ABSTRACT
New data from the years 2012 to 2015 from the Danish National Fungemia Surveillance are reported, and epidemiological trends are investigated in a 12-year perspective (2004 to 2015). During 2012 to 2015, 1,900 of 1,939 (98%) fungal bloodstream isolates were included. The average incidence was 8.4/100,000 inhabitants, and this appears to represent a stabilizing trend after the increase to 10.1/100,000 in 2011. The incidence was higher in males than females (10.0 versus 6.8) and in patients above 50 years, and those changes were mainly driven by an increasing incidence among 80-to-89-year-old males (65.3/100,000 in 2014 to 2015). The proportion of Candida albicans isolates decreased from 2004 to 2015 (64.4% to 42.4%) in parallel with a doubling of the proportion of Candida glabrata isolates (16.5% to 34.6%, P < 0.0001). C. glabrata was more common among females (34.0% versus 30.4% in males). Following an increase in 2004 to 2011, the annual drug use stabilized during the last 2 to 3 years of that time period but remained higher than in other Nordic countries. This was particularly true for the fluconazole and itraconazole use in the primary health care sector, which exceeded the combined national levels of use of these compounds in each of the other Nordic countries. Fluconazole susceptibility decreased (68.5%, 65.2%, and 60.6% in 2004 to 2007, 2008 to 2011, and 2012 to 2015, respectively, P < 0.0001), and echinocandin resistance emerged in Candida (0%, 0.6%, and 1.7%, respectively, P < 0.001). Amphotericin B susceptibility remained high (98.7%). Among 16 (2.7%) echinocandin-resistant C. glabrata isolates (2012 to 2015), 13 harbored FKS mutations and 5 (31%) were multidrug resistant. The epidemiological changes and the increased incidence of intrinsic and acquired resistance emphasize the importance of continued surveillance and of strengthened focus on antifungal stewardship.
KEYWORDS: fungemia, candidemia, surveillance, population-based, epidemiology, antifungal consumption, echinocandin resistance, azole resistance, multidrug resistance, Candida glabrata
INTRODUCTION
Candidemia remains a threat to susceptible patients and continues to carry a high crude 30-day mortality rate of 30% to 40% (1–3). The mortality is linked to infecting species, comorbidity, underlying disease, age, and time to initiation of appropriate treatment (3, 4). However, as diagnosis of invasive candidiasis is often delayed, diligent attention to intravascular devices is important and empirical treatment recommendations must be founded on reliable contemporary epidemiology (5). Institutional studies are affected by the referral practices and departments served. In general, population-based studies are less prone to bias, especially when the study base becomes large and representative of entire countries. Nationwide surveillance data are available from Australia, Scotland, Finland, Iceland, Norway, Sweden, and Denmark (DK) (6–18). In Denmark, an increasing incidence of candidemia was demonstrated during 8 years, reaching 10.1/100,000 inhabitants in 2011 (18). Although increasing incidence rates have also been found elsewhere, the Danish incidence is notably high compared to other reported contemporary nationwide incidence rates of 2.4 to 5.7/100,000 inhabitants (12–16).
Besides increasing incidence rates, another common observation is the changing species distribution to non-albicans species and particularly to Candida glabrata in the Northern Hemisphere, Australia, and Taiwan and C. parapsilosis in southern Europe and South America (3, 12, 13, 19, 20) at the expense of C. albicans. The increase in C. glabrata levels has been coupled with an increased use of azoles, to which C. glabrata is intrinsically less susceptible (18, 21, 22).
The echinocandins were introduced in Europe and the United States beginning in 2001 and were incorporated into candidemia management guidelines as the first-line treatment in 2009 to 2012 (23–27). Following the increase in the use of the echinocandins, reports of acquired resistance among several different species, and especially C. glabrata, have been published (28–31), thereby warranting continued surveillance and monitoring. The aim of this population-based nationwide study was to update and assess patterns of the incidence and susceptibility of fungemia in Denmark during a 12-year period.
(Preliminary data were presented as a short oral poster session at the 27th ECCMID in Vienna 2017.)
MATERIALS AND METHODS
Population and surveillance in 2012 to 2015.
Incidence rates per 100,000 inhabitants each year were calculated using the population data corresponding to 1 January (www.statistikbanken.dk). The Danish population increased from 5,580,516 to 5,659,715 inhabitants (1.4%) from the years 2012 to 2015. The mean was used for calculation of incidence rates for periods of >1 year. Data corresponding to the national annual number of admissions and bed days were available from The Danish Health Data Authority (www.eSundhed.dk). The local study representative reported population, numbers of admissions, and numbers of bed days for hospitals within the geographical capture area serviced by each center of clinical microbiology. All Danish residents have access to universal tax-supported free-of-charge health care. All centers of clinical microbiology have specific geographic capture areas as specified previously (18); however, due to public health reforms, the number of centers was reduced from 13 to 11 (centers 3 and 4 were administratively merged in 2012 [geographically separated blood culturing sites remained] and centers 12 and 13 were fully merged in 2013). The original numbering of the centers was retained to allow direct comparison with previous reports (17, 18).
Isolates were referred to the National Reference Mycology Laboratory for species verification and susceptibility testing (see below). Completeness was ensured through comparison with local electronic laboratory records. Among 1,939 isolates, a total of 39 (2.0%) (14 C. albicans; 14 C. glabrata; 2 C. tropicalis; 2 C. parapsilosis; 1 each of C. krusei, C. lusitaniae, and C pellliculosa; and 2 unidentified Candida species isolates) were not referred and were therefore excluded from the susceptibility paragraphs.
Two blood culture (BC) systems were used, namely, BacT/Alert (bioMérieux, Marcy l'Etoile, France) and Bactec (Becton Dickinson, Franklin Lakes, NJ, USA), accounting for the detection of 77.9% and 22.1% of cases, respectively. For fungemia patients with successive fungal bloodstream infections over time, subsequent episodes were included if they occurred at least 21 days apart or were caused by a different species consistent with previous reports (17, 18, 32).
Species identification.
Identification methods, including the use of matrix-assisted laser desorption/ionization time of flight mass spectrometry (Bruker, Bremen, Germany), were performed as previously described (18), with the addition of DNA sequencing as described below.
Susceptibility testing.
Susceptibility testing was done contemporaneously for the referred isolates and included tests of susceptibility to amphotericin B, voriconazole, and isavuconazole (98.0% of the isolates); anidulafungin and micafungin (97.9% of the isolates); and fluconazole (97.6% of isolates) according to the EUCAST definitive document E.Def 7.3 (33). Exceptions were amphotericin B (prior to January 2015), for which the Etest (bioMérieux, Herlev, Denmark) and RPMI 1640 2% glucose agar buffered with MOPS (morpholinepropanesulfonic acid; SSI Diagnostika, Hillerød, Denmark) were used. Stock solutions (5,000 mg/liter in dimethyl sulfoxide [DMSO] [Sigma-Aldrich, Brøndby, Denmark]) were used of the following compounds (manufacturer names): fluconazole and amphotericin B (Sigma-Aldrich), anidulafungin and voriconazole (Pfizer A/S, Ballerup, Denmark), micafungin (Astellas Pharma Inc., Tokyo, Japan), and isavuconazole (Basilea, Pharmaceutica Ltd., Basel, Switzerland). C. parapsilosis ATCC 22019 or C. krusei ATCC 6258 or both were included as quality controls in each run (34). Susceptibility classification was performed according to established or proposed EUCAST breakpoints and epidemiological cutoff values (ECOFFs) (see Table S1 in the supplemental material) (35–38). Finally, amphotericin B (susceptibility MIC, ≤1 mg/liter) and fluconazole (susceptibility MIC, ≤2 mg/liter) were used for testing the remaining species to illustrate the overall susceptibility of those species or groups of fungi, but the data should not be interpreted as representing exact values corresponding to clinical susceptibility or resistance.
Molecular identification and FKS gene sequence analysis (for selected isolates).
Sequencing of internal transcribed spacer regions ITS1 and ITS2 (ITS) (18) and the translation elongation factor (TEF) (for Fusarium) was performed as previously described (39). DNA sequence analysis of echinocandin target hot spots in FKS1 (and, for C. glabrata, in FKS2 also) was performed for resistant isolates, and the sequences obtained were compared to relevant reference sequences, including GenBank accession no. JX899422, for C. kefyr (40).
Consumption of antifungal compounds.
Information concerning overall use of antifungal agents in Denmark in 2000 to 2015 was retrieved from the Danish Medicines Agency (www.medstat.dk). Posaconazole tablets and intravenous (i.v.) formulations have been marketed in Denmark since 2014. The licensed maintenance dose of these formulations is 300 mg/day compared to 800 mg/day for the oral suspension. To reflect the actual number of individual dosages given in 2014 to 2015, a corrected use was calculated (0.3 g enterotablet or i.v. infusion and 0.8 g oral solution, which translated to 1 defined daily dose [DDD]). The data corresponding to antifungal use (DDD/1,000 inhabitants/year) in Norway, Sweden, and Finland were retrieved from www.legemiddelforbruk.no, www.socialstyrelsen.se, and www.fimea.fi, respectively. Posaconazole formulation information was not available, and unadjusted posaconazole DDDs were used for comparisons of data from the Nordic countries and Denmark.
Statistics.
A chi-square test or Fisher's exact test was used for comparisons of proportions, and the Chi-square test for trend was used for evaluation of changes in species distribution over the 12-year surveillance period. Calculations were performed using GraphPad Prism version 6.04 (GraphPad Software Inc., La Jolla, CA, USA). For 12 episodes (10 in 2004 and 1 each in 2005 and 2007), the gender was unknown. When possible, episodes were allotted evenly to genders in specific 10-year age groups, but single cases within age groups in four instances were excluded from analysis of gender- and age-specific incidence rates, conducted with linear Poisson regression/incidence ratio rate calculations (package: epitools). P values of <0.05 (two-tailed test) were considered statistically significant. Binomial univariate logistic regression was used to investigate associations between species distribution and year, age, gender, and BC system using R (R Foundation for Statistical Computing, Vienna, Austria) (https://www.R-project.org/). For this analysis and to ensure independence of observations, only incident cases were included. Covariates with a P value of <0.1 were investigated further in a binomial multivariate analysis. Year, age, gender, and BC system were all retained in the multivariate analysis and independent significant findings displayed. The P value also calculated for data that excluded the main tertiary referral hospital serviced by center 1-RH.
RESULTS
Current epidemiology (2012 to 2015) in a 12-year perspective.
A total of 1,939 isolates from 1,883 unique episodes and 1,813 patients were included in the period 2012 to 2015. The mean and median ages of patients were 65 years (range, 0 to 98 years) and 69 years (interquartile range, 58 to 77 years), respectively. Overall, 59.7% of patients were males and that proportion increased over the 12 years (P = 0.01) (Tables 1 and 2). In a 12-year perspective, both the ages of the candidemia patients and the proportion of males increased (Table 2).
TABLE 1.
Characteristics of the national fungemia surveillance scheme on incidence rates, age, gender, and species distribution in 2012 to 2015
| Characteristic | Value(s) |
||||
|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2012–2015 | |
| No. of isolates | 492 | 523 | 441 | 483 | 1939 |
| No. of episodes | 479 | 508 | 429 | 467 | 1883 |
| No. of patients | 461 | 490 | 419 | 443 | 1813 |
| Mean patient age (95% CI) | 66.1 (64.5–67.7) | 64.7 (63.2–66.3) | 64.8 (63.1–66.5) | 65.7 (63.1–66.5) | 65.3 (64.5–66.2) |
| Median patient age (interquartile range) | 69 (58–78) | 68 (59–77) | 69 (58–77) | 69 (59–77) | 69 (58–77) |
| Gender (% male) | 55.1 | 57.3 | 63.5 | 63.7 | 59.7 |
| Episode rate | |||||
| Per 100,000 inhabitants | 8.58 | 9.07 | 7.62 | 8.25 | 8.38 |
| Per 1,000 discharges | 0.35 | 0.37 | 0.31 | 0.34 | 0.34 |
| Per 10,000 bed days | 1.05 | 1.14 | 0.97 | 1.08 | 1.06 |
| No. (%) of isolates | |||||
| C. albicans | 230 (47) | 268 (51) | 225 (51) | 205 (42) | 928 (47.9) |
| C. dubliniensis | 13 (3) | 12 (2) | 20 (5) | 26 (5) | 71 (3.7) |
| C. glabrata | 170 (35) | 155 (30) | 125 (28) | 167 (35) | 617 (31.8) |
| C. krusei | 19 (4) | 19 (4) | 20 (5) | 17 (4) | 75 (3.9) |
| C. parapsilosis | 16 (3) | 18 (3) | 10 (2) | 20 (4) | 64 (3.3) |
| C. tropicalis | 18 (4) | 23 (4) | 15 (3) | 27 (6) | 83 (4.3) |
| Candida speciesa | 18 (4) | 19 (4) | 17 (4) | 15 (3) | 69 (3.6) |
| Other fungib | 8 (2) | 9 (2) | 9 (2) | 6 (1) | 32 (1.7) |
C. lusitaniae (n = 19); C. kefyr (13); C. fermentati (8); C. pelliculosa (6); C. guilliermondii (5); C. inconspicua (4); C. orthopsilosis (3); C. magnoliae and C. nivariensis (2 each); C. fabianii, C. metapsilosis, C. norvegensis, C. palmioleophila, and C. utilis (1 each). Two Candida isolates not referred for species identification were also included.
S. cerevisiae (11); Fusarium dimerum and Cryptococcus neoformans (4 each); Fusarium solani (3); Fusarium oxysporum, Rhodotorula mucilaginosa, and Saprochaete clavata (2 each); A. fumigatus, Talaromyces marneffei, Trichosporon asahii, and Williopsis saturnus (1 each).
TABLE 2.
Numbers of isolates, demographics of patients, and blood culture system use during three 4-year periods in 2012 to 2015
| Parameter | Value(s) |
P value (time trend) | ||
|---|---|---|---|---|
| 2004–2007 | 2008–2011 | 2012–2015 | ||
| No. of isolatesa | 1,932 | 2,049 | 1,939 | |
| No. of episodes | 1,874 | 1,994 | 1,883 | |
| No. of patients | 1,795 | 1,895 | 1,813 | |
| Mean age (95% CI) | 62.4 (61.6–63.3) | 63.3 (62.5–64.1) | 65.3 (64.5–66.2) | <0.0001 |
| Median age (25% quartile) | 66 (55–75) | 66 (56–75) | 69 (58–77) | ND |
| Male gender (%) | 56.4 | 59.2 | 59.7 | 0.01 |
| Episode rate | ||||
| Per 100,000 inhabitants | 8.64 | 9.03 | 8.38 | 0.34 |
| Per 1,000 discharges | 0.39 | 0.38 | 0.34 | <0.0001 |
| Per 10,000 bed days | 0.90b | 1.03 | 1.06 | <0.0001 |
| Proportion of isolates from Bactec (%) | 45.0 | 36.2 | 22.0 | <0.0001 |
For information pertaining to the isolates included, please see Table 1 (data for 2012 to 2015) and refer to references 17 and 18. ND, not determined. The chi-square trend test was used for the statistical analyses, apart from the statistical analyses for age and episode rates, where a linear logistic regression analysis was employed.
The value corresponding to the number of bed days in 2004 was not available, and the figure from 2005 was used for 2004.
The average episode rate was 8.38/100,000 inhabitants (range, 7.6 to 9.1) in 2012 to 2015 and was stable overall over the 12 years, although a significant increase in the incidence rate was evident from 2004 to 2011 (P = 0.001). The population grew by 4.9% from 2004 to 2015 and the number of discharges increased by 20.2%, but the number of bed days decreased by 18.5% (2005 to 2015). Consequently, the incidence rate/1,000 admissions declined whereas the incidence rate/10,000 bed days increased (Table 2). The rates of incidence varied across the centers in 2012 to 2015, increasing from 3.1/100,000 inhabitants to 13.1/100,000 inhabitants, from 0.2/1,000 discharges to 0.7/1,000 discharges, and from 0.6/10,000 somatic bed days to 1.8/10,000 somatic bed days (see Table S2 in the supplemental material).
The highest incidence rates in 2012 to 2015 were seen at the extremes of age, with 9.5 per 100,000 inhabitants in the <1-year-old age group and 17.2, 31.4, 39.9, and 21.2 per 100,000 inhabitants in the 60-to-69-, 70-to-79-, 80-to-89-, and ≥90-year-old age groups, respectively. Moreover, the incidence rate was significantly higher in males than in females (10.0 versus 6.8 [incidence rate ratio {IRR}, 1.5; 95% confidence interval {CI}, 1.3 to 1.6]) (Fig. 1). In the 12-year perspective, decreasing age-specific incidence rates were observed in all of the age groups that were ≥50 years of age except in the group of those ≥80 years of age (Fig. 1). Whereas the overall incidence rate for females decreased over the three 4-year periods (P = 0.05), the incidence rate for males remained stable, with a significant increase in the group of those between 80 and 89 years of age (Fig. 1).
FIG 1.
Age- and gender-specific incidence rates per 100,000 population (pop.) in three 4-year intervals (2004 to 2015). The overall incidence rate for males was stable, whereas the overall incidence rate for females declined. Males had a slight decrease in the age group of 50 to 59 years (P = 0.04) and a significant increase in the age group of 80 to 89 years (P < 0.0001). Females had significant decreases in the age groups of 40 to 49 years and 60 to 69 years (and an increase only in the low-incidence age group of 20 to 29 years).
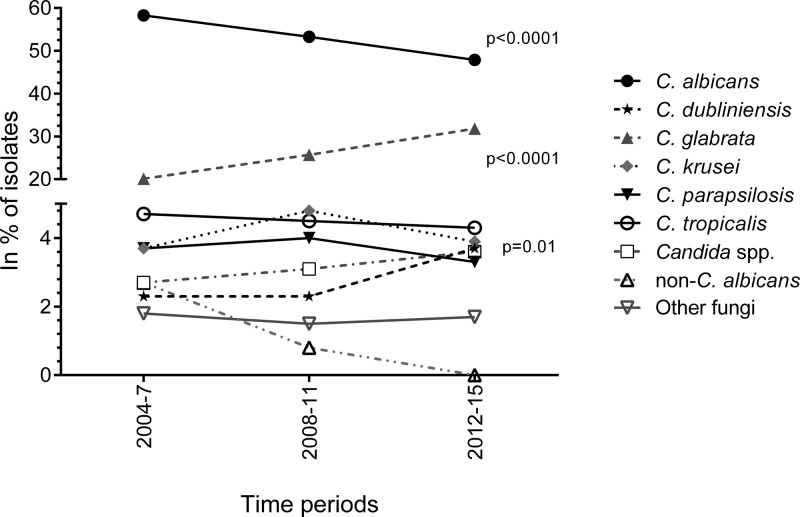
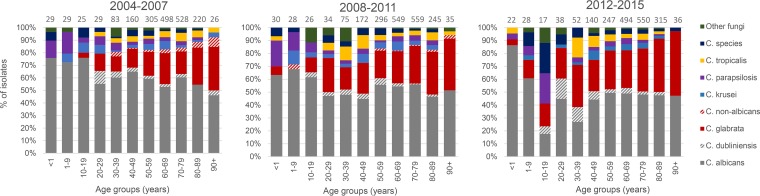
C. albicans accounted for the majority (47.9%) of the isolates in 2012 to 2015, followed by C. glabrata (31.8%). Among C. tropicalis, C. krusei, C. dubliniensis, and C. parapsilosis isolates, each species accounted for ≤4.3%. Sixty-nine (3.6%) of the isolates belonged to other Candida species and 32 (1.7%) to other fungal genera, among which 11 were mold isolates and another 11 Saccharomyces cerevisiae isolates (Table 1). Polyfungal episodes (n = 53) involved 5.6% of the isolates. Over the 12 years, the proportion of C. albicans decreased (P < 0.0001) whereas the proportion of C. glabrata increased (P < 0.0001) (Fig. 2). This development was observed despite conservatively assigning non-C. albicans as C. glabrata among the isolates from the period 2004 to 2009. The proportion of C. dubliniensis isolates increased significantly from 2.3% to 3.6% (P = 0.01), whereas no change was detected for other species (Fig. 2). The increase in the proportion of C. glabrata isolates was significant for age groups 1 to 9, 30 to 39, 50 to 59, and 70 to 79 years, again despite assigning those early isolates identified only as non-albicans to C. glabrata (P < 0.03 for all groups; Fig. 3).
FIG 2.
Species distribution (shown as percentages of isolates) over three 4-year periods during 2004 to 2015. The presented percentages are based on isolate numbers during the 4-year periods. Significant P values from a Chi-square test for trend are presented. (For C. dubliniensis, a P value of 0.01 was found due to an increase in isolates from 2014 to 2015.)
FIG 3.
Species distribution of bloodstream infections among age groups in 2012 to 2015.
The species distribution in 2012 to 2015 varied by age, by gender, and by center (see Table S2 and S3). Correlations between species and age, gender, BC system, and calendar year were investigated for all incident cases (with only the first episode included in the full 12-year period) in a univariate and multivariate logistic regression analysis (Table 3). A decrease in C. albicans infection episodes and an increase in C. glabrata and C. dubliniensis infection episodes over time were found. C. glabrata was positively associated with female sex, whereas the opposite was the case for C. tropicalis. C. albicans, C. parapsilosis, C. dubliniensis, and “other fungi” were associated with younger patients, whereas the odds of being infected with C. glabrata increased with age. Candidemia involving C. glabrata was positively associated with BacT/Alert, whereas the opposite was true for C. parapsilosis, C. krusei, and the isolates classified as “other fungi” (multivariate analysis). For the isolates classified as “other fungi,” by excluding the data from center 1-RH, this association disappeared.
TABLE 3.
Binomial logistic regression analysis of variables associated with changing species distribution in Denmark in 2004 to 2015a
| Species (no. of isolates) and parameter | Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Pb | Odds ratio | 95% CI | P | Pb | |
| C. albicans (2,967) | ||||||||
| Calendar yr | 0.94 | 0.93–0.96 | <0.001 | <0.001 | 0.95 | 0.94–0.97 | <0.001 | <0.001 |
| (Yr) | 0.92 | 0.89–0.94 | <0.001 | <0.001 | ||||
| Age (Yr) | 0.995 | 0.992–0.998 | <0.001 | 0.001 | 0.995 | 0.992–0.998 | 0.002 | 0.002 |
| C. glabrata (1,369) | ||||||||
| Calendar yr | 1.08 | 1.06–1.10 | <0.001 | <0.001 | 1.06 | 1.04–1.08 | <0.001 | <0.001 |
| (Yr) | 1.11 | 1.07–1.15 | <0.001 | <0.001 | ||||
| Blood culture system (Bactec) | 0.65 | 0.57–0.75 | <0.001 | <0.001 | 0.78 | 0.68–0.90 | <0.001 | 0.004 |
| Gender (Female) | 1.15 | 1.01–1.30 | 0.032 | 0.023 | 1.17 | 1.03–1.33 | 0.014 | 0.009 |
| Age (Yr) | 1.02 | 1.02–1.03 | <0.001 | <0.001 | 1.02 | 1.02–1.02 | <0.001 | <0.001 |
| C. tropicalis (237) | ||||||||
| Gender (Female) | 0.74 | 0.56–0.97 | 0.034 | 0.018 | 0.74 | 0.56–0.97 | 0.033 | 0.018 |
| C. parapsilosis (171) | ||||||||
| BC system (Bactec) | 1.87 | 1.38–2.54 | <0.001 | 0.005 | 1.70 | 1.24–2.34 | 0.001 | 0.045 |
| Age (Yr) | 0.98 | 0.98–0.99 | <0.001 | <0.001 | 0.98 | 0.98–0.99 | <0.001 | <0.001 |
| C. krusei (219) | ||||||||
| BC system (Bactec) | 2.00 | 1.52–2.62 | <0.001 | <0.001 | 2.07 | 1.56–2.74 | <0.001 | <0.001 |
| C. dubliniensis (149) | ||||||||
| Calendar yr (Yr) | 1.06 | 1.01–1.12 | 0.014 | 0.010 | 1.07 | 1.02–1.13 | 0.007 | 0.001 |
| Age (Yr) | 0.99 | 0.982–0.997 | 0.007 | 0.009 | 0.989 | 0.981–0.997 | 0.008 | 0.005 |
| Other fungi (83) | ||||||||
| BC system (Bactec) | 1.87 | 1.21–2.89 | 0.005 | 0.046 | 1.71 | 1.09–2.69 | 0.019 | 0.10 |
| Age (Yr) | 0.98 | 0.97–0.99 | <0.001 | <0.001 | 0.98 | 0.97–0.99 | <0.001 | 0.001 |
Only incident cases were included, and only significant findings are displayed. For the multivariate analysis, year, age, gender, and BC system were all included in the model. 95% CI, 95% confidence intervals. Due to interactions between calendar year data and BC system data, where “Calendar yr” data and “(Yr)” data appear in contiguous rows, the year factor data are split as follows: Calendar yr, BacT/Alert; (Yr), Bactec.
The indicated P values exclude the main tertiary hospital of Rigshospitalet (Centre 1-RH).
Susceptibility.
For the six most common Candida species, susceptibility patterns largely correlated with those predicted by the species identification (Table 4). However, acquired resistance in Candida was occasionally detected, and the results determined per drug class are detailed below.
TABLE 4.
Pattern of susceptibility to six antifungal compounds of Danish fungemia isolates collected in 2012 to 2015a
| Species and compound (no. of isolates) | No. of isolates with the given MIC (mg/liter) |
S |
R |
Non-wt |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.008 | 0.015 | 0.032 | 0.064 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | No. of isolates | % of isolates | No. of isolates | % of isolates | No. of isolates | % of isolates | |
| C. albicans (914) | |||||||||||||||||||||
| Amphotericin B | — | 4 | 67 | 155 | 356 | 331 | 1 | — | — | — | — | — | — | 914 | 100 | 0 | 0.0 | 0 | 0.0 | ||
| Anidulafungin | — | 874 | 40 | — | — | — | — | — | — | 914 | 100 | 0 | 0.0 | 0 | 0.0 | ||||||
| Micafungin | 880 | 29 | 5 | — | — | — | — | — | — | 909 | 99.5 | 5 | 0.5 | 5 | 0.5 | ||||||
| Fluconazole | — | — | — | — | 520 | 349 | 34 | 5 | 2 | 1 | 3 | — | — | 910 | 99.6 | 4 | 0.4 | 6 | 0.7 | ||
| Voriconazole | — | — | 909 | 1 | 1 | 1 | 2 | — | — | — | — | 911 | 99.7 | 3 | 0.3 | 3 | 0.3 | ||||
| Isavuconazole | — | — | 911 | 1 | 2 | — | — | — | — | ND | ND | ND | ND | 3 | 0.3 | ||||||
| C. dubliniensis (71) | |||||||||||||||||||||
| Amphotericin B | — | 16 | 18 | 15 | 20 | 2 | — | — | — | — | — | — | 71 | 100 | 0 | 0.0 | 0 | 0.0 | |||
| Anidulafungin | — | 43 | 25 | 3 | — | — | — | — | — | — | 68 | 95.8 | 3 | 4.2 | 3 | 4.2 | |||||
| Micafungin | 43 | 24 | 4 | — | — | — | — | — | — | 67 | 94.4 | 4 | 5.6 | 4 | 5.6 | ||||||
| Fluconazole | — | — | — | — | 28 | 19 | 13 | 7 | 1 | 2 | 1 | — | — | 68 | 95.8 | 3 | 4.2 | 4 | 5.6 | ||
| Voriconazole | — | — | 68 | 1 | 1 | 1 | — | — | — | — | 69 | 97.2 | 2 | 2.8 | 2 | 2.8 | |||||
| Isavuconazole | — | — | 70 | 1 | — | — | — | — | ND | ND | ND | ND | 1 | 1.4 | |||||||
| C. glabrata (603) | |||||||||||||||||||||
| Amphotericin B | — | 1 | 5 | 13 | 86 | 128 | 218 | 143 | 9 | — | — | — | — | — | — | 594 | 98.5 | 9 | 1.5 | 9 | 1.5 |
| Anidulafungin | — | 129 | 200 | 258 | 6 | 5 | 1 | 2 | 2 | — | — | — | — | — | — | 587 | 97.3 | 16 | 2.7 | 16 | 2.7 |
| Micafungin | 434 | 132 | 25 | 3 | 2 | 1 | 3 | 1 | 2 | — | — | — | — | — | — | 591 | 98.0 | 12 | 2.0 | 12 | 2.0 |
| Fluconazole | — | — | — | — | 1 | 1 | 22 | 164 | 278 | 53 | 15 | 14 | 38 | 17 | 0 | 0.0 | 55 | 9.1 | 55 | 9.1 | |
| Voriconazole | — | — | 108 | 248 | 157 | 13 | 10 | 18 | 31 | 17 | 1 | — | — | — | — | IE | IE | IE | IE | 49 | 8.1 |
| Isavuconazole | — | — | 426 | 78 | 20 | 6 | 17 | 24 | 17 | 15 | 0 | — | — | — | — | ND | ND | ND | ND | ND | ND |
| C. krusei (73) | |||||||||||||||||||||
| Amphotericin B | — | 1 | 4 | 30 | 32 | 6 | — | — | — | — | — | — | 67 | 91.8 | 6 | 8.2 | 6 | 8.2 | |||
| Anidulafungin | — | 11 | 16 | 41 | 4 | 1 | — | — | — | — | — | — | 68 | 93.2 | 5 | 6.8 | 5 | 6.8 | |||
| Micafungin | 15 | 46 | 11 | 1 | — | — | — | — | — | — | IE | IE | IE | E | 1 | 1.4 | |||||
| Fluconazole | — | — | — | — | 1 | 17 | 55 | — | — | 0 | 0.0 | 73 | 100 | ND | ND | ||||||
| Voriconazole | — | — | 16 | 40 | 15 | 1 | 1 | — | — | — | — | IE | IE | IE | IE | 1 | 1.4 | ||||
| Isavuconazole | — | — | 21 | 19 | 24 | 7 | 2 | — | — | — | — | ND | ND | ND | ND | ND | ND | ||||
| C. parapsilosis (62) | |||||||||||||||||||||
| Amphotericin B | — | 6 | 34 | 20 | 2 | — | — | — | — | — | — | 62 | 100.0 | 0 | 0.0 | 0 | 0.0 | ||||
| Anidulafungin | — | 2 | 7 | 37 | 16 | — | — | — | — | — | — | 0 | 0.0 | ND | ND | ND | ND | ||||
| Micafungin | 1 | 13 | 35 | 13 | — | — | — | — | — | — | 0 | 0.0 | ND | ND | ND | ND | |||||
| Fluconazole | — | — | — | — | 5 | 31 | 16 | 3 | 3 | 1 | 1 | 2 | — | — | 55 | 88.7 | 4 | 6.5 | 7 | 11.3 | |
| Voriconazole | — | — | 53 | 5 | 2 | 1 | 1 | — | — | — | — | 60 | 96.8 | 2 | 3.2 | 2 | 3.2 | ||||
| Isavuconazole | — | — | 61 | 1 | — | — | — | — | ND | ND | ND | ND | 1 | 1.6 | |||||||
| C. tropicalis (81) | |||||||||||||||||||||
| Amphotericin B | — | 26 | 41 | 14 | — | — | — | — | — | — | 81 | 100 | 0 | 0.0 | 0 | 0.0 | |||||
| Anidulafungin | — | 33 | 33 | 13 | 1 | 1 | — | — | — | — | — | — | 79 | 97.5 | 2 | 2.5 | 2 | 2.5 | |||
| Micafungin | 23 | 28 | 24 | 5 | 1 | — | — | — | — | — | — | IE | IE | IE | IE | 1 | 1.2 | ||||
| Fluconazole | — | — | — | — | 10 | 22 | 26 | 15 | 1 | 2 | 1 | 2 | 2 | — | — | 74 | 91.4 | 5 | 6.2 | 7 | 8.6 |
| Voriconazole | — | — | 66 | 4 | 4 | 3 | 1 | 2 | 1 | — | — | — | — | 74 | 91.4 | 7 | 8.6 | 7 | 8.6 | ||
| Isavuconazole | — | — | 76 | 2 | 2 | 1 | — | — | — | — | ND | ND | ND | ND | 5 | 6.2 | |||||
| Candida spp. (65) | |||||||||||||||||||||
| Amphotericin B | — | 6 | 12 | 19 | 19 | 6 | 3 | — | — | — | — | — | — | 62 | 95.4 | 3 | 4.6 | ND | ND | ||
| Anidulafungin | — | 12 | 9 | 17 | 8 | 2 | 7 | 7 | 3 | — | — | — | — | — | — | ND | ND | ND | ND | ND | ND |
| Micafungin | 2 | 6 | 12 | 23 | 4 | 3 | 9 | 3 | 3 | — | — | — | — | — | — | ND | ND | ND | ND | ND | ND |
| Fluconazole | — | 4 | 11 | 16 | 3 | 6 | 8 | 3 | 6 | 8 | — | — | 40 | 61.5 | 17 | 26.2 | ND | ND | |||
| Voriconazole | — | 35 | 6 | 14 | 3 | 5 | 1 | 1 | — | — | — | — | ND | ND | ND | ND | ND | ND | |||
| Isavuconazole | — | 46 | 6 | 6 | 2 | 1 | 3 | 1 | — | — | — | — | ND | ND | ND | ND | ND | ND | |||
| Other fungi (31)b | |||||||||||||||||||||
| Amphotericin B | — | 2 | 4 | 5 | 7 | 7 | 6 | — | — | — | — | — | — | 25 | 80.6 | 2 | 6.5 | ND | ND | ||
| Anidulafungin | — | 4 | 5 | 2 | 0 | 0 | 18 | — | — | — | — | — | — | ND | ND | ND | ND | ND | ND | ||
| Micafungin | 1 | 1 | 7 | 2 | 0 | 0 | 18 | — | — | — | — | — | — | ND | ND | ND | ND | ND | ND | ||
| Fluconazole | — | — | — | — | 7 | 4 | 5 | 7 | — | — | 0 | 0.0 | 16 | 69.6 | ND | ND | |||||
| Voriconazole | — | — | 4 | 8 | 5 | 2 | 1 | 2 | 7 | 2 | — | — | — | — | ND | ND | ND | ND | ND | ND | |
| Isavuconazole | — | — | 3 | 7 | 4 | 1 | 3 | 3 | 1 | 1 | 8 | — | — | — | — | ND | ND | ND | ND | ND | ND |
| Overall (1,900)c | |||||||||||||||||||||
| Amphotericin B | — | 17 | 27 | 103 | 284 | 574 | 666 | 205 | 24 | — | — | — | — | — | — | 1,876 | 98.7 | 24 | 1.3 | ND | ND |
| Anidulafungin | — | 1,102 | 323 | 336 | 24 | 11 | 16 | 46 | 40 | — | — | — | — | — | — | ND | ND | ND | ND | ND | ND |
| Micafungin | 1,382 | 219 | 71 | 47 | 59 | 18 | 25 | 39 | 38 | — | — | — | — | — | — | ND | ND | ND | ND | ND | ND |
| Fluconazole | — | — | — | — | 562 | 407 | 121 | 68 | 177 | 298 | 64 | 48 | 147 | — | — | 1,147 | 60.6 | 177 | 9.4 | ND | ND |
| Voriconazole | — | — | 1,239 | 269 | 202 | 66 | 33 | 23 | 35 | 26 | 7 | — | — | — | — | ND | ND | ND | ND | ND | ND |
| Isavuconazole | — | — | 1,614 | 111 | 58 | 16 | 23 | 30 | 18 | 18 | 12 | — | — | — | — | ND | ND | ND | ND | ND | ND |
The EUCAST breakpoints and ECOFFs (see Table S1) were used for the classifications as follows: S, susceptible; R, resistant; Non-wt, non-wild type (as the MIC was above the ECOFF). Empty cells indicate that there were no isolates for which the MIC had the indicated value. —, the indicated concentration of that particular drug was not tested. Bold numbers indicate isolates for which the MIC was above the ECOFF, and numbers that are bold and underlined indicate isolates that were resistant. For Candida species and “Other fungi,” an amphotericin B breakpoint of 1 mg/liter was used. For “Other fungi,” a fluconazole breakpoint of 2 mg/liter was used. Those two cutoff values represent only an indication of the susceptibility profile for the given species/group of isolates (and a conservative estimate of the proportion of cases that are likely good targets for the compound in question). “IE” indicates there is insufficient evidence to suggest that the species is a good target for therapy with the drug; no susceptibility classification is offered. ND, not determined.
For the echinocandins and fluconazole, 29 and 23 isolates were tested, respectively.
For the echinocandins and fluconazole, 1,898 and 1,892 isolates were tested, respectively.
Azoles.
Overall, significantly fewer of the isolates were fluconazole susceptible in 2012 to 2015 (1,147/1,892; 60.6%) than in 2008 to 2011 (1,137/1,745; 65.2%) and 2004 to 2007 (972/1,420; 68.5%) (P < 0.0001 [trend test]). Among the C. albicans, C. dubliniensis, C. parapsilosis, and C. tropicalis isolates, 2.1% (24/1,128) were non-wild type (non-wt) for fluconazole and 1.4% (16/1,128) were resistant (0.4% C. albicans, 4.2% C. dubliniensis, 6.5% C. parapsilosis, and 6.2% C. tropicalis) (Table 4). Two of four fluconazole-resistant C. parapsilosis isolates were voriconazole resistant. Six of seven fluconazole-nonsusceptible C. tropicalis isolates had a trailing phenotype, making MIC determination difficult due to 50% growth inhibition over a broad range of MIC values (which was also the case for voriconazole and isavuconazole). Taking the results together, 14 (1.2%) isolates of the four species were resistant/non-wt for voriconazole and 10 (0.9%) were non-wt for isavuconazole, and none were fluconazole susceptible.
For C. glabrata, a bimodal fluconazole MIC distribution was observed, with peaks at MIC values of 4 mg/liter and 64 mg/liter; 9.1% were resistant (MIC, >32 mg/liter). This proportion declined from the years 2012 to 2013 to the years 2014 to 2015 (11.4% versus 6.6%, P = 0.04). Finally, 8.1% of C. glabrata isolates were also non-wt for voriconazole and 1.4% of C. krusei isolates were non-wt for voriconazole.
Echinocandins.
Acquired echinocandin resistance increased compared to the previous years: 29/1,754 (1.7%) of the isolates were resistant compared to 10/1,581 (0.6%) and 0/1,294 (0%) in the years 2008 to 2011 and the years 2004 to 2007, respectively (P < 0.001).
The 29 Candida isolates displaying acquired anidulafungin resistance in 2012 to 2015 consisted of 4.2% C. dubliniensis, 2.7% C. glabrata, 6.8% C. krusei, 2.5% C. tropicalis, and 23% (3/13) C. kefyr isolates (Table 4). FKS sequencing detected hot spot alterations in 13/16 C. glabrata, 1/5 C. krusei (with the remaining 4 having L701M outside the hot spot which has not been found uniformly associated with echinocandin resistance in our laboratory), 0/3 C. dubliniensis, 2/2 C. tropicalis, and 1/3 C. kefyr isolates. Five of the 16 (31%) C. glabrata isolates were fluconazole cross resistant and thus multidrug resistant. For data on less-common species, see Text S1 in the supplemental material.
Amphotericin B.
Acquired amphotericin B resistance was found in 1.3% of fungemia isolates and 1.0% (18/1,851) of Candida isolates in 2012 to 2015 (Table 4); those isolates included 1.5% C. glabrata isolates, 8.2% C. krusei isolates, two C. nivariensis isolates, and one C. norvegensis isolate. In all instances, the MIC was 2 mg/liter and thus 1 dilution step above the breakpoint.
Antifungal consumption.
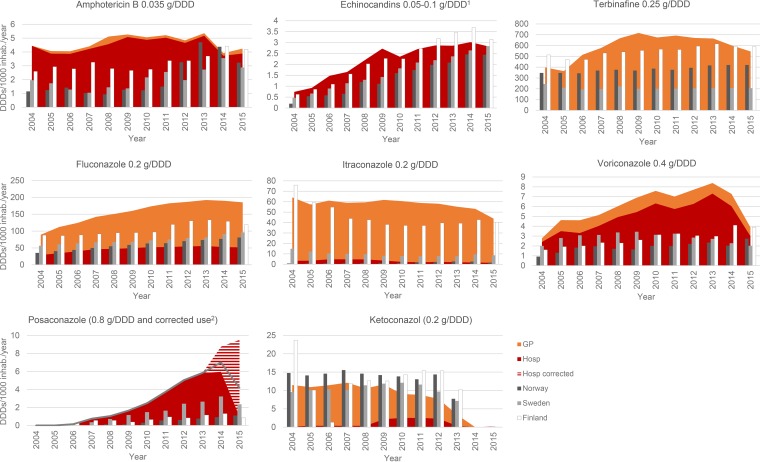
The antifungal consumption in Denmark increased over the first 10 years of observation but stabilized or decreased during the last 2 to 3 years (except for posaconazole) (17, 18) (Fig. 4). Most of the fluconazole, ketoconazole, itraconazole, and terbinafine used (69.9%, 87.9%, 94.7%, and 99.8%, respectively) was used in the primary health care sector in the years 2004 to 2015.
FIG 4.
Annual consumption of systemic antifungal compounds in DDDs/1,000 inhabitants (inhab.) in 2004 to 2015. Hospital (Hosp) use data are shown in red, and data corresponding to use in the primary health care sector data are shown in orange. Data corresponding to the total annual consumption in Norway, Sweden, and Finland are inserted for comparison (dark gray, light gray, and white bars, respectively). DDD1, The DDD was 50 mg for caspofungin, whereas the DDD was 0.1 g for micafungin and anidulafungin. corrected use2, for posaconazole, an enterotablet and an i.v. formulation were introduced on the Danish market in 2014. The licensed daily dose of these new formulations is 300 mg/day (after a loading dose), whereas the treatment dose of the suspension is 800 mg/day. The official defined DDD was 0.8 g for all three formulations (recently [in 2017] changed to 0.3 g). We have translated DDDs to reflect the actual use (for enterotablets and the i.v. formulation, 1 DDD of 0.3 g [red/white stripes]; for oral suspensions, 1 DDD of 0.8 g [solid red]). The total value for use in the Nordic countries and DK (uncorrected; gray line) is a DDD of 0.8 g.
Denmark had a higher consumption of systemic antifungal drugs per 1,000 inhabitants than other Nordic countries (for 2015, a total of 790 DDDs [DK] versus 512, 321, and 762 DDDs [Norway, Sweden, and Finland]) and, in particular, higher consumption of the azoles (with an increase of 44% to 174%; 237 DDDs [DK] versus 87, 109, and 164 DDDs [Norway, Sweden, and Finland, respectively]) despite a continued annual increase in fluconazole consumption until 2012 (Finland) or 2015 (Norway and Sweden). Caspofungin was the main echinocandin used. Anidulafungin was introduced in 2009 and accounted for 6% to 13% of the echinocandin use until 2013 and 25% in 2015.
DISCUSSION
The Danish fungemia incidence rate declined slightly in 2012 to 2015 after an increase in the preceding 8 years. Whether this result represents merely annual variations or an actual declining incidence is not yet known. In the other Nordic countries and Scotland, incidence rates had been increasing during the 1990s and the early 2000s but for most part appear now to have stabilized around a lower level than that seen in Denmark (7–11, 13–16, 41) (see Fig. S1 in the supplemental material). Outliers include Australia, with a low incidence rate but a modest increase in numbers of cases of infection, and metropolitan Spain, with a notable increase to 8.1/100,000 partly driven by a doubling of the rate incidence among children <1 year old (3, 12). A study of U.S. community hospital discharge records of invasive candidiasis among >1-month-old children also demonstrated a minor decrease from 2005 to 2012 for both genders (42). This finding was corroborated by a population-based study from two metropolitan areas in the United States, where their unprecedentedly high incidence rates declined from 2008 to 2013 (for Atlanta, 14.1/100,000 to 9.5/100,000; for Baltimore, 30.9/100,000 to 14.4/100,000). This change was found in almost all age groups but was limited to patients with central venous catheters (85%) and was hypothesized to be related to the introduction of an infection control bundle focusing on i.v. catheter management (43).
A recent study has examined the observed differences in incidence rates in the years 2010 to 2011 between the Nordic countries. Denmark had a higher prevalence of malignant hematological disease than the other Nordic countries, but no demographic differences could justify the higher rate. Despite similar overall rates of antibacterial consumption (DDDs/1,000 inhabitants/day), the levels of use of penicillin, piperacillin-tazobactam, metronidazole, carbapenem, and colistin were significantly higher in Denmark (16). Metronidazole and broad-spectrum beta-lactams are associated with a profound impact on gastrointestinal (GI) flora, thereby potentially selecting for yeast (44, 45). Moreover, a higher use of broad-spectrum antibiotics and the increasing utilization of BC reported in Denmark (particularly in the ≥65-year-old population) may be markers of Danish patients being more severely ill or of the introduction of sepsis packages (including timely diagnostics) (46).
Despite the high overall incidence rate, a more diverse picture was observed among children and the elderly. The incidence rate (10.8/100,000) has remained constant in the <1-year-old population in the 12-year perspective and was comparable to rates reported from Norway, Finland, and England and Wales (6.6/100,000 to 11/100,000) (8, 15, 47) but was low in a global perspective (20 to >90/100,000) (3, 6, 14, 48, 49). Such differences suggest that socioeconomic factors and infection control practices may play an important role, as also suggested in the study of isolates from Atlanta and Baltimore (43). In contrast, the incidence rate in the elderly population was higher than in any population-based study in Caucasians (3, 6, 8, 14, 15, 48, 49) and was exceeded only by the rate seen with the isolates from the mixed populations of Atlanta and Baltimore (43). The high Danish rates in the elderly were mainly driven by a significantly higher and increasing rate in males aged 80 to 89 years. The reason for this is unclear. Colon cancer and hematological malignancies are recognized risk factors for candidemia. Both malignancies are more common in males and increase in incidence with advancing age, and the total prevalences of gastrointestinal tract cancers (including pancreas cancer and liver cancer) as well as of large groups of hematological diseases (leukemia and non-Hodgkin lymphoma) increased more in males during the decade of 2004 to 2014 (http://www.ancr.nu).
The previously observed species shift toward infections by non-albicans species and particularly toward C. glabrata infections continued over the years 2012 to 2015. This trend has been observed in several population-based candidemia surveys (3, 12, 13, 20, 50). The increase in the proportion of C. glabrata infections has happened concomitantly with an increase in azole use in Denmark and with the population getting older. Sweden, Norway, Finland, and Iceland, although they share demographic characteristics, have not witnessed the same increase in the proportion of C. glabrata infections during the last decade of surveillance. Despite an increase in systemic azole use in all four countries, the overall use was substantially lower in the other Nordic countries (Fig. 4) (14). In 2015, the overall systemic azole consumption in Norway was comparable to the use of fluconazole alone in Denmark in 2004. In contrast, the use of topical azoles for vaginitis was twice as high in Norway as in Denmark (an average of 375 versus 213 DDD/1,000 inhabitants/year). We speculate whether the increasing selection pressure mediated by systemic azoles over the 12-year period facilitated the increase in the proportion of C. glabrata infections in the Danish setting.
The age-dependent species distribution of C. parapsilosis and C. glabrata and the influence of blood culture systems on the detection of C. glabrata were confirmed in a multivariate analysis (15, 17, 18, 51). Although the gradual change from Bactec to BacT/Alert may have contributed to the increase in the C. glabrata data, no centers have changed to BacT/Alert since January 2011. Less importance has been placed on the impact of gender in relation to C. glabrata. The presence of C. glabrata was correlated with females and was especially common in those above the age of 40 years, an observation also made in our previous study (18) but not to our knowledge reported elsewhere. One reason for this could be the gender inequality in antifungal consumption in the primary health care sector. Prior fluconazole use has been shown to be associated with the emergence of C. glabrata (18, 22, 52, 53). Fluconazole is the main azole used in Denmark, and the majority is administered in the primary care sector. In this setting (years 2012 to 2015), two-thirds of the fluconazole sold was prescribed for the age group of 20 to 65 years, with a 4.8 female/male ratio of DDDs/1,000 inhabitants/day. Genotyping studies have confirmed that the infecting organism derives from the colonizing flora (54). We therefore hypothesize that the considerable use of fluconazole in adult females in the primary health care sector may play a role in the overrepresentation of C. glabrata in adult females. Consequently, the recommended use of topical azoles rather than systemic treatment whenever possible has been reinforced in the 2012 national guidelines (55). We did not see an increase in the incidence of C. krusei infections. Although this species is inherently resistant to fluconazole and is potentially selected for by azole treatment, it is also less pathogenic (56). No nationwide study has, to our knowledge, reported an increase in C. krusei proportions, reflecting the knowledge that infections occur primarily in a well-defined subset of patients, most of whom are already recipients of prophylaxis.
Fluconazole nonsusceptibility was detected in more than one-third of the isolates, mainly driven by an increase in the incidence of C. glabrata infections. Of note, 9.1% of C. glabrata isolates were fluconazole and voriconazole cross resistant and unlikely to respond to even high dosages of azoles. The rate of nonsusceptibility to fluconazole in C. tropicalis (8.6%) was primarily a consequence of heavy trailing growth and affected all azoles equally, impeding precise and reproducible MIC determinations. Less than 50% trailing is commonly observed with C. tropicalis and was not associated with differential levels of clinical efficacy of fluconazole in 21 C. tropicalis cases included in a multivariate analysis based on the CANDIPOP study. The overall mortality rate for these patients was less than 10%, suggesting that the majority were not severely ill (3, 57, 58). Therefore, it still remains to be elucidated if isolates displaying heavy trailing are indeed good targets for fluconazole treatment, particularly in the setting of severe disease.
Acquired echinocandin resistance remained low but was found to have increased. All isolates with an MIC that was elevated ≥2 dilution steps above the breakpoint had an FKS hot spot mutation, whereas this was the case in only 4/16 isolates with an MIC that was 1 dilution step above the breakpoint. The majority of the isolates with hot spot mutations were C. glabrata, and 31% were fluconazole cross resistant, as reported elsewhere (31, 59, 60). Studies reported from the United States demonstrated increasing echinocandin resistance rates in C. glabrata (60, 61). In this context, it is worrying that we are now seeing emerging resistance in 2.7% of our C. glabrata isolates, particularly when no isolates were found in 2004 to 2007 and only 1.4% of the isolates during 2008 to 2012. Furthermore, we are now seeing 2.5% of isolates with confirmed resistance in C. tropicalis and FKS mutations in C. krusei and C. kefyr. Echinocandin resistance has been associated with prior therapy and, in C. glabrata, particularly with the presence of mutations in the DNA mismatch repair gene MSH2 (62). The emergence of echinocandin resistance in Denmark follows a significant increase in echinocandin consumption from 2004 to 2015. These observations suggest that longer-term administration of echinocandin, including its empirical use, should be minimized if possible (63, 64).
In contrast to the increasing resistance observed for azoles and echinocandins, 98% of all isolates were amphotericin B susceptible, which is in agreement with previous reports showing broad activity and no indication of acquired resistance (18).
In conclusion, Denmark remains a high-incidence country for fungemia where less than two-thirds of the isolates are now fully fluconazole susceptible and where acquired echinocandin resistance is on the increase. Continued epidemiological surveillance is important, and efforts should be directed toward improving diagnostics and lowering the antifungal selection pressure, including regulation of fluconazole use in the primary health care sector.
Supplementary Material
ACKNOWLEDGMENTS
We thank Birgit Brandt for excellent laboratory assistance.
We thank Gilead, Basilea, and Astellas for unrestricted research grants supporting the national fungemia surveillance.
We have no specific conflicts of interest related to this paper but declare the following: K.M.T.A. has received travel grants from Pfizer and Gilead; H.K.J. has been supported as a clinical research stipend by The Novo Nordisk Foundation but declares to have no competing interests; L.L. has received travel grants from MSD and Pfizer; L.N. has received travel grants from Roche, Astellas, and MSD; R.K.H. has received research grant from Gilead and travel grants from Astellas, MSD, and Pfizer; M.C.A. has received research grants or speaker honoraria from Amplyx, Astellas, Basilea, Cidara, F2G, Gilead, MSD, Novartis, Pfizer, and T2Biosystems. M.C.A. is the current chairman for EUCAST-AFST and has served before this on advisory boards for MSD (until 2014) and Pfizer (until 2012).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01564-17.
For a commentary on this article, see https://doi.org/10.1128/JCM.01907-17.
REFERENCES
- 1.Lausch KR, Søgard M, Rosenvinge FS, Johansen HK, Boysen T, Røder BL, Mortensen KL, Nielsen L, Lemming LE, Olesen B, Leitz C, Kristensen L, Dzajic E, Østergård L, Schønheyder HC, Arendrup MC. 2017. Behind candidaemia: risk factors and outcome in a high-incidence nationwide setting, abstr OS0587 Abstr 27th ECCMID, 22 to 25 April 2017, Vienna, Austria. [Google Scholar]
- 2.Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, Fontanet A, Bretagne S, Dromer F. 2014. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med 40:1303–1312. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puig-Asensio M, Padilla B, Garnacho-Montero J, Zaragoza O, Aguado JM, Zaragoza R, Montejo M, Muñoz P, Ruiz-Camps I, Cuenca-Estrella M, Almirante B. 2014. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect 20:O245–O254. doi: 10.1111/1469-0691.12380. [DOI] [PubMed] [Google Scholar]
- 4.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CY, Huang LJ, Wang WS, Chen TL, Yen CC, Yang MH, Hsiao LT, Liu CY, Chen PM, Chiou TJ. 2009. Candidemia in cancer patients: impact of early removal of non-tunneled central venous catheters on outcome. J Infect 58:154–160. doi: 10.1016/j.jinf.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Slavin M, Nguyen Q, Marriott D, Playford EG, Ellis D, Sorrell T. 2006. Active surveillance of candidemia, Australia. Emerg Infect Dis 12:1508–1516. doi: 10.3201/eid1210.060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odds FC, Hanson MF, Davidson AD, Jacobsen MD, Wright P, Whyte JA, Gow NAR, Jones BL. 2007. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol 56:1066–1075. doi: 10.1099/jmm.0.47239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poikonen E, Lyytikäinen O, Anttila V-J, Koivula I, Lumio J, Kotilainen P, Syrjälä H, Ruutu P. 2010. Secular trend in candidemia and the use of fluconazole in Finland, 2004–2007. BMC Infect Dis 10:312. doi: 10.1186/1471-2334-10-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asmundsdóttir LR, Erlendsdóttir H, Gottfredsson M. 2002. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J Clin Microbiol 40:3489–3492. doi: 10.1128/JCM.40.9.3489-3492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandven P, Bevanger L, Digranes A, Haukland HH, Mannsaker T, Gaustad P. 2006. Candidemia in Norway (1991 to 2003): results from a nationwide study. J Clin Microbiol 44:1977–1981. doi: 10.1128/JCM.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ericsson J, Chryssanthou E, Klingspor L, Johansson AG, Ljungman P, Svensson E, Sjölin J. 2013. Candidaemia in Sweden: a nationwide prospective observational survey. Clin Microbiol Infect 19:E218–E221. doi: 10.1111/1469-0691.12111. [DOI] [PubMed] [Google Scholar]
- 12.Chapman B, Slavin M, Marriott D, Halliday C, Kidd S, Arthur I, Bak N, Heath CH, Kennedy K, Morrissey CO, Sorrell TC, van Hal S, Keighley C, Goeman E, Underwood N, Hajkowicz K, Hofmeyr A, Leung M, Macesic N, Botes J, Blyth C, Cooley L, George CR, Kalukottege P, Kesson A, McMullan B, Baird R, Robson J, Korman TM, Pendle S, Weeks K, Liu E, Cheong E, Chen S. 2017. Changing epidemiology of candidaemia in Australia. J Antimicrob Chemother 72:1270–1270. doi: 10.1093/jac/dkx047. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson EM, Hanson MF, Williams C, Munro CA, Jones BJ, Ramage G. 2016. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection—Scotland, 2012–2013. Clin Microbiol Infect 22:87–93. doi: 10.1016/j.cmi.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asmundsdottir LR, Erlendsdottir H, Gottfredsson M. 2013. Nationwide study of candidemia, antifungal use, and antifungal drug resistance in Iceland, 2000 to 2011. J Clin Microbiol 51:841–848. doi: 10.1128/JCM.02566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesstvedt L, Gaustad P, Andersen CT, Haarr E, Hannula R, Haukland HH, Hermansen N-O, Larssen KW, Mylvaganam H, Ranheim TE, Sandven P, Nordøy I, Kanestrøm A, Grub C, Onken A, Thielsen C, Skaare D, Tofteland S, Sønsteby L-J, Hjetland R, Hide R, Vik E, Kümmel A, Åsheim S. 2015. Twenty-two years of candidaemia surveillance: results from a Norwegian national study. Clin Microbiol Infect 21:938–945. doi: 10.1016/j.cmi.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Hesstvedt L, Arendrup MC, Poikonen E, Klingpor L, Friman V, Nordøy I. 2017. Differences in epidemiology of candidaemia in the Nordic countries - what is to blame? Mycoses 60:11–19. doi: 10.1111/myc.12535. [DOI] [PubMed] [Google Scholar]
- 17.Arendrup MC, Bruun B, Christensen JJ, Fuursted K, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Moller J, Nielsen L, Rosenvinge FS, Roder B, Schonheyder HC, Thomsen MK, Truberg K. 2011. National surveillance of fungemia in Denmark (2004 to (2009). J Clin Microbiol 49:325–334. doi: 10.1128/JCM.01811-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendrup MC, Dzajic E, Jensen RH, Johansen HK, Kjaldgaard P, Knudsen JD, Kristensen L, Leitz C, Lemming LE, Nielsen L, Olesen B, Rosenvinge FS, Røder BL, Schønheyder HC. 2013. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect 19:e343–e353. doi: 10.1111/1469-0691.12212. [DOI] [PubMed] [Google Scholar]
- 19.Guinea J. 2014. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20(Suppl 6):5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Z-L, Lin C-C, Chu W-L, Yang Y-L, Lo H-J. 2016. The distribution and drug susceptibilities of clinical Candida species in TSARY 2014. Diagn Microbiol Infect Dis 86:399–404. doi: 10.1016/j.diagmicrobio.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. 2012. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 73:45–48. doi: 10.1016/j.diagmicrobio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F. 2011. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother 55:532–538. doi: 10.1128/AAC.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Herbrecht R, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Verweij PE, Viscoli C, Ullmann AJ. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 18:38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 24.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18:19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 25.Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Donnelly JP, Garbino J, Groll AH, Hope WW, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Cuenca-Estrella M. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 18:53–67. doi: 10.1111/1469-0691.12041. [DOI] [PubMed] [Google Scholar]
- 26.Lortholary O, Petrikkos G, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Groll AH, Herbrecht R, Hope WW, Jensen HE, Kullberg BJ, Lass-Flörl C, Meersseman W, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infect 18:68–77. doi: 10.1111/1469-0691.12042. [DOI] [PubMed] [Google Scholar]
- 27.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg B-J, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D, Grenouillet F, Cassaing S, Baixench M-T, Bretagne S, Dromer F, Lortholary O. 2012. Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg Infect Dis 18:86–90. doi: 10.3201/eid1801.110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staab JF, Neofytos D, Rhee P, Jiménez-Ortigosa C, Zhang SX, Perlin DS, Marr KA. 2014. Target enzyme mutations confer differential echinocandin susceptibilities in Candida kefyr. Antimicrob Agents Chemother 58:5421–5427. doi: 10.1128/AAC.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen RH, Johansen HK, Arendrup MC. 2013. Stepwise development of a homozygous S80P substitution in Fks1p, conferring echinocandin resistance in Candida tropicalis. Antimicrob Agents Chemother 57:614–617. doi: 10.1128/AAC.01193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. 2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 50:1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arendrup MC, Fuursted K, Gahrn-Hansen B, Jensen IM, Knudsen JD, Lundgren B, Schønheyder HC, Tvede M. 2005. Seminational surveillance of fungemia in Denmark: notably high rates of fungemia and numbers of isolates with reduced azole susceptibility. J Clin Microbiol 43:4434–4440. doi: 10.1128/JCM.43.9.4434-4440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arendrup MC, Guinea J, Cuenca-Estrella M, Meletiadis J, Mouton JW, Lagrou K, Howard SJ, and the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST)*. 2015. EUCAST definitive document E.DEF 7.3. Method for the determination of broth dilution minimum Inhibitory concentrations of antifungal agents for yeasts. www.eucast.org.
- 34.Arendrup MC, Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, Howard SJ, Arendrup MC, Meletiadis J, Howard SJ, Mouton J, Guinea J, Lagrou K, Arikan-Akdagli S, Barchiesi F, Hamal P, Järv H, Lass-Flörl C, Mares M, Matos T, Muehlethaler K, Rogers TR, Torp Andersen C, Verweij P. 2016. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect 22:571.e1–571.e4. doi: 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Lass-Flörl C, Arendrup MC, Rodriguez-Tudela J-L, Cuenca-Estrella M, Donnelly P, Hope W. 2011. EUCAST technical note on amphotericin B. Clin Microbiol Infect 17:E27–E29. doi: 10.1111/j.1469-0691.2011.03644.x. [DOI] [PubMed] [Google Scholar]
- 36.Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST)*. 2008. EUCAST technical note on voriconazole. Clin Microbiol Infect 14:985–987. doi: 10.1111/j.1469-0691.2008.02087.x. [DOI] [PubMed] [Google Scholar]
- 37.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW; European Committee on Antimicrobial Susceptibility Testing - Subcommittee on Antifungal Susceptibility Testing (EUCAST-AFST). 2014. EUCAST technical note on Candida and micafungin, anidulafungin and fluconazole. Mycoses 57:377–379. [DOI] [PubMed] [Google Scholar]
- 38.Howard SJ, Lass-Florl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. 2013. Determination of isavuconazole susceptibility of aspergillus and Candida species by the EUCAST method. Antimicrob Agents Chemother 57:5426–5431. doi: 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Donnell K, Sutton DA, Rinaldi MG, Sarver BA, Balajee SA, Schroers HJ, Summerbell RC, Robert VA, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee YH, Kang S, Park B, Geiser DM. 2010. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol 48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arendrup MC, Garcia-Effron G, Lass-Florl C, Lopez AG, Rodriguez-Tudela J-L, Cuenca-Estrella M, Perlin DS. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and IsoSensitest media. Antimicrob Agents Chemother 54:426–439. doi: 10.1128/AAC.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poikonen E, Lyytikäinen O, Anttila V-J, Ruutu P. 2003. Candidemia in Finland, 1995–1999. Emerg Infect Dis 9:985–990. doi: 10.3201/eid0908.030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strollo S, Lionakis MS, Adjemian J, Steiner CA, Prevots DR. 2016. Epidemiology of hospitalizations associated with invasive candidiasis, United States, 2002–2012. Emerg Infect Dis 23:7–13. doi: 10.3201/eid2301.161198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cleveland AA, Harrison LH, Farley MM, Hollick R, Stein B, Chiller TM, Lockhart SR, Park BJ. 2015. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. PLoS One 10:e0120452. doi: 10.1371/journal.pone.0120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben-Ami R, Olshtain-Pops K, Krieger M, Oren I, Bishara J, Dan M, Wiener-Well Y, Weinberger M, Zimhony O, Chowers M, Weber G, Potasman I, Chazan B, Kassis I, Shalit I, Block C, Keller N, Kontoyiannis DP, Giladi M. 2012. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob Agents Chemother 56:2518–2523. doi: 10.1128/AAC.05947-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rafii F, Sutherland JB, Cerniglia CE. 2008. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther Clin Risk Manag 4:1343–1358. doi: 10.2147/TCRM.S4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gubbels S, Nielsen J, Voldstedlund M, Kristensen B, Schønheyder HC, Vandenbroucke-Grauls CMJE, Arpi M, Björnsdóttir MK, Knudsen JD, Dessau RB, Jensen TG, Kjældgaard P, Lemming L, Møller JK, Hansen DS, Mølbak K. 2015. Utilization of blood cultures in Danish hospitals: a population-based descriptive analysis. Clin Microbiol Infect 21:344.e13–344.e21. doi: 10.1016/j.cmi.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Oeser C, Lamagni T, Heath PT, Sharland M, Ladhani S. 2013. The epidemiology of neonatal and pediatric candidemia in England and Wales, 2000–2009. Pediatr Infect Dis J 32:23–26. doi: 10.1097/INF.0b013e318275612e. [DOI] [PubMed] [Google Scholar]
- 48.Laupland KB. 2005. Invasive Candida species infections: a 5 year population-based assessment. J Antimicrob Chemother 56:532–537. doi: 10.1093/jac/dki258. [DOI] [PubMed] [Google Scholar]
- 49.Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, Mirza SA, Phelan M, Morgan J, Lee-Yang W, Ciblak MA, Benjamin LE, Thomson Sanza L, Huie S, Yeo SF, Brandt ME, Warnock DW. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol 42:1519–1527. doi: 10.1128/JCM.42.4.1519-1527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 50:3435–3442. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horvath LL, George BJ, Murray CK, Harrison LS, Hospenthal DR. 2004. Direct comparison of the BACTEC 9240 and BacT/ALERT 3D automated blood culture systems for Candida growth detection. J Clin Microbiol 42:115–118. doi: 10.1128/JCM.42.1.115-118.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bassetti M, Ansaldi F, Nicolini L, Malfatto E, Molinari MP, Mussap M, Rebesco B, Bobbio Pallavicini F, Icardi G, Viscoli C. 2009. Incidence of candidaemia and relationship with fluconazole use in an intensive care unit. J Antimicrob Chemother 64:625–629. doi: 10.1093/jac/dkp251. [DOI] [PubMed] [Google Scholar]
- 53.Jensen RH, Johansen HK, Søes LM, Lemming LE, Rosenvinge FS, Nielsen L, Olesen B, Kristensen L, Dzajic E, Astvad KMT, Arendrup MC. 2015. Posttreatment antifungal resistance among colonizing Candida isolates in candidemia patients: results from a systematic multicenter study. Antimicrob Agents Chemother 60:1500–1508. doi: 10.1128/AAC.01763-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brillowska-Dabrowska A, Bergmann O, Jensen IM, Jarløv JO, Arendrup MC. 2010. Typing of Candida isolates from patients with invasive infection and concomitant colonization. Scand J Infect Dis 42:109–113. doi: 10.3109/00365540903348336. [DOI] [PubMed] [Google Scholar]
- 55.Saunte DML, Hald M, Lindskov R, Foged EK, Svejgaard EL, Arendrup MC. 2012. Guidelines for superficielle svampeinfektioner. Danish Society of Dermatology. http://dds.nu/wp-content/uploads/2012/08/Guidelines-for-superficielle-svampeinfektioner_version-2.pdf Accessed 17 February 2018.
- 56.Arendrup M, Horn T, Frimodt-Møller N. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30:286–291. doi: 10.1007/s15010-002-2131-0. [DOI] [PubMed] [Google Scholar]
- 57.Marcos-Zambrano LJ, Escribano P, Sánchez-Carrillo C, Bouza E, Guinea J. 2016. Scope and frequency of fluconazole trailing assessed using EUCAST in invasive Candida spp. isolates. Med Mycol 54:733–739. doi: 10.1093/mmy/myw033. [DOI] [PubMed] [Google Scholar]
- 58.Rueda C, Puig-Asensio M, Guinea J, Almirante B, Cuenca-Estrella M, Zaragoza O; CANDIPOP Project from GEIH-GEMICOMED (SEIMC) and REIPI. 2017. Evaluation of the possible influence of trailing and paradoxical effects on the clinical outcome of patients with candidemia. Clin Microbiol Infect 23:49.e1–49.e8. doi: 10.1016/j.cmi.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 59.Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, Derado G, Pham CD, Lockhart SR, Smith RM. 2015. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, 2008–2014. Open Forum Infect Dis 2:ofv163. doi: 10.1093/ofid/ofv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimbeck AJ, Iqbal N, Ahlquist AM, Farley MM, Harrison LH, Chiller T, Lockhart SR. 2010. FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from U.S. population-based surveillance. Antimicrob Agents Chemother 54:5042–5047. doi: 10.1128/AAC.00836-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Healey KR, Zhao Y, Perez WB, Lockhart SR, Sobel JD, Farmakiotis D, Kontoyiannis DP, Sanglard D, Taj-Aldeen SJ, Alexander BD, Jimenez-Ortigosa C, Shor E, Perlin DS. 2016. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat Commun 7:11128. doi: 10.1038/ncomms11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD. 2010. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol 48:2373–2380. doi: 10.1128/JCM.02390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bordallo-Cardona MÁ, Escribano P, de la Pedrosa EG, Marcos-Zambrano LJ, Cantón R, Bouza E, Guinea J. 2017. In vitro exposure to increasing micafungin concentrations easily promotes echinocandin resistance in Candida glabrata isolates. Antimicrob Agents Chemother 61:e01542–16. doi: 10.1128/AAC.01542-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.