specific CMI after the resolution of the first episode of CMV DNAemia, suggesting that performing the QFN-CMV assay after the resolution of the first infectious episode could be useful to identify patients at higher risk of viral relapses. In conclusion, the results of the present study suggest that, despite the results of the QFN-CMV assay being limited primarily to CD8+ responses (20 of the 22 peptides used for T-cell stimulation were 8 to 10 amino acids long), unlike other approaches assessing both CD8+ and CD4+ T cell responses, it may be a useful tool in the clinical management of infection in HT recipients at specific time points posttransplant by helping to implement a personalized anti-CMV strategy and guide therapeutic decision-making. To our knowledge, there has been only one relevant interventional study in which Kumar and colleagues used QFN-CMV results to make changes to the clinical care of solid-organ transplant recipients. Despite the several limitations of Kumar's study, pointed out by the investigators themselves, the use of the QFN-CMV measurements proved to be feasible and safe in clinical decision-making (8). In order to obtain further evidence regarding the usefulness of this immunological tool, additional studies using a prospective combination of virological monitoring and immunological monitoring for investigations of posttransplant routine clinical practice are needed.
ACKNOWLEDGMENTS
We thank our Linguistic Consultant, Lucy Scioscia, for editing the English language text.
The study was partially supported by an unrestricted grant from Novartis Farma. Qiagen kindly provided reagents for the QuantiFERON-CMV assay. Luciano Potena consulted for Novartis Farma. The rest of us declare no conflict of interest.
A.C. acquired and interpreted data as well as drafted the paper. L.B., D.S., G.T., E.P., G.P., and L.G. contributed to the acquisition and analysis of the data. F.G. clinically managed the patients during the posttransplant period and provided the respective clinical information. D.G. performed statistical analysis. L.P. designed the project and clinically managed the patients during the posttransplant period. T.L. designed and supervised the project and critically revised the manuscript. All of us discussed the results, commented on the manuscript at all stages of development, and provided final approval of the submitted version.
Footnotes
For a commentary on this article, see https://doi.org/10.1128/JCM.02009-17.
REFERENCES
- 1.Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, Kalpoe J, Lisboa L, Ely L, Kaul DR, Schwartz BS, Morris MI, Ison MG, Yen-Lieberman B, Sebastian A, Assi M, Humar A. 2013. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis 56:817–824. doi: 10.1093/cid/cis993. [DOI] [PubMed] [Google Scholar]
- 2.Durante-Mangoni E, Andini R, Pinto D, Iossa D, Molaro R, Agrusta F, Casillo R, Grimaldi M, Utili R. 2015. Effect of the immunosuppressive regimen on the incidence of cytomegalovirus infection in 378 heart transplant recipients: a single centre, prospective cohort study. J Clin Virol 68:37–42. doi: 10.1016/j.jcv.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A; Transplantation Society International CMV Consensus Group. 2013. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 4.Roman A, Manito N, Campistol JM, Cuervas-Mons V, Almenar L, Arias M, Casafont F, del Castillo D, Crespo-Leiro MG, Delgado JF, Herrero JI, Jara P, Morales JM, Navarro M, Oppenheimer F, Prieto M, Pulpón LA, Rimola A, Serón D, Ussetti P; ATOS working group. 2014. The impact of the prevention strategies on the indirect effects of CMV infection in solid organ transplant recipients. Transplant Rev (Orlando) 28:84–91. doi: 10.1016/j.trre.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Weseslindtner L, Kerschner H, Steinacher D, Nachbagauer R, Kundi M, Jaksch P, Simon B, Hatos-Agyi L, Scheed A, Klepetko W, Puchhammer-Stöckl E. 2012. Prospective analysis of human cytomegalovirus DNAemia and specific CD8+ T cell responses in lung transplant recipients. Am J Transplant 12:2172–2180. doi: 10.1111/j.1600-6143.2012.04076.x. [DOI] [PubMed] [Google Scholar]
- 6.Crough T, Khanna R. 2009. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calarota SA, Aberle JH, Puchhammer-Stöckl E, Baldanti F. 2015. Approaches for monitoring of non virus-specific and virus-specific T-cell response in solid organ transplantation and their clinical applications. J Clin Virol 70:109–119. doi: 10.1016/j.jcv.2015.07.299. [DOI] [PubMed] [Google Scholar]
- 8.Kumar D, Mian M, Singer L, Humar A. 16 June 2017. An interventional study using cell mediated immunity to personalize therapy for cytomegalovirus infection after transplantation. Am J Transplant doi: 10.1111/ajt.14347. [DOI] [PubMed] [Google Scholar]
- 9.Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, Bell S, Gailbraith A, McNeil K, Jones S, Khanna R. 2007. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON®-CMV. Transpl Infect Dis 9:165–170. doi: 10.1111/j.1399-3062.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 10.Chiereghin A, Piccirilli G, Turello G, Squarzoni D, Pavia C, Gabrielli L, Landini MP, Lazzarotto T. 18 October 2016. Monitoring of cytomegalovirus (CMV) infection in solid organ transplant recipients: quantitation of CMV DNAemia by two Real-Time PCR assays. Microbiol Med 31:5963. doi: 10.4081/mm.2016.5963. [DOI] [Google Scholar]
- 11.Delgado JF, Manito N, Almenar L, Crespo-Leiro M, Roig E, Segovia J, Vázquez de Prada JA, Lage E, Palomo J, Campreciós M, Arizón JM, Rodríguez-Lambert JL, Blasco T, de la Fuente L, Pascual D, Rábago G. 2011. Risk factors associated with cytomegalovirus infection in heart transplant patients: a prospective, epidemiological study. Transpl Infect Dis 13:136–144. doi: 10.1111/j.1399-3062.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 12.Mendez-Eirin E, Paniagua-Martín MJ, Marzoa-Rivas R, Barge-Caballero E, Grille-Cancela Z, Cañizares A, Naya-Leira C, Gargallo-Fernández P, Castro-Beiras A, Crespo-Leiro M. 2012. Cumulative incidence of cytomegalovirus infection and disease after heart transplantation in the last decade: effect of preemptive therapy. Transplant Proc 44:2660–2662. doi: 10.1016/j.transproceed.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Potena L, Solidoro P, Patrucco F, Borgese L. 2016. Treatment and prevention of cytomegalovirus infection in heart and lung transplantation: an update. Expert Opin Pharmacother 17:1611–1622. doi: 10.1080/14656566.2016.1199684. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Ruiz M, Kumar D, Humar A. 2014. Clinical immune-monitoring strategies for predicting infection risk in solid organ transplantation. Clin Transl Immunol 3:e12. doi: 10.1038/cti.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tey SK, Kennedy GA, Cromer D, Davenport MP, Walker S, Jones LI, Crough T, Durrant ST, Morton JA, Butler JP, Misra AK, Hill GR, Khanna R. 2013. Clinical assessment of anti-viral CD8+ T cell immune monitoring using Quantiferon-CMV® assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One 8:e74744. doi: 10.1371/journal.pone.0074744. [DOI] [PMC free article] [PubMed] [Google Scholar]


