ABSTRACT
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has proved to be a useful diagnostic method for identifying conventional bacteria. In the case of mycobacteria, a good protein extraction protocol is essential in order to obtain reliable identification results. To date, no such protocol has been definitively established. The aim of this study was to compare the manufacturer's recommended protein extraction protocol (protocol A) with two novel protocols (protocols B and C), which apply different freezing temperatures and mechanical disruption times using an automatic tissue homogenizer. A total of 302 clinical isolates, comprising 41 nontuberculous mycobacteria (NTM) species, were grown in parallel on solid and liquid media and analyzed: 174 isolates were slow-growing mycobacteria (SGM) and 128 isolates were rapid-growing mycobacteria (RGM). Overall, MALDI-TOF MS identified a higher number of NTM isolates from solid than from liquid media, especially with protocol C (83.4 and 68.2%, respectively; P < 0.05). From solid media, this protein extraction method identified 57.9 and 3.9% more isolates than protocols A (P < 0.001) and B (P < 0.05), respectively. In the case of liquid media, protocol C identified 49.7 and 6.3% more isolates than protocols A and B, respectively (P < 0.001). With regard to the growth rate, MALDI-TOF MS identified more RGM isolates than SGM isolates in all of the protocols studied. In conclusion, the application of freezing and automatic tissue homogenizer improved protein extraction of NTM and boosted identification rates. Consequently, MALDI-TOF MS, which is a cheap and simple method, could be a helpful tool for identifying NTM species in clinical laboratories.
KEYWORDS: identification, MALDI-TOF, mass spectrometry, nontuberculous mycobacteria, protein extraction protocol
INTRODUCTION
Currently, there are 186 recognized species in the Mycobacterium genus (see the list of mycobacterial names with standing in nomenclature [http://www.bacterio.net/mycobacterium.html]). Nontuberculous mycobacteria (NTM) account for most of these species, and their isolation has been increasing over recent years. Moreover, many NTM species have been associated with important human infections in immunocompetent and immunocompromised patients (1–4). The American Thoracic Society and the Infectious Disease Society of America (ATS/IDSA) therefore recommend the identification of NTM isolates to the species level when possible (5). This is important given that treatment for NTM can depend on the species isolated (5, 6).
The identification and differentiation of mycobacteria are complicated and can be carried out using a range of diagnostic techniques. For many years, conventional identification by phenotypic characteristics was used; however, this method was not only slow and laborious (7) but was also unable to distinguish some species from others. Given this poor accuracy, identification now tends to be performed by commercial molecular techniques based on simple and sensitive PCR-reverse hybridization methods. However, these are limited to a small number of species (8), with the remaining species needing to be identified by genetic sequencing of several genes like partial 16S rRNA, hsp65, the internal transcribed spacer (ITS) between 16S and 23S, and rpoB (9–11). Unfortunately, although it provides highly reliable results, sequencing requires expertise and a specialized laboratory.
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has proven to be a rapid, cost-effective, and accurate technique for conventional bacterium and yeast identification (12, 13). Mycobacteria require special processing before analysis by mass spectrometry. First, an inactivation is needed to secure manipulation of the microorganisms. Second, the characteristics of mycobacterial cell walls (high lipid content) mean that protein extraction treatment is necessary, using chemical reagents and mechanical lysis, to achieve high-quality spectra and obtain reliable identification by MALDI-TOF MS (14). Although different approaches to sample processing have been explored, there is no consensus on the best extraction protocol for identifying mycobacteria. Moreover, other possibilities remain unconsidered, such as the application of a freezing-thawing step in the protein extraction protocol. It has also been observed that the analysis of mycobacteria grown on different culture media (liquid or solid) can affect the rates of identification by MALDI-TOF MS, and this also warrants further consideration (15–17).
The aim of this study was to evaluate and compare two protein extraction methods based on freezing-thawing, in comparison with the manufacturer's protocol, for identifying NTM from liquid and solid cultures by MALDI-TOF MS.
MATERIALS AND METHODS
Mycobacterial strains.
A total of 302 clinical isolates, obtained from different patients and covering 41 NTM species, were analyzed in the Department of Microbiology of the Hospital Universitari de Bellvitge-IDIBELL (Barcelona, Spain). The species included were as follows: 174 slow-growing mycobacteria (SGM) encompassing 20 different species and 128 rapid-growing mycobacteria (RGM) covering 21 species (Table 1).
TABLE 1.
Comparison of species identifications by MALDI-TOF MS, GenoType, and sequencinga
| Mycobacterium type (no. of isolates [n = 302]) | Result |
||
|---|---|---|---|
| GenoType Mycobacterium CM/AS | MALDI-TOF MS | Sequencing | |
| Slow-growing mycobacteria | |||
| 9 | Mycobacterium sp. | M. arupense | M. arupense |
| 30 | M. avium | M. avium | NA |
| 2 | M. celatum | M. celatum | NA |
| 11 | M. intracellulare | M. chimaera | M. chimaera |
| 2 | M. intracellulare | M. colombiense | M. colombiense |
| 1 | Mycobacterium sp. | M. conspicuum | M. conspicuum |
| 23 | M. gordonae | M. gordonae | NA |
| 1 | Mycobacterium sp. | NI | M. heraklionense |
| 1 | M. interjectum | NI | NA |
| 32 | M. intracellulare | M. intracellulare | NA |
| 20 | M. kansasii | M. kansasii | NA |
| 3 | Mycobacterium sp. | M. kumamotonense | M. kumamotonense |
| 1 | M. lentiflavum | M. lentiflavum | NA |
| 10 | M. marinum | M. marinum | NA |
| 1 | Mycobacterium sp. | NI | M. paraterrae |
| 1 | M. scrofulaceum | NI | NA |
| 1 | M. shimoidei | NI | NA |
| 3 | M. szulgai | M. szulgai | NA |
| 21 | M. xenopi | M. xenopi | NA |
| 1 | Mycobacterium sp. | NI | M. yongonense |
| Rapid-growing mycobacteria | |||
| 14 | M. abscessus | M. abscessus | NA |
| 1 | Mycobacterium sp. | M. algericum | M. algericum |
| 3 | M. mucogenicum | M. aubagnense | M. aubagnense |
| 2 | Mycobacterium sp. | M. canariasense | M. canariasense |
| 36 | M. chelonae | M. chelonae | NA |
| 1 | M. fortuitum | M. conceptionense, M. senegalense | M. conceptionense |
| 2 | Mycobacterium sp. | M. elephantis | M. elephantis |
| 19 | M. fortuitum | M. fortuitum | NA |
| 1 | Mycobacterium sp. | NI | M. frederiksbergense |
| 1 | M. goodii | M. goodii | NA |
| 1 | Mycobacterium sp. | NI | M. madagascariense |
| 13 | M. fortuitum | M. mageritense | M. mageritense |
| 14 | M. mucogenicum | M. mucogenicum | NA |
| 6 | M. peregrinum | M. peregrinum | NA |
| 1 | M. phlei | M. phlei | NA |
| 5 | M. fortuitum | M. porcinum | M. porcinum |
| 1 | M. peregrinum | M. septicum | M. septicum |
| 3 | M. peregrinum | M. setense | M. setense |
| 1 | M. smegmatis | M. smegmatis | NA |
| 2 | Mycobacterium sp. | M. thermoresistibile | M. thermoresistibile |
| 1 | M. fortuitum | M. wolinskyi | M. wolinskyi |
NA, not applicable; NI, not identified.
Growth conditions.
All strains were cultured in liquid medium (MGIT; Becton Dickinson, Towson, MD) and solid medium (Löwenstein-Jensen; bioMérieux, Marcy l'Etoile, France). The liquid medium was incubated at 37°C in the Bactec MGIT 960 system (Becton Dickinson) and analyzed between 3 and 5 days after positivity. The solid medium was incubated at 37°C in a 7.5% CO2 atmosphere, except for RGM and M. marinum (incubated at 30°C) and M. xenopi (incubated at 42°C).
PCR-reverse hybridization.
All clinical isolates were identified by PCR-reverse hybridization using the commercial system GenoType Mycobacterium CM/AS (HAIN Lifescience, Nehren, Germany). This assay consisted of multiplex targeting species-specific DNA regions (23S rRNA) and reverse hybridization with oligonucleotide probes immobilized on a nitrocellulose strip. The GenoType Mycobacterium CM permits simultaneous molecular genetic identification of the Mycobacterium tuberculosis complex and 13 of the most common NTM species, while the GenoType Mycobacterium AS identifies 16 additional NTM species. The assay was performed as recommended by the manufacturer's instructions.
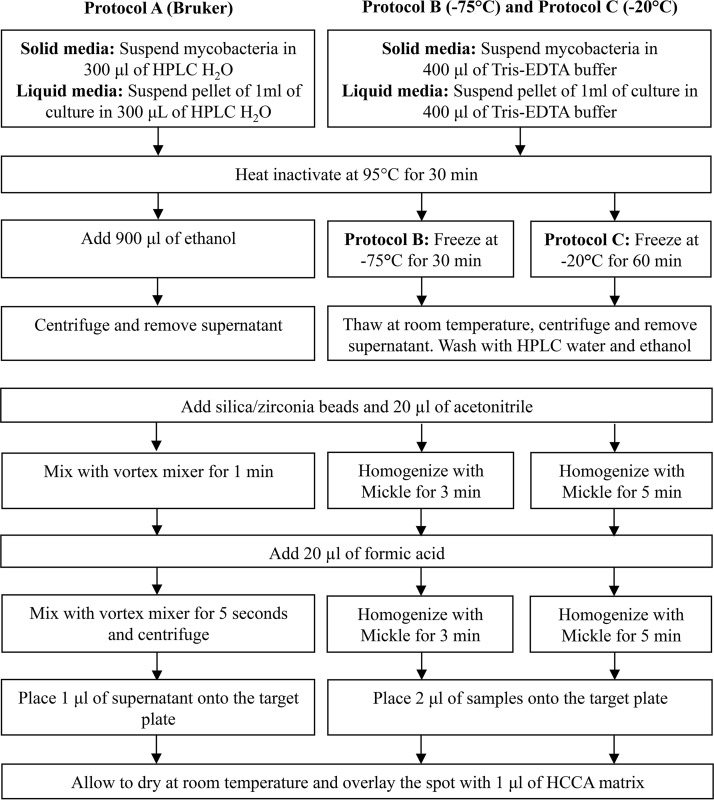
Protein extraction protocols. (i) Protocol A (Bruker MycoEx v3.0).
The first MALDI-TOF MS protocol for identifying NTM used the ethanol-formic acid extraction method recommended by the manufacturer (MycoEx v3.0) (Fig. 1). Briefly, for the liquid medium, 1 ml was centrifuged at 13,000 rpm for 2 min, the supernatant was removed, and the pellet was resuspended in 300 μl of water for high-performance liquid chromatography (HPLC). For the solid medium, several colonies were harvested and suspended in 300 μl of HPLC water and vortex mixed for a short time. In both cases, the samples were inactivated at 95°C for 30 min. After that step, 900 μl of absolute ethanol was added into the tube, followed by mixing and centrifugation at 13,000 rpm for 2 min, before the supernatant was discarded. The residual ethanol was evaporated at room temperature. The tip of a small spatula of silica/zirconia beads (0.5-mm-diameter beads; BioSpec Products, Bartlesville, OK) was added with 20 μl of acetonitrile (Sigma-Aldrich, St. Louis, MO) and intensively vortex mixed for 1 min. Then, 20 μl of 70% (vol/vol) formic acid (Sigma-Aldrich) was added, and the samples were vortex mixed for 5 s and finally centrifuged at 13,000 rpm for 2 min. The overall time for this procedure was approximately 1 h.
FIG 1.
Flow chart of the three protein extraction protocols tested. HCCA, α-cyano-4-hydroxycinnamic acid; HPLC, high-performance liquid chromatography.
(ii) Protocol B.
We used a freezing-based protein extraction protocol (Fig. 1). For liquid medium, 1 ml was centrifuged at 13,000 rpm for 2 min, and the supernatant was discarded; later, 400 μl of Tris-EDTA buffer was added. For solid medium, several colonies were harvested and suspended in 400 μl of Tris-EDTA buffer and briefly vortex mixed. The samples from both media were inactivated at 95°C for 30 min. Protein extraction was performed by freezing samples at −75°C for 30 min. The samples were then thawed at room temperature and centrifuged at 13,000 rpm for 2 min, and the supernatant was discarded. The next step was to add 800 μl of HPLC water and to repeat centrifugation at 13,000 rpm for 2 min, again discarding the supernatant. Then, 800 μl of absolute ethanol was added to the tube and centrifuged at 13,000 rpm for 2 min, discarding the supernatant. The residual ethanol was evaporated at room temperature. The tip of a small spatula of 0.5-mm-diameter silica/zirconia beads was added with 20 μl of acetonitrile. The mycobacterial cells were mechanically disrupted in a Mickle tissue disintegrator (Cavey Laboratory Engineering Co., Ltd., Gomshall, United Kingdom) for 3 min. Finally, 20 μl of 70% formic acid was added, and the samples were homogenized for 3 min by the Mickle disintegrator. The overall time for this procedure was approximately 90 min.
(iii) Protocol C.
The second freezing-based protein extraction method (Fig. 1) followed the same procedure used for protocol B, but with two changes: the samples were frozen at −20°C for 60 min and homogenized with the Mickle tissue disintegrator for 5 min. The overall time for this procedure was approximately 2 h.
MALDI-TOF MS analysis.
After protein extraction, we deposited 1 μl of protocol A samples and 2 μl of protocol B and protocol C samples by duplicate on the MALDI-TOF MS target plate (Bruker Daltonics, Bremen, Germany) and allowed them to air dry at room temperature. Then, 1 μl of the matrix solution, comprising a saturated solution of α-cyano-4-hydroxycinnamic acid (HCCA; Bruker Daltonics) in 50% acetonitrile and 2.5% trifluoroacetic acid (Sigma-Aldrich), was added to each spot on the MALDI-TOF MS target plate. The plates were again allowed to dry at room temperature. The Bacterial Test Standard (Escherichia coli extract; Bruker Daltonics) was used for equipment calibration and as a positive control before each analysis. The target plate was inserted into the MALDI-TOF microflex LT (Bruker Daltonics), and spectra were obtained over a mass/charge (m/z) ratio of 2,000 to 20,000 Da using FlexControl v3.0 software, which included the Mycobacteria Library v3.0 database. The accelerating voltage of extracted ions was 20 kV. The spots were measured in automatic mode using groups of 40 nitrogen laser shots at 337 nm and 60 Hz, with a total of 240 laser shots collected per spot. The software assigned a score from 0 to 3 and classified results into three categories: reliable (species level; ≥2), probable (genus level; 1.7 to 1.9), and nonidentifiable (<1.7). The acceptable cutoff used for identification of specimens by MALDI-TOF MS was a score of ≥1.7.
Sequencing.
Partial sequencing of the 16S rRNA was performed in cases of discrepancy between the GenoType Mycobacterium CM/AS and MALDI-TOF MS results. When this test was unreliable, we performed additional sequencing of hsp65 or ITS genes. A sequence similarity of ≥99% with the database was used as the final identification.
Statistical analysis.
The differences in NTM identification rates for all protein extraction protocols in both culture media were analyzed using the two-tailed McNemar test for paired samples. The two growth rate groups were compared by chi-square test. A P value below 0.05 was considered statistically significant for all analyses.
RESULTS
Comparison of the protein extraction protocols for NTM identification by MALDI-TOF MS.
When protocol C was applied to the solid medium, protocol C identified 252 (83.4%) isolates (57.9% more mycobacterial isolates than protocol A [P < 0.001] and 3.9% more than protocol B [P > 0.05]). In liquid medium, protocol C identified 206 (68.2%) isolates (49.7% more mycobacterial isolates than protocol A [P < 0.001] and 6.3% more than protocol B [P < 0.001]; Table 2).
TABLE 2.
MALDI-TOF MS results for each culture medium and protein extraction protocols A, B, and C based on the score cutoff used (≥1.7)
| Species group (n) and culture mediuma | Protocol A |
Protocol B |
Protocol C |
CIb (P) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1.7 | ≥1.7 | ≥1.7 (CI) | <1.7 | ≥1.7 | ≥1.7 (CI) | <1.7 | ≥1.7 | ≥1.7 (CI) | A vs B | A vs C | B vs C | |
| RGM (128) | ||||||||||||
| LIQ (%) | 93 (72.7) | 35 (27.3) | 33 (25.8) | 30 (23.4) | 98 (76.6) | 93 (72.6) | 24 (18.8) | 104 (81.2) | 101 (78.9) | <0.001 | <0.001 | 0.1814 |
| SOL (%) | 85 (66.4) | 43 (33.6) | 42 (32.8) | 22 (17.2) | 106 (82.8) | 106 (82.8) | 15 (11.7) | 113 (88.3) | 113 (88.3) | <0.001 | <0.001 | 0.2109 |
| SGM (174) | ||||||||||||
| LIQ (%) | 153 (87.9) | 21 (12.1) | 21 (12.1) | 85 (48.9) | 89 (51.1) | 89 (51.1) | 72 (41.4) | 102 (58.6) | 102 (58.6) | <0.001 | <0.001 | <0.001 |
| SOL (%) | 140 (80.5) | 34 (19.5) | 34 (19.5) | 40 (23) | 134 (77) | 134 (77) | 35 (20.1) | 139 (79.9) | 139 (79.9) | <0.001 | <0.001 | 0.4990 |
| Total (302) | ||||||||||||
| LIQ (%) | 246 (81.5) | 56 (18.5) | 54 (17.9) | 115 (38.1) | 187 (61.9) | 182 (60.3) | 96 (31.8) | 206 (68.2) | 203 (67.2) | <0.001 | <0.001 | 0.013 |
| SOL (%) | 225 (74.5) | 77 (25.5) | 76 (25.2) | 62 (20.5) | 240 (79.5) | 240 (79.5) | 50 (16.6) | 252 (83.4) | 252 (83.4) | <0.001 | <0.001 | 0.148 |
RGM, rapid-growing mycobacteria; SGM, slow-growing mycobacteria; LIQ, liquid medium; SOL, solid medium.
CI, correctly identified according to molecular methods (GenoType and/or sequencing).
Results based on culture media and growth rate group.
The mean number of isolates identified by MALDI-TOF MS (score ≥ 1.7) for the three protocols was 150 (49.7%) for liquid medium and 190 (62.9%) for solid medium (P < 0.05; Table 2). By growth rate groups of NTM, the mean numbers of isolates identified by mass spectrometry (score ≥ 1.7) were 83/128 (64.8%) for RGM and 87/174 (50%) for SGM (P < 0.05; Table 2).
Identification discordances between MALDI-TOF MS and GenoType results.
Of the 41 species tested, 20 (48.8%) were identified by PCR-reverse hybridization and 38 (92.7%) by MALDI-TOF MS. From the 38 species, five (13.2%) were assigned to the correct species but had a score of <1.7. Some isolates only identified as Mycobacterium genus by GenoType were correctly identified by MALDI-TOF MS to the species level. This was the case for M. algericum, M. arupense, M. canariasense, M. elephantis, M. kumamotonense, and M. thermoresistibile. On the other hand, several species were classified by GenoType inside a group, while MALDI-TOF MS identified them correctly to the species level. This applied to M. mageritense, M. conceptionense, M. porcinum, and M. wolinskyi, which were identified as M. fortuitum by GenoType. Another example was that of M. septicum and M. setense, which were classified as M. peregrinum by this PCR-reverse hybridization system. In the same way, M. colombiense and M. chimaera, which were correctly identified by MALDI-TOF MS, were identified as M. intracellulare by GenoType. Similarly, M. aubagnense was classified as M. mucogenicum by this technique.
Conversely, several species were not identified by either GenoType or MALDI-TOF MS, including M. frederiksbergense, M. heraklionense, M. madagascariense, M. paraterrae, and M. yongonense. Final identification of these strains was confirmed by sequencing. Among the isolates analyzed by MALDI-TOF MS, a total of six from the 302 isolates presented a misidentification in at least one of the three protein extraction protocols (Table 3).
TABLE 3.
Identification discordances obtained by MALDI-TOF MS between different culture media and protein extraction protocols
| Isolatea | Medium | Identification (score) |
||
|---|---|---|---|---|
| Protocol A | Protocol B | Protocol C | ||
| M. setense | Solid | M. porcinum (1.866) | M. setense (1.668) | M. setense (1.912) |
| M. mageritense | Liquid | M. mageritense (1.783) | M. porcinum (1.955) | M. mageritense (2.076) |
| M. conceptionense | Liquid | M. conceptionense (1.647) | M. senegalense (2.179) | M. senegalense (2.237) |
| M. setense | Liquid | NPb | M. porcinum (1.718) | M. porcinum (1.766) |
| M. setense | Liquid | M. porcinum (1.790) | M. peregrinum (1.902) | M. setense (1.775) |
| M. setense | Liquid | M. porcinum (1.797) | M. peregrinum (1.916) | M. porcinum (1.816) |
NTM isolates identified by reference method (sequencing).
NP, no peaks found.
DISCUSSION
The implementation of MALDI-TOF MS in clinical microbiology laboratories has markedly improved bacterial identification by increasing diagnostic accuracy, increasing the speed of diagnosis, and reducing costs. This system proved quite robust for the identification of mycobacteria from solid media. In contrast, identification in liquid media remains a challenge due to the low concentration of biomass; the continued improvement and optimization of protein extraction protocols are necessary in order to obtain better-quality spectra. The two novel extraction methods evaluated (protocol B and protocol C) identified more isolates compared to the standard procedure (protocol A; Table 2). This can be explained by two main differences in critical steps. First, the freezing of samples suggests that cold application can break the cellular wall without damaging proteins, thereby ensuring that suitable profiles are obtained. Previous reports also support this approach, indicating that freezing of processed samples did not reduce the quality of the obtained spectra (18). Second, the use of an automatic tissue homogenizer could have led to a more efficient rupture of the mycobacterial cell wall than could be achieved by vortex mixing (16, 19); in addition, using silica/zirconia beads may have facilitated the mechanical disruption of clumps (14). Instead of the application of the automatic tissue homogenizer or only the vortex mixing, other studies have evaluated the use of sonication for cell disruption and have reported reliable results (20, 21). It is important to note that protocol C was better than protocol B, suggesting that a longer freezing time and a longer mechanical disruption time may both improve protein extraction.
In this study, the number of isolates identified by MALDI-TOF MS was higher in solid medium than in liquid medium, with statistically significant differences among the three protein extraction methods. This is consistent with previous reports (15, 22, 23) in which these differences were attributed to spectral acquisition failure by low concentrations of available biomass from the liquid medium. This is probably because liquid-culture systems are more sensitive than solid culture to the detection of fewer microorganisms at the time of positivity. In contrast, other studies have reported no significant differences by medium type (16, 24), which could be explained because analyses were performed several days after culture positivity in some isolates. Thus, an extended incubation might increase the mycobacterial biomass and therefore the sensitivity of MALDI-TOF MS. More RGM than SGM strains were also identified by mass spectrometry in this study, an observation consistent with previous research (25).
There were some discrepancies in species identification between MALDI-TOF MS and PCR-reverse hybridization (GenoType; Table 1), and these can be attributed to various factors. First, some species were not included in the test probes of GenoType (M. algericum, M. arupense, M. canariasense, M. conspicuum, M. elephantis, M. frederiksbergense, M. heraklionense, M. kumamotonense, and M. thermoresistibile), while they were included in the MALDI-TOF MS database. Second, other species were identified inside a group by GenoType because they are phylogenetically too closely related to be discriminated by this method; this includes the M. fortuitum complex (which includes M. mageritense, M. conceptionense, M. porcinum, M. peregrinum, M. septicum and M. setense), the M. mucogenicum group (including M. aubagnense), and MAC (M. avium complex) species related to M. intracellulare (including M. chimaera and M. colombiense). Although M. wolinskyi was identified as M. fortuitum by GenoType, it actually pertains to M. smegmatis group.
The nonidentifiable results (score < 1.7) obtained for some isolates by MALDI-TOF MS can be explained by the low concentration of mycobacteria in cultures or by the loss of material during the washing steps of the protein extraction protocol. Therefore, at the end of the process, the protein concentration will have been too low for detection by mass spectrometry. Another reason is that some species are not included in the current Mycobacteria Library database, such as M. madagascariense, M. paraterrae, and M. yongonense, which precluded identification by MALDI-TOF MS (26). In addition, some species have only one reference or a small number of references in the database, which may have led to low scores when analyzed.
Despite the high accuracy of MALDI-TOF MS for identifying mycobacteria with a cutoff score of ≥1.7, there were a few misidentifications in this study (Table 3). The misclassification of one M. mageritense strain as M. porcinum by protocol B (liquid medium) can be explained by the phylogenetically close relationship between the species (27). The same explanation accounts for the misidentification of some M. setense strains (three from liquid medium and one from solid medium) as M. porcinum and M. peregrinum (28). These were classified as probable (score, 1.7 to 1.9) by MALDI-TOF MS. However, M. conceptionense was classified (score, ≥2) as M. senegalense by MALDI-TOF MS because it is more closely related (29). Interestingly, M. setense and M. conceptionense species had only one reference in the Mycobacteria Library (version v3.0), suggesting that the addition of more references in an upcoming database could allow for higher scores and could prevent misidentifications. With all of these findings, a cutoff score of ≥1.7 may be acceptable for most species studied here, with the exception of some species related to the M. fortuitum group, such as M. setense and M. conceptionense, in which it might generate confusing results.
In summary, the results observed in this study suggest that freezing and mechanical disruption of samples are essential for improving protein extraction from mycobacteria and yield better identification results with MALDI-TOF MS. Therefore, this simple, reliable, and inexpensive technique could be used to help identify many NTM species in clinical microbiology laboratories.
REFERENCES
- 1.Prevots DR, Marras TK. 2015. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falkinham JO., III 2016. Current epidemiologic trends of the nontuberculous mycobacteria (NTM). Curr Environ Health Rep 3:161–167. doi: 10.1007/s40572-016-0086-z. [DOI] [PubMed] [Google Scholar]
- 3.Adjemian J, Frankland TB, Daida YG, Honda JR, Olivier KN, Zelazny A, Honda S, Prevots DR. 2017. Epidemiology of nontuberculous mycobacterial lung disease and tuberculosis, Hawaii, USA. Emerg Infect Dis 23:439–447. doi: 10.3201/eid2303.161827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcaide F, Peña MJ, Pérez-Risco D, Camprubi D, Gonzalez-Luquero L, Grijota-Camino MD, Dorca J, Santin M. 2017. Increasing isolation of rapidly growing mycobacteria in a low-incidence setting of environmental mycobacteria, 1994-2015. Eur J Clin Microbiol Infect Dis 36:1425–1432. doi: 10.1007/s10096-017-2949-0. [DOI] [PubMed] [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 6.Esteban J, García-Pedrazuela M, Muñoz-Egea MC, Alcaide F. 2012. Current treatment of nontuberculous mycobacteriosis: an update. Expert Opin Pharmacother 13:967–986. doi: 10.1517/14656566.2012.677824. [DOI] [PubMed] [Google Scholar]
- 7.Sinner P, Stenger S, Richter E, Brown-Elliott B, Wallace R, Wengenack N. 2015. Mycobacterium: Laboratory Characteristics of Slowly Growing Mycobacteria, p 570–594. In Jorgensen J, Pfaller M, Carroll K, Funke G, Landry M, Richter S, Warnock D (ed), Manual of clinical microbiology, 11th ed ASM Press, Washington, DC. [Google Scholar]
- 8.Makinen J, Marjamaki M, Marttila H, Soini H. 2006. Evaluation of a novel strip test, GenoType Mycobacterium CM/AS, for species identification of mycobacterial cultures. Clin Microbiol Infect 12:481–483. doi: 10.1111/j.1469-0691.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- 9.Ringuet H, Akoua-Koffi C, Honore S, Varnerot A, Vincent V, Berche P, Gaillard JL, Pierre-Audigier C. 1999. hsp65 sequencing for identification of rapidly growing mycobacteria. J Clin Microbiol 37:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth A, Fischer M, Hamid ME, Michalke S, Ludwing W, Mauch H. 1998. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J Clin Microbiol 36:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BJ, Lee SH, Lyu MA, Kim SJ, Bai GH, Chae GT, Kim EC, Cha CY, Kook YH. 1999. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J Clin Microbiol 37:1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel R. 2015. MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem 61:100–111. doi: 10.1373/clinchem.2014.221770. [DOI] [PubMed] [Google Scholar]
- 13.Singhal N, Kumar M, Kanaujia PK, Virdi JS. 2015. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleeb PG, Drake SK, Murray PR, Zelazny AM. 2011. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 49:1790–1794. doi: 10.1128/JCM.02135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinlan P, Phelan E, Doyle M. 2015. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF MS) mass spectrometry (MS) for the identification of mycobacteria from MBBacT ALERT 3D liquid cultures and Lowenstein-Jensen (LJ) solid cultures. J Clin Pathol 68:229–235. doi: 10.1136/jclinpath-2014-202374. [DOI] [PubMed] [Google Scholar]
- 16.Tudó G, Monté MR, Vergara A, López A, Hurtado JC, Ferrer-Navarro M, Vila J, Gonzalez-Martin J. 2015. Implementation of MALDI-TOF MS technology for the identification of clinical isolates of Mycobacterium spp. in mycobacterial diagnosis. Eur J Clin Microbiol Infect Dis 34:1527–1532. doi: 10.1007/s10096-015-2381-2. [DOI] [PubMed] [Google Scholar]
- 17.Balada-Llasat JM, Kamnoj K, Pancholi P. 2013. Identification of mycobacteria from solid and liquid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry in the clinical laboratory. J Clin Microbiol 51:2875–2879. doi: 10.1128/JCM.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunne WM Jr, Doing K, Miller E, Miller E, Moreno E, Baghli M, Mailler S, Girard V, Van Belkum A, Deol P. 2014. Rapid inactivation of Mycobacterium and Nocardia species before identification using matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 52:3654–3659. doi: 10.1128/JCM.01728-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Totty H, Miller E, Moreno E, Dunne WM Jr, Deol P. 2016. Comparison of mechanical disruption techniques for rapid inactivation of Mycobacterium and Nocardia species before identification using matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. J Clin Microbiol 54:2626–2627. doi: 10.1128/JCM.01096-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor JA, Lynch-Healy M, Corcoran D, O'Reilly B, O'Mahony J, Lucey B. 2016. Improved matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based identification of Mycobacterium spp. by use of a novel two-step cell disruption preparatory technique. J Clin Microbiol 54:495–496. doi: 10.1128/JCM.02998-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon M, Alcaide F, Amlerová J, Bou-Arévalo G, Coll P, Corcoran D, Fangous SM, Gorton R, González-Álvarez I, Hrabák J, Hery-Arnaud G, Ingebretsen A, Lucey B, Marekoviċ I, O'Connor J, O'Mahony J, O'Reilly B, Orth-Hoeller D, Oviaño-García M, Palacios J, Pranada A, Rodríguez-Temporal D, Ruiz-Serrano M, Seagar L, Tudó G, Van den Bossche A, Rodríguez-Sánchez B. 2017. Multicenter study on the improved identification of non-tuberculous mycobacteria with MALDI-TOF MS using three different preprocessing protocols, abstr P-0085. Abstr 27th Eur Congr Clin Microbiol Infect Dis. [Google Scholar]
- 22.van Eck K, Faro D, Watternberg M, de Jong A, Kuipers S, van Ingen J. 2016. Matrix-assisted laser desorption ionization-time of flight mass spectrometry fails to identify nontuberculous mycobacteria from primary cultures of respiratory samples. J Clin Microbiol 54:1915–1917. doi: 10.1128/JCM.00304-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotz A, Ferroni A, Beretti JL, Dauphin B, Carbonnelle E, Veziris N, Heym B, Jarlier V, Gaillard JL, Pierre-Audigier C, Frapy E, Berche P, Nassif X, Bille E. 2010. Rapid identification of mycobacterial whole cell in solid and liquid culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 48:4481–4486. doi: 10.1128/JCM.01397-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerhrmann J, Schoerding AK, Murali R, Wessel S, Koehling HL, Mosel F, Buer J. 2016. Performance of Vitek MS in identifying nontuberculous mycobacteria from MGIT liquid medium and Lowenstein-Jensen solid medium. Diagn Microbiol Infect Dis 84:43–47. doi: 10.1016/j.diagmicrobio.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Sánchez B, Ruiz-Serrano MJ, Marín M, López-Roa P, Rodríguez-Créixems M, Bouza E. 2015. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of nontuberculous mycobacteria from clinical isolates. J Clin Microbiol 53:2737–2740. doi: 10.1128/JCM.01380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Temporal D, Perez-Risco D, Struzka EA, Mas M, Alcaide F. 2017. Impact of updating the MALDI-TOF MS database on the identification of nontuberculous mycobacteria. J Mass Spectrom 52:597–602. doi: 10.1002/jms.3944. [DOI] [PubMed] [Google Scholar]
- 27.Domenech P, Jimenez MS, Menendez MC, Bull TJ, Samper S, Manrique A, Garcia MJ. 1997. Mycobacterium mageritense sp. nov. Int J Syst Bacteriol 47:535–540. doi: 10.1099/00207713-47-2-535. [DOI] [PubMed] [Google Scholar]
- 28.Lamy B, Marchandin H, Hamitouche K, Laurent F. 2008. Mycobacterium setense sp. nov., a Mycobacterium fortuitum-group organism isolated from a patient with soft tissue infection and osteitis. Int J Syst Evol Microbiol 58:486–490. doi: 10.1099/ijs.0.65222-0. [DOI] [PubMed] [Google Scholar]
- 29.Adékambi T, Stein A, Carvajal J, Raoult D, Drancourt M. 2006. Description of Mycobacterium conceptionense sp. nov., a Mycobacterium fortuitum group organism isolated from a posttraumatic osteitis inflammation. J Clin Microbiol 44:1268–1273. doi: 10.1128/JCM.44.4.1268-1273.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]